Synthesis and Characterization of Fluorite-Type La2Ce2O7 Plasma Sprayable Powder for TBCs Application
Abstract
:1. Introduction
2. Materials and Methods
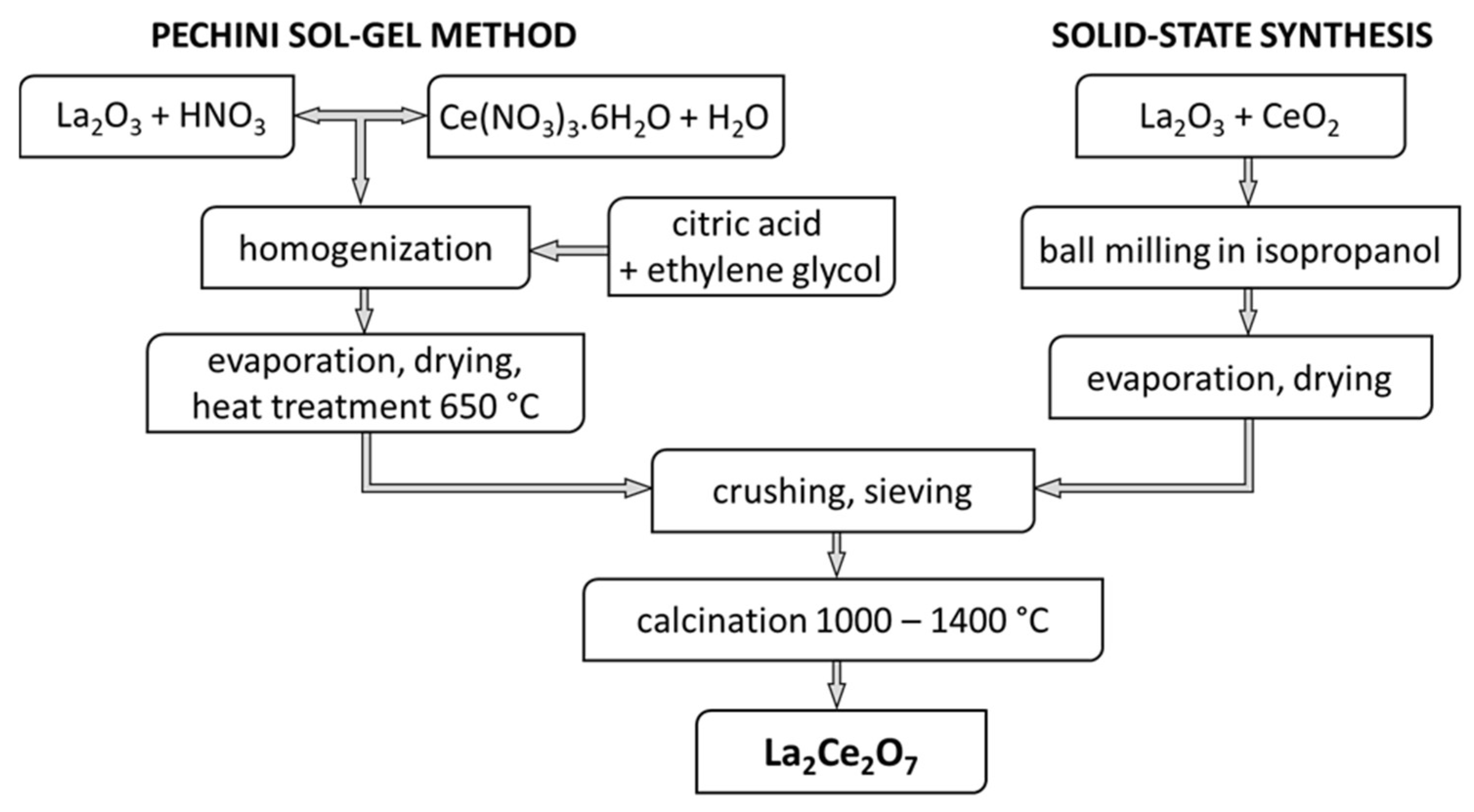
2.1. Powder Synthesis
2.2. Characterization Methods
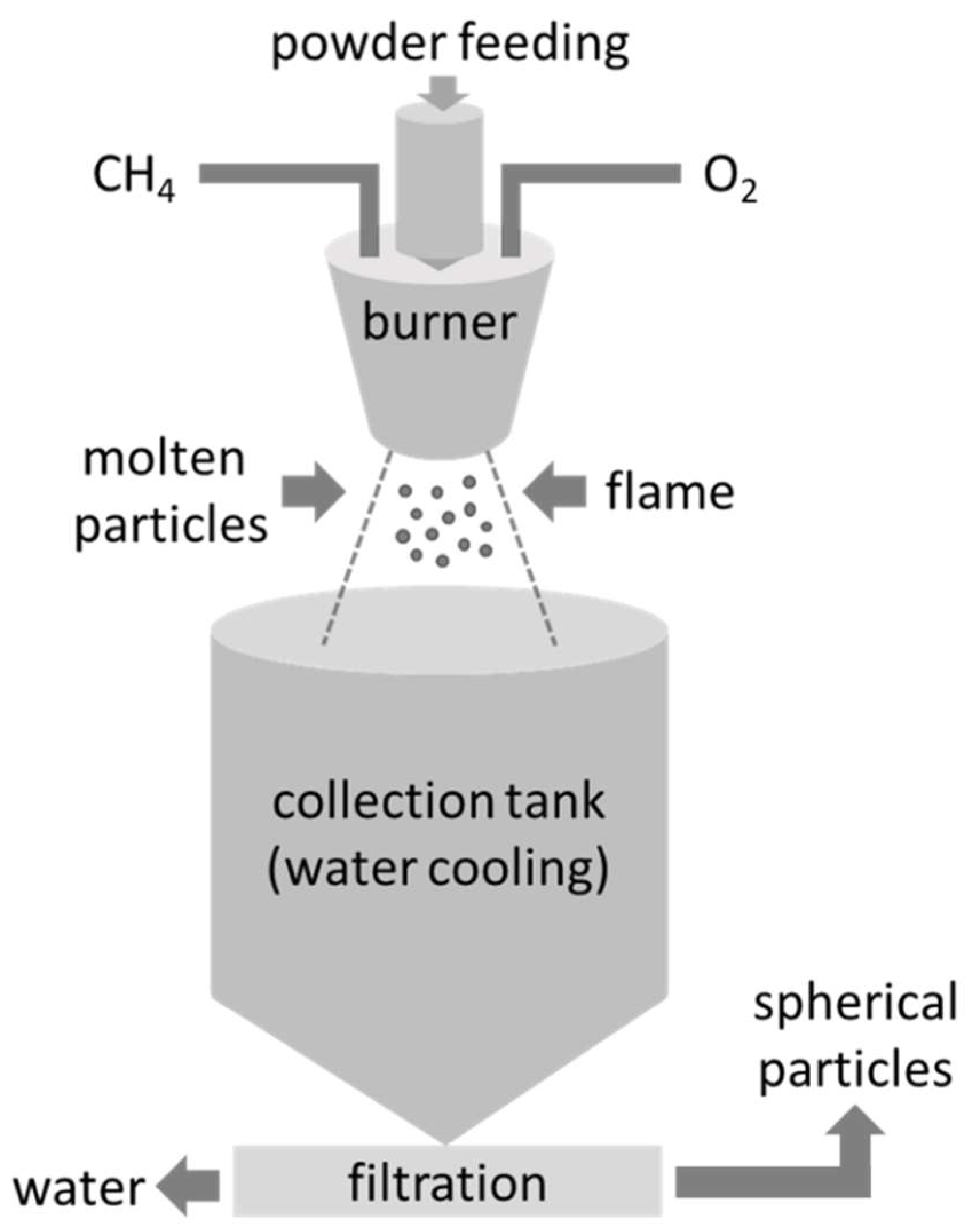
2.3. Spheroidization of LC Powder
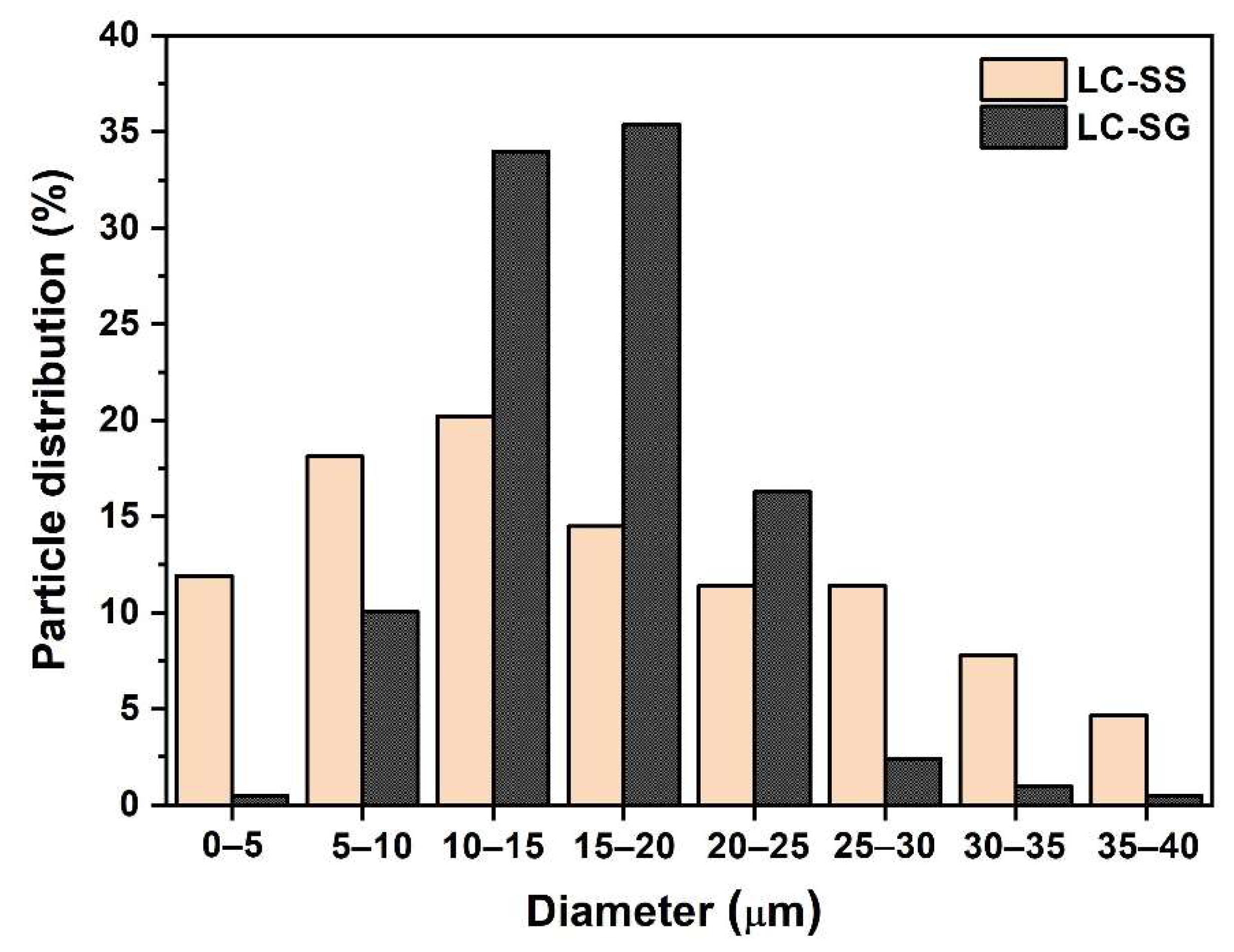
3. Results and Discussion
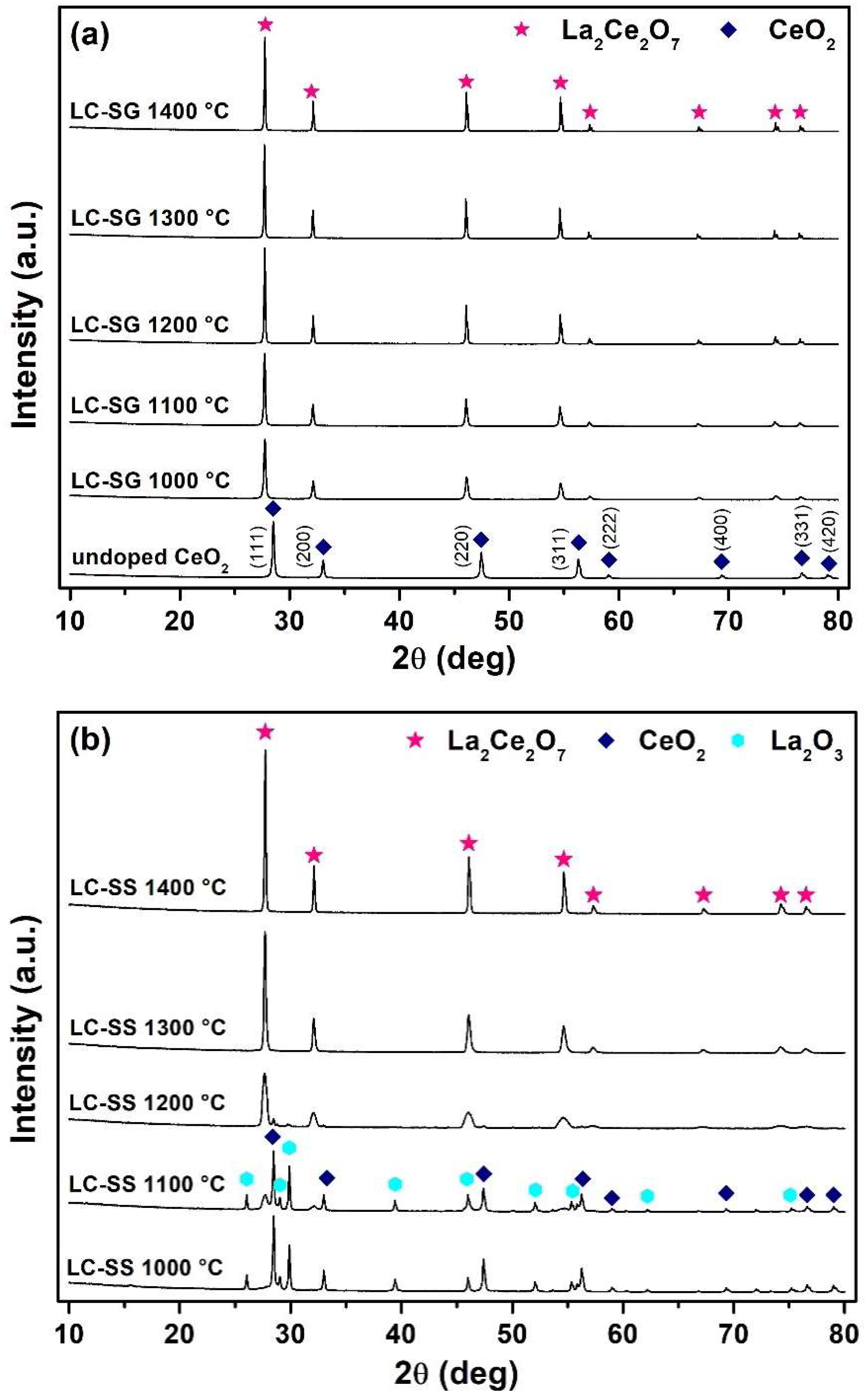
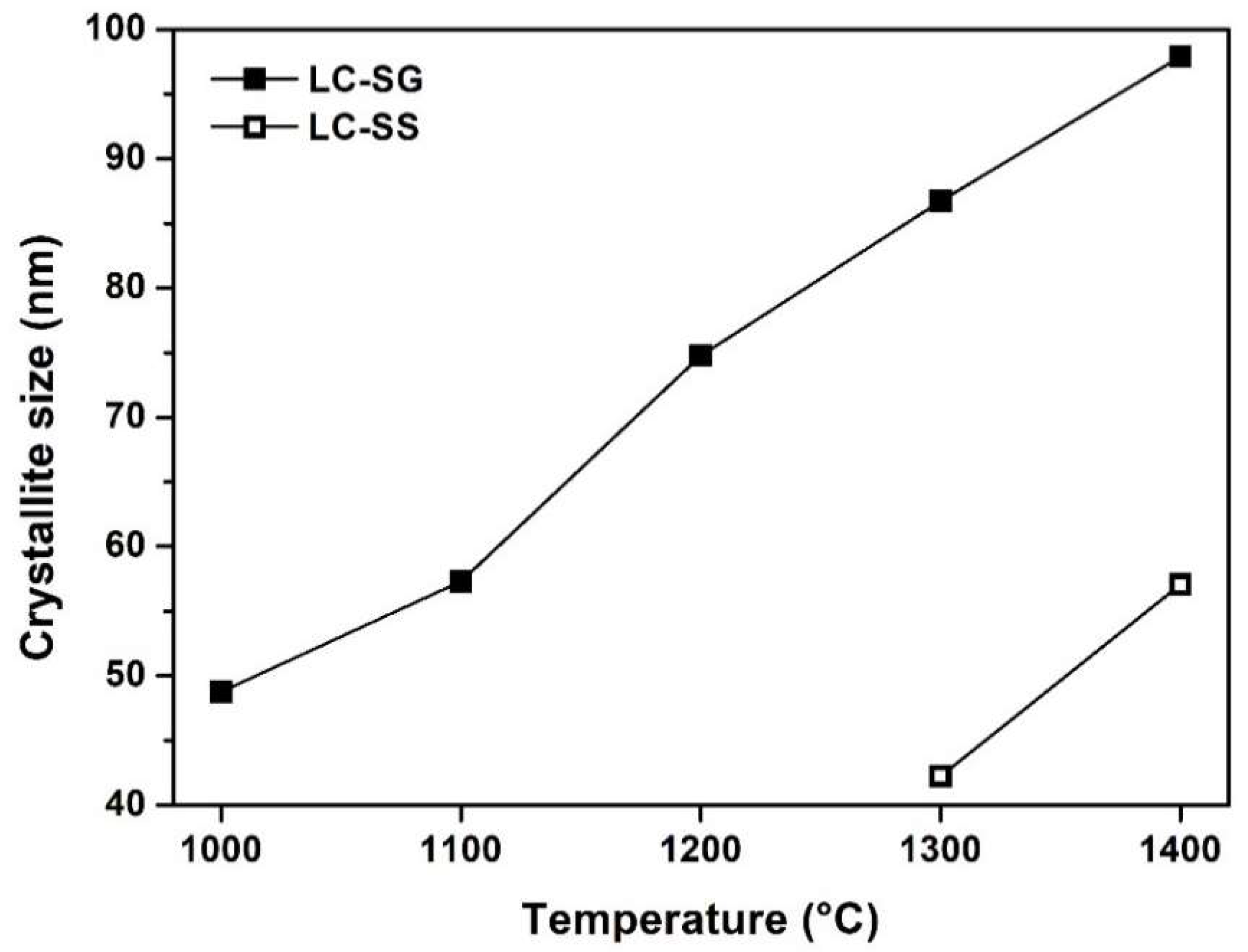
3.1. Characterization of Prepared Powders
3.2. Spheroidization of LC Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tinwala, H.; Shah, D.V.; Menghani, J.; Pati, R. Synthesis of La2Ce2O7 nanoparticles by co-precipitation method and its characterization. J. Nanosci. Nanotechnol. 2014, 14, 6072–6076. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, H.; Gong, S. Thermal shock resistance and mechanical properties of La2Ce2O7 thermal barrier coatings with segmented structure. Ceram. Int. 2009, 35, 2639–2644. [Google Scholar] [CrossRef]
- Sung Bae, J.; Kil Choo, W.; Hee Lee, C. The crystal structure of ionic conductor LaxCe1−xO2−x/2. J. Eur. Ceram. Soc. 2004, 24, 1291–1294. [Google Scholar] [CrossRef]
- Trovarelli, A.; de Leitenburg, C.; Boaro, M.; Dolcetti, G. The utilization of ceria in industrial catalysis. Catal. Today 1999, 50, 353–367. [Google Scholar] [CrossRef]
- Zhu, Z.; Yan, L.; Liu, H.; Sun, W.; Zhang, Q.; Liu, W. A mixed electronic and protonic conducting hydrogen separation membrane with asymmetric structure. Int. J. Hydrogen Energy 2012, 37, 12708–12713. [Google Scholar] [CrossRef]
- Cao, X.; Vassen, R.; Fischer, W.; Tietz, F.; Jungen, W.; Stöver, D. Lanthanum-cerium oxide as a thermal barrier-coating material for high-temperature applications. Adv. Mater. 2003, 15, 1438–1442. [Google Scholar] [CrossRef]
- Kang, Y.X.; Bai, Y.; Fan, W.; Yuan, T.; Gao, Y.; Bao, C.G.; Li, B.Q. Thermal cycling performance of La2Ce2O7/50 vol.% YSZ composite thermal barrier coating with CMAS corrosion. J. Eur. Ceram. Soc. 2018, 38, 2851–2862. [Google Scholar] [CrossRef]
- Ma, W.; Gong, S.; Xu, H.; Cao, X. The thermal cycling behavior of Lanthanum–Cerium Oxide thermal barrier coating prepared by EB–PVD. Surf. Coatings Technol. 2006, 200, 5113–5118. [Google Scholar] [CrossRef]
- Ma, W.; Gong, S.; Xu, H.; Cao, X. On improving the phase stability and thermal expansion coefficients of lanthanum cerium oxide solid solutions. Scr. Mater. 2006, 54, 1505–1508. [Google Scholar] [CrossRef]
- Gao, L.; Guo, H.; Gong, S.; Xu, H. Plasma-sprayed La2Ce2O7 thermal barrier coatings against calcium-magnesium-alumina-silicate penetration. J. Eur. Ceram. Soc. 2014, 34, 2553–2561. [Google Scholar] [CrossRef]
- Vanpoucke, D.E.P.; Bultinck, P.; Cottenier, S.; Van Speybroeck, V.; Van Driessche, I. Density functional theory study of La2Ce2O7: Disordered fluorite versus pyrochlore structure. Phys. Rev. B-Condens. Matter Mater. Phys. 2011, 84, 054110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.X.; Tracy, C.L.; Lang, M.; Ewing, R.C. Stability of fluorite-type La2Ce2O7 under extreme conditions. J. Alloys Compd. 2016, 674, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Dehkharghani, A.M.F.; Rahimipour, M.R.; Zakeri, M. Crystal Structure and Lattice Parameter Investigation of La 3 + Substituted CeO2 in LaxCe1−xO2−X/2 Synthesized by Solid-State Method. Adv. Ceram. Prog. 2020, 6, 43–48. [Google Scholar]
- Besikiotis, V.; Knee, C.S.; Ahmed, I.; Haugsrud, R.; Norby, T. Crystal structure, hydration and ionic conductivity of the inherently oxygen-deficient La2Ce2O7. Solid State Ion. 2012, 228, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, C.; Cheng, Y.; Chi, F. Influence of different surfactants on crystal growth behavior and sinterability of La2Ce2O7 solid solution. Ceram. Int. 2014, 40, 4305–4310. [Google Scholar] [CrossRef]
- Khademinia, S.; Behzad, M. Lanthanum cerate (La2Ce2O7): Hydrothermal synthesis, characterization and optical properties. Int. Nano Lett. 2015, 5, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Hongsong, Z.; Zhengying, W.; Yongde, Z.; Gang, L.; Zhenjun, L. Preparation, Characterization of A2Ce2O7 (A = La and Gd) and Their Photo-Catalytic Properties. Energy Environ. Focus 2015, 4, 324–329. [Google Scholar] [CrossRef]
- Weng, S.F.; Wang, Y.H.; Lee, C.S. Autothermal steam reforming of ethanol over La2Ce2−xRuxO7 (x = 0-0.35) catalyst for hydrogen production. Appl. Catal. B Environ. 2013, 134, 359–366. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhu, Y.P.; Zhang, W.G. Preparation of La2Ce2O7 Nano-Powders by Molten Salts Method. Adv. Mater. Res. 2009, 79, 337–340. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yi, H.; Che, J.W.; Liang, G.Y. Phase, compositional, structural, and chemical stability of La2Ce2O7 after high temperature heat treatment. Ceram. Int. 2019, 45, 5030–5035. [Google Scholar] [CrossRef]
- Pati, R.K.; Lee, I.C.; Gaskell, K.J.; Ehrman, S.H. Precipitation of nanocrystalline CeO2 using triethanolamine. Langmuir 2009, 25, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Wang, Y.; Cheng, Y.; Zou, B.; Fan, X.; Yang, J.; Cao, X. Synthesis of monodispersed La2Ce2O7 nanocrystals via hydrothermal method: A study of crystal growth and sintering behavior. Int. J. Refract. Met. Hard Mater. 2012, 31, 242–246. [Google Scholar] [CrossRef]
- Dehkharghani, A.M.F.; Rahimipour, M.R.; Zakeri, M. Improving the thermal shock resistance and fracture toughness of synthesized La2Ce2O7 thermal barrier coatings through formation of La2Ce2O7/YSZ composite coating via air plasma spraying. Surf. Coat. Technol. 2020, 399, 126174. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, J.; Song, W.; Zhou, X.; Dong, S.; Duo, S.; Wang, J.; Yang, X.; Jiang, J.; Deng, L.; et al. Composition, mechanical properties and thermal cycling performance of YSZ toughened La2Ce2O7 composite thermal barrier coatings. Ceram. Int. 2020, 46, 6641–6651. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. US Patent No. 3330697, 11 July 1967. [Google Scholar]
- Hassanzadeh-Tabrizi, S.A.A. Synthesis and luminescence properties of YAG:Ce nanopowder prepared by the Pechini method. Adv. Powder Technol. 2012, 23, 324–327. [Google Scholar] [CrossRef]
- Kakihana, M. “Sol-Gel” preparation of high temperature superconducting oxides. J. Sol.-Gel Sci. Technol. 1996, 6, 7–55. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Schwartz, S.; Jungen, W.; Tietz, F.; Stöever, D. Spray-drying of ceramics for plasma-spray coating. J. Eur. Ceram. Soc. 2000, 20, 2433–2439. [Google Scholar] [CrossRef]
- Praveen, K.; Sivakumar, S.; Ananthapadmanabhan, P.V.; Shanmugavelayutham, G. Lanthanum cerate thermal barrier coatings generated from thermal plasma synthesized powders. Ceram. Int. 2018, 44, 6417–6425. [Google Scholar] [CrossRef]
- Pakseresht, A.H.; Rahimipour, M.R.; Vaezi, M.R.; Salehi, M. Thermal plasma spheroidization and spray deposition of barium titanate powder and characterization of the plasma sprayable powder. Mater. Chem. Phys. 2016, 173, 395–403. [Google Scholar] [CrossRef]
- Kraxner, J. Utility Model of Equipment for the Production of Solid and Hollow Glass and Glass Ceramic Microspheres by Flame Synthesis. SK Patent 8673, 20 January 2020. [Google Scholar]
- Zhang, H.; Wang, J.; Dong, S.; Yuan, J.; Zhou, X.; Duo, S.; Chen, S.; Huo, P.; Jiang, J.; Deng, L.; et al. Mechanical properties and thermal cycling behavior of Ta2O5 doped La2Ce2O7 thermal barrier coatings prepared by atmospheric plasma spraying. J. Alloys Compd. 2019, 785, 1068–1076. [Google Scholar] [CrossRef]
- Bezerra Lopes, F.W.; de Souza, C.P.; Vieira de Morais, A.M.; Dallas, J.-P.; Gavarri, J.-R. Determination of RE2Ce2O7 pyrochlore phases from monazite–allanite ores. Hydrometallurgy 2009, 97, 167–172. [Google Scholar] [CrossRef]
- Hong-song, Z.; Xiao-ge, C.; Gang, L.; Xin-Li, W.; Xu-dan, D. Influence of Gd2O3 addition on thermophysical properties of La 2Ce2O7 ceramics for thermal barrier coatings. J. Eur. Ceram. Soc. 2012, 32, 3693–3700. [Google Scholar] [CrossRef]
- Katta, L.; Sudarsanam, P.; Thrimurthulu, G.; Reddy, B.M. Doped nanosized ceria solid solutions for low temperature soot oxidation: Zirconium versus lanthanum promoters. Appl. Catal. B Environ. 2010, 101, 101–108. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178–185. [Google Scholar] [CrossRef]
- Chromčíková, M.; Hruška, B.; Nowicka, A.; Svoboda, R.; Liška, M. Role of modifiers in the structural interpretation of the glass transition behavior in MgO/BaO-Al2O3-P2O5 glasses. J. Non. Cryst. Solids 2021, 573, 121114. [Google Scholar] [CrossRef]
- Vašková, H. A powerful tool for material identification: Raman spectroscopy. Int. J. Math. Model. Methods Appl. Sci. 2011, 5, 1205–1212. [Google Scholar]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and X-ray studies of Ce1−xRExO2−y, where RE = La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Joulia, A.; Vardelle, M.; Rossignol, S. Synthesis and thermal stability of Re2Zr2O7, (Re=La, Gd) and La2(Zr1−xCex)2O7−δ compounds under reducing and oxidant atmospheres for thermal barrier coatings. J. Eur. Ceram. Soc. 2013, 33, 2633–2644. [Google Scholar] [CrossRef]
- Parchovianská, I.; Parchovianský, M.; Nowicka, A.; Prnová, A.; Pakseresht, A.H. Solid state synthesis and characterization of La2Ce2O7 powder as a candidate material for thermal barrier coatings. In Proceedings of the 14th International Conference on Solid State Chemistry, Trenčín, Slovakia, 13–17 June 2021. [Google Scholar]
- Guo, H.; Wang, Y.; Wang, L.; Gong, S. Thermo-physical properties and thermal shock resistance of segmented La2Ce2O7/YSZ thermal barrier coatings. J. Therm. Spray Technol. 2009, 18, 665–671. [Google Scholar] [CrossRef]
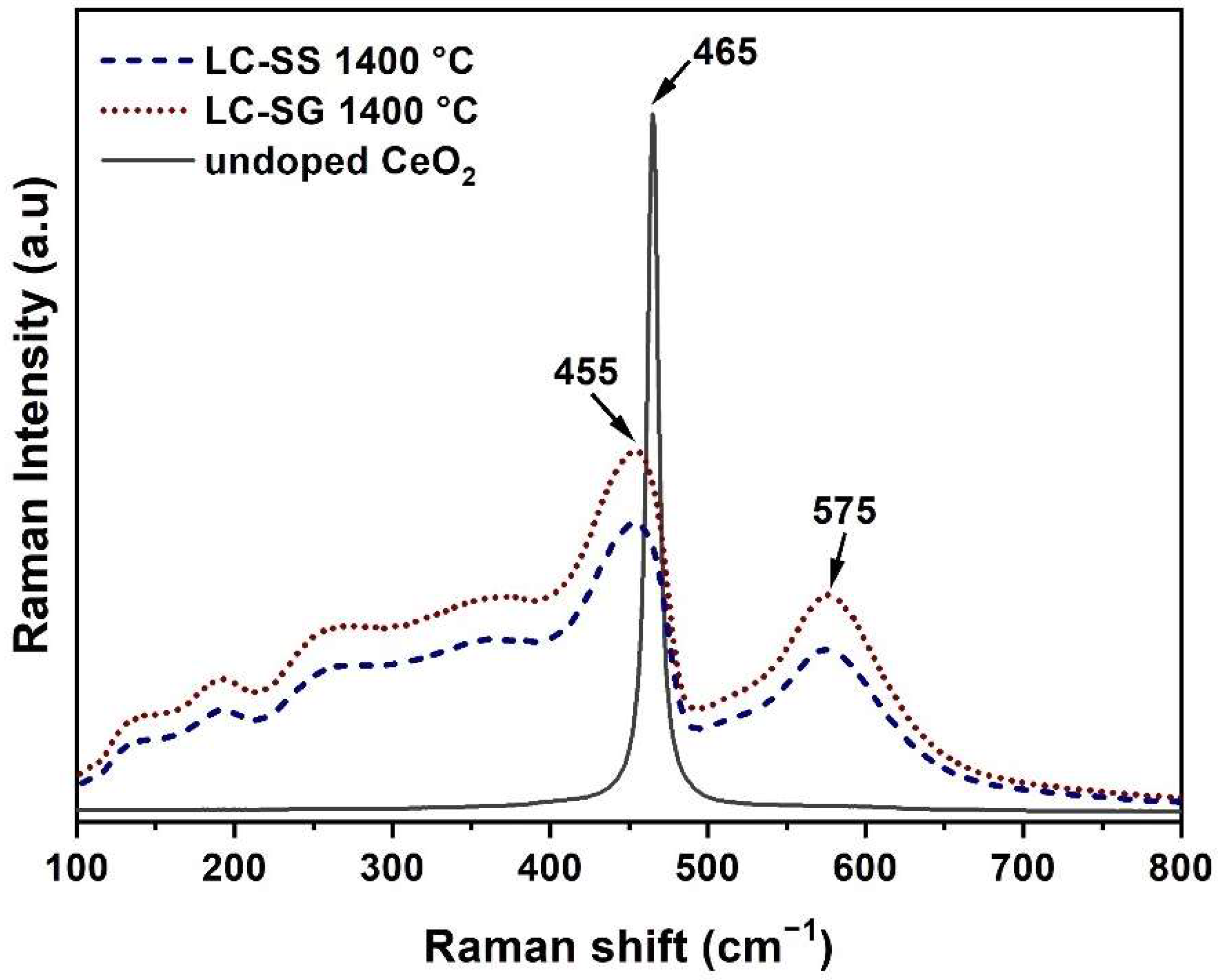
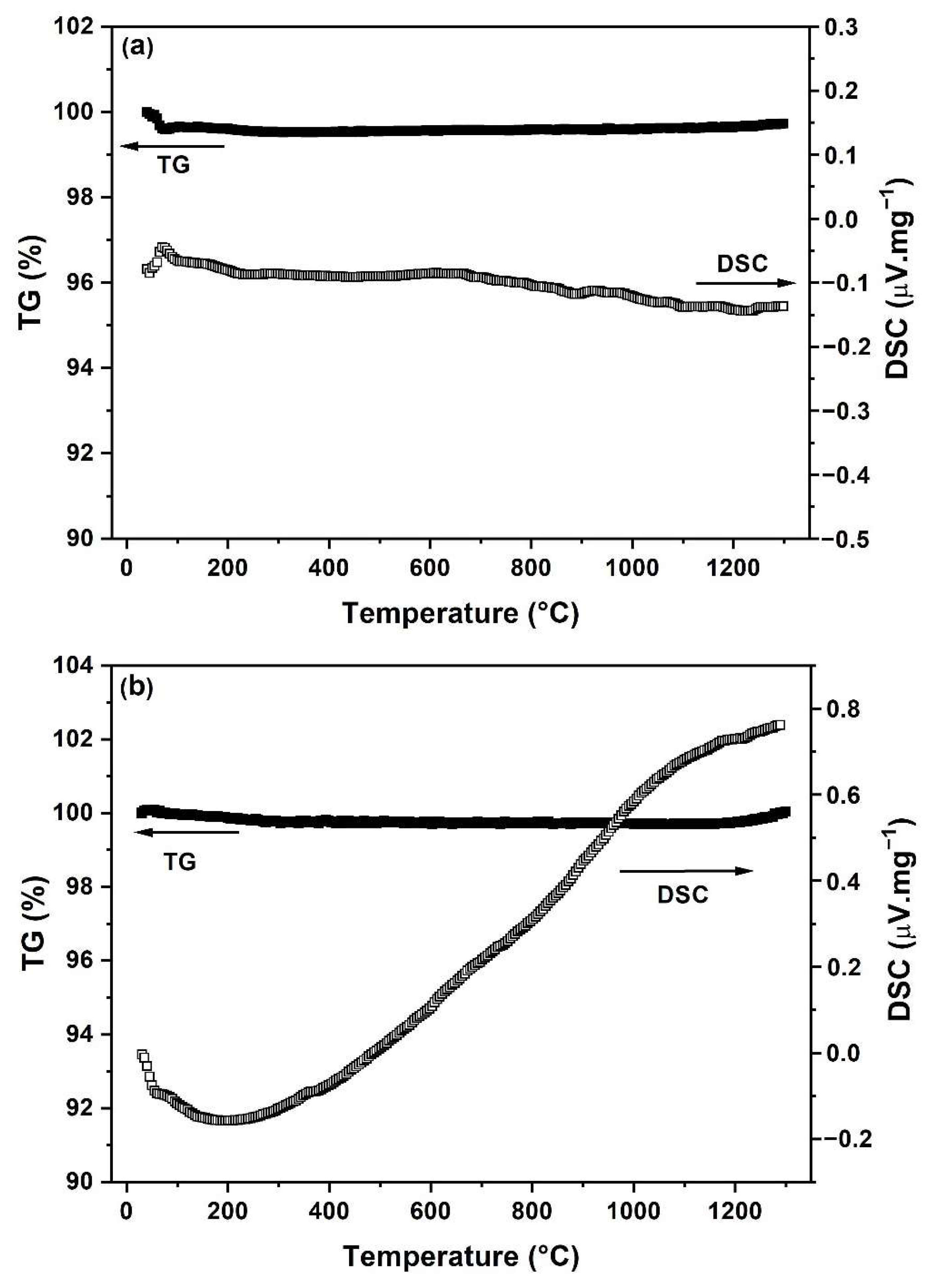
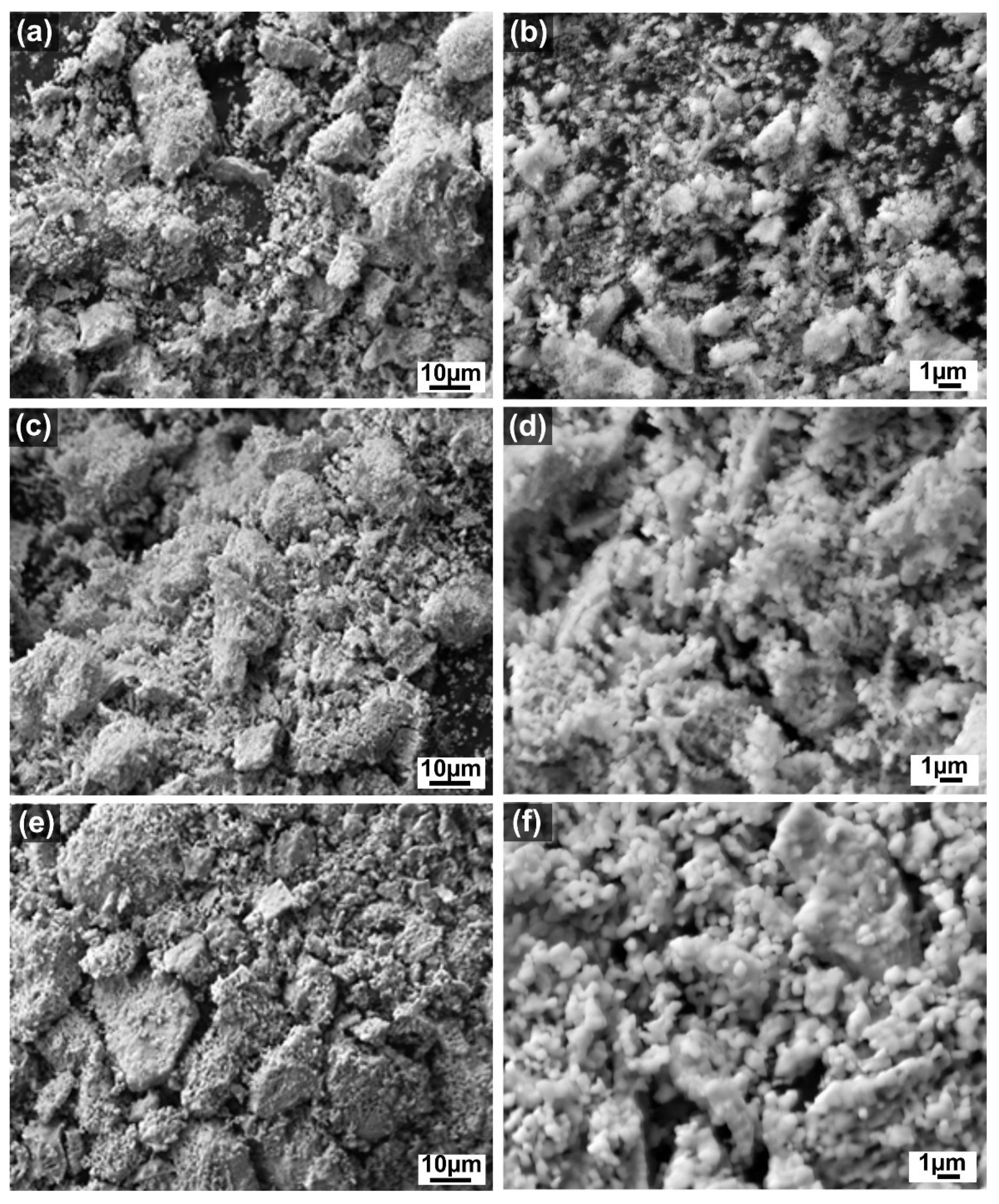
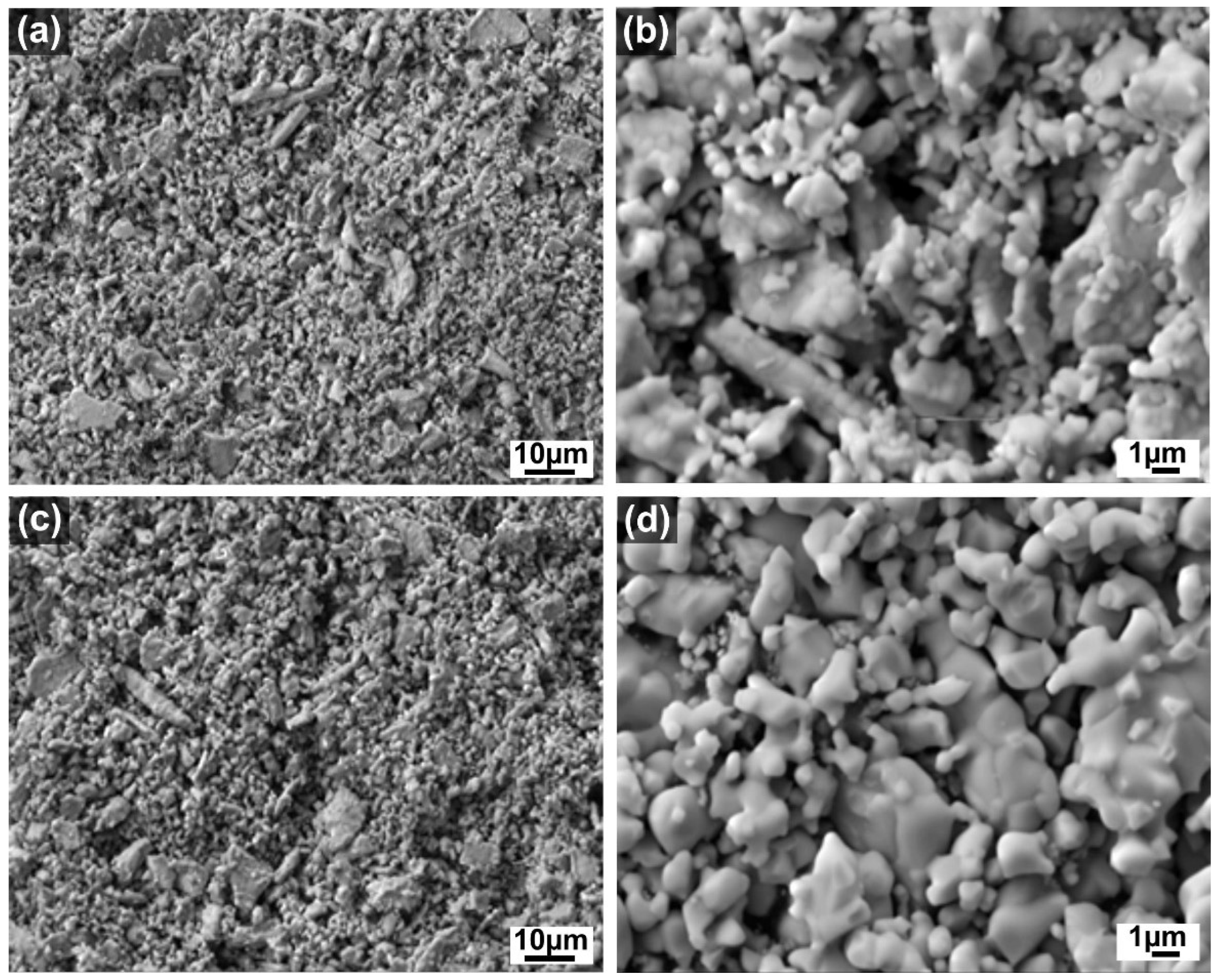


| Sample | Crystallite Size (nm) | Lattice Parameter a (nm) | La (at %) | Ce (at %) | O (at %) |
|---|---|---|---|---|---|
| LC-SG | 98 | 0.55729 | 18.4 | 18.8 | 62.8 |
| LC-SS | 57 | 0.55712 | 18.7 | 18.9 | 62.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parchovianská, I.; Parchovianský, M.; Pecušová, B.; Hanzel, O.; Pakseresht, A. Synthesis and Characterization of Fluorite-Type La2Ce2O7 Plasma Sprayable Powder for TBCs Application. Materials 2022, 15, 4007. https://doi.org/10.3390/ma15114007
Parchovianská I, Parchovianský M, Pecušová B, Hanzel O, Pakseresht A. Synthesis and Characterization of Fluorite-Type La2Ce2O7 Plasma Sprayable Powder for TBCs Application. Materials. 2022; 15(11):4007. https://doi.org/10.3390/ma15114007
Chicago/Turabian StyleParchovianská, Ivana, Milan Parchovianský, Beáta Pecušová, Ondrej Hanzel, and Amirhossein Pakseresht. 2022. "Synthesis and Characterization of Fluorite-Type La2Ce2O7 Plasma Sprayable Powder for TBCs Application" Materials 15, no. 11: 4007. https://doi.org/10.3390/ma15114007
APA StyleParchovianská, I., Parchovianský, M., Pecušová, B., Hanzel, O., & Pakseresht, A. (2022). Synthesis and Characterization of Fluorite-Type La2Ce2O7 Plasma Sprayable Powder for TBCs Application. Materials, 15(11), 4007. https://doi.org/10.3390/ma15114007







