Study on Corrosion Resistance and Conductivity of TiMoN Coatings with Different Mo Contents under Simulated PEMFC Cathode Environment
Abstract
:1. Introduction
2. Experimental Methods
2.1. Sample Pretreatment
2.2. Preparation of TiMoN Coatings
2.3. Surface Characterization
2.4. Electrochemical Measurement
2.5. Interfacial Contact Resistance Measurements
2.6. Water Contact Angle Measurement
3. Result and Discussion
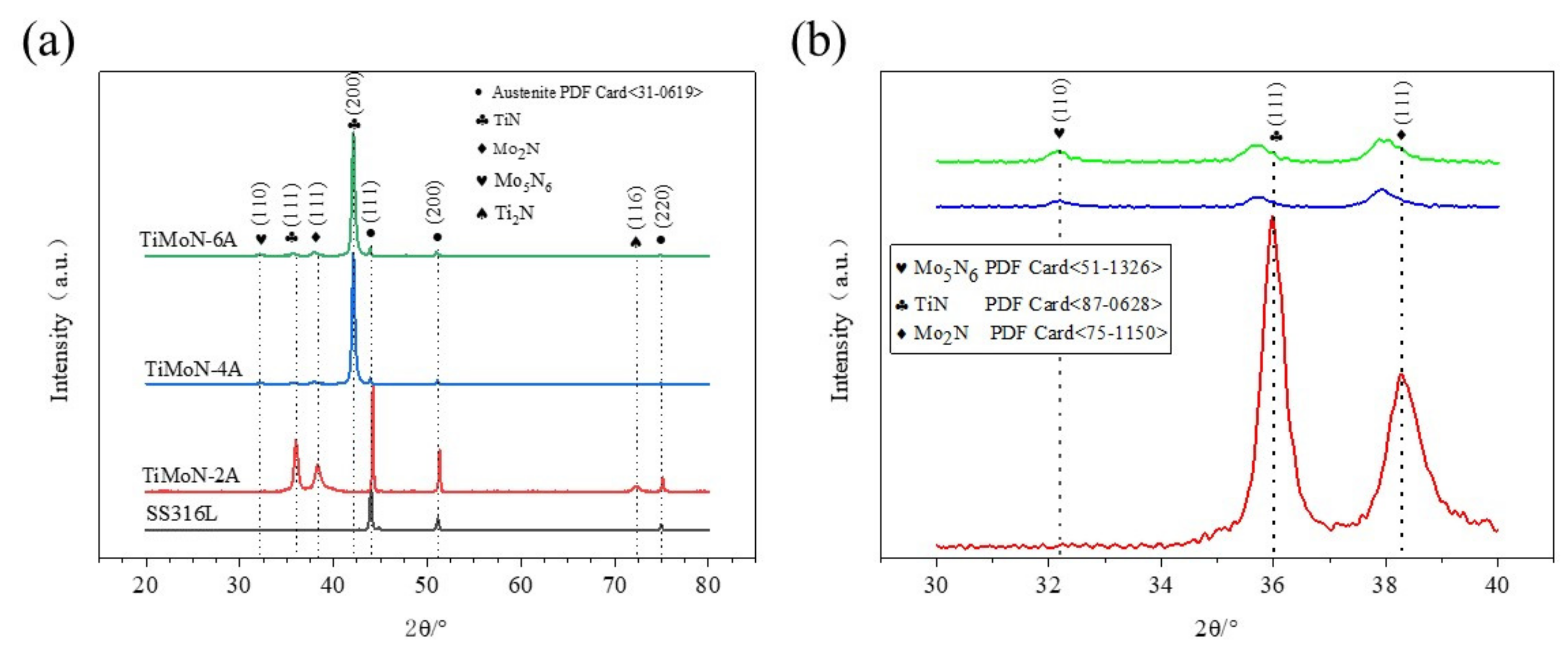
3.1. Phase Characterization
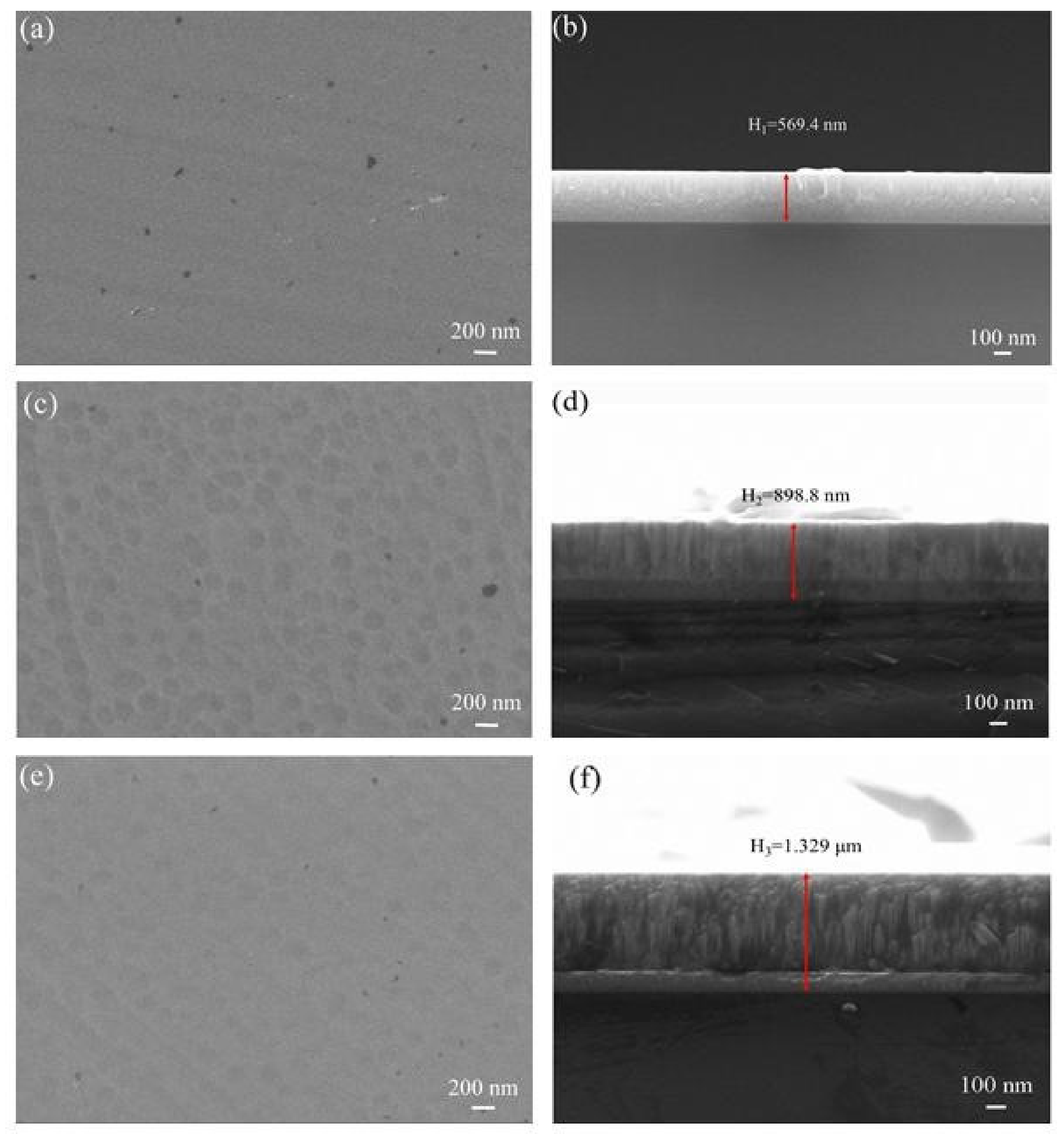
3.2. Microstructural Characterization
3.3. Electrochemical Corrosion Evaluation
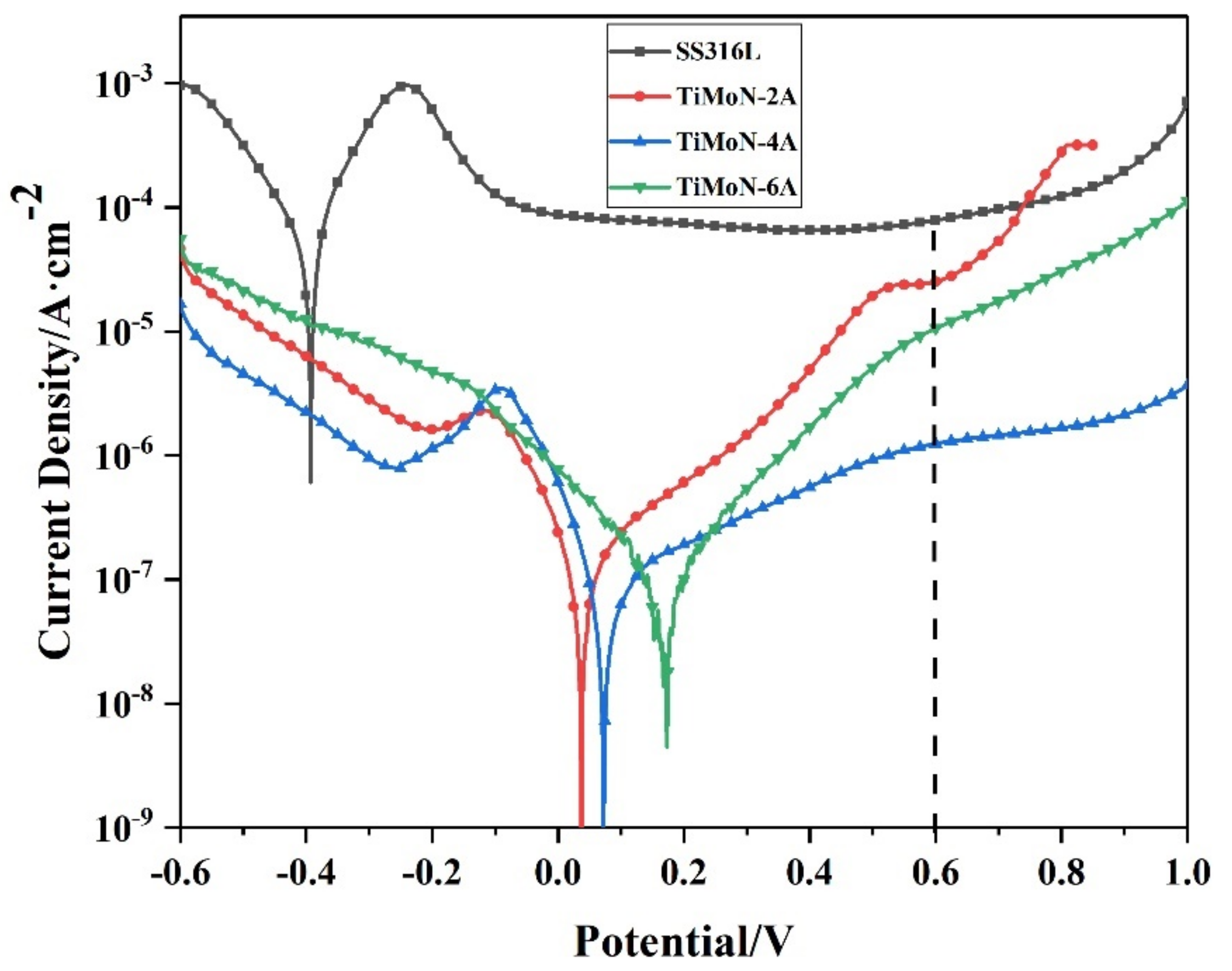
3.3.1. Potential Polarization Readings
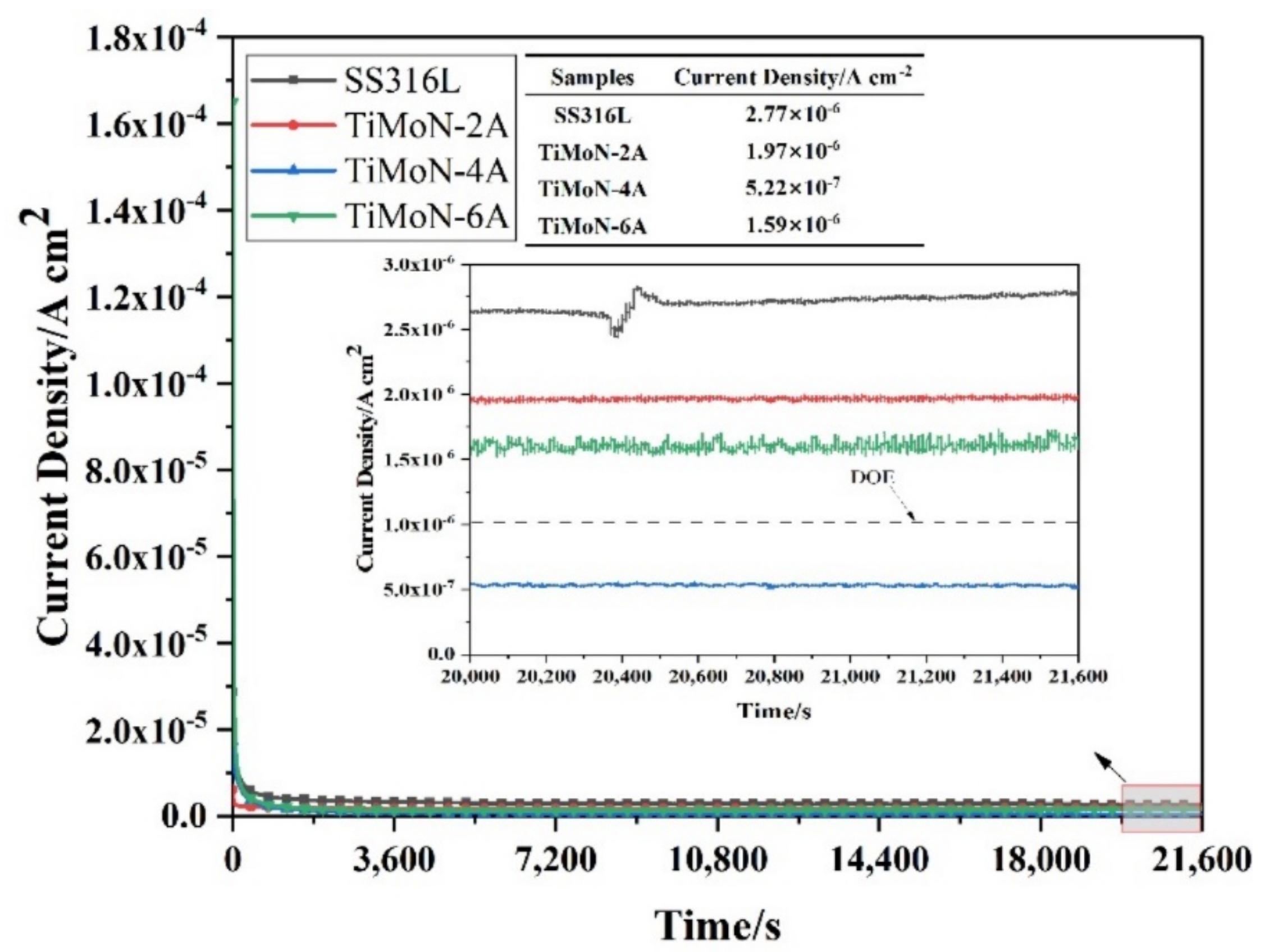
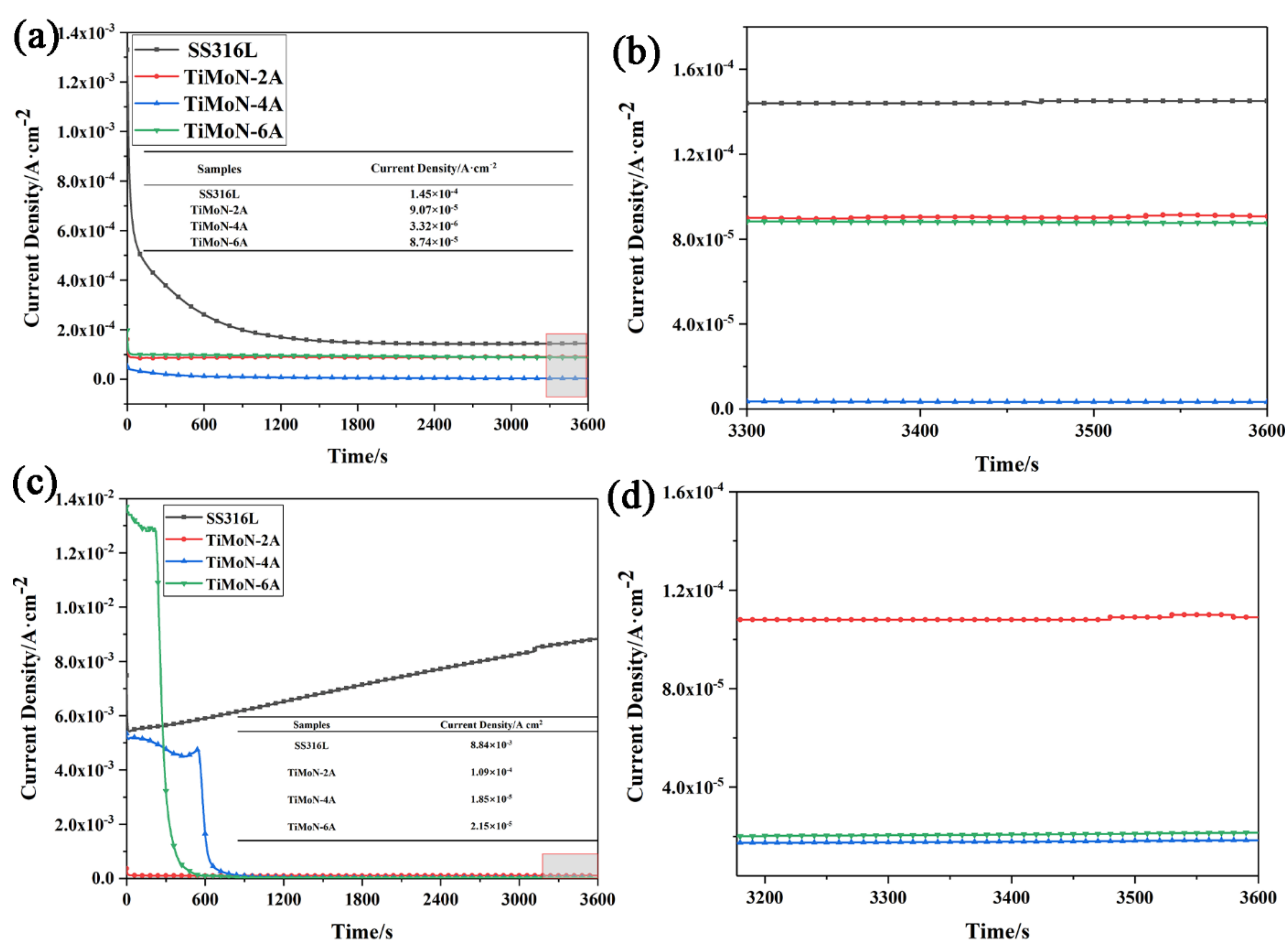
3.3.2. Potentiostatic Polarization Readings
3.3.3. High Potential Polarization Readings
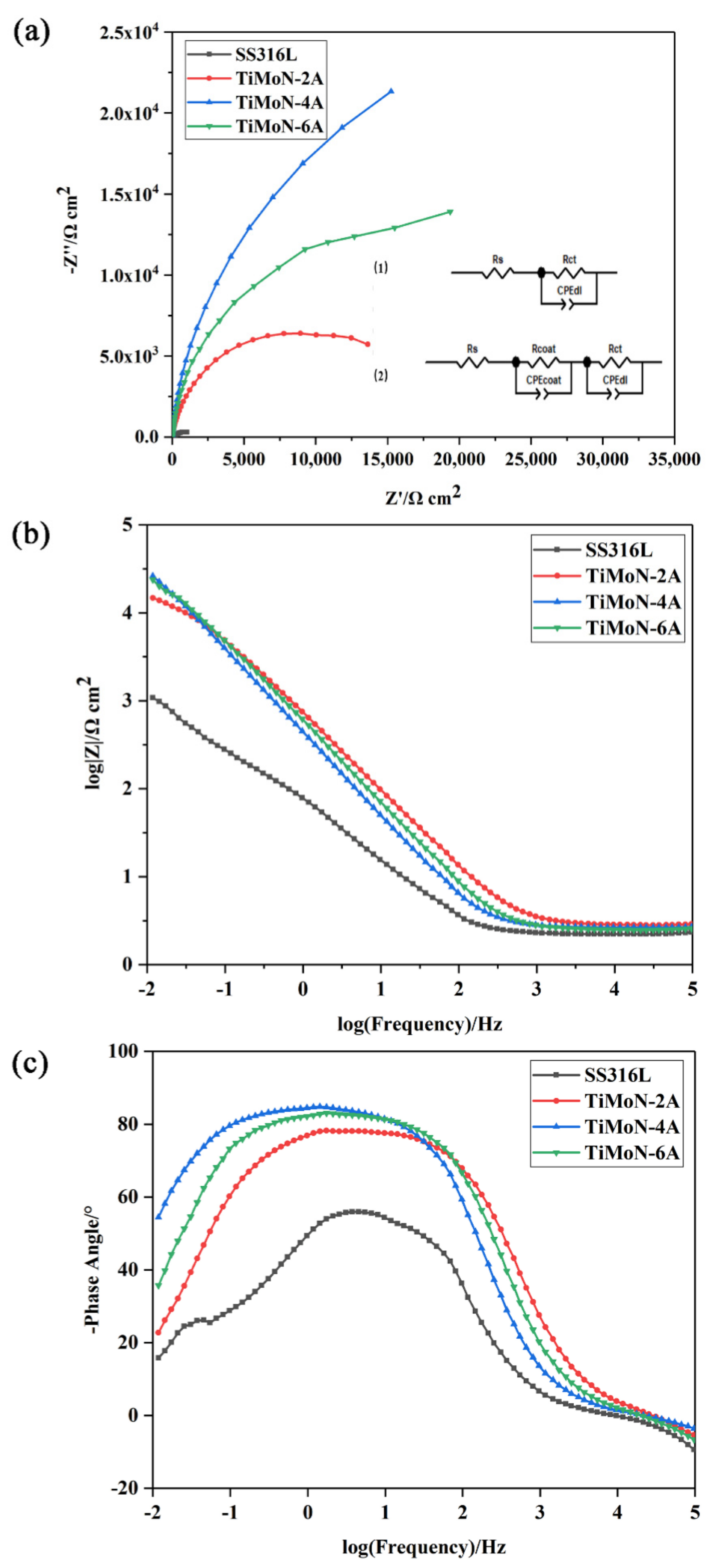
3.3.4. Electrochemical Impedance Spectroscopy (EIS) Measurements
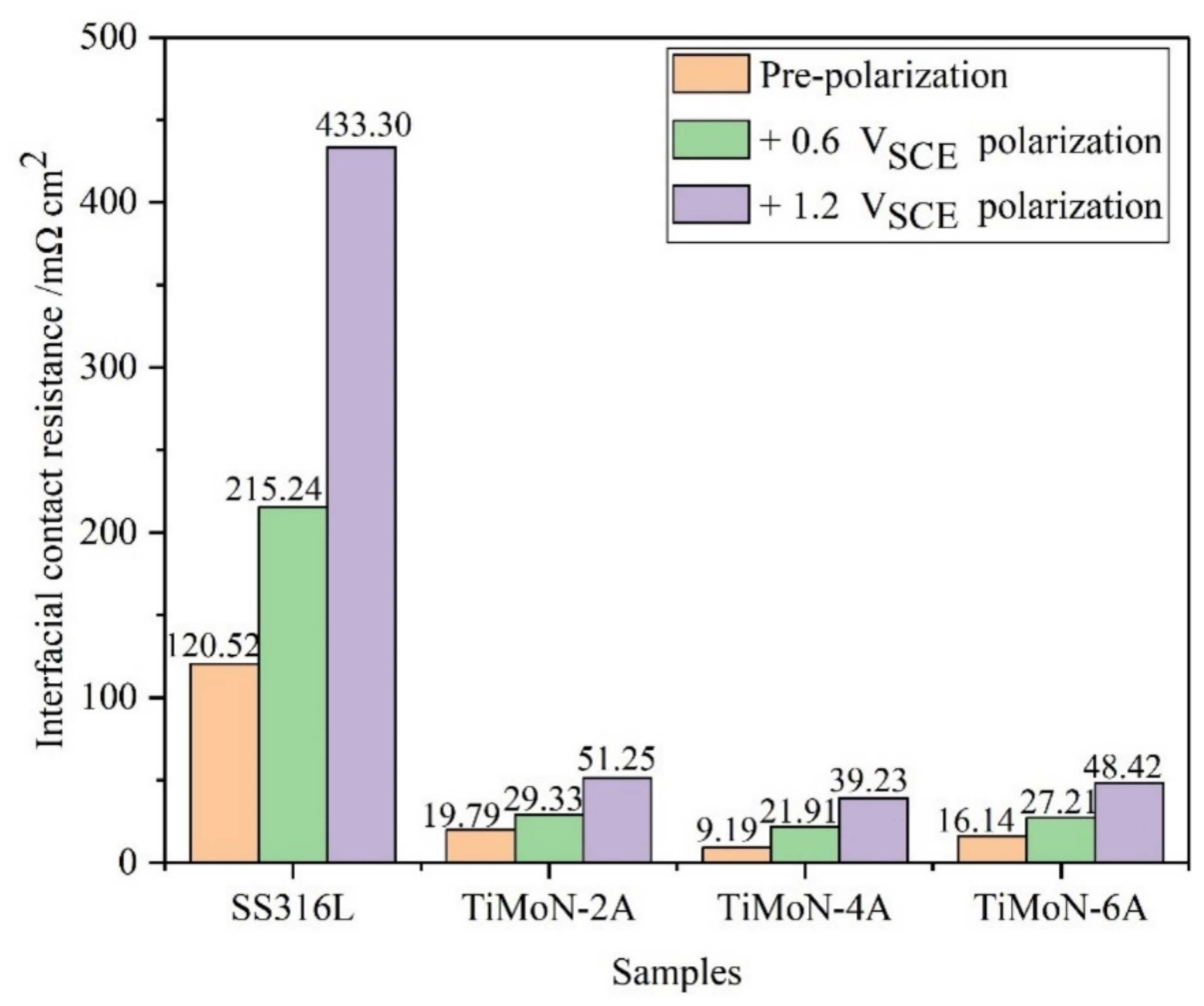
3.4. ICR Measurements
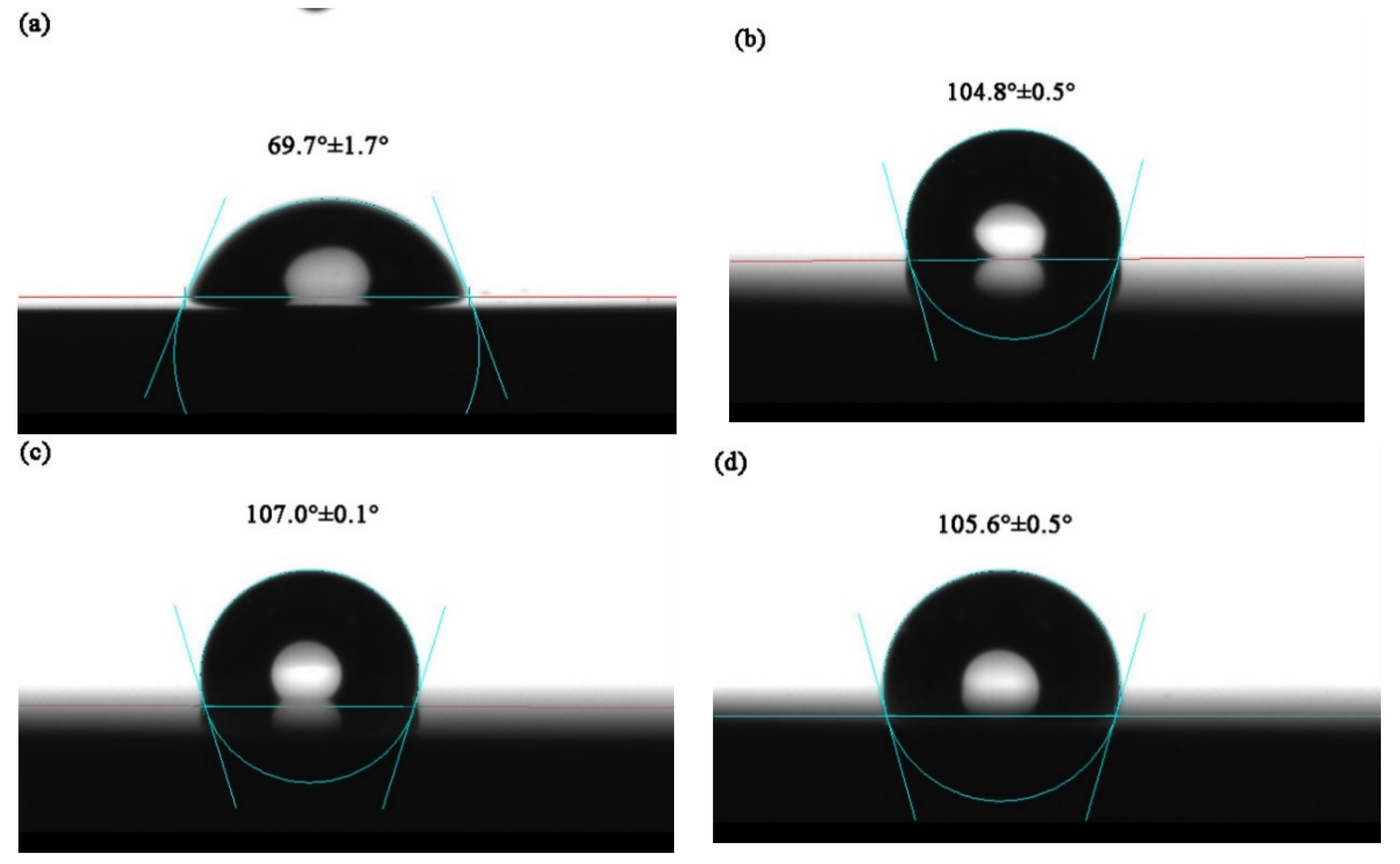
3.5. Water Contact Angle Measurements
4. Conclusions
- (1)
- From the XRD analysis, When the Mo target current is 2A, the phases of TiMoN coating are mainly TiN (111) and Mo2N (111), and the weak phase Ti2N (116). With the increase in Mo target current (4A and 6A), the phase structure of TiMoN coating changes. The phase structure of TiMoN coating is mainly TiN (200), and the phase of Mo5N6 is generated. In addition, according to the texture coefficient and lattice lattice parameters, it is proved that the change of Mo element causes the solution strengthening of TiMoN coating, and Mo atom enters TiN solid solution;
- (2)
- Combined with FE-SEM surface and cross-sectional micro-morphology analysis, When the Mo target current is 2A, the microstructure of TiMoN coating is uniform but relatively thin. With the current of Mo target increases, the surface morphology of TiMoN coating becomes rough, and the thickness increases. The deposition rate increases from 6.33 nm/min to 14.77 nm/min by calculation;
- (3)
- Potentiodynamic polarization and potentiostatic polarization tests show that the Icorr of TiMoN coatings is better than SS316L substrate. At the same time, the Icorr of TiMoN coatings meets DOE 2020 targets (≤1 × 10−6 A·cm−2). Among them, TiMoN-4A coating has the lowest corrosion current density, which is 9.33 × 10−8 A·cm−2 and 5.22 × 10−7 A·cm−2, respectively. The high-potential polarization test shows that TiMoN-4A coating has the lowest Icorr at high potential (+1.0 VSCE, +1.2 VSCE). Meanwhile, the surface morphology of TiMoN-4A coating has no obvious change, indicating that TiMoN-4A coating has good corrosion resistance under high-potential corrosion;
- (4)
- The results of EIS show that the semi-arc resistance of TiMoN coating increases obviously, indicating that the corrosion resistance is enhanced. Meanwhile, the phase angle of TiMoN coating is close to 90° in the range of middle- and low-frequency region, indicating that the TiMoN coatings have two-time constants;
- (5)
- The ICR results show that the ICR of TiMoN coatings is significantly lower than SS316L. Meanwhile, the ICR of TiMoN-4A coating is the lowest of 9.19 mΩ·cm2, which meets the DOE 2020 targets (≤10 mΩ·cm2);
- (6)
- The water contact angle test results show that the water contact angle of TiMoN coatings is significantly higher than 90°, which indicates that TiMoN coatings have good hydrophobicity. Among them, the TiMoN-4A coating has the largest water contact angle and the best hydrophobicity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, J.; Zhang, P.; Han, Y.; Wang, H.; Wang, X.; Yu, Y.; Sun, J. Investigation on electrochemical behavior and surface conductivity of titanium carbide modified Ti bipolar plate of PEMFC. Int. J. Hydrog. Energy 2020, 45, 10050–10058. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Chen, L.; Luo, J.; Pang, P.; Zhang, X.; Liao, B.; Ying, M. Corrosion behavior and interfacial conductivity of amorphous hydrogenated carbon and titanium carbide composite (a-C: H/TiC) films prepared on titanium bipolar plates in PEMFCs. Diam. Relat. Mater. 2021, 120, 108628–108637. [Google Scholar] [CrossRef]
- Yang, L.X.; Liu, R.J.; Wang, Y.; Liu, H.J.; Zeng, C.L.; Fu, C. Growth of nanocrystalline β-Nb2N coating on 430 ferritic stainless steel bipolar plates of PEMFCs by disproportionation reaction of Nb(IV) ions in molten salt. Corros. Sci. 2020, 174, 108862–108872. [Google Scholar] [CrossRef]
- Hu, B.; Chang, F.-L.; Xiang, L.-Y.; He, G.-J.; Cao, X.-W.; Yin, X.-C. High performance polyvinylidene fluoride/graphite/multi-walled carbon nanotubes composite bipolar plate for PEMFC with segregated conductive networks. Int. J. Hydrog. Energy 2021, 46, 25666–25676. [Google Scholar] [CrossRef]
- Mi, B.; Chen, Z.; Wang, Q.; Li, Y.; Qin, Z.; Wang, H. Properties of C-doped CrTiN films on the 316L stainless steel bipolar plate for PEMFC. Int. J. Hydrog. Energy 2021, 46, 32645–32654. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Zhang, R.; Jiang, T.; Peng, L. Fabrication of micro channels for titanium PEMFC bipolar plates by multistage forming process. Int. J. Hydrog. Energy 2021, 46, 11092–11103. [Google Scholar] [CrossRef]
- Khatir, F.A.; Barzegari, M.M.; Talebi-Ghadikolaee, H.; Seddighi, S. Integration of design of experiment and finite element method for the study of geometrical parameters in metallic bipolar plates for PEMFCs. Int. J. Hydrog. Energy 2021, 46, 39469–39482. [Google Scholar] [CrossRef]
- Simaafrookhteh, S.; Khorshidian, M.; Momenifar, M. Fabrication of multi-filler thermoset-based composite bipolar plates for PEMFCs applications: Molding defects and properties characterizations. Int. J. Hydrog. Energy 2020, 45, 14119–14132. [Google Scholar] [CrossRef]
- Gao, P.; Xie, Z.; Wu, X.; Ouyang, C.; Lei, T.; Yang, P.; Liu, C.; Wang, J.; Ouyang, T.; Huang, Q. Development of Ti bipolar plates with carbon/PTFE/TiN composites coating for PEMFCs. Int. J. Hydrog. Energy 2018, 43, 20947–20958. [Google Scholar] [CrossRef]
- Kumagai, M.; Myung, S.-T.; Kuwata, S.; Asaishi, R.; Yashiro, H. Corrosion behavior of austenitic stainless steels as a function of pH for use as bipolar plates in polymer electrolyte membrane fuel cells. Electrochim. Acta 2008, 53, 4205–4212. [Google Scholar] [CrossRef]
- Wang, L.; Kang, B.; Gao, N.; Du, X.; Jia, L.; Sun, J. Corrosion behaviour of austenitic stainless steel as a function of methanol concentration for direct methanol fuel cell bipolar plate. J. Power Sources 2014, 253, 332–341. [Google Scholar] [CrossRef]
- Huang, P.; Chen, Z.; Zhang, J.; Wu, M.; Liu, Y.; Zhang, F.; Chen, Y.; Chen, X. Stainless steel bipolar plate fuel cell with different flow field structures prepared by laser additive manufacturing. Int. J. Heat Mass Transf. 2022, 183, 122186–122199. [Google Scholar] [CrossRef]
- Lu, J.L.; Abbas, N.; Tang, J.N.; Tang, J.; Zhua, G.M. Synthesis and characterization of conductive ceramic MAX-phase coatings for metal bipolar plates in simulated PEMFC environments. Corros. Sci. 2019, 158, 108106–108114. [Google Scholar] [CrossRef]
- Leng, Y.; Ming, P.; Yang, D.; Zhang, C. Stainless steel bipolar plates for proton exchange membrane fuel cells: Materials, flow channel design and forming processes. J. Power Sources 2020, 451, 227783–227806. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, J.; Hu, M.; Li, X. Investigation of high potential corrosion protection with titanium carbonitride coating on 316 L stainless steel bipolar plates. Corros. Sci. 2021, 191, 109757–109769. [Google Scholar] [CrossRef]
- Jin, J.; Zheng, D.; Liu, H. The corrosion behavior and mechanical properties of CrN/Ni-P multilayer coated mild steel as bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2017, 42, 28883–28897. [Google Scholar] [CrossRef]
- Wang, S.; Hou, M.; Zhao, Q.; Jiang, Y.; Wang, Z.; Li, H.; Fu, Y.; Shao, Z. Ti/(Ti,Cr)N/CrN multilayer coated 316L stainless steel by arc ion plating as bipolar plates for proton exchange membrane fuel cells. J. Energy Chem. 2017, 26, 168–174. [Google Scholar] [CrossRef]
- Yi, P.; Zhu, L.; Dong, C.; Xiao, K. Corrosion and interfacial contact resistance of 316L stainless steel coated with magnetron sputtered ZrN and TiN in the simulated cathodic environment of a proton-exchange membrane fuel cell. Surf. Coat. Technol. 2019, 363, 198–202. [Google Scholar] [CrossRef]
- Lin, M.-T.; Wan, C.-H.; Wu, W. Comparison of corrosion behaviors between SS304 and Ti substrate coated with (Ti,Zr)N thin films as Metal bipolar plate for unitized regenerative fuel cell. Thin Solid Film. 2013, 544, 162–169. [Google Scholar] [CrossRef]
- Wang, L.; Tao, Y.; Zhang, Z.; Wang, Y.; Feng, Q.; Wang, H.; Li, H. Molybdenum carbide coated 316L stainless steel for bipolar plates of proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2019, 44, 4940–4950. [Google Scholar] [CrossRef]
- Fan, H.-Q.; Shi, D.-D.; Wang, X.-Z.; Luo, J.-L.; Zhang, J.-Y.; Li, Q. Enhancing through-plane electrical conductivity by introducing Au microdots onto TiN coated metal bipolar plates of PEMFCs. Int. J. Hydrog. Energy 2020, 45, 29442–29448. [Google Scholar] [CrossRef]
- Penga, S.; Xua, J.; Lic, Z.; Jiang, S.; Munroee, P.; Xie, Z.-H.; Luh, H. A reactive-sputter-deposited TiSiN nanocomposite coating for the protection of metallic bipolar plates in proton exchange membrane fuel cells. Ceram. Int. 2020, 46, 2743–2757. [Google Scholar] [CrossRef]
- Wanga, L.; Northwooda, D.O.; Niea, X.; Housdenb, J.; Spainb, E.; Leylandc, A.; Matthewsc, A. Corrosion properties and contact resistance of TiN, TiAlN and CrN coatings in simulated proton exchange membrane fuel cell environments. J. Power Sources 2010, 195, 3814–3821. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Yogesh, K.; Rajam, K.S. Deposition of TiAlN coatings using reactive bipolar-pulsed direct current unbalanced magnetron sputtering. Vacuum 2009, 83, 427–434. [Google Scholar] [CrossRef]
- Mani, S.P.; Agilan, P.; Kalaiarasan, M.; Ravichandran, K.; Rajendran, N.; Meng, Y. Effect of multilayer CrN/CrAlN coating on the corrosion and contact resistance behavior of 316L SS bipolar plate for high temperature proton exchange membrane fuel cell. J. Mater. Sci. Technol. 2022, 97, 134–146. [Google Scholar] [CrossRef]
- Jin, J.; He, Z.; Zhao, X. Formation of a protective TiN layer by liquid phase plasma electrolytic nitridation on Ti-6Al-4V bipolar plates for PEMFC. Int. J. Hydrog. Energy 2020, 45, 12489–12500. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, B.; Wang, Z.; Shi, J.; Yang, C.; Dong, D.; Wang, X. Thermal stabilization of nanocrystalline promoting conductive corrosion resistance of TiN–Ag films for metal bipolar plates. Vacuum 2022, 195, 110631–110646. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, Q.; Huang, B. Synthesis and properties of novel N/Ta-co-doped TiO2 coating on titanium in simulated PEMFC environment. J. Alloy. Compd. 2021, 879, 160470–160477. [Google Scholar] [CrossRef]
- Jin, J.; He, Z.; Zhao, X. Effect of Al content on the corrosion resistance and conductivity of metal nitride coating in the cathode environment of PEMFCs. Mater. Chem. Phys. 2020, 245, 122739–122751. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, W.; Zhang, L. Crystalline Size Effects on Texture Coefficient, Electrical and Optical Properties of Sputter-deposited Ga-doped ZnO Thin Films. J. Mater. Sci. Technol. 2015, 31, 175–181. [Google Scholar] [CrossRef]
- Barranco, J.; Barreras, F.l.; Lozano, A.; Lopez, A.M.; Roda, V.; Martin, J.s.; Maza, M.; Fuentes, G.G.; Almandoz, E. Cr and Zr/Cr nitride CAE-PVD coated aluminum bipolar plates for polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2010, 35, 11489. [Google Scholar] [CrossRef]
- Nam, N.D.; Ahn, J.H.; Lee, N.E.; Kim, J.G. Electrochemical evaluation of the reliability of plasma-polymerized methylcyclohexane films. Mater. Res. Bull. 2010, 45, 269–274. [Google Scholar] [CrossRef]
- Kumar, A.; Ricketts, M.; Hirano, S. Ex situ evaluation of nanometer range gold coating on stainless steel substrate for automotive polymer electrolyte membrane fuel cell bipolar plate. J. Power Sources 2010, 195, 1401–1407. [Google Scholar] [CrossRef]
- Jin, J.; Liu, H.; Zheng, D.; Zhu, Z. Effects of Mo content on the interfacial contact resistance and corrosion properties of CrN coatings on SS316L as bipolar plates in simulated PEMFCs environment. Int. J. Hydrog. Energy 2018, 43, 10048–10060. [Google Scholar] [CrossRef]
- Jin, J.; Hu, M.; Zhao, X. Investigation of incorporating oxygen into TiN coating to resist high potential effects on PEMFC bipolar plates in vehicle applications. Int. J. Hydrog. Energy 2020, 45, 23310–23326. [Google Scholar] [CrossRef]
- Gilewicz, A.; Warcholinski, B. Deposition and characterisation of Mo2N/CrN multilayer coatings prepared by cathodic arc evaporation. Surf. Coat. Technol. 2015, 279, 126–133. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.; Liu, Z.; Yan, Y.; Chen, Y. Surface microstructure and performance of TiN monolayer film on titanium bipolar plate for PEMFC. Int. J. Hydrog. Energy 2021, 46, 31382–31390. [Google Scholar] [CrossRef]
- Wanga, T.; Zhang, J.; Li, Y.; Gaob, F.; Zhang, G. Self-lubricating TiN/MoN and TiAlN/MoN nano-multilayer coatings for drilling of austenitic stainless steel. Ceram. Int. 2019, 45, 24248–24253. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Ye, C.-P.; Shi, D.-D.; Fan, H.-Q.; Li, Q. Potential polarization accelerated degradation of interfacial electrical conductivity for Au/TiN coated 316L SS bipolar plates used in polymer electrolyte membrane fuel cells. Corros. Sci. 2021, 189, 109624. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Luo, H.; Muneshwar, T.; Fan, H.-Q.; Cadien, K.; Luo, J.-L. Zr2N2O Coating-Improved Corrosion Resistance for the Anodic Dissolution Induced by Cathodic Transient Potential. ACS Appl. Mater. Interfaces 2018, 10, 40111–40124. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Yi, P.; Peng, L.; Lai, X. Niobium doped amorphous carbon film on metallic bipolar plates for PEMFCs: First principle calculation, microstructure and performance. Int. J. Hydrog. Energy 2019, 44, 3144–3156. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Y. Preparation and performances of modified Ti4O7 doped polypyrrole coating for metallic bipolar plates. Corros. Sci. 2021, 190, 109703–109714. [Google Scholar] [CrossRef]
- Liu, S.; Pan, T.J.; Pan, T.J.; Wang, R.F.; Yue, Y.; Shen, J. Anti-corrosion and conductivity of the electrodeposited graphene/polypyrrole composite coating for metallic bipolar plates. Prog. Org. Coat. 2019, 136, 105237–105246. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Luo, J.; Zhang, F.; Wang, C.; Dong, S.; Ma, Y.; Liang, Z.; Lin, C. Enhanced corrosion protection by Al surface immobilization of in-situ grown layered double hydroxide films co-intercalated with inhibitors and low surface energy species. Corros. Sci. 2020, 164, 108340. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Pan, Y.; Li, J. Effect of Cr on the passive film formation mechanism of steel rebar in saturated calcium hydroxide solution. Appl. Surf. Sci. 2016, 389, 1182–1191. [Google Scholar] [CrossRef]
- Ojea, A.M.; Ogwub, A.A.; Rahmana, S.U.; Ojea, A.I.; Tsendzughula, N. Effect of temperature variation on the corrosion behaviour and semiconducting properties of the passive film formed on chromium oxide coatings exposed to saline solution. Corros. Sci. 2019, 154, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Yi, P.; Zhang, W.; Bi, F.; Peng, L.; Lai, X. Enhanced Corrosion Resistance and Interfacial Conductivity of TiCx/a-C Nanolayered Coatings via Synergy of Substrate Bias Voltage for Bipolar Plates Applications in PEMFCs. ACS Appl. Mater. Interfaces 2018, 10, 19087–19096. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, H.; Li, R.; Geng, J.; Shao, Z. Nb–Cr–C coated titanium as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2022, 520, 230797–230805. [Google Scholar] [CrossRef]


| Cr | Ni | Mo | Mn | Si | P | S | C | Fe |
|---|---|---|---|---|---|---|---|---|
| 20.0–21.0 | 6.0–7.0 | 1.5 | 1.5 | 1.0 | 0.04 | 0.03 | 0.03 | Balance |
| Deposition Process | Ar Gas Flow Rate/Sccm | N2 Gas Flow Rate/Sccm | Ti Target Current/A | Mo Target Current/A | Bias Voltage/V | Deposition Time/s |
|---|---|---|---|---|---|---|
| 1. Surface cleaning | 40 | 0 | 0.3 | 0.3 | −500 | 1800 |
| 2. Ti layer deposited | 40 | 0 | 6 | 0.3 | −80 | 1800 |
| 3. TiMoN coating | 40 | 30 | 6 | 2, 4, 6 | −80 | 5400 |
| Samples | Mo5N6 | TiN | Ti2N | Mo2N | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lattice Constant/nm | ||||||||||||
| a | b | c | a | b | c | a | b | c | a | b | c | |
| TiMoN-2A | / | / | / | 0.2956 | 0.2956 | 0.4756 | 0.4188 | 0.4188 | 0.8048 | 0.4210 | 0.4210 | 0.8060 |
| TiMoN-4A | 0.4893 | 0.4893 | 1.106 | 0.4244 | 0.4244 | 0.4244 | / | / | / | 0.4140 | 0.4140 | 0.8805 |
| TiMoN-6A | 0.7605 | 0.7605 | 1.062 | 0.4320 | 0.4320 | 0.4320 | / | / | / | 0.4210 | 0.4210 | 0.8060 |
| Samples | Crystal Plane | ||||
|---|---|---|---|---|---|
| Mo5N6 (111) | TiN (111) | Mo2N (111) | TiN (200) | ||
| TiMoN-2A | TC (hkl) | / | 0.735 | 0.524 | / |
| TiMoN-4A | 0.171 | 0.054 | 0.130 | 1.356 | |
| TiMoN-6A | 0.153 | 0.041 | 0.118 | 1.287 | |
| Samples | Ecorr/V | Icorr/A·cm−2 | Βa/V dec−1 | βc/V dec−1 | Rp/ohm | I0.6V/A·cm−2 | Pi/% |
|---|---|---|---|---|---|---|---|
| SS316L | −0.397 | 4.48 × 10−5 | 0.126 | 0.084 | 4.48 × 102 | 7.81 × 10−5 | / |
| TiMoN-2A | 0.029 | 1.42 × 10−7 | 0.096 | 0.267 | 2.16 × 106 | 2.51 × 10−6 | 99.68 |
| TiMoN-4A | 0.081 | 9.33 × 10−8 | 0.094 | 0.416 | 3.57 × 106 | 1.25 × 10−6 | 99.79 |
| TiMoN-6A | 0.169 | 1.34 × 10−7 | 0.210 | 0.213 | 3.43 × 106 | 1.06 × 10−6 | 99.70 |
| Samples | Rs/Ω·cm2 | CPEcoat-T/cm−2S−nΩ | CPEcoat-P/cm−2S−nΩ | Rcoat/Ω cm | CPEdl-T/cm−2S−nΩ | CPEdl-P/cm−2S−nΩ | Rct/Ω·cm2 |
|---|---|---|---|---|---|---|---|
| SS316L | 2.10 | / | / | / | 3.44 × 10−3 | 0.732 | 900.9 |
| TiMoN-2A | 2.83 | 1.82 × 10−3 | 0.873 | 714.7 | 3.09 × 10−4 | 0.888 | 15,596 |
| TiMoN-4A | 2.65 | 2.46 × 10−3 | 0.818 | 1021 | 4.45 × 10−4 | 0.997 | 41,572 |
| TiMoN-6A | 2.51 | 1.76 × 10−3 | 0.853 | 1107 | 3.50 × 10−4 | 0.959 | 26,748 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Cao, R.; Zhang, J.; Tao, Y.; Tian, X.; Kou, X. Study on Corrosion Resistance and Conductivity of TiMoN Coatings with Different Mo Contents under Simulated PEMFC Cathode Environment. Materials 2022, 15, 3766. https://doi.org/10.3390/ma15113766
Jin J, Cao R, Zhang J, Tao Y, Tian X, Kou X. Study on Corrosion Resistance and Conductivity of TiMoN Coatings with Different Mo Contents under Simulated PEMFC Cathode Environment. Materials. 2022; 15(11):3766. https://doi.org/10.3390/ma15113766
Chicago/Turabian StyleJin, Jie, Rui Cao, Jinzhou Zhang, Yi Tao, Xu Tian, and Xianli Kou. 2022. "Study on Corrosion Resistance and Conductivity of TiMoN Coatings with Different Mo Contents under Simulated PEMFC Cathode Environment" Materials 15, no. 11: 3766. https://doi.org/10.3390/ma15113766
APA StyleJin, J., Cao, R., Zhang, J., Tao, Y., Tian, X., & Kou, X. (2022). Study on Corrosion Resistance and Conductivity of TiMoN Coatings with Different Mo Contents under Simulated PEMFC Cathode Environment. Materials, 15(11), 3766. https://doi.org/10.3390/ma15113766





