Bio-Synthesis of Aspergillus terreus Mediated Gold Nanoparticle: Antimicrobial, Antioxidant, Antifungal and In Vitro Cytotoxicity Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials Used
2.2. Preparation of Crude Extract of A. terreus AREF023
2.3. Biosynthesis of Gold Nanoparticle GNP023
2.4. Characterization Studies of the Synthesised Gold Nanoparticle GNP023
2.4.1. UV-Visible Spectroscopy and Light Scattering (DLS) Study
2.4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. X-ray Diffraction (XRD) Analysis
2.4.5. UPLC–MS Analysis of the A. terreus AREF023 Biomass
2.5. Bioactivities of Gold Nanoparticle GNP023
2.5.1. Antibacterial Activity of Gold Nanoparticles GNP023
2.5.2. Antifungal Activity of Gold Nanoparticles GNP023 against Phytopathogens
2.5.3. ABTS Scavenging Activity
2.5.4. DNA Nick Assay
2.5.5. Cell Cytotoxicity/Viability Assay
3. Results and Discussion
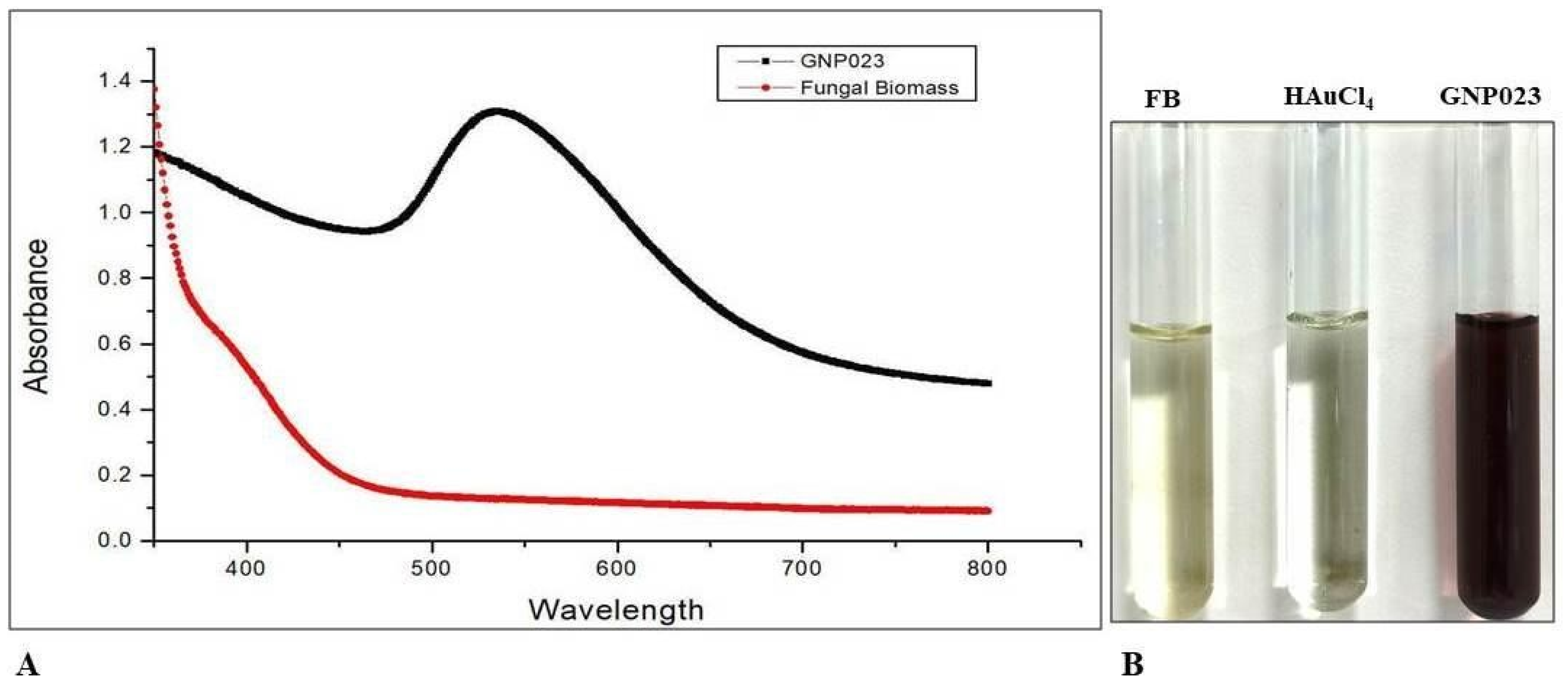
3.1. Bio-Fabrication of Gold Nanoparticles from A. terreus AREF023
3.2. Characterization Studies of the Synthesised Gold Nanoparticle GNP023
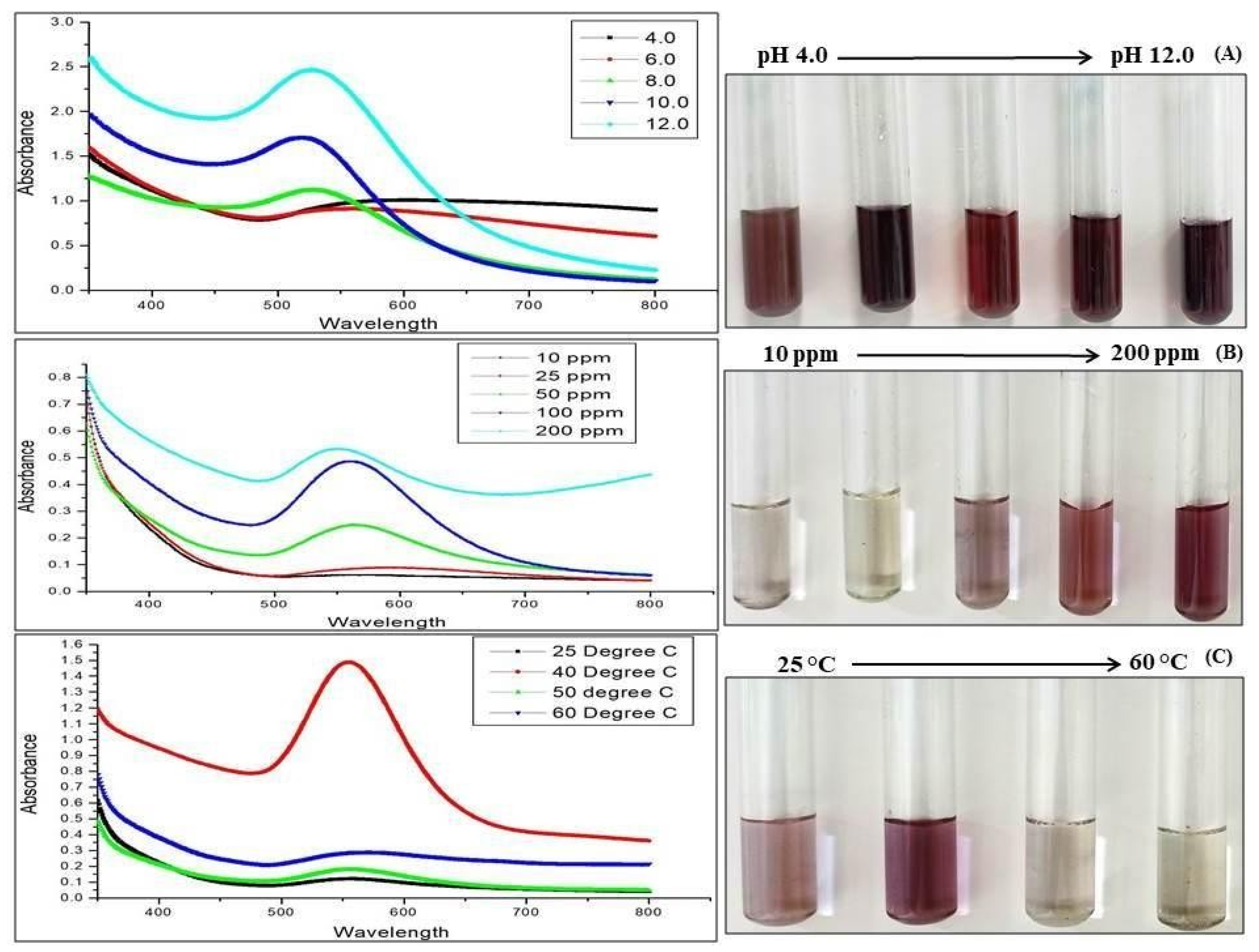
3.2.1. UV-Visible Spectroscopy
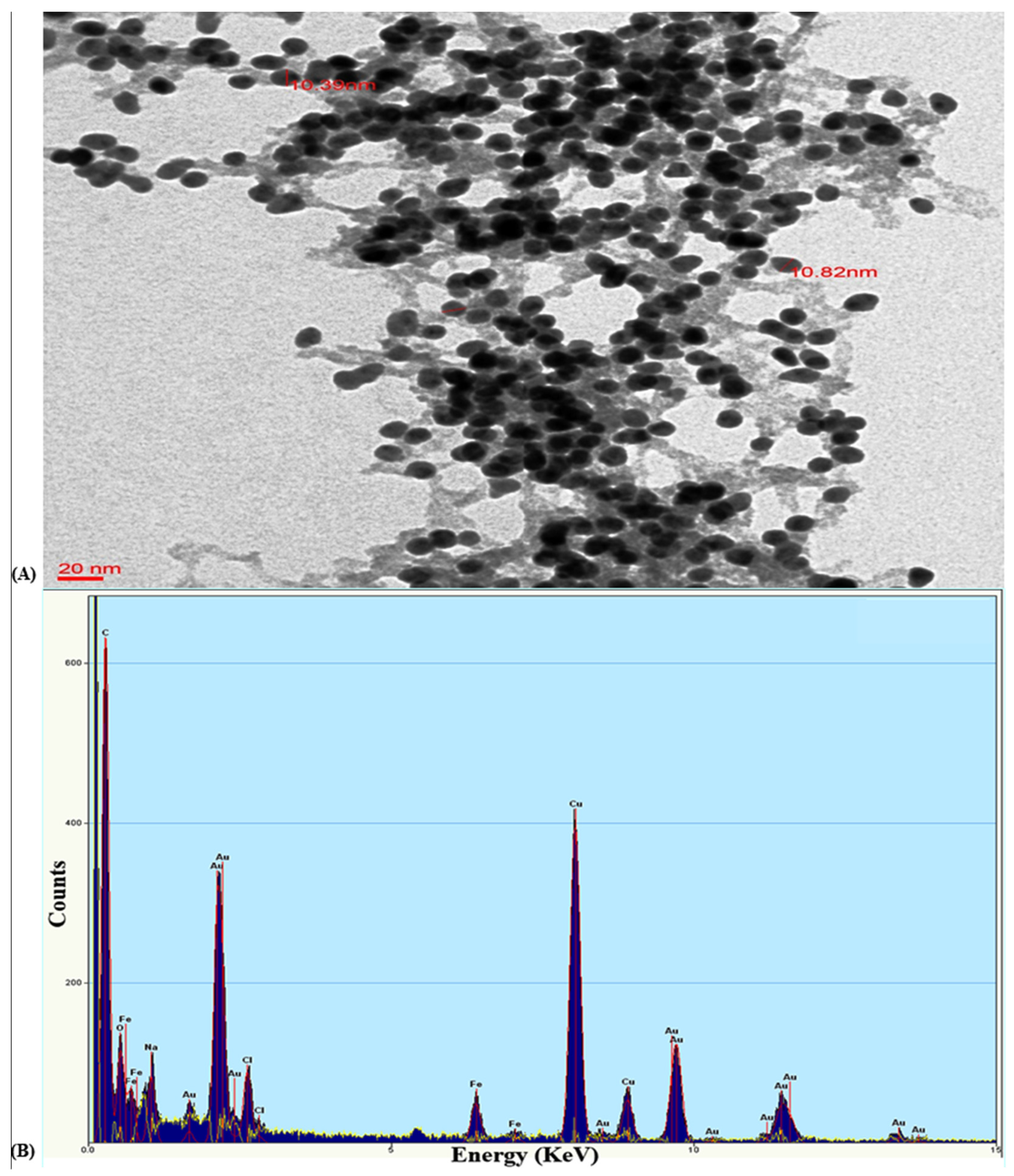
3.2.2. TEM and EDAX Analysis of the Gold Nanoparticle GNP023
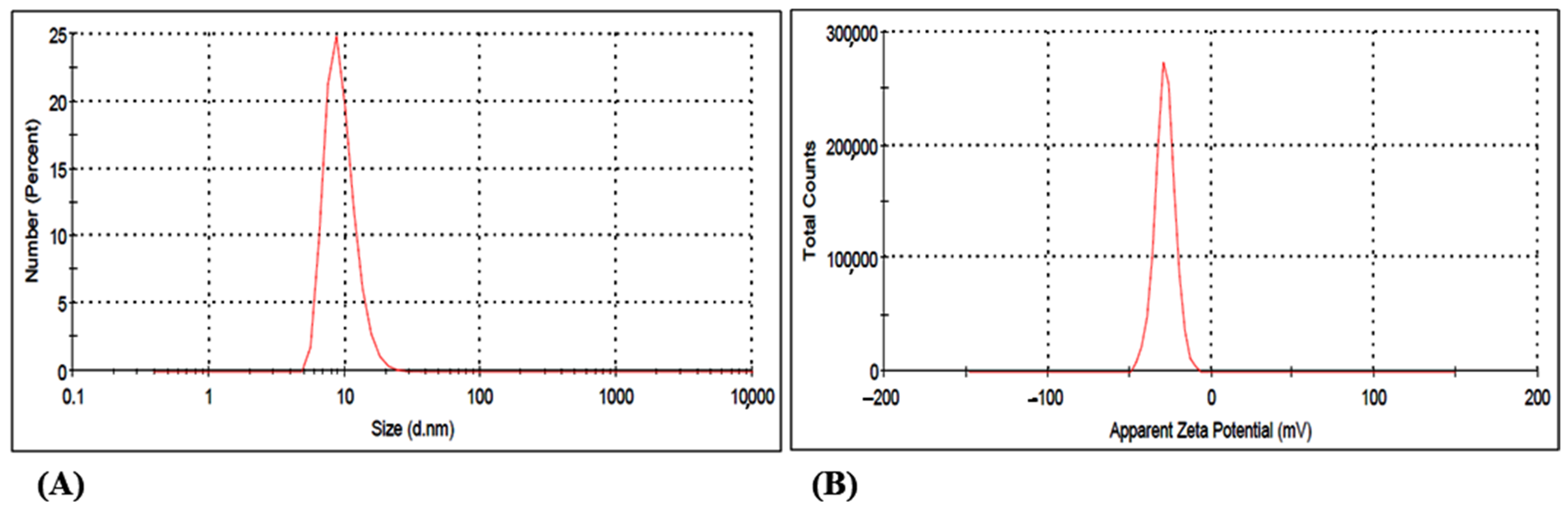
3.2.3. DLS Study of the Gold Nanoparticle GNP023
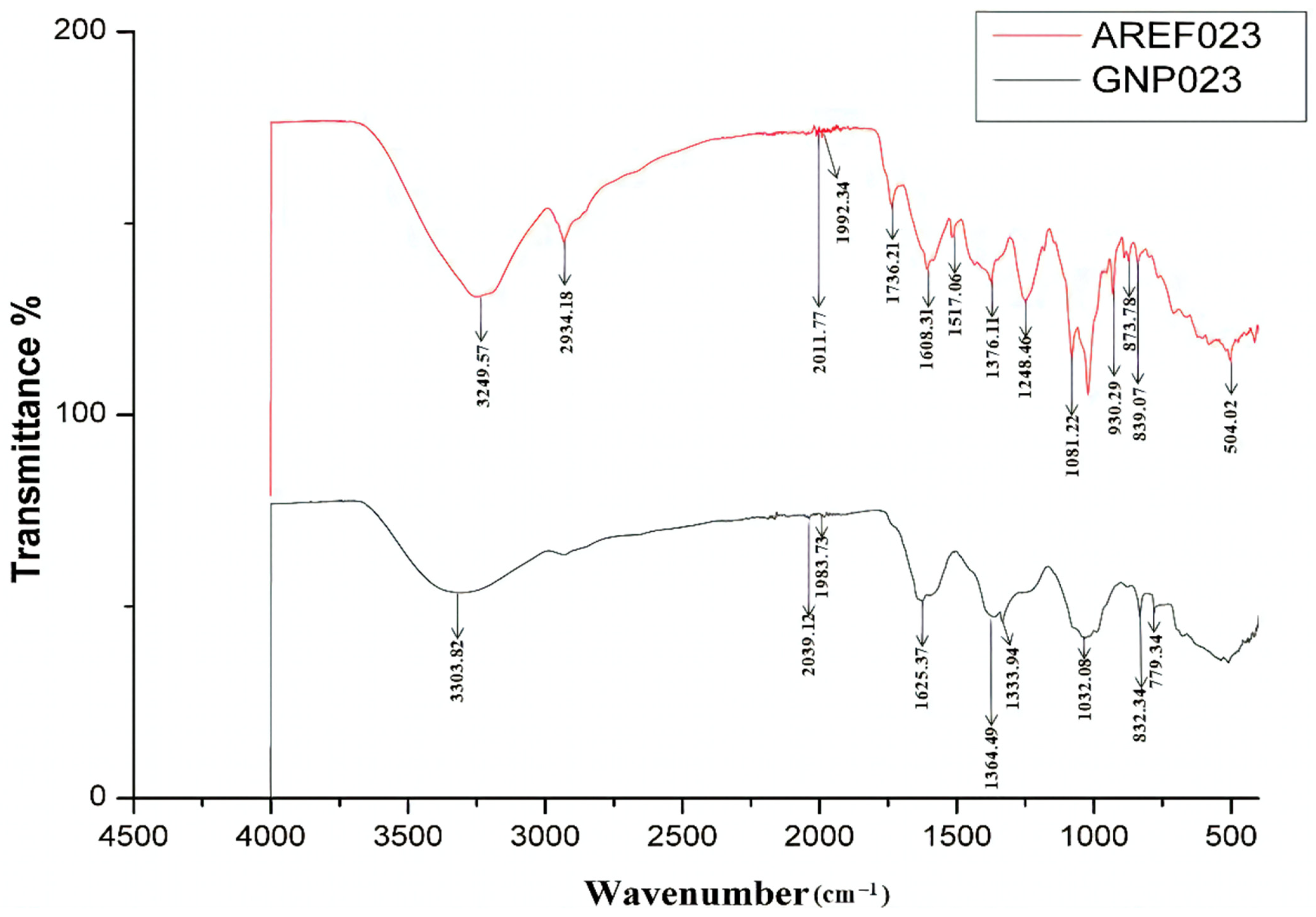
3.2.4. FTIR Analysis of the Gold Nanoparticle GNP023
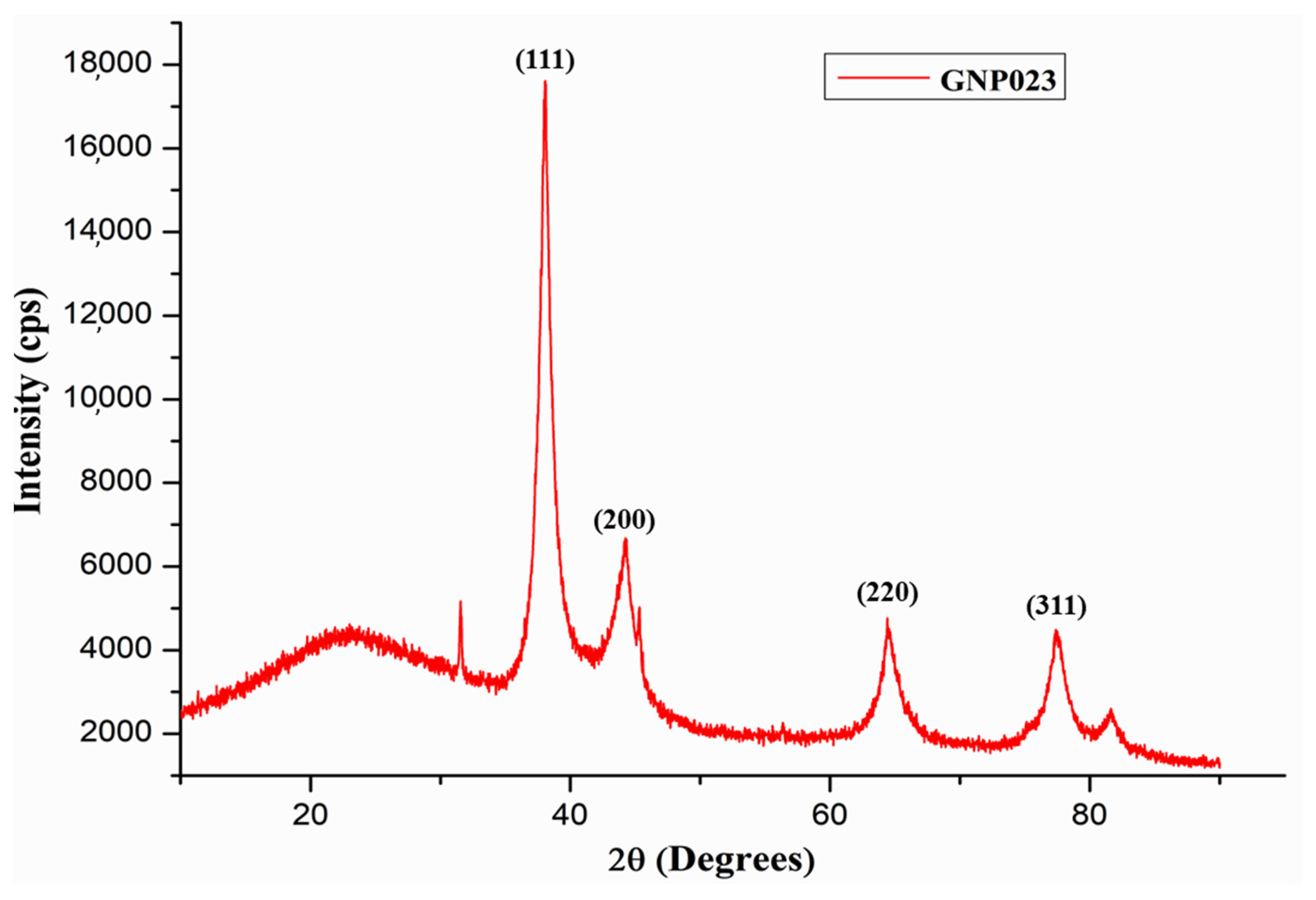
3.2.5. XRD Characterization of the Gold Nanoparticle GNP023
3.3. UHPLC–MS Profiling of the Compounds Present in the Extract of A. terreus AREF023
3.4. Bioactivities of the Synthesised Gold Nanoparticle GNP023
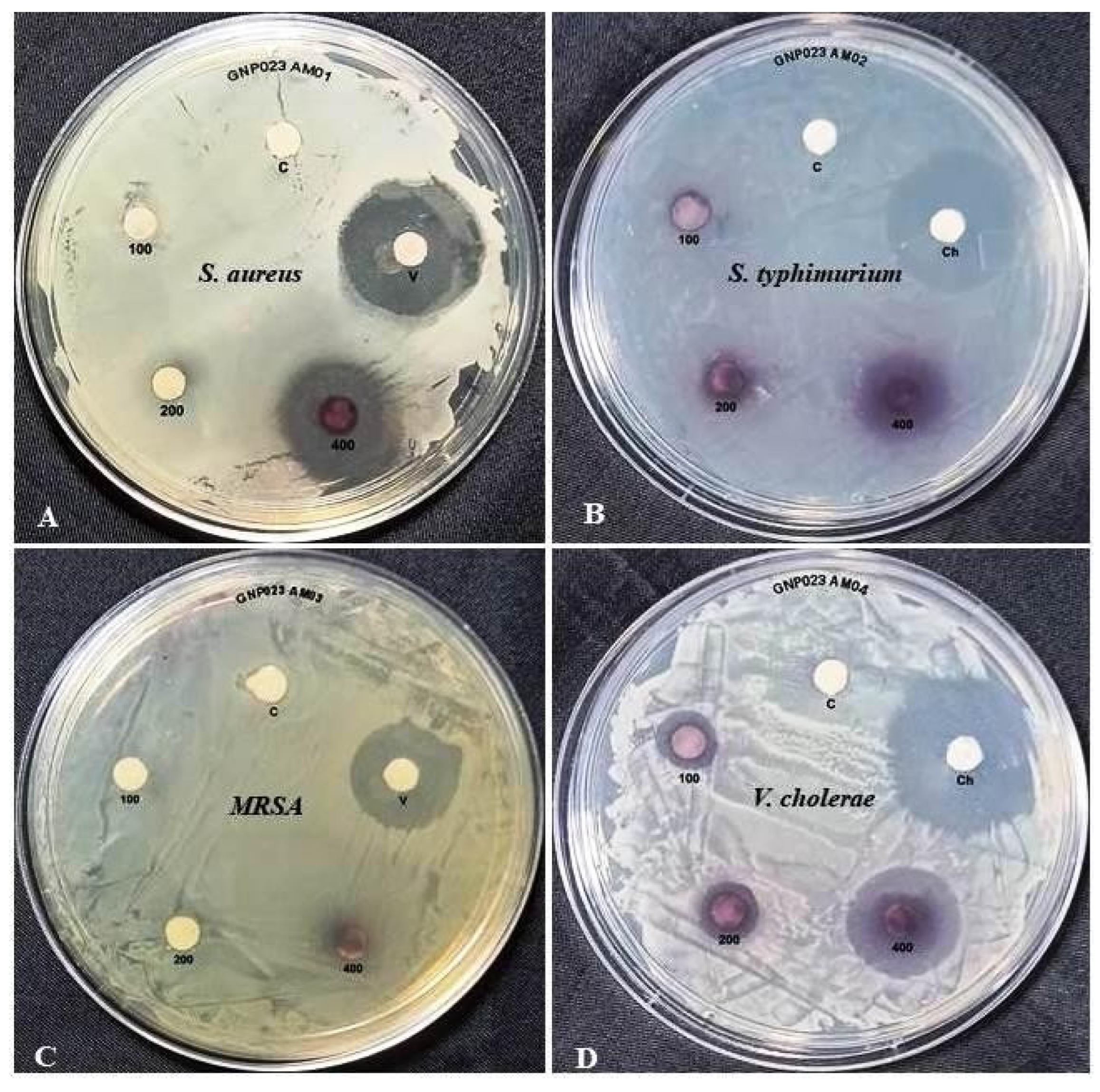
3.4.1. Antimicrobial Activity of the Gold Nanoparticle GNP023
3.4.2. Evaluation of Antifungal Activity of Gold Nanoparticles GNP023
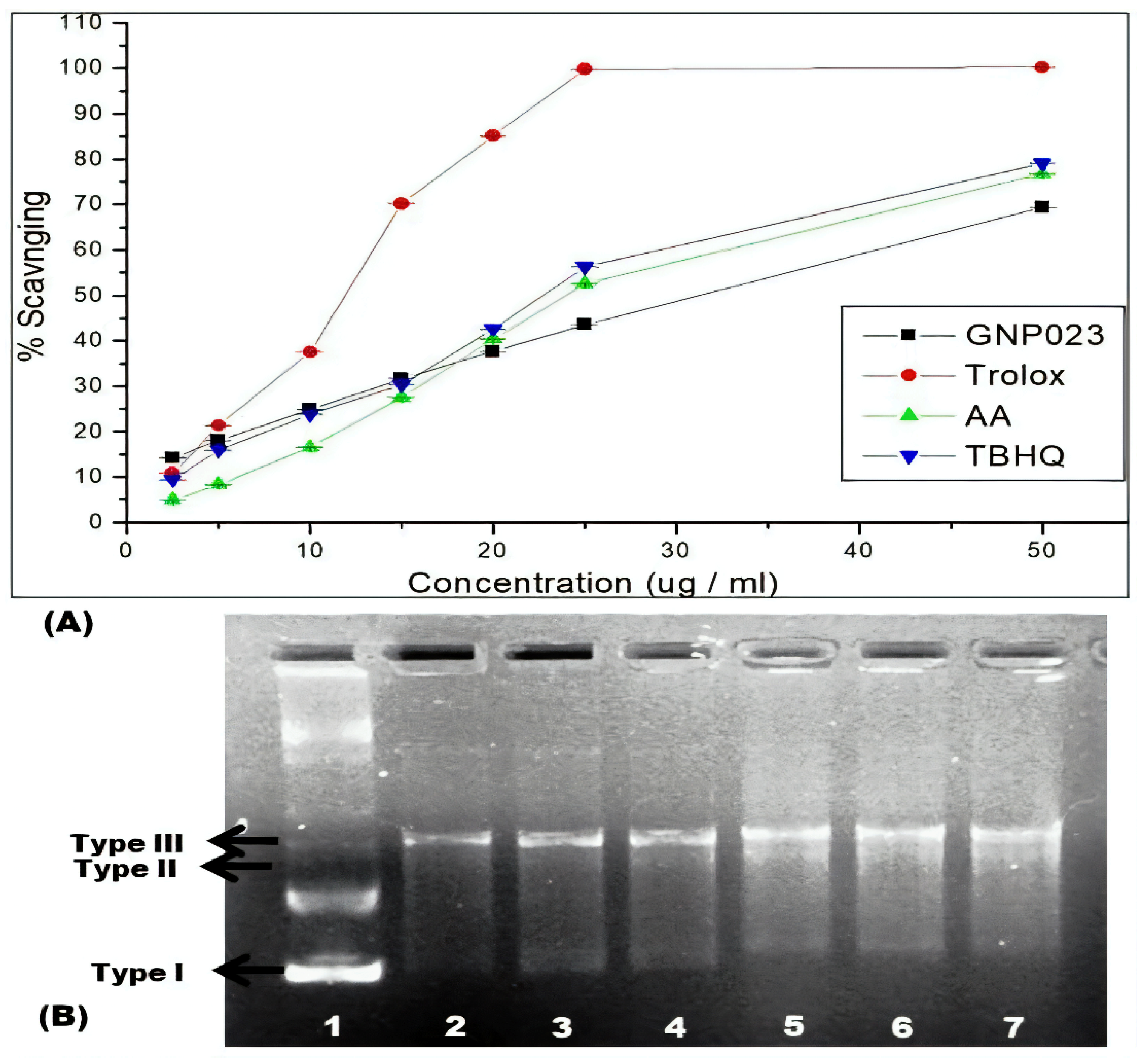
3.4.3. Protective Effect of Gold Nanoparticle GNP023 against ABTS Radical in Antioxidant Assay and Hydroxyl Radical in DNA Nick Assay
3.4.4. Cell Cytotoxicity/Viability Assay of the Gold Nanoparticle GNP023
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid |
| EDX | Energy-dispersive X-ray |
| FTIR | Fourier-Transform Infrared GNP–gold nanoparticle |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide NB–nutrient broth |
| SPR | Surface Plasmon Resonance |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
References
- Elegbede, J.A.; Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Oladipo, I.C.; Aina, D.A.; Beukes, L.S.; Gueguim-Kana, E.B. Biofabrication of gold nanoparticles using xylanases through valorization of corncob by Aspergillus niger and Trichoderma longibrachiatum: Antimicrobial, antioxidant, anticoagulant and thrombolytic activities. Waste Biomass Valorization 2020, 11, 781–791. [Google Scholar] [CrossRef]
- Ojo, S.A.; Lateef, A.; Azeez, M.A.; Oladejo, S.M.; Akinwale, A.S.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Gueguim-Kana, E.B.; et al. Biomedical and catalytic applications of gold and silver-gold alloy nanoparticles biosynthesized using cell-free extract of Bacillus safensis LAU 13: Antifungal, dye degradation, anti-coagulant and thrombolytic activities. IEEE Trans. Nanobiosci. 2016, 15, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Eng, S.-K.; Pusparajah, P.; Mutalib, N.-S.A.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Välimaa, A.-L.; Tilsala-Timisjärvi, A.; Virtanen, E. Rapid detection and identification methods for Listeria monocytogenes in the food chain—A review. Food Control. 2015, 55, 103–114. [Google Scholar] [CrossRef]
- Heiman, K.E.; Mody, R.K.; Johnson, S.D.; Griffin, P.M.; Hannah Gould, L. Escherichia coli O157 outbreaks in the United States, 2003–2012. Emerg. Infect. Dis. 2015, 21, 1293. [Google Scholar] [CrossRef]
- Kothary, M.H.; Babu, U.S. Infective dose of foodborne pathogens in volunteers: A review. J. Food Saf. 2001, 21, 49–68. [Google Scholar] [CrossRef]
- Chukwu, E.E.; Nwaokorie, F.O.; Coker, A.O.; Avila-Campos, M.J.; Solis, R.L.; Llanco, L.A.; Ogunsola, F.T. Detection of toxigenic Clostridium perfringens and Clostridium botulinum from food sold in Lagos, Ni geria. Anaerobe 2016, 42, 176–181. [Google Scholar] [CrossRef]
- Deacon, J.W.; Berry, L.A. Biocontrol of soil-borne plant pathogens: Concepts and their application. Pestic. Sci. 1993, 37, 417–426. [Google Scholar] [CrossRef]
- Mishra, R.C.; Goel, M.; Colin, B.; Deshmukh, S.K. Endophytic fungi-an untapped source of potential antioxidants. Curr. Bioact. Compd. 2020, 16, 944–964. [Google Scholar] [CrossRef]
- Mishra, R.C.; Kalra, R.; Dwivedi, N.; Goel, M. Exploring endophytes using “Omics”: An approach for sustainable production of bioactive metabolites. In Mycoremediation and Environmental Sustainability; Springer: Heidelberg, Germany, 2021; pp. 349–376. [Google Scholar]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Zawrah, M.; El-Moez, S.; Center, D. Antimicrobial activities of gold nanoparticles against major foodborne pathogens. Life Sci. J. 2011, 8, 37–44. [Google Scholar]
- Patra, P.; Shouvik, M.; Nitai, D.; Arunava, G. Biochemical-, biophysical-, and microarray-based antifungal evaluation of the buffer-mediated synthesized nano zinc oxide: An in vivo and in vitro toxicity study. Langmuir 2012, 28, 16966–16978. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Khan, S.; Fu, P. Fungus-(Alternaria sp.) mediated silver nanoparticles synthesis, characterization, and screening of antifungal activity against some phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Samrot, A.V.; Bhavya, K.S.; Sahithya, C.S.; Sowmya, N. Evaluation of toxicity of chemically synthesised gold nanoparticles against Eudrilus eugeniae. J. Clust. Sci. 2018, 29, 1217–1225. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial silver. Met. -Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.-H.; Kim, H.-J.; Seong, C. A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol. J. 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Ramani, R.; Parischa, R.; Ajayakumar, P.V.; Alam, M.; et al. Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Asharani, P.V.; Yi, L.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Qadri, M.; Johri, S.; Shah, B.; Khajuria, A.; Sidiq, T.; Lattoo, S.; Abdin, M.; Riyaz-Ul-Hassan, S. Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. SpringerPlus 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fu, Y.; Luo, M.; Zu, Y.; Wang, W.; Zhao, C.; Gu, C. Endophytic fungi from pigeon pea [Cajanus cajan (L.) Millsp.] produce antioxidant cajaninstilbene acid. J. Agric. Food Chem. 2012, 60, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.C.; Kalra, R.; Dilawari, R.; Deshmukh, S.K.; Barrow, C.J.; Goel, M. Characterization of an endophytic strain Talaromyces assiutensis, CPEF04 with evaluation of production medium for extracellular red pigments having antimicrobial and anticancer properties. Front. Microbiol. 2021, 12, 1578. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI document M100–S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Aguilar-Méndez, M.A.; Martín-Martínez, E.S.; Ortega-Arroyo, L.; Cobián-Portillo, G.; Sánchez-Espíndola, E. Synthesis and characterization of silver nanoparticles: Effect on phytopathogen Colletotrichum gloesporioides. J. Nanoparticle Res. 2011, 13, 2525–2532. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, C.; Tang, M.; Yang, Z.; Jia, W.; Ma, Y.; Jia, P.; Pei, D.; Wang, H. Nanotoxicity of silver nanoparticles on HEK293T cells: A combined study using biomechanical and biological techniques. ACS Omega 2018, 3, 6770–6778. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, V. Endophytes as potential nanofactories. Int. J. Chem. Environ. Biol. Sci. 2013, 1, 488–491. [Google Scholar]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Imran, M.; Khan, Z.U.H.; Tahir, K.; Khan, A.U.; Raza, M.; Khana, Q.; Yuan, Q. Size dependent catalytic activities of green synthesized gold nanoparticles and electro-catalytic oxidation of catechol on gold nanoparticles modified electrode. RSC Adv. 2015, 5, 99364–99377. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Binupriya, A.R.; Sathishkumar, M.; Vijayaraghavan, K.; Yun, S.-I. Bioreduction of trivalent aurum to nano-crystalline gold particles by active and inactive cells and cell-free extract of Aspergillus oryzae var. viridis. J. Hazard. Mater. 2010, 177, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.K.; Bonde, P.P.; Ingle, A.; Marcato, P.D. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’souza, S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Parial, D.; Patra, H.K.; Dasgupta, A.K.; Pal, R. Screening of different algae for green synthesis of gold nanoparticles. Eur. J. Phycol. 2012, 47, 22–29. [Google Scholar] [CrossRef]
- Singh, A.K.; Talat, M.; Singh, D.P.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- Ke, Y.; Aboody, M.S.A.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef]
- Song, J.Y.; Jang, H.-K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Verma, V.C.; Singh, S.K.; Solanki, R.; Prakash, S. Biofabrication of anisotropic gold nanotriangles using extract of endophytic Aspergillus clavatus as a dual functional reductant and stabilizer. Nanoscale Res. Lett. 2011, 6, 16. [Google Scholar] [CrossRef]
- Varghese, S.; Akshaya, C.; Jisha, M. Unravelling the bioprospects of mycoendophytes residing in withania somnifera for productive pharmaceutical applications. Biocatal. Agric. Biotechnol. 2021, 37, 102172. [Google Scholar] [CrossRef]
- Aswani, P.; Tijith, K.; George, T.; Shanavas, J. Characterization of bioactive metabolites of endophytic Fusarium solani isolated from Withania somnifera. J. Biol. Act. Products Nat. 2017, 7, 411–426. [Google Scholar]
- Fujimoto, H.; Nakayama, Y.; Yamazaki, M. Identification of immunosuppressive components of a mushroom, Lactarius flavidulus. Chem. Pharm. Bull. 1993, 41, 654–658. [Google Scholar] [CrossRef][Green Version]
- Chávez, M.I.; Soto, M.; Taborga, L.; Díaz, K.; Olea, A.F.; Bay, C.; Peña-Cortés, H.; Espinoza, L. Synthesis and in vitro antifungal activity against Botrytis cinerea of geranylated phenols and their phenyl acetate derivatives. Int. J. Mol. Sci. 2015, 16, 19130–19152. [Google Scholar] [CrossRef] [PubMed]
- Iorizzi, M.; Lanzotti, V.; Ranalli, G.; De Marino, S.; Zollo, F. Antimicrobial furostanol saponins from the seeds of Capsicum annuum L. var. acuminatum. J. Agric. Food Chem. 2002, 50, 4310–4316. [Google Scholar] [CrossRef]
- de Rodríguez, D.J.; Angulo-Sánchez, J.L.; Hernández-Castillo, F.D. An overview of the antimicrobial properties of Mexican medicinal plants. Adv. Phytomed. 2006, 3, 325–377. [Google Scholar]
- Ibrahim, S.R.M.; Mohamed, G.A.; Haidari, R.A.A.; Zayed, M.F.; El-Kholy, A.A.; Elkhayat, E.S.; Ross, S.A. Fusarithioamide B, a new benzamide derivative from the endophytic fungus Fusarium chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorganic Med. Chem. 2018, 26, 786–790. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Chamakura, K.; Perez-Ballestero, R.; Luo, Z.; Bashir, S.; Liu, J. Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloids Surf. B Biointerfaces 2011, 84, 88–96. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Zada, S.; Ahmad, A.; Khan, S.; Iqbal, A.; Ahmad, S.; Ali, H.; Fu, P. Biofabrication of gold nanoparticles by Lyptolyngbya JSC-1 extract as super reducing and stabilizing agents: Synthesis, characterization and antibacterial activity. Microb. Pathog. 2018, 114, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Wieczorek, H. The V-type H+ ATPase: Molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006, 209, 577–589. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, K.H.; Su, X. Study of single-stranded DNA binding protein-nucleic acids interactions using unmodified gold nanoparticles and its application for detection of single nucleotide polymorphisms. Anal. Chem. 2011, 83, 4251–4257. [Google Scholar] [CrossRef]
- Adebayo, A.E.; Oke, A.M.; Lateef, A.; Oyatokun, A.A.; Abisoye, O.D.; Adiji, I.P.; Fagbenro, D.O.; Amusan, T.V.; Badmus, J.A.; Asafa, T.B.; et al. Biosynthesis of silver, gold and silver–gold alloy nanoparticles using Persea americana fruit peel aqueous extract for their biomedical properties. Nanotechnol. Environ. Eng. 2019, 4, 13. [Google Scholar] [CrossRef]
- Leba, L.-J.; Brunschwig, C.; Saout, M.; Martial, K.; Vulcain, E.; Bereau, D.; Robinson, J.-C. Optimization of a DNA nicking assay to evaluate Oenocarpus bataua and Camellia sinensis antioxidant capacity. Int. J. Mol. Sci. 2014, 15, 18023–18039. [Google Scholar] [CrossRef]



| Analyte No. | Tentative Allotment of Compounds Based on METLIN | Chemical Formula | Parent Ion (m/z) (Positive Ion Mode [M + H] | Peak Intensity | Mass Recorded (METLIN) | METLIN ID |
|---|---|---|---|---|---|---|
| 1 | Levothyroxine sodium anhydrous | C15H11I4NO4 | 799.6769 | 8.05 × 105 | 798.6686 | 66850 |
| 2 | Sitoindoside I | C51H90O7 | 815.6778 | 1.84 × 106 | 814.6687 | 87222 |
| 3 | 3,3′-Difluoro-4-[(6-methyloctyl)oxy]-4′-undecyl-1,1′-biphenyl | C32H48F2O | 487.3727 | 3.81 × 106 | 486.3673 | 894417 |
| 4 | Flavidulol C | C34H42O4 | 515.3141 | 6.69 × 105 | 514.3083 | 93747 |
| 5 | Capsicoside C3 | C44H70O17 | 871.4661 | 1.49 × 106 | 870.4613 | 95394 |
| 6 | tert-Butyl {5-[6-(benzyloxy)naphthalen-2-yl]-2,2-dimethyl-1,3-dioxan-5-yl}carbamate | C28H33NO5 | 464.2419 | 1.60 × 106 | 463.2359 | 862873 |
| 7 | N-(2-Hydroxyethyl)-3,5-bis(octadecyloxy)benzamide | C45H83NO4 | 702.6394 | 1.36 × 105 | 701.6322 | 909645 |
| Diameter of Zone of Inhibition (mm) * against Pathogenic Bacteria | ||||
|---|---|---|---|---|
| Concentrations (µg/mL) | S. aureus | S. typhimurium | Methicillin resistant S. aureus | V. cholerae |
| DMSO (control) | 0 | 0 | 0 | 0 |
| GNP023—100 | 0 | 0 | 0 | 5.2 ± 0.18 |
| GNP023—200 | 4.1 ± 0.07 | 0 | 0 | 6.1 ± 0.12 |
| GNP023—400 | 8.58 ± 0.28 | 0 | 6.1 ± 0.12 | 9.31 ± 0.14 |
| Vancomycin (25 µg/ disk) | 10.06 ± 0.16 | - | 9.53 ± 0.23 | - |
| Chloramphenicol (25 µg/disk) | - | 10.51 ± 0.23 | - | 10.53 ± 0.21 |
| Pathogenic Fungal Strain | Percentage Inhibition (%) * Mycelial Growth after GNP023 (µg/mL) Treatment | ||||
|---|---|---|---|---|---|
| Control | 25 µg/mL | 50 µg/mL | 100 µg/mL | 200 µg/mL | |
| Rhizoctonia solani | 0 | 10.75 ± 0.22 | 31.96 ± 0.42 | 36.24 ± 0.55 | 52.5 ± 0.47 |
| Fusarium oxysporum | 0 | 18.28 ± 0.46 | 34.51 ± 0.38 | 56.30 ± 0.45 | 65.46 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, R.C.; Kalra, R.; Dilawari, R.; Goel, M.; Barrow, C.J. Bio-Synthesis of Aspergillus terreus Mediated Gold Nanoparticle: Antimicrobial, Antioxidant, Antifungal and In Vitro Cytotoxicity Studies. Materials 2022, 15, 3877. https://doi.org/10.3390/ma15113877
Mishra RC, Kalra R, Dilawari R, Goel M, Barrow CJ. Bio-Synthesis of Aspergillus terreus Mediated Gold Nanoparticle: Antimicrobial, Antioxidant, Antifungal and In Vitro Cytotoxicity Studies. Materials. 2022; 15(11):3877. https://doi.org/10.3390/ma15113877
Chicago/Turabian StyleMishra, Rahul Chandra, Rishu Kalra, Rahul Dilawari, Mayurika Goel, and Colin J. Barrow. 2022. "Bio-Synthesis of Aspergillus terreus Mediated Gold Nanoparticle: Antimicrobial, Antioxidant, Antifungal and In Vitro Cytotoxicity Studies" Materials 15, no. 11: 3877. https://doi.org/10.3390/ma15113877
APA StyleMishra, R. C., Kalra, R., Dilawari, R., Goel, M., & Barrow, C. J. (2022). Bio-Synthesis of Aspergillus terreus Mediated Gold Nanoparticle: Antimicrobial, Antioxidant, Antifungal and In Vitro Cytotoxicity Studies. Materials, 15(11), 3877. https://doi.org/10.3390/ma15113877







