Surface Kinetic Mechanisms of Epitaxial Chemical Vapour Deposition of 4H Silicon Carbide Growth by Methyltrichlorosilane-H2 Gaseous System
Abstract
:1. Introduction
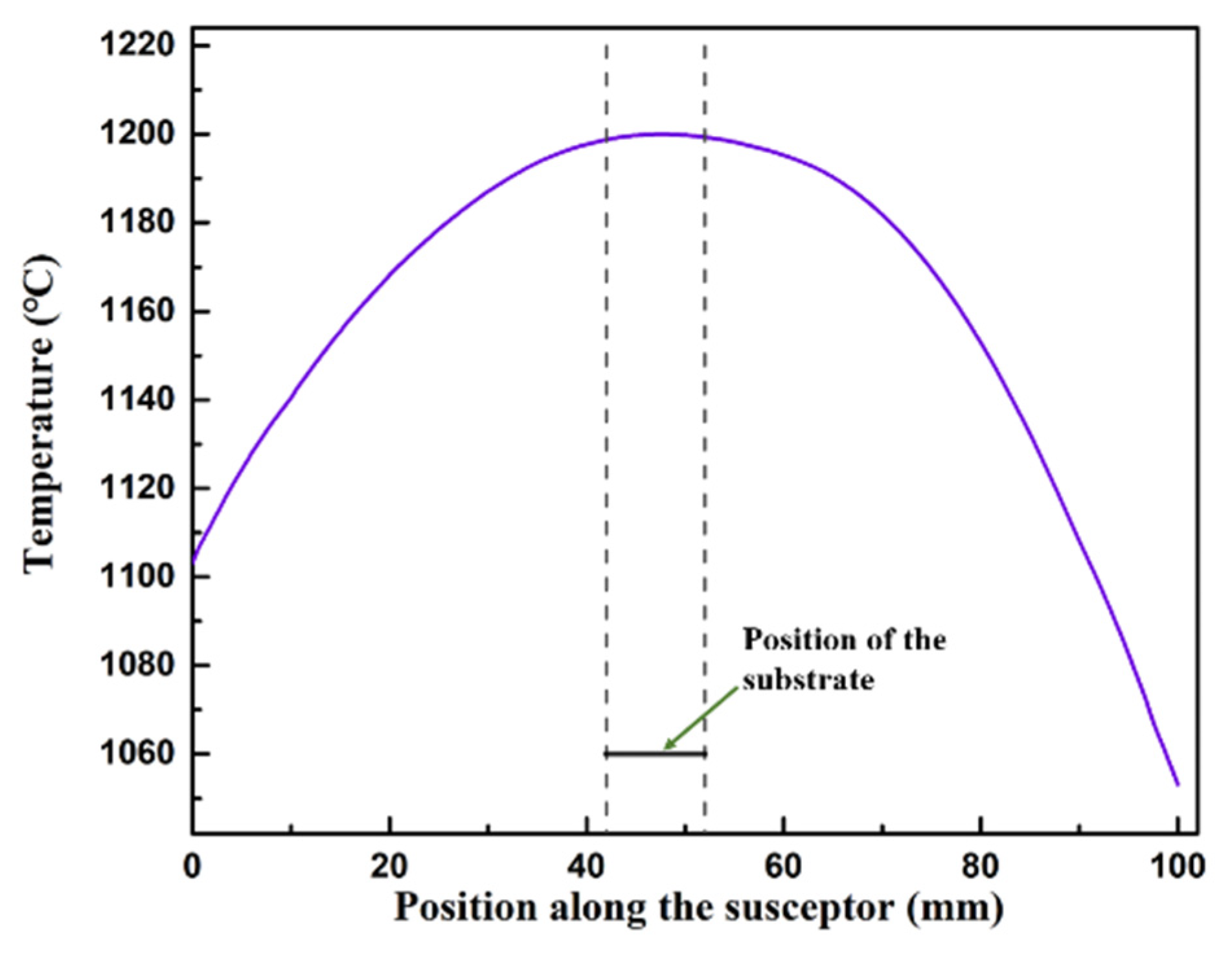
2. Numerical Modeling
3. Results and Discussion
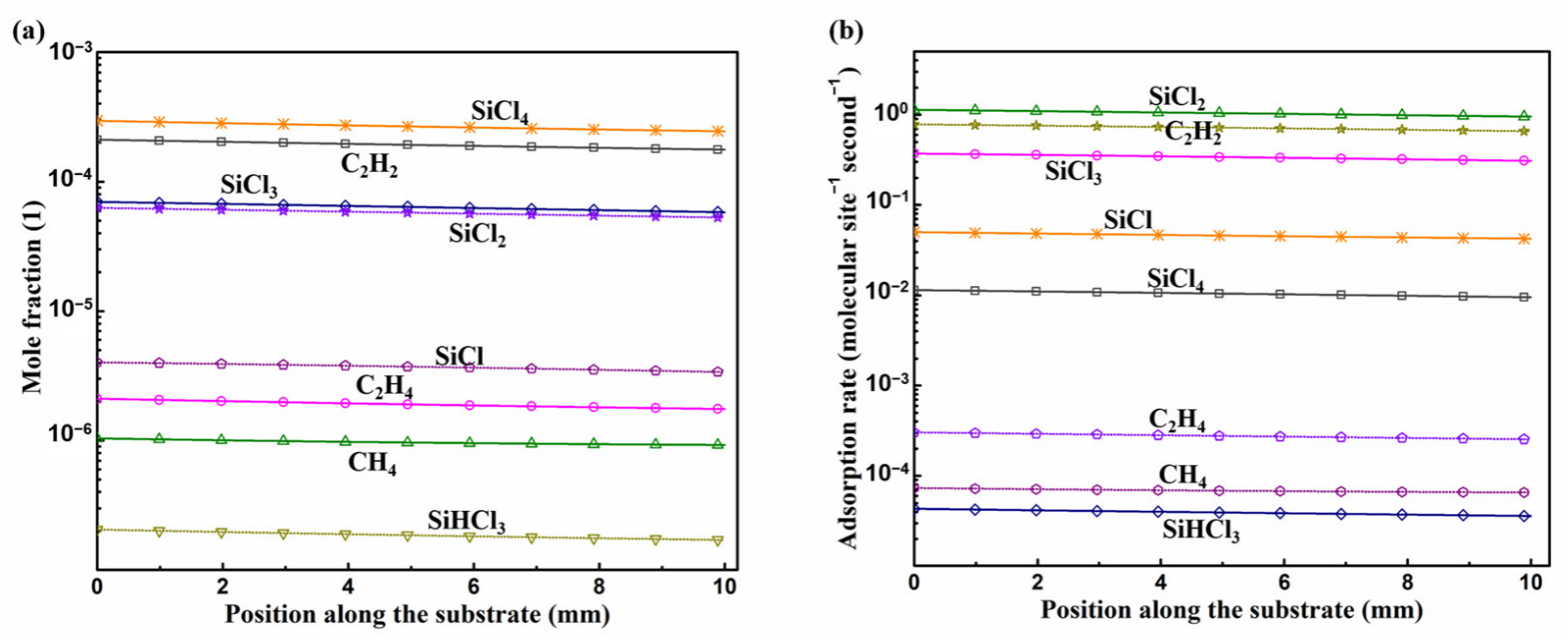
3.1. Gas Phase Reaction
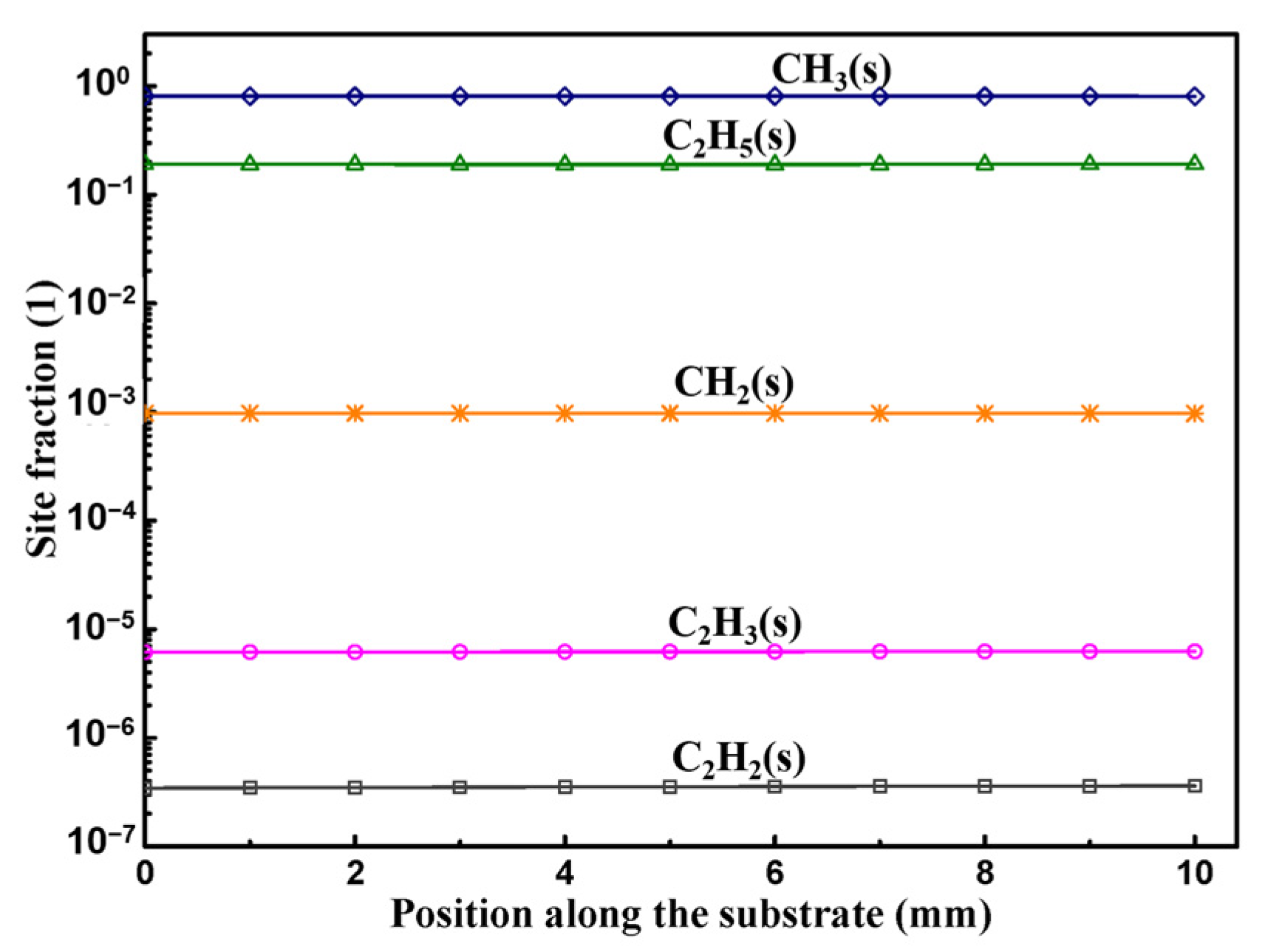
3.2. Surface Reactions on Si Face
3.3. Surface Reactions on Si Face
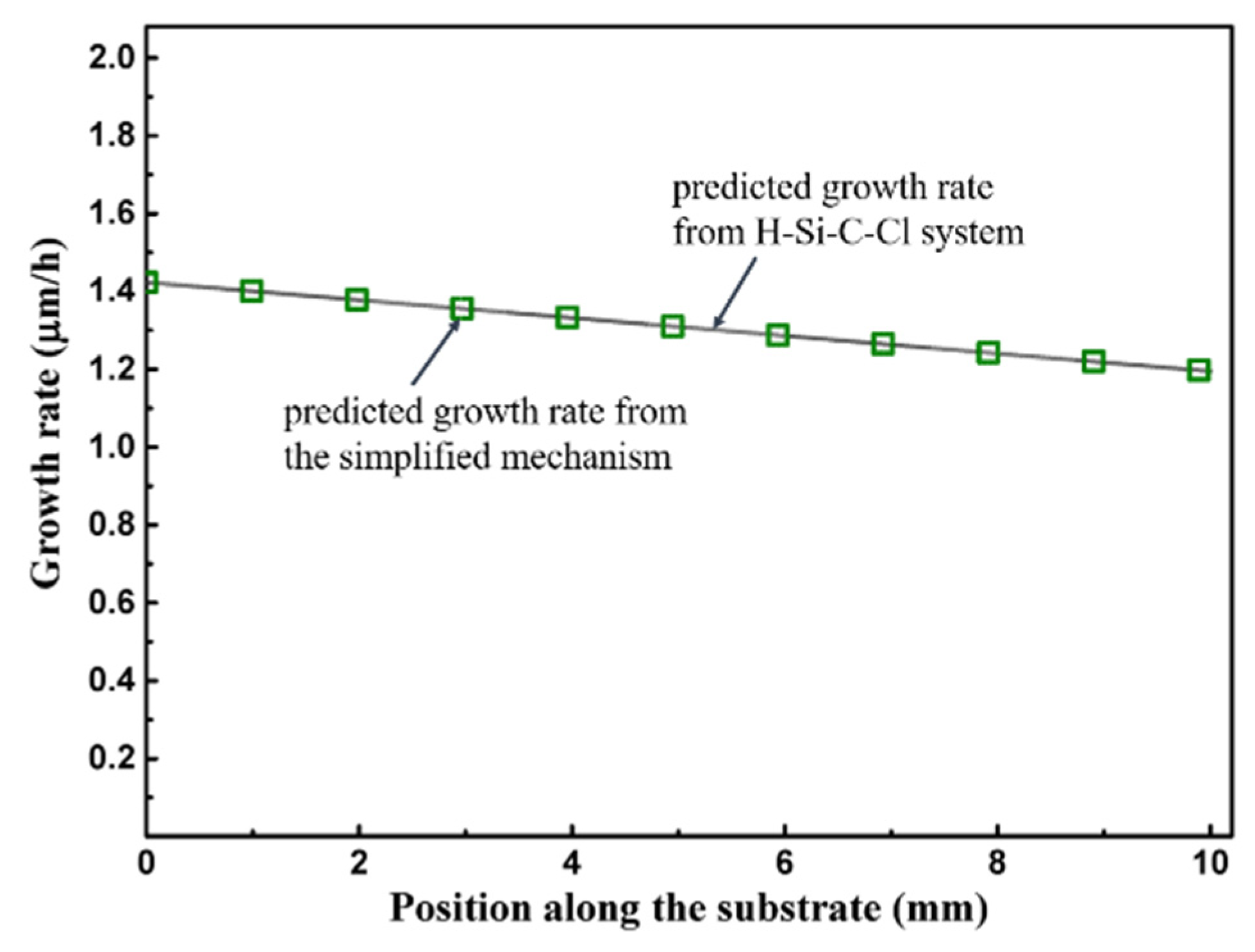
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Foti, G. Silicon carbide: From amorphous to crystalline material. Appl. Surf. Sci. 2001, 184, 20–26. [Google Scholar] [CrossRef]
- Guillermet, A.F. Analysis of Thermochemical Properties and Phase-Stability in the Zirconium Carbon System. J. Alloys Compd. 1995, 217, 69–89. [Google Scholar] [CrossRef]
- Raghunathan, R.; Alok, D.; Baliga, B.J. High-Voltage 4h-Sic Schottky-Barrier Diodes. IEEE Electr. Device Lett. 1995, 16, 226–227. [Google Scholar] [CrossRef]
- Itoh, A.; Kimoto, T.; Matsunami, H. High-Performance of High-Voltage 4h-Sic Schottky-Barrier Diodes. IEEE Electr. Device Lett. 1995, 16, 280–282. [Google Scholar] [CrossRef]
- Lofgren, P.M.; Ji, W.; Hallin, C.; Gu, C.Y. Modeling of silicon carbide epitaxial growth in hot-wall chemical vapor deposition processes. J. Electrochem. Soc. 2000, 147, 164–175. [Google Scholar] [CrossRef]
- Leone, S.; Kordina, O.; Henry, A.; Nishizawa, S.; Danielsson, O.; Janzen, E. Gas-Phase Modeling of Chlorine-Based Chemical Vapor Deposition of Silicon Carbide. Cryst. Growth Des. 2012, 12, 1977–1984. [Google Scholar] [CrossRef]
- Kimoto, T. Material science and device physics in SiC technology for high-voltage power devices. Jpn. J. Appl. Phys. 2015, 54. [Google Scholar] [CrossRef]
- Snead, L.L.; Nozawa, T.; Ferraris, M.; Katoh, Y.; Shinavski, R.; Sawan, M. Silicon carbide composites as fusion power reactor structural materials. J. Nucl. Mater. 2011, 417, 330–339. [Google Scholar] [CrossRef]
- Naslain, R.R. SiC-matrix composites: Nonbrittle ceramics for thermo-structural application. Int. J. Appl. Ceram. Technol. 2005, 2, 75–84. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.B.; Yang, H.; Du, S.Y.; Huang, Q. The critical issues of SiC materials for future nuclear systems. Scripta Mater 2018, 143, 149–153. [Google Scholar] [CrossRef]
- Danielsson, O.; Henry, A.; Janzen, E. Growth rate predictions of chemical vapor deposited silicon carbide epitaxial layers. J. Cryst. Growth 2002, 243, 170–184. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Kee, R.J. A Model of Silicon-Carbide Chemical Vapor-Deposition. J. Electrochem. Soc. 1991, 138, 841–852. [Google Scholar] [CrossRef]
- Meziere, J.; Ucar, M.; Blanquet, E.; Pons, M.; Ferret, P.; Di Cioccio, L. Modeling and simulation of SiC CVD in the horizontal hot-wall reactor concept. J. Cryst. Growth 2004, 267, 436–451. [Google Scholar] [CrossRef]
- Nishizawa, S.; Pons, M. Growth and doping modeling of SiC-CVD in a horizontal hot-wall reactor. Chem. Vapor Depos. 2006, 12, 516–522. [Google Scholar] [CrossRef]
- Crippa, D.; Valente, G.L.; Ruggiero, A.; Neri, L.; Reitano, R.; Calcagno, L.; Foti, G.; Mauceri, M.; Leone, S.; Pistone, G.; et al. New achievements on CVD based methods for SIC epitaxial growth. Mater. Sci. Forum 2005, 483, 67–71. [Google Scholar] [CrossRef]
- La Via, F.; Galvagno, G.; Foti, G.; Mauceri, M.; Leone, S.; Pistone, G.; Abbondanza, G.; Veneroni, A.; Masi, M.; Valente, G.L.; et al. 4H SiC epitaxial growth with chlorine addition. Chem. Vapor Depos. 2006, 12, 509–515. [Google Scholar] [CrossRef]
- La Via, F.; Galvagno, G.; Roccaforte, F.; Giannazzo, F.; Di Franco, S.; Ruggiero, A.; Reitano, R.; Calcagno, L.; Foti, G.; Mauceri, M.; et al. High growth rate process in a SiC horizontal CVD reactor using HCl. Microelectron. Eng. 2006, 83, 48–50. [Google Scholar] [CrossRef]
- Wang, R.; Ma, R.H. An integrated model for halide chemical vapor deposition of silicon carbide epitaxial films. J. Cryst. Growth 2008, 310, 4248–4255. [Google Scholar] [CrossRef]
- Wang, R.; Ma, R.H.; Dudley, M. Reduction of Chemical Reaction Mechanism for Halide-Assisted Silicon Carbide Epitaxial Film Deposition. Ind. Eng. Chem. Res. 2009, 48, 3860–3866. [Google Scholar] [CrossRef]
- Kimoto, T. Bulk and epitaxial growth of silicon carbide. Prog. Cryst. Growth Charact. Mater. 2016, 62, 329–351. [Google Scholar] [CrossRef]
- Pedersen, H.; Leone, S.; Kordina, O.; Henry, A.; Nishizawa, S.; Koshka, Y.; Janzen, E. Chloride-Based CVD Growth of Silicon Carbide for Electronic Applications. Chem. Rev. 2012, 112, 2434–2453. [Google Scholar] [CrossRef] [PubMed]
- Burk, A.A.; Rowland, L.B. Reduction of unintentional aluminum spikes at SiC vapor phase epitaxial layer substrate interfaces. Appl. Phys. Lett. 1996, 68, 382–384. [Google Scholar] [CrossRef]
- Hallin, C.; Bakin, A.S.; Owman, F.; Martensson, P.; Kordina, O.; Janzen, E. Study of the hydrogen etching of silicon carbide substrates. Inst. Phys. Conf. Ser. 1996, 142, 613–616. [Google Scholar]
- Veneroni, A.; Masi, M. Gas-phase and surface kinetics of epitaxial silicon carbide growth involving chlorine-containing species. Chem. Vapor Depos. 2006, 12, 562–568. [Google Scholar] [CrossRef]
- Josiek, A.; Langlais, F. Residence-time dependent kinetics of CVD growth of SiC in the MTS/H-2 system. J. Cryst. Growth 1996, 160, 253–260. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.G. Chemical vapor deposition of SiC at different molar ratios of hydrogen to methyltrichlorosilane. J. Cent. South Univ. Technol. 2009, 16, 730–737. [Google Scholar] [CrossRef]
- Stinton, D.P.; Hembree, D.M.; More, K.L.; Sheldon, B.W.; Besmann, T.M.; Headinger, M.H.; Davis, R.F. Matrix Characterization of Fiber-Reinforced Sic Matrix Composites Fabricated by Chemical-Vapor Infiltration. J. Mater. Sci. 1995, 30, 4279–4285. [Google Scholar] [CrossRef]
- Wang, L.J.; Chen, Z.K.; Wang, B.W.; Li, Y.; Zhang, R.Q.; Liu, G.L.; He, Z.B.; Fu, D.G.; Wang, H.R.; Xiong, X. Effect of free carbon on micro-mechanical properties of a chemically vapor deposited SiC coating. Ceram. Int. 2018, 44, 17118–17123. [Google Scholar] [CrossRef]
- Peng, J.; Jolly, B.; Mitchell, D.J.; Haynes, J.A.; Shin, D. Computational thermodynamic study of SiC chemical vapor deposition from MTS-H-2. J. Am. Ceram. Soc. 2021, 104, 3726–3737. [Google Scholar] [CrossRef]
- Mollick, P.K.; Venugopalan, R.; Srivastava, D. CFD coupled kinetic modeling and simulation of hot wall vertical tubular reactor for deposition of SiC crystal from MTS. J. Cryst. Growth 2017, 475, 97–109. [Google Scholar] [CrossRef]
- Cavallotti, C.; Rossi, F.; Ravasio, S.; Masi, M. A Kinetic Analysis of the Growth and Doping Kinetics of the SiC Chemical Vapor Deposition Process. Ind. Eng. Chem. Res. 2014, 53, 9076–9087. [Google Scholar] [CrossRef]
- Cagliostro, D.E.; Riccitiello, S.R.; Carswell, M.G. Analysis of the Pyrolysis Products of Dimethyldichlorosilane in the Chemical Vapor-Deposition of Silicon-Carbide in Argon. J. Am. Ceram. Soc. 1990, 73, 607–614. [Google Scholar] [CrossRef]
- Cagliostro, D.E.; Riccitiello, S.R. Model for the Formation of Silicon-Carbide from the Pyrolysis of Dichlorodimethylsilane in Hydrogen: 1, Silicon Formation from Chlorosilanes. J. Am. Ceram. Soc. 1993, 76, 39–48. [Google Scholar] [CrossRef]
- Cagliostro, D.E.; Riccitiello, S.R. Model for the Formation of Silicon-Carbide from the Pyrolysis of Dichlorodimethylsilane in Hydrogen.2. Silicon-Carbide Formation from Silicon and Methane. J. Am. Ceram. Soc. 1993, 76, 49–53. [Google Scholar] [CrossRef]
- Loumagne, F.; Langlais, F.; Naslain, R. Reactional Mechanisms of the Chemical-Vapor-Deposition of Sic-Based Ceramics from Ch3sicl3/H-2 Gas Precursor. J. Cryst. Growth 1995, 155, 205–213. [Google Scholar] [CrossRef]
- Fischman, G.S.; Petuskey, W.T. Thermodynamic Analysis and Kinetic Implications of Chemical Vapor-Deposition of Sic from Si-C-Cl-H Gas Systems. J. Am. Ceram. Soc. 1985, 68, 185–190. [Google Scholar] [CrossRef]
- Brennfleck, K.; Schneweis, S.; Weiss, R. In-situ-spectroscopic monitoring for SiC-CVD process control. J. Phys. IV 1999, 9, 1041–1048. [Google Scholar] [CrossRef]
- Papasouliotis, G.D.; Sotirchos, S.V. On the Homogeneous Chemistry of the Thermal-Decomposition of Methyltrichlorosilane—Thermodynamic Analysis and Kinetic Modeling. J. Electrochem. Soc. 1994, 141, 1599–1611. [Google Scholar] [CrossRef]
- Deng, J.L.; Su, K.H.; Wang, X.; Zeng, Q.F.; Cheng, L.F.; Xu, Y.D.; Zhang, L.T. Thermodynamics of the gas-phase reactions in chemical vapor deposition of silicon carbide with methyltrichlorosilane precursor. Theor. Chem. Acc. 2009, 122, 1–22. [Google Scholar] [CrossRef]
- Deng, J.L.; Su, K.H.; Zeng, Q.F.; Wang, X.; Cheng, L.F.; Xu, Y.D.; Zhang, L.T. Thermodynamics of the Production of Condensed Phases in the CVD of Methyltrichlorosilane Pyrolysis. Chem. Vapor Depos. 2009, 15, 281–290. [Google Scholar] [CrossRef]
- Fiorucci, A.; Moscatelli, D.; Masi, M. Homoepitaxial silicon carbide deposition processes via chlorine routes. Surf. Coat. Technol. 2007, 201, 8825–8829. [Google Scholar] [CrossRef]
- Guan, K.; Gao, Y.; Zeng, Q.F.; Luan, X.G.; Zhang, Y.; Cheng, L.F.; Wu, J.Q.; Lu, Z.Y. Numerical modeling of SiC by low-pressure chemical vapor deposition from methyltrichlorosilane. Chin. J. Chem. Eng. 2020, 28, 1733–1743. [Google Scholar] [CrossRef]
- Guan, K.; Zeng, Q.F.; Liu, Y.S.; Luan, X.G.; Lu, Z.Y.; Wu, J.Q. A multiscale model for CVD growth of silicon carbide. Comput. Mater. Sci. 2021, 196, 110512. [Google Scholar] [CrossRef]
- Danielsson, O.; Karlsson, M.; Sukkaew, P.; Pedersen, H.; Ojamae, L. A Systematic Method for Predictive In Silico Chemical Vapor Deposition. J. Phys. Chem. C 2020, 124, 7725–7736. [Google Scholar] [CrossRef] [Green Version]
- Sukkaew, P.; Danielsson, O.; Kordina, O.; Janzen, E.; Ojamae, L. Ab Initio Study of Growth Mechanism of 4H-SiC: Adsorption and Surface Reaction of C2H2, C2H4, CH4, and CH3. J. Phys. Chem. C 2017, 121, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Sukkaew, P.; Kalered, E.; Janzen, E.; Kordina, O.; Danielsson, O.; Ojamae, L. Growth Mechanism of SiC Chemical Vapor Deposition: Adsorption and Surface Reactions of Active Si Species. J. Phys. Chem. C 2018, 122, 648–661. [Google Scholar] [CrossRef] [Green Version]
- Ravasio, S.; Masi, M.; Cavallotti, C. Analysis of the Gas Phase Reactivity of Chlorosilanes. J. Phys. Chem. A 2013, 117, 5221–5231. [Google Scholar] [CrossRef]
- Song, B.T.; Gao, B.; Han, P.F.; Yu, Y.; Tang, X. Numerical Simulation of Gas Phase Reaction for Epitaxial Chemical Vapor Deposition of Silicon Carbide by Methyltrichlorosilane in Horizontal Hot-Wall Reactor. Materials 2021, 14, 7532. [Google Scholar] [CrossRef]
- Kleijn, C.R. Computational modeling of transport phenomena and detailed chemistry in chemical vapor deposition—a benchmark solution. Thin Solid Films 2000, 365, 294–306. [Google Scholar] [CrossRef]
- Fukushima, Y.; Sato, N.; Funato, Y.; Sugiura, H.; Hotozuka, K.; Momose, T.; Shimogaki, Y. Multi-Scale Analysis and Elementary Reaction Simulation of SiC-CVD Using CH3SiCl3/H-2. Ecs J Solid State Sc 2013, 2, P492–P497. [Google Scholar] [CrossRef]
- Pedersen, H.; Leone, S.; Henry, A.; Darakchieva, V.; Carlsson, P.; Gallstrom, A.; Janzen, E. Very high crystalline quality of thick 4H-SiC epilayers grown from methyltrichlorosilane (MTS). Phys. Status Solidi-Rapid Res. Lett. 2008, 2, 188–190. [Google Scholar] [CrossRef]
- Pedersen, H.; Leone, S.; Henry, A.; Beyer, F.C.; Darakchieva, V.; Janen, E. Very High Growth Rate of 4H-SiC using MTS as Chloride-based Precursor. Mater. Sci. Forum 2009, 600–603, 115–118. [Google Scholar] [CrossRef]


| Forward Rate Constant | Reverse Rate Constant | ||
|---|---|---|---|
| 1200 ℃ | 1200 ℃ | ||
| Surface site equilibrium reactions | |||
| RS1 | H(g) + H(s) → H2(g) + OSi(s) | 6.75 × 107 a,# | - |
| RS2 | H2(g) + OSi(s) → H(g) + H(s) | 5.29 × 104 a,# | - |
| RS3 | H (g) + OSi(s) → H(s) | 1.29 × 108 c,# | - |
| RS4 | H(s) + H(s) → H2(g) + 2OSi(s) | 1.25 × 105 c,@ | - |
| Adsorption reactions of active C species on the Si surface | |||
| RS5 | CH3(g) + H(s) → CH3(s) + H(g) | 2.45 a,# | - |
| RS6 | CH4(g) + H(s) → CH3(s) + H2(g) | 2.13 × 10−8 a,# | - |
| RS7 | C2H2(g) + H(s) → C2H3(s) | 2.4 × 10−3 a,# | - |
| RS8 | C2H4(g) + H(s) → C2H5(s) | 1.25 × 10−6 a,# | - |
| RS9 | CH4(g) + OSi(s) → CH3(s) + H(g) | 5.58 × 10−2 a,# | - |
| RS10 | C2H2(g) + OSi(s) → C2H2(s) | 3.02 × 105 a,# | - |
| RS11 | C2H4(g) + OSi(s) → C2H4(s) | 1.2 × 105 a,# | - |
| RS12 | CH3(g) + OSi(s) → CH3(s) | 1.4 × 107 c,# | - |
| RS13 | CH3(s) + H(g) → CH2(s) + H2(g) | 1.7 × 105 a,# | - |
| RS14 | CH2(s) + H2(g) → CH3(s) + H(g) | 6.7 × 102 a,# | - |
| RS15 | H(g) + CH2(s) → CH3(s) | 1.29 × 108 c,# | - |
| RS16 | H(g) + CH(s)-CH2(s) → CH2(s) + CH2(s) | 1.29 × 108 c,# | - |
| Surface species reactions on the Si surface | |||
| RS17 | CH2(s) + H(s) → CH3(s) + OSi(s) | 6.32 × 108 b,@ | - |
| RS18 | C2H2(s) + H(s) → C2H3(s) + OSi(s) | 1.9 × 1011 b,@ | - |
| RS19 | C2H3(s) + OSi(s) → CH(s)-CH2(s) | 3.54 × 109 b,@ | - |
| RS20 | C2H4(s) + H(s) → C2H5(s) + OSi(s) | 6.83 × 1010 b,@ | - |
| RS21 | C2H5(s) + OSi(s) → CH2(s) + CH3(s) | 1.09 × 104 b,@ | - |
| Growth reactions | |||
| RS22 | Si(g) + CH2(s) → H2(g) + OSi(s) + SiC(b) | 2.36 × 107 c,# | - |
| RS23 | Si(g) + CH3(s) → H2(g) + H(s) + SiC(b) | 4 × 106 c,# | - |
| RS24 | SiH(g) + CH2(s) → H2(g) + H(s) + SiC(b) | 2.32 × 107 c,# | - |
| RS25 | SiH(g) + CH3(s) → H2(g) + H(g) + H(s) + SiC(b) | 1.15 × 103 c,# | - |
| Adsorption reactions of active Si species on the C surface | |||
| RS26 | SiCl(g) + CH3(s) → SiHCl-CH2(s) | 2.63 × 101 a,# | - |
| RS27 | SiHCl(g) + C2H4(s) + OSi(s) → SiHCl-(CH2)2(s) | 1.03 × 10−4 a,# | - |
| RS28 | SiCl2(g) + C2H4(s) + OSi(s) → SiCl2-(CH2)2(s) | 4.44 × 10−6 a,# | - |
| Surface species reactions on the C surface | |||
| RS29 | SiHCl-CH2(s) + CH3(s) → SiHCl-(CH2)2(s) + H(g) | 2.74 × 105 b,@ | - |
| RS30 | SiHCl-(CH2)2(s) + CH3(s) → SiH-(CH2)3(s) + HCl(g) | 1.37 × 104 b,@ | 4.65 × 10−1 b,# |
| RS31 | SiHCl-(CH2)2(s) + CH3(s) → SiCl-(CH2)3(s) + H2(g) | 1.84 × 103 b,@ | 1.24 × 10−4 b,# |
| H atom abstraction reactions | |||
| RS32 | SiH-(CH2)3(s) + H(g) → Si-(CH2)3(s) + H2(g) | 2.11 × 108 b,# | 1.48 × 105 b,# |
| RS33 | SiCl-(CH2)3(s) + H(g) → Si-(CH2)3(s) + HCl(g) | 1.35 × 105 b,# | 4.8 × 104 b,# |
| 1 Surface Site Occupied | 2 Surface Sites Occupied | 3 Surface Sites Occupied |
|---|---|---|
| H(s), CH2(s), CH3(s), C2H2(s), C2H3(s), C2H4(s), C2H5(s), SiHCl-CH2(s) | CH(s)-CH2(s), SiCl2-(CH2)2(s), SiHCl-(CH2)2(s) | SiCl-(CH2)3(s), SiH-(CH2)3(s), Si-(CH2)3(s) |
| Sticking Coefficient | ||||
|---|---|---|---|---|
| On H(s) | On OSi(s) | On CH3(s) | On C2H4(s) | |
| CH3 | 1.7 × 10−7 a | 1 c | - | - |
| CH4 | 1.5 × 10−15 a | 4 × 10−9 a | - | - |
| C2H2 | 2.2 × 10−10 a | 2.8 × 10−2 a | - | - |
| C2H4 | 1.2 × 10−13 a | 1.1 × 10−2 a | - | - |
| SiCl | - | - | 3.7 × 10−6 b | - |
| SiHCl | - | - | - | 1.5 × 10−11 b |
| SiCl2 | - | - | - | 7.9 × 10−13 b |
| Adsorbed Reactions of Active C Species | Sticking Coefficient a | |
| RE1 | CH(g) + OSi(s) → CH(s) | 0.01 |
| RE2 | CH2(g) + OSi(s) → C(s) + H2(g) | 0.01 |
| RE3 | CH4(g) + OSi(s) → C(s) + 2H2(g) | 5 × 10−5 |
| RE4 | C2H2(g) + 2OSi(s) → 2C(s) + H2(g) | 0.02 |
| RE5 | C2H3(g) + 2OSi(s) → C(s) + CH(s) + H2(g) | 0.03 |
| RE6 | C2H4(g) + 2OSi(s) → 2C(s) + 2H2(g) | 0.0016 |
| RE7 | C2H5(g) + 2OSi(s) → C(s) + CH(s) + 2H2(g) | 0.03 |
| RE8 | C2H6(g) + 2OSi(s) → 2C(s) + 3H2(g) | 0.0016 |
| Adsorbed reactions of active Si/Cl species | Sticking coefficient a | |
| RE9 | SiHCl3(g) + 2OSi(s) + 2OC(s) → SiCl(s) + H(s) + 2ClSi(s) | 0.01 |
| RE10 | SiHCl3(g) + OSi(s) + 3OC(s) → SiCl(s) + H(s) + ClSi(s) + ClC(s) | 0.01 |
| RE11 | SiH3Cl(g) + 2OC(s) → SiCl(s) + H(s) + H2(g) | 0.01 |
| RE12 | SiH2Cl2(g) + OSi(s) + 3OC(s) → SiCl(s) + 2H(s) + ClSi(s) | 0.01 |
| RE13 | SiHCl(g) + OC(s) → Si(s) + HCl(g) | 0.02 |
| RE14 | SiCl4(g) + 2OSi(s) + 2OC(s) → SiCl(s) + ClC(s) + 2ClSi(s) | 0.01 |
| RE15 | SiCl3(g) + OSi(s) + 2OC(s) → SiCl(s) + ClC(s) + ClSi(s) | 0.02 |
| RE16 | SiCl3(g) + 3OC(s) → SiCl(s) + 2ClC(s) | 0.02 |
| RE17 | SiCl3(g) + 2OSi(s) + OC(s) → SiCl(s) + 2ClSi(s) | 0.02 |
| RE18 | SiCl2(g) + OSi(s) + OC(s) → SiCl(s) + ClSi(s) | 0.02 |
| RE19 | SiCl2(g) + 2OC(s) → SiCl(s) + ClC(s) | 0.02 |
| RE20 | SiCl(g) + OC(s) → SiCl(s) | 0.01 |
| RE21 | HCl(g) + OSi(s) + OC(s) → H(s) + ClSi(s) | 0.02 |
| RE22 | HCl(g) + 2OC(s) → H(s) + ClC(s) | 0.02 |
| Cl abstraction reactions | Rate constant # | |
| RE23 | HCl(g) + SiCl(s) → SiCl2(g) + H(g) + OC(s) | 1.34 × 106 c |
| RE24 | ClC(s) + H(g) → HCl(g) + OC(s) | 1.19 × 108 b |
| RE25 | ClSi(s) + H(g) → HCl(g) + OSi(s) | 1.19 × 108 b |
| RE26 | 2ClC(s) + SiCl2(g) → SiCl4(g) + 2OC(s) | 3 × 10−5 b |
| RE27 | 2ClC(s) + H2(g) → 2HCl(g) + 2OC(s) | 1.22 × 10−10 c |
| RE28 | 2ClSi(s) + H2(g) → 2HCl(g) + 2OSi(s) | 5.96 × 10−12 c |
| RE29 | ClSi(s) + ClC(s) + H2(g) → 2HCl(g) + OSi(s) + OC(s) | 2.69 × 10−11 b |
| Surface species reactions | Rate constant @ | |
| RE30 | SiCl(s) + ClC(s) → SiCl2(g) + 2OC(s) | 9.18 × 107 b |
| RE31 | SiCl(s) + ClSi(s) → SiCl2(g) + OC(s) + OSi(s) | 6.8 × 10−1 b |
| RE32 | 2SiCl(s) → SiCl2(g) + Si(s) + OC(s) | 6.8 × 10−1 b |
| RE33 | SiCl(s) + H(s) → HCl(g) + Si(s) + OC(s) | 2.06 × 101 b |
| RE34 | Si(s) + ClSi(s) → SiCl(s) + OSi(s) | 2.03 × 108 b |
| RE35 | Si(s) + ClC(s) → SiCl(s) + OC(s) | 2.03 × 108 b |
| RE36 | ClSi(s) + H(s) → HCl(g) + OSi(s) + OC(s) | 6.76 × 103 b |
| RE37 | ClC(s) + H(s) → HCl(g) + 2OC(s) | 3.05 × 104 b |
| RE38 | H(s) + H(s) → H2(g) + 2OC(s) | 1.55 × 108 b |
| Growth reactions | Rate constant @ | |
| RE39 | SiCl(s) + C(s) → SiC(b) + Cl(g) + OC(s) + OSi(s) | 2.03 × 108 b |
| RE40 | Si(s) + C(s) → SiC(b) + OC(s) + OSi(s) | 2.03 × 108 b |
| RE41 | SiCl(s) + CH(s) → SiC(b) + HCl(g) + OC(s) + OSi(s) | 2.03 × 108 b |
| RE42 | Si(s) + CH(s) → SiC(b) + H(g) + OC(s) + OSi(s) | 2.03 × 108 b |
| CH4(g) + OSi(s) → C(s) + 2H2(g) | C2H2(g) + 2OSi(s) → 2C(s) + H2(g) |
| C2H4(g) + 2OSi(s) → 2C(s) + 2H2(g) | H(s) + H(s) → H2(g) + 2OC(s) |
| SiHCl3(g) + 2OSi(s) + 2OC(s) → SiCl(s) + H(s) + 2ClSi(s) | SiHCl3(g) + OSi(s) + 3OC(s) → SiCl(s) + H(s) + ClSi(s) + ClC(s) |
| SiHCl(g) + OC(s) → Si(s) + HCl(g) | SiCl4(g) + 2OSi(s) + 2OC(s) → SiCl(s) + ClC(s) + 2ClSi(s) |
| SiCl3(g) + OSi(s) + 2OC(s) → SiCl(s) + ClC(s) + ClSi(s) | SiCl3(g) + 3OC(s) → SiCl(s) + 2ClC(s) |
| SiCl3(g) + 2OSi(s) + OC(s) → SiCl(s) + 2ClSi(s) | SiCl2(g) + OSi(s) + OC(s) → SiCl(s) + ClSi(s) |
| SiCl2(g) + 2OC(s) → SiCl(s) + ClC(s) | SiCl(g) + OC(s) → SiCl(s) |
| HCl(g) + OSi(s) + OC(s) → H(s) + ClSi(s) | HCl(g) + 2OC(s) → H(s) + ClC(s) |
| HCl(g) + SiCl(s) → SiCl2(g) + H(g) + OC(s) | ClC(s) + H(g) → HCl(g) + OC(s) |
| ClSi(s) + H(g) → HCl(g) + OSi(s) | 2ClC(s) + SiCl2(g) → SiCl4(g) + 2OC(s) |
| SiCl(s) + ClC(s) → SiCl2(g) + 2OC(s) | SiCl(s) + ClSi(s) → SiCl2(g) + OC(s) + OSi(s) |
| 2SiCl(s) → SiCl2(g) + Si(s) + OC(s) | SiCl(s) + H(s) → HCl(g) + Si(s) + OC(s) |
| Si(s) + ClSi(s) → SiCl(s) + OSi(s) | Si(s) + ClC(s) → SiCl(s) + OC(s) |
| ClSi(s) + H(s) → HCl(g) + OSi(s) + OC(s) | ClC(s) + H(s) → HCl(g) + 2OC(s) |
| SiCl(s) + C(s) → SiC(b) + Cl(g) + OC(s) + OSi(s) | Si(s) + CH(s) → SiC(b) + H(g) + OC(s) + OSi(s) |
| Si(s) + C(s) → SiC(b) + OC(s) + OSi(s) | Si(s) + C(s) → SiC(b) + OC(s) + OSi(s) |
| SiCl(s) + CH(s) → SiC(b) + HCl(g) + OC(s) + OSi(s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Gao, B.; Han, P.; Yu, Y. Surface Kinetic Mechanisms of Epitaxial Chemical Vapour Deposition of 4H Silicon Carbide Growth by Methyltrichlorosilane-H2 Gaseous System. Materials 2022, 15, 3768. https://doi.org/10.3390/ma15113768
Song B, Gao B, Han P, Yu Y. Surface Kinetic Mechanisms of Epitaxial Chemical Vapour Deposition of 4H Silicon Carbide Growth by Methyltrichlorosilane-H2 Gaseous System. Materials. 2022; 15(11):3768. https://doi.org/10.3390/ma15113768
Chicago/Turabian StyleSong, Botao, Bing Gao, Pengfei Han, and Yue Yu. 2022. "Surface Kinetic Mechanisms of Epitaxial Chemical Vapour Deposition of 4H Silicon Carbide Growth by Methyltrichlorosilane-H2 Gaseous System" Materials 15, no. 11: 3768. https://doi.org/10.3390/ma15113768
APA StyleSong, B., Gao, B., Han, P., & Yu, Y. (2022). Surface Kinetic Mechanisms of Epitaxial Chemical Vapour Deposition of 4H Silicon Carbide Growth by Methyltrichlorosilane-H2 Gaseous System. Materials, 15(11), 3768. https://doi.org/10.3390/ma15113768







