Approach to the Fatigue and Cellular Behavior of Superficially Modified Porous Titanium Dental Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Fatigue Behavior of the Porous Dental Implants Studied
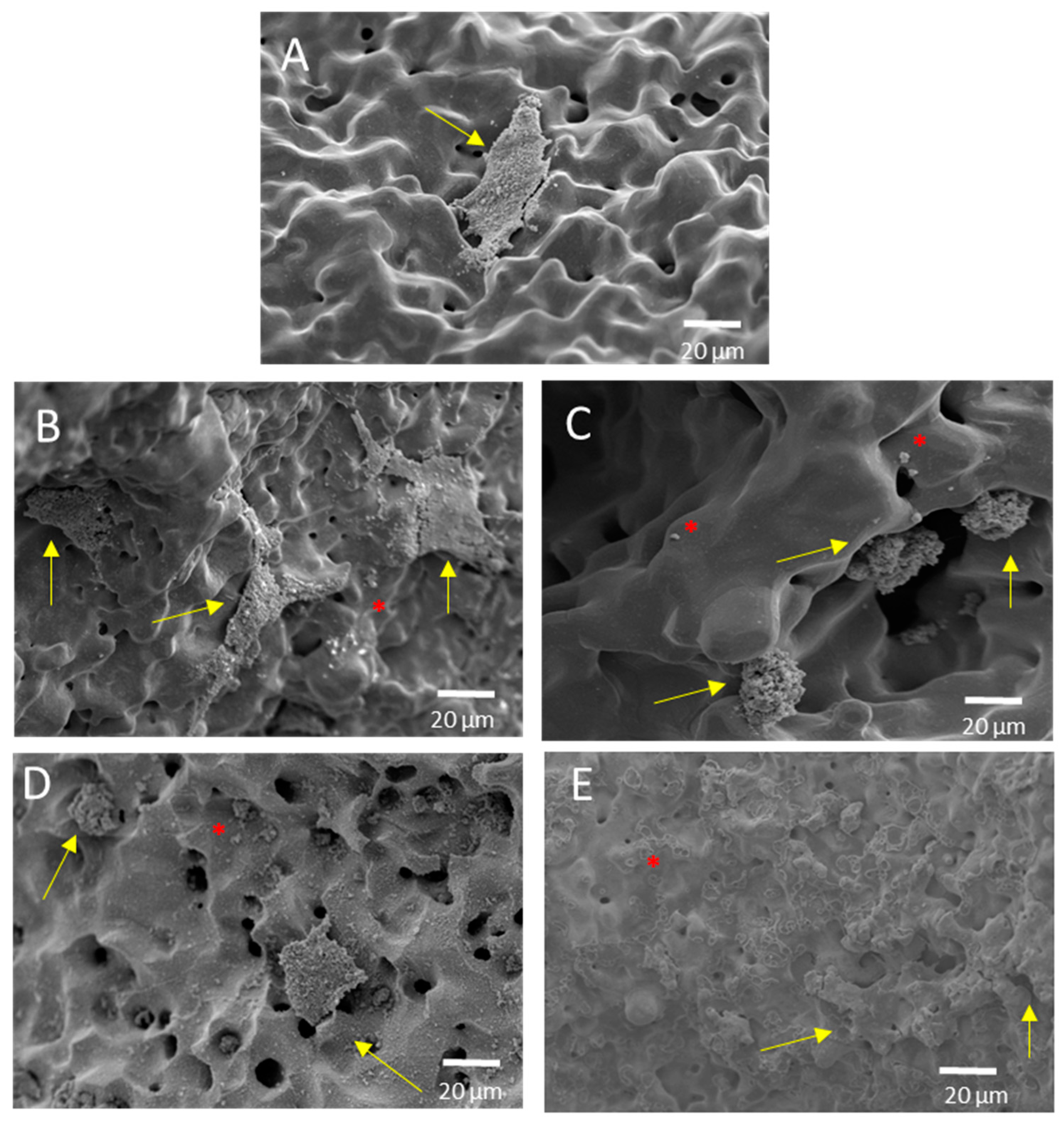
2.2. Cellular Characterization of Superficially Modified Porous Dental Implants
2.2.1. In Vitro Cell Culture
2.2.2. Cell Differentiation by Alkaline Phosphatase (ALP) Evaluation
2.2.3. Cell Morphology
2.2.4. Statistical Analysis
3. Results and Discussion
- (i)
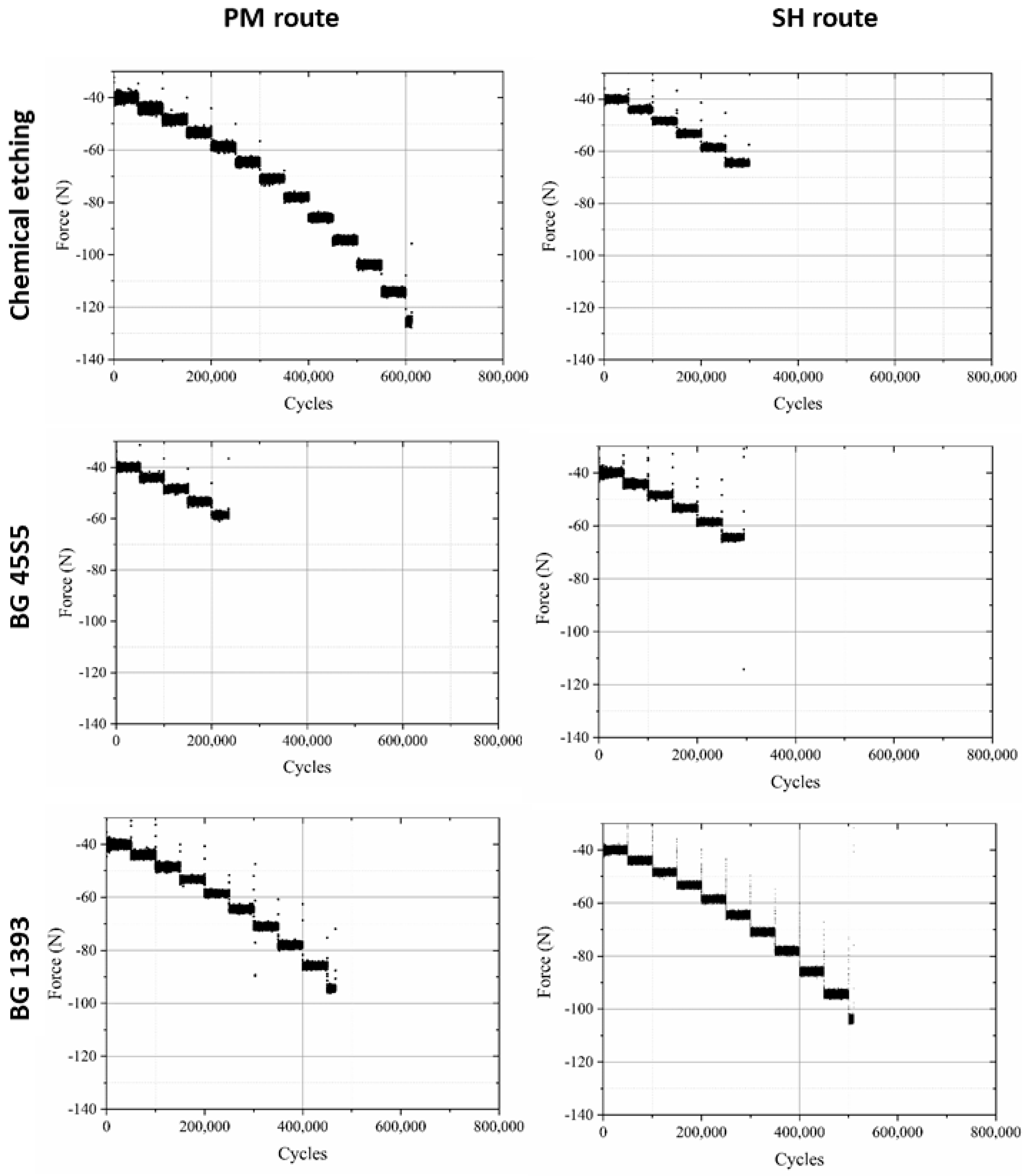
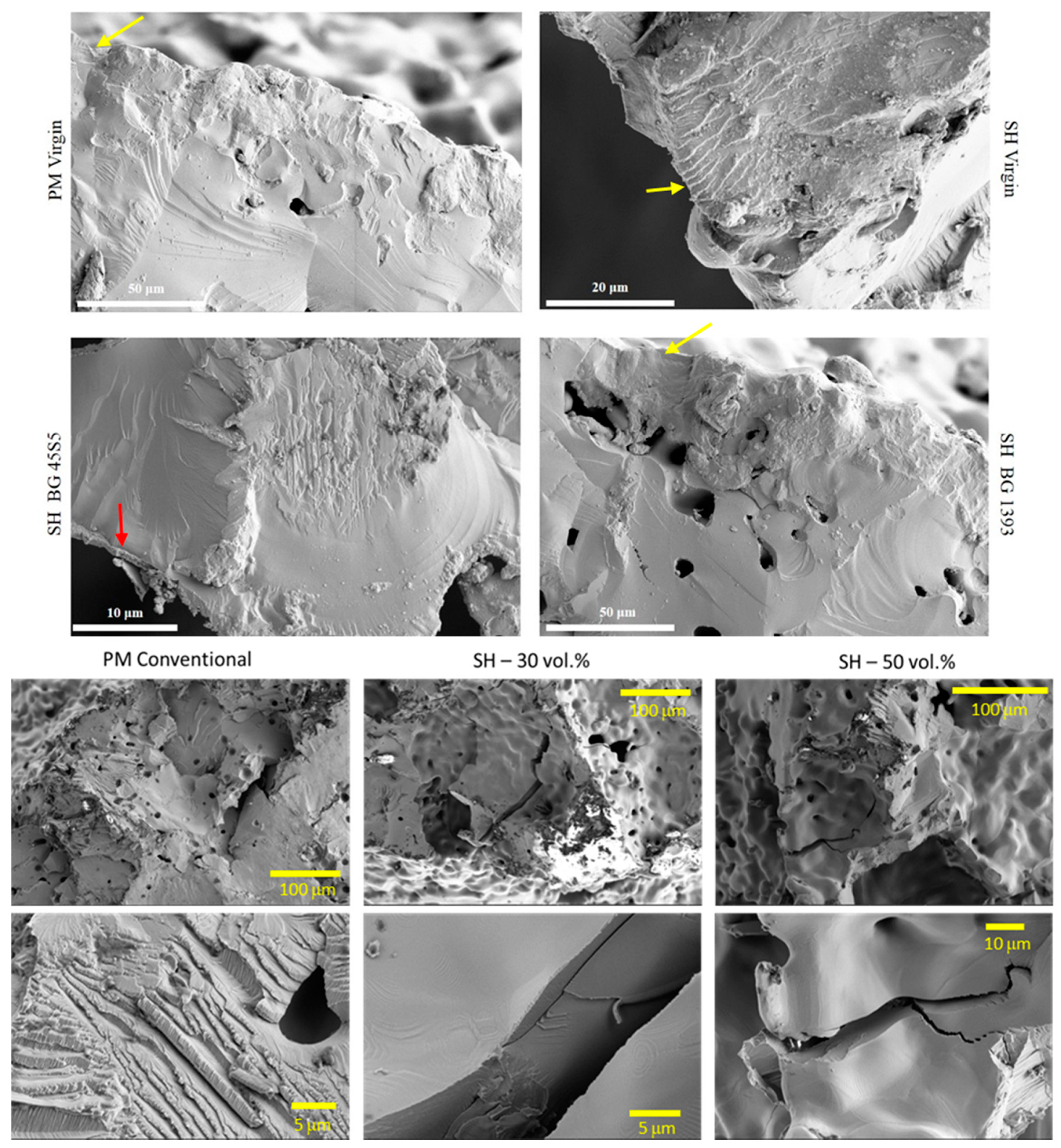
- Modified PM implants: The chemically etched implant and the bioactive glass BG 1393 coated implant presented a higher fatigue resistance than the virgin PM implant, while this was less for the implant coated with BG 45S5. On the other hand, the improvement in the fatigue life of the chemically etched implant may be associated with the formation of a more stable oxide layer on the surface of the implant, usually rutile. This oxide hardened the surface, and thus hindered the movement of dislocations and/or nucleation of micro-cracks under cyclic loads [41]. Comparable results were already reported by Apachitei et al. [42]. They studied in detail the effect of plasma electrolytic oxidation coatings on the fatigue properties of Ti6Al4V and Ti6Al7Nb alloys under physiological conditions (Hank’s solution at 37 °C) in order to describe the fact that oxidized Ti6Al7Nb alloys exhibit an improved fatigue behavior if compared to oxidized Ti6Al4V alloys, independently from the coating thickness. Furthermore, the best fatigue behavior of the implant coated with BG 1393 could be explained by its better adhesion with the Ti implant [43,44] compared to BG 45S5. This fact could be associated with the best compatibility between its thermal expansion coefficients [45]. Furthermore, the temperature used during the coating treatment (exceeding the melting temperature of BG 1393) allowed its infiltration into the macro-pores (see Figure 6).
- (ii)
- Modified SH implants: As previously described, the fatigue behavior of SH virgin implants was conditioned by the role of macro-pores (associated with the use of spacer particles). However, the resistance under cyclical loads of the modified implants clearly depended on what happened on their surface and how it took place. In this context, after chemical etching, the macro-pores were larger and more irregular, justifying the sudden drop in mechanical strength (see Figure 6). Furthermore, the intrinsic micro-porosity of the BG 45S5 coating and its poor adherence (see the red arrow in Figure 7) compromised their use for this type of solicitation. Finally, despite the good infiltration and adherence of BG 1393, the presence of pre-existing microcracks—originating in the macro-pores after the thermal treatment of this coating—could explain its resistance to fatigue (see Figure 6).
- (1)
- surface hardening (virgin c.p. Ti implants);
- (2)
- the nucleation and accumulation of damage to the treated surface, chemically or in the interlayer of the coating-implant joint; and
- (3)
- the subcritical growth of pre-existing micro-cracks in the coating.
4. Conclusions
- (1)
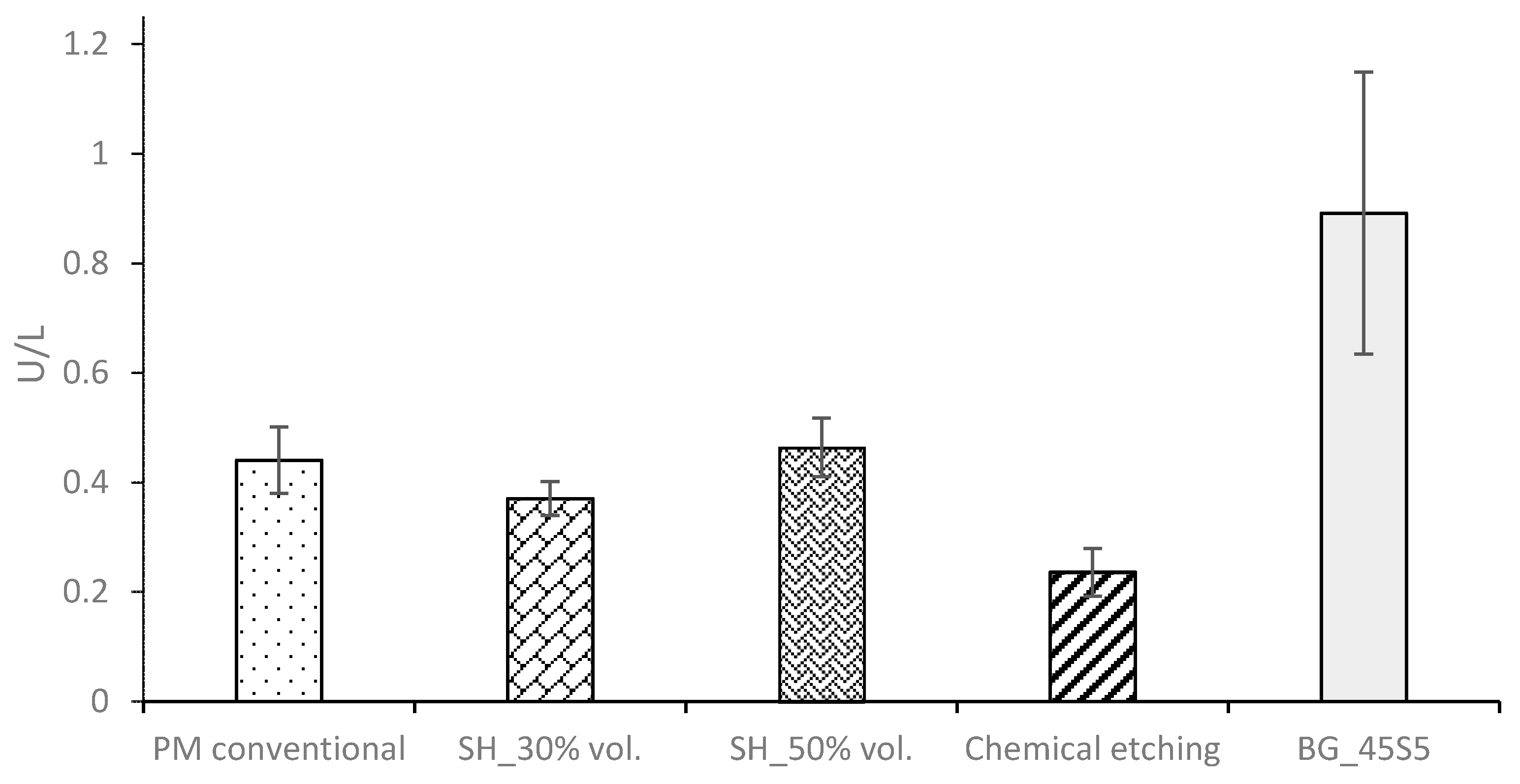
- The virgin SH dental implants have a lower fatigue resistance than those obtained by the conventional PM route. The macro-pores control the crack nucleation process, although they can also hinder the propagation of cracks (stop-hole mechanism—the tip of the crack is blunted). On the other hand, the roughness of the walls of these implants favors the adhesion of osteoblasts. Furthermore, an increase in the behavior (ALP activity or cells differentiation) of the in vitro cell cultures is observed after the surface modifications, and the differences between the treatments used are not statistically significant.
- (2)
- The high micro-porosity of the BG 45S5 coating compromised the fatigue behavior of the implant, being 17% less than the value corresponding to PM dental implants without surface treatment. In the case of SH 30 vol.% implants, it also decreased by 65% compared to the virgin implant. On the other hand, the fatigue resistance of conventional PM implants coated with BG 1393 improves by 25%. This increase may be related to the improved infiltration and/or better thermal compatibility (coefficients of expansion) between Ti and the BG 1393. Finally, the increase of the fatigue resistance of the superficially chemically etched porous dental implant (38% vs. PM virgin) is related to the formation of a hard layer of titanium oxide formed during the chemical treatment of the surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Pałka, K.; Pokrowiecki, R. Porous titanium implants: A review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Ghouse, S.; Reznikov, N.; Boughton, O.R.; Babu, S.; Ng, K.G.; Blunn, G.; Cobb, J.P.; Stevens, M.M.; Jeffers, J.R. The design and in vivo testing of a locally stiffness-matched porous scaffold. Appl. Mater. Today 2019, 15, 377–388. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Beta titanium alloys: The lowest elastic modulus for biomedical applications: A review. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2014, 8, 726. [Google Scholar]
- Biesiekierski, A.; Lin, J.; Li, Y.; Ping, D.; Yamabe-Mitarai, Y.; Wen, C. Investigations into Ti–(Nb, Ta)–Fe alloys for biomedical applications. Acta Biomater. 2016, 32, 336–347. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Sevostyanov, M.A.; Konushkin, S.V.; Losertová, M.; Ivannikov, A.Y.; Kolmakov, A.G.; Izmailov, A.Y. Manufacturing and study of mechanical properties, structure and compatibility with biological objects of plates and wire from new Ti-25Nb-13Ta-5Zr alloy. Metals 2020, 10, 1584. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Giner, M.; Chicardi, E.; Costa, A.d.F.; Santana, L.; Vázquez-Gámez, M.Á.; García-Garrido, C.; Colmenero, M.A.; Olmo-Montes, F.J.; Torres, Y.; Montoya-García, M.J. Biocompatibility and cellular behavior of tinbta alloy with adapted rigidity for the replacement of bone tissue. Metals 2021, 11, 130. [Google Scholar] [CrossRef]
- Goharian, A.; Abdullah, M.R. 7—Bioinert Metals (Stainless Steel, Titanium, Cobalt Chromium). In Trauma Plating Systems; Goharian, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–142. [Google Scholar]
- Olmedo, M.M.; Godino, F.I.; Liétor, P.F.; Iglesias, F.C. Corrosion and fracture analysis in screws of dental implants prostheses. New coatings. Eng. Fail. Anal. 2017, 82, 657–665. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2019, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of dental implant surface modifications on osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [Green Version]
- Civantos, A.; Domínguez, C.; Pino, R.J.; Setti, G.; Pavón, J.J.; Martínez-Campos, E.; Garcia, F.J.G.; Rodríguez, J.A.; Allain, J.P.; Torres, Y. Designing bioactive porous titanium interfaces to balance mechanical properties and in vitro cells behavior towards increased osseointegration. Surf. Coat. Technol. 2019, 368, 162–174. [Google Scholar] [CrossRef]
- Lobato, J.; Hussain, N.S.; Botelho, C.; Maurício, A.; Lobato, J.; Lopes, M.; Afonso, A.; Ali, N.; Santos, J. Titanium dental implants coated with Bonelike®: Clinical case report. Thin Solid Film. 2006, 515, 279–284. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef]
- Torres, Y.; Sarria, P.; Gotor, F.J.; Gutiérrez, E.; Peon, E.; Beltrán, A.M.; González, J.E. Surface modification of Ti-6Al-4V alloys manufactured by selective laser melting: Microstructural and tribo-mechanical characterization. Surf. Coat. Technol. 2018, 348, 31–40. [Google Scholar] [CrossRef]
- Sarma, J.; Kumar, R.; Sahoo, A.K.; Panda, A. Enhancement of material properties of titanium alloys through heat treatment process: A brief review. Mater. Today Proc. 2020, 23, 561–564. [Google Scholar] [CrossRef]
- Gherde, C.; Dhatrak, P.; Nimbalkar, S.; Joshi, S. A comprehensive review of factors affecting fatigue life of dental implants. Mater. Today Proc. 2021, 43, 1117–1123. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D. Identification of failure mechanisms in retrieved fractured dental implants. Eng. Fail. Anal. 2014, 38, 58–65. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J.J.P. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Rojo, R.; Prados-Privado, M.; Reinoso, A.J.; Prados-Frutos, J.C. Evaluation of Fatigue Behavior in Dental Implants from In Vitro Clinical Tests: A Systematic Review. Metals 2018, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis update: Risk indicators, diagnosis, and treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, D.; Alphin, M.S. Influence of geometric design variable and bone quality on stress distribution for zirconia dental implants-A 3D finite element analysis. Comput. Modeling Eng. Sci. 2018, 117, 125–141. [Google Scholar] [CrossRef]
- Hedayati, R.; Hosseini-Toudeshky, H.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A. Computational prediction of the fatigue behavior of additively manufactured porous metallic biomaterials. Int. J. Fatigue 2016, 84, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fatemi, A. Surface roughness effect on multiaxial fatigue behavior of additive manufactured metals and its modeling. Theor. Appl. Fract. Mech. 2019, 103, 102260. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Ternero, F.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; Montealegre-Meléndez, I.; Arévalo, C.; García-Moreno, F.; Torres, Y. Improvement of the balance between a reduced stress shielding and bone ingrowth by bioactive coatings onto porous titanium substrates. Surf. Coat. Technol. 2018, 338, 32–37. [Google Scholar] [CrossRef]
- Trueba, P.; Navarro, C.; Rodríguez-Ortiz, J.A.; Beltrán, A.M.; García-García, F.J.; Torres, Y. Fabrication and characterization of superficially modified porous dental implants. Surf. Coat. Technol. 2021, 408, 126796. [Google Scholar] [CrossRef]
- AENOR UNE-EN ISO 14801; Dentistry—Implants—Dynamic Fatigue Test for Endosseous Dental Implants. ISO: Geneva, Switzerland, 2007.
- Miner, M.A. Cumulative Damage in Fatigue. J. Appl. Mech. 1945, 12, A159–A164. [Google Scholar] [CrossRef]
- Topoliński, T.; Cichański, A.; Mazurkiewicz, A.; Nowicki, K. Applying a stepwise load for calculation of the SN curve for trabecular bone based on the linear hypothesis for fatigue damage accumulation. Mater. Sci. Forum 2012, 726, 39–42. [Google Scholar] [CrossRef]
- Nielsen, L.F. On strength of porous material: Simple systems and densified systems. Mater. Struct. 1998, 31, 651–661. [Google Scholar] [CrossRef]
- Lascano, S.; Arévalo, C.; Montealegre-Melendez, I.; Muñoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous titanium for biomedical applications: Evaluation of the conventional powder metallurgy frontier and space-holder technique. Appl. Sci. 2019, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Trueba, P.; Beltrán, A.M.; Bayo, J.M.; Rodríguez-Ortiz, J.A.; Larios, D.F.; Alonso, E.; Dunand, D.C.; Torres, Y. Porous titanium cylinders obtained by the freeze-casting technique: Influence of process parameters on porosity and mechanical behavior. Metals 2020, 10, 188. [Google Scholar] [CrossRef] [Green Version]
- Black, J.; Hastings, G. Handbook of Biomaterial Properties; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Collings, E. The Physical Metallurgy of Titanium Alloys; ASM Series in Metal Processin; ASM International: Novelty, OH, USA, 1984; Volume 3, ISBN-13: 978-0871701817. [Google Scholar]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue characteristics of metallic biomaterials. Int. J. Fatigue 2007, 29, 992–1000. [Google Scholar] [CrossRef]
- Pramanik, A.; Islam, M.; Basak, A.; Littlefair, G. Machining and tool wear mechanisms during machining titanium alloys. Adv. Mater. Res. 2013, 651, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Apachitei, I.; Lonyuk, B.; Fratila-Apachitei, L.; Zhou, J.; Duszczyk, J. Fatigue response of porous coated titanium biomedical alloys. Scr. Mater. 2009, 61, 113–116. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Ternero, F.; Rodríguez-Ortiz, J.A.; Heise, S.; Boccaccini, A.R.; Lebrato, J.; Torres, Y. Bioactive coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 349, 584–592. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Begines, B.; Alcudia, A.; Rodríguez-Ortiz, J.A.; Torres, Y. Biofunctional and tribomechanical behavior of porous titanium substrates coated with a bioactive glass bilayer (45S5–1393). ACS Appl. Mater. Interfaces 2020, 12, 30170–30180. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Sola, A. Coefficient of thermal expansion of bioactive glasses: Available literature data and analytical equation estimates. Ceram. Int. 2011, 37, 2963–2972. [Google Scholar] [CrossRef]
- Hisham Zainal Ariffin, S.; Manogaran, T.; Zarina Zainol Abidin, I.; Megat Abdul Wahab, R.; Senafi, S. A perspective on stem cells as biological systems that produce differentiated osteoblasts and odontoblasts. Curr. Stem Cell Res. Ther. 2017, 12, 247–259. [Google Scholar] [CrossRef] [PubMed]


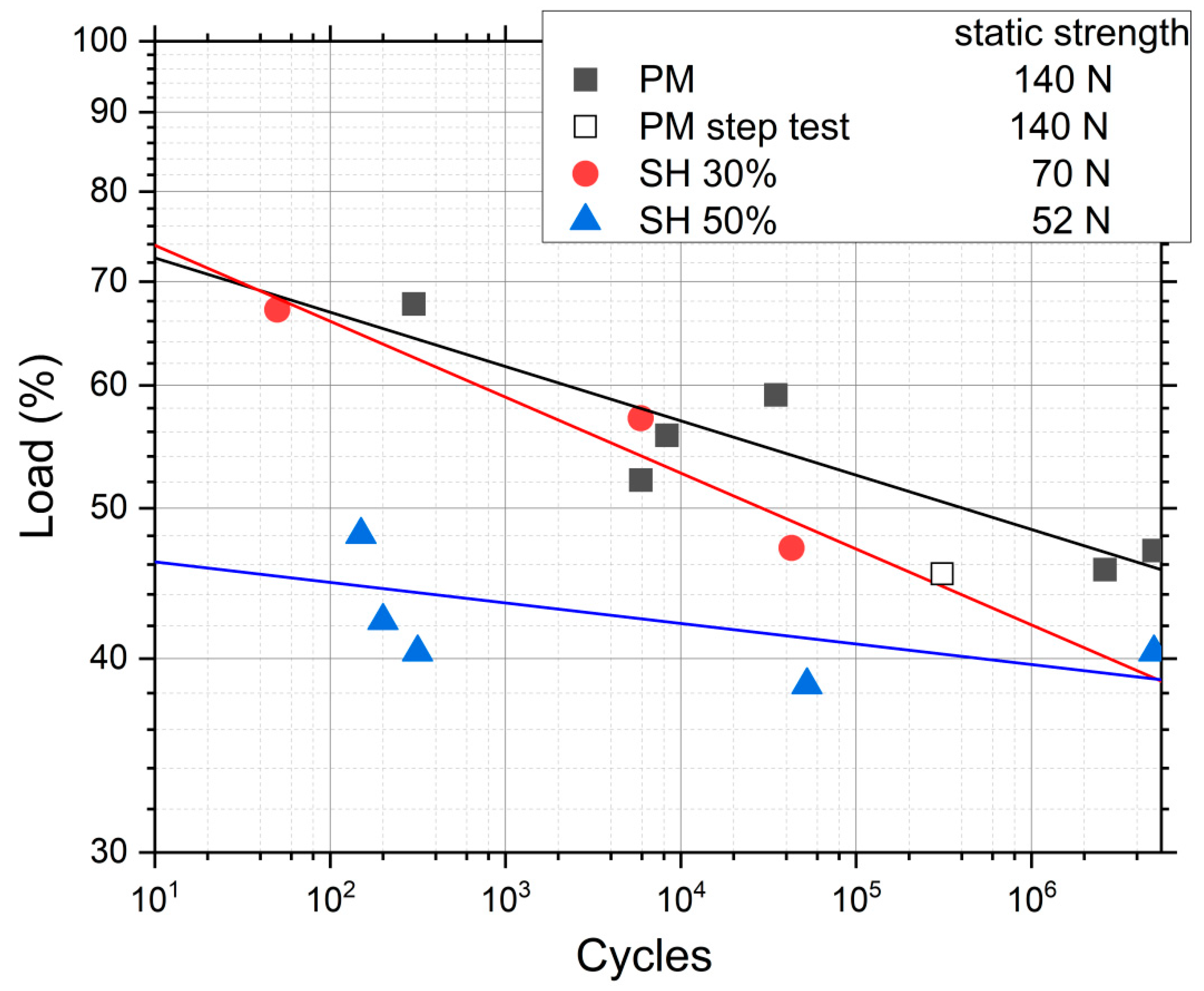


| Fracture Load (N) | Nominal Stress (MPa) | Estimated Mechanical Behavior [34,35,36] | ||||
|---|---|---|---|---|---|---|
| EN (GPa) | Ed (GPa) | σy (MPa) | ||||
| PM | 140 ± 3 | 191 ± 1 | 90.9 ± 0.5 | 86.2 ± 0.6 | 638 ± 5 | |
| SH | 30 vol.% | 70 ± 4 | 95.5 ± 1.5 | 44.6 ± 0.9 | 45.8 ± 1.0 | 200 ± 8 |
| 50 vol.% | 52 ± 6 | 71 ± 2 | 30.3 ± 1.1 | 35.6 ± 1.0 | 135 ± 14 | |
| PM | SH 30 vol.% | SH 50 vol.% | |||
|---|---|---|---|---|---|
| Cycles | MPa | Cycles | MPa | CYCLES | MPa |
| 300 | 129.3 | 50 | 64.1 | 150 | 34.1 |
| 34,820 | 113.0 | 5900 | 54.6 | 200 | 30.0 |
| 8324 | 106.4 | 42,855 | 45.0 | 52,403 | 27.3 |
| 5900 | 99.6 | - | - | 315 | 28.7 |
| 310,056 * | 86.6 * | - | - | 5 × 106 | 28.7 |
| 5 × 106 | 89.7 | - | - | ||
| 2.6 × 106 | 87.2 | - | - | ||
| Porous Dental Implants | Maximum Fatigue Load (N) | Nominal Stress (MPa) | Equivalent Maximum Stress (MPa) (See Figure 5) | Number of Total Cycles | Estimated Fatigue Strength at 105 Cycles (MPa) | |
|---|---|---|---|---|---|---|
| Virgin | PM | 70.9 | 96.7 | 86.6 | 310,056 | 90.1 |
| Chemical Etching | PM | 114.1 | 155.7 | 150.5 | 611,850 | 160.4 |
| SH | 64.4 | 87.9 | 81 | 298,754 | 85.5 | |
| BG 45S5 | PM | 58.6 | 79.9 | 75 | 235,260 | 77.3 |
| SH | 64.4 | 87.9 | 80.8 | 294,670 | 85.2 | |
| BG 1393 | PM | 94.3 | 128.7 | 115.3 | 466,920 | 121.7 |
| SH | 103.7 | 141.5 | 120.3 | 510,240 | 130.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trueba, P.; Navarro, C.; Giner, M.; Rodríguez-Ortiz, J.A.; Montoya-García, M.J.; Delgado-Pujol, E.J.; Rodríguez-Albelo, L.M.; Torres, Y. Approach to the Fatigue and Cellular Behavior of Superficially Modified Porous Titanium Dental Implants. Materials 2022, 15, 3903. https://doi.org/10.3390/ma15113903
Trueba P, Navarro C, Giner M, Rodríguez-Ortiz JA, Montoya-García MJ, Delgado-Pujol EJ, Rodríguez-Albelo LM, Torres Y. Approach to the Fatigue and Cellular Behavior of Superficially Modified Porous Titanium Dental Implants. Materials. 2022; 15(11):3903. https://doi.org/10.3390/ma15113903
Chicago/Turabian StyleTrueba, Paloma, Carlos Navarro, Mercè Giner, José A. Rodríguez-Ortiz, María José Montoya-García, Ernesto J. Delgado-Pujol, Luisa M. Rodríguez-Albelo, and Yadir Torres. 2022. "Approach to the Fatigue and Cellular Behavior of Superficially Modified Porous Titanium Dental Implants" Materials 15, no. 11: 3903. https://doi.org/10.3390/ma15113903
APA StyleTrueba, P., Navarro, C., Giner, M., Rodríguez-Ortiz, J. A., Montoya-García, M. J., Delgado-Pujol, E. J., Rodríguez-Albelo, L. M., & Torres, Y. (2022). Approach to the Fatigue and Cellular Behavior of Superficially Modified Porous Titanium Dental Implants. Materials, 15(11), 3903. https://doi.org/10.3390/ma15113903








