External Sulfate Attack on Cementitious Binders: Limitations and Effects of Sample Geometry on the Quantification of Expansion Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Analytics
2.3. Finite Element Modelling
2.4. Estimating the Maximum Crystallisation Pressure
3. Results and Discussion
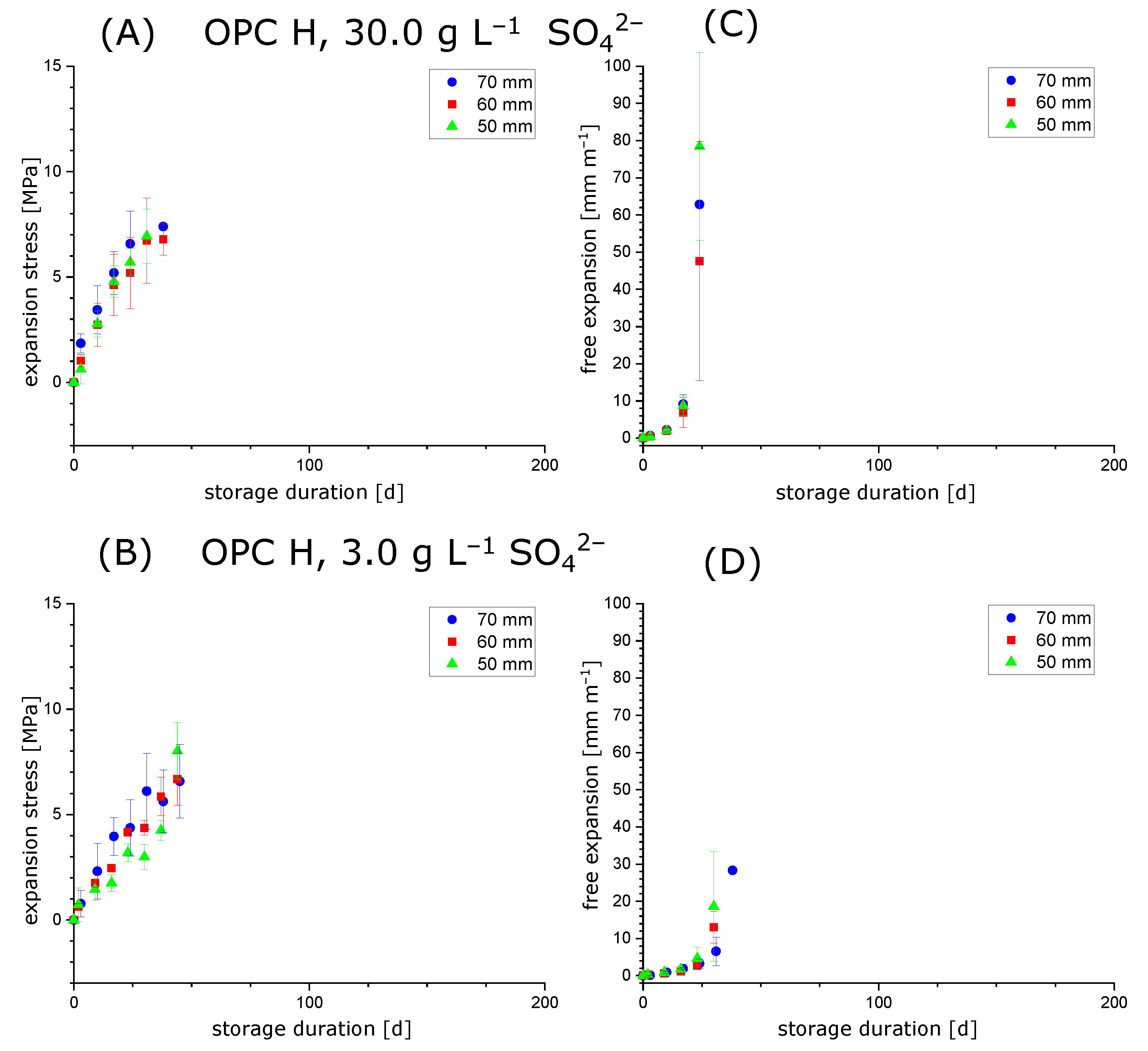
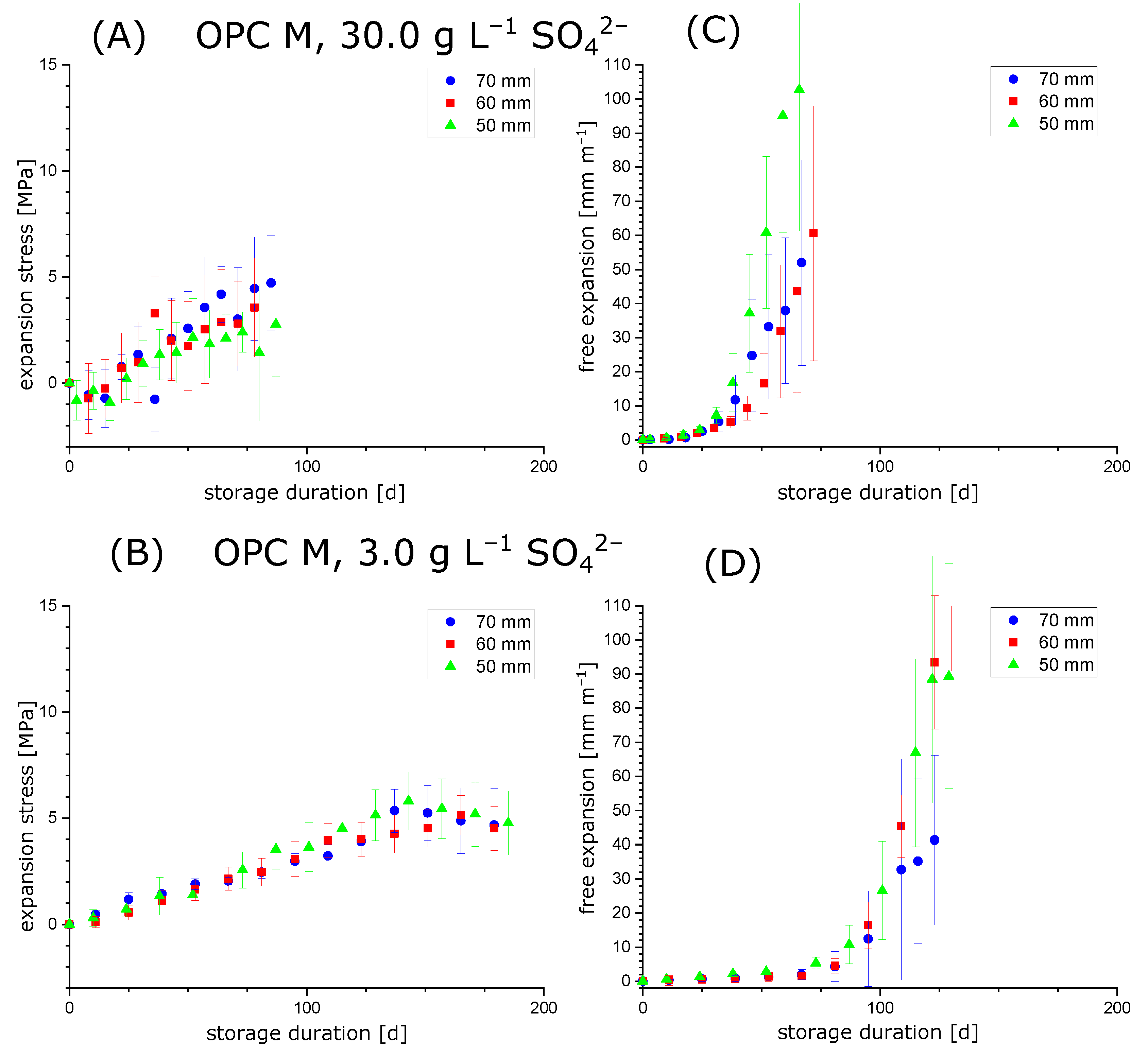
3.1. Comparison of Maximum Crystallisation Pressure and Maximum Expansion Stress
3.2. Testing the Condition
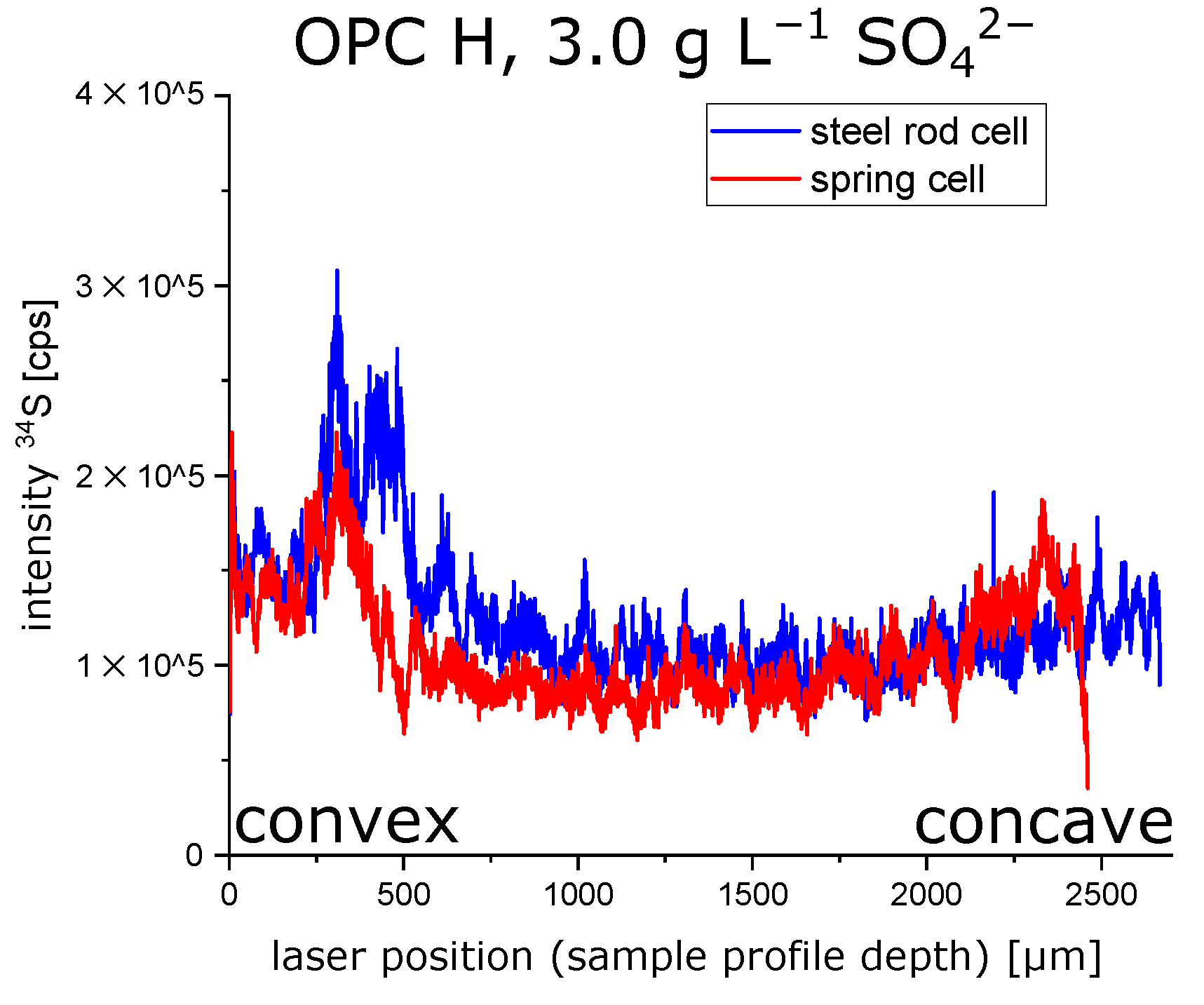
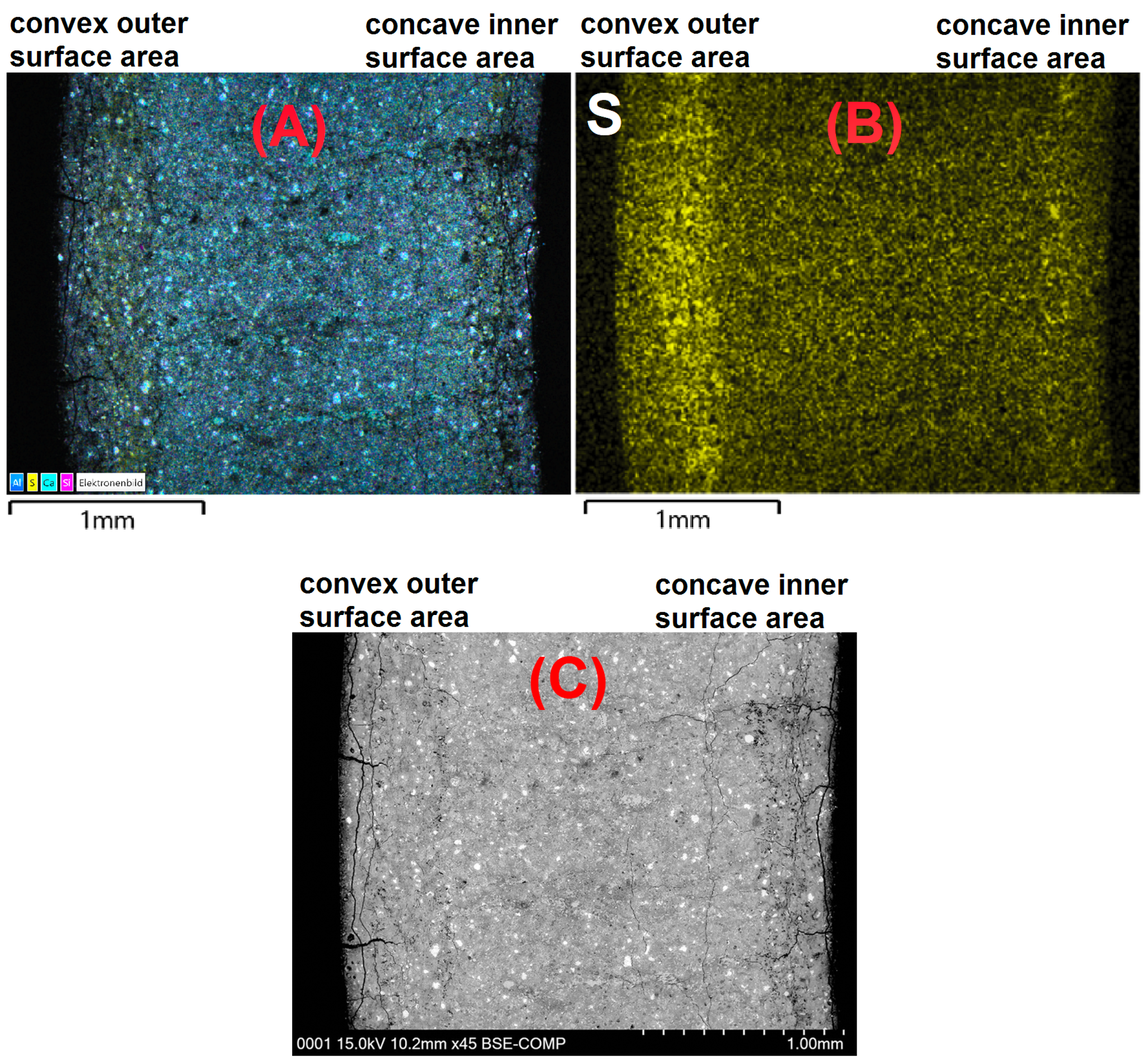
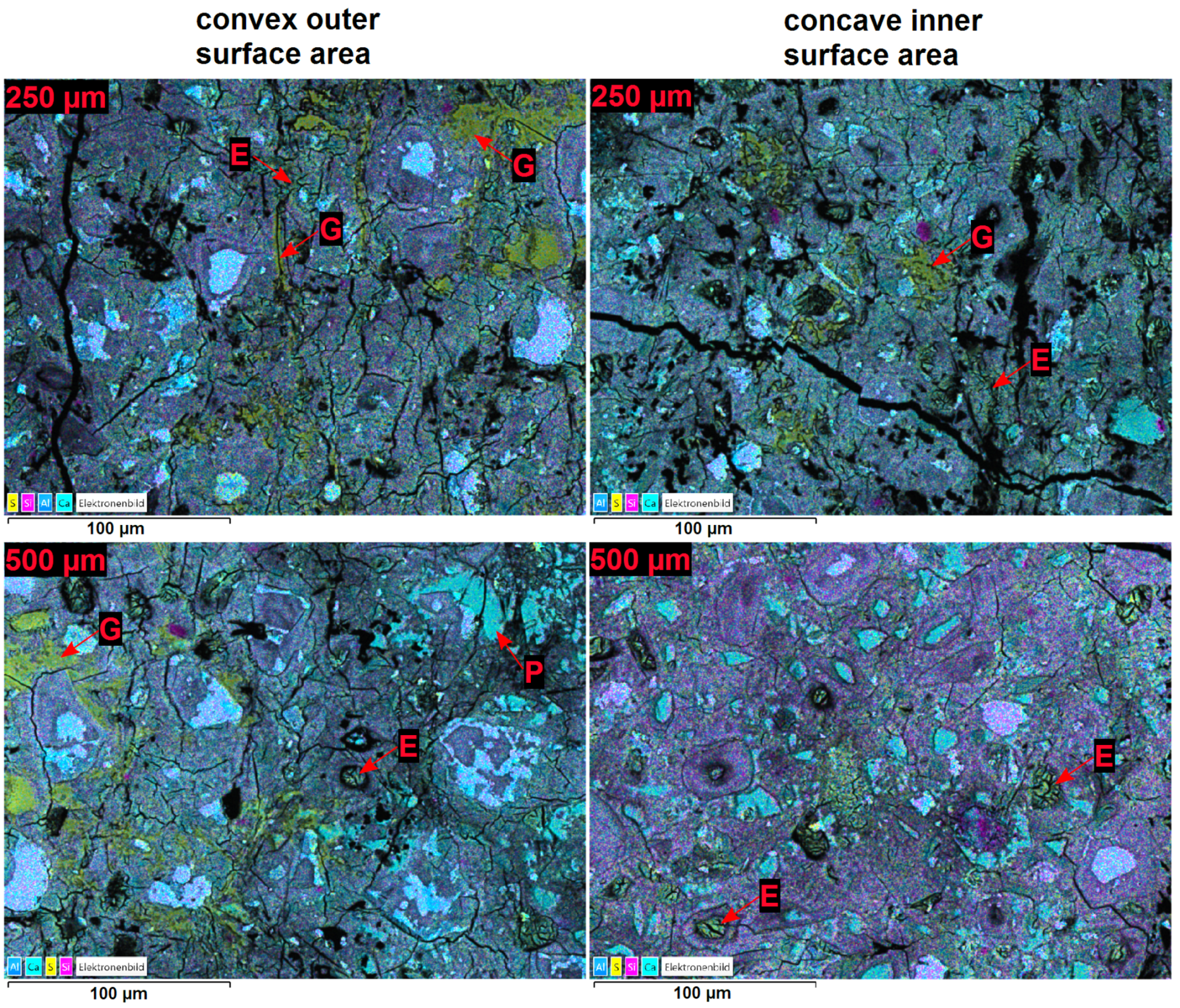
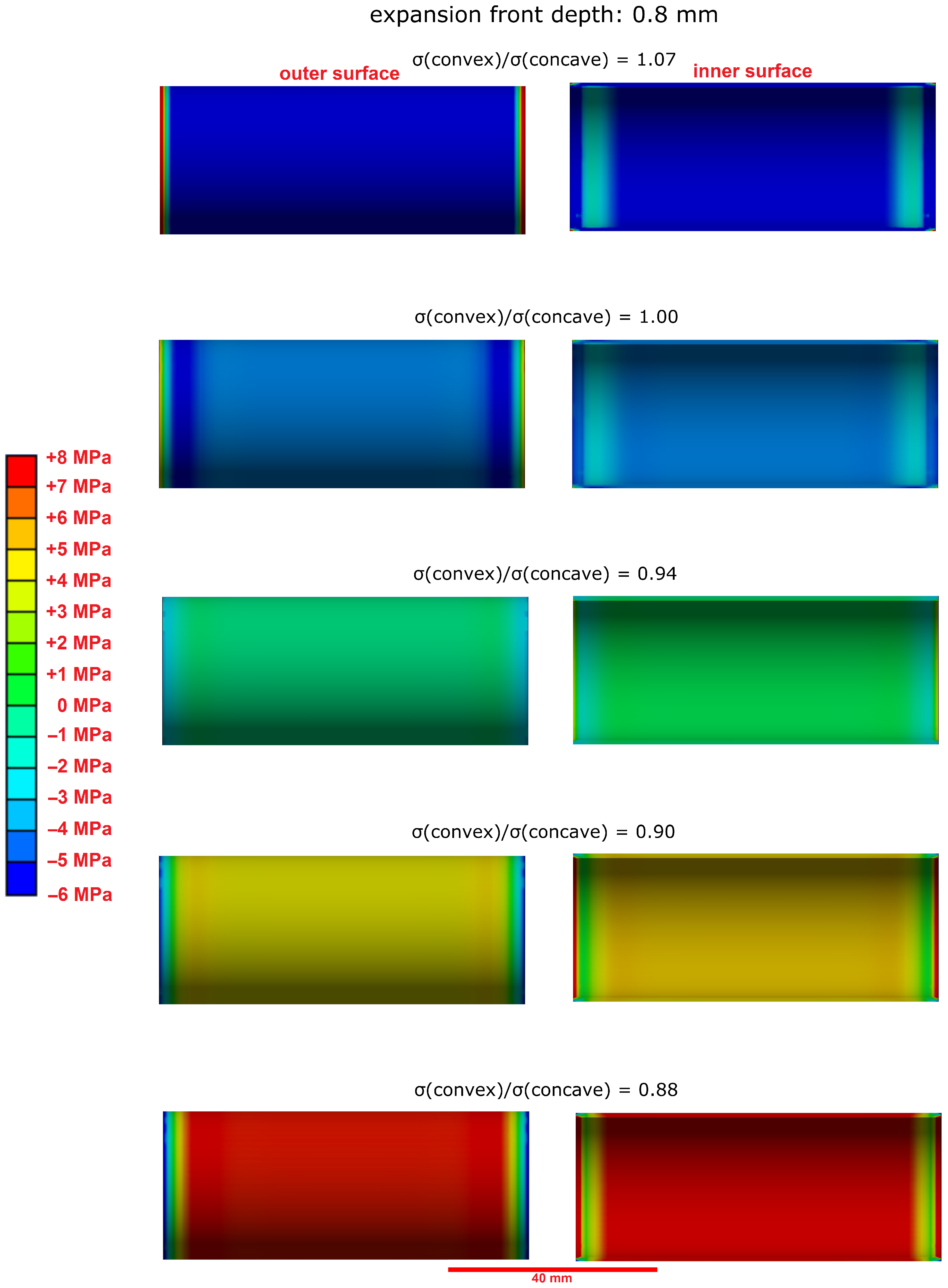
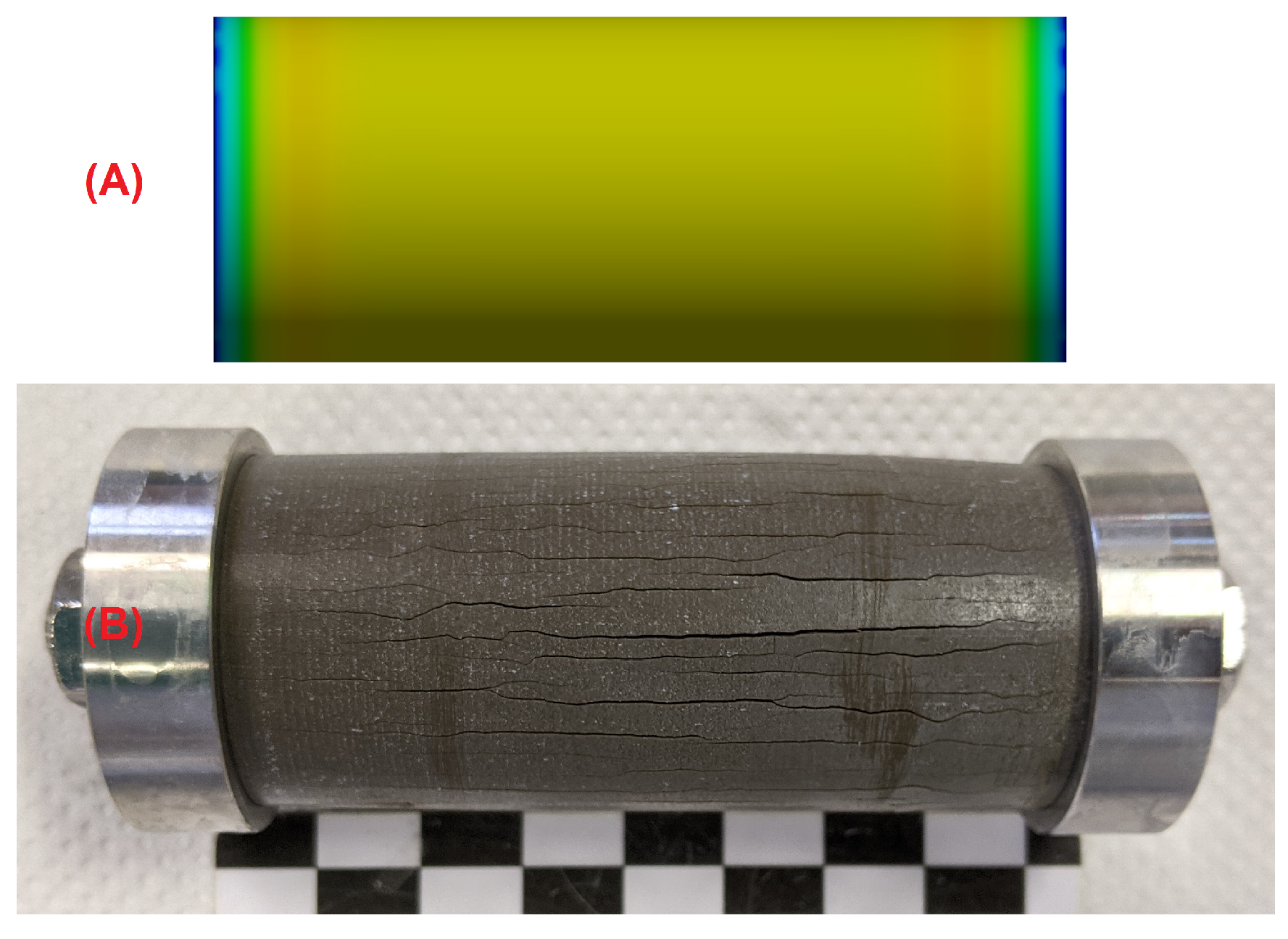
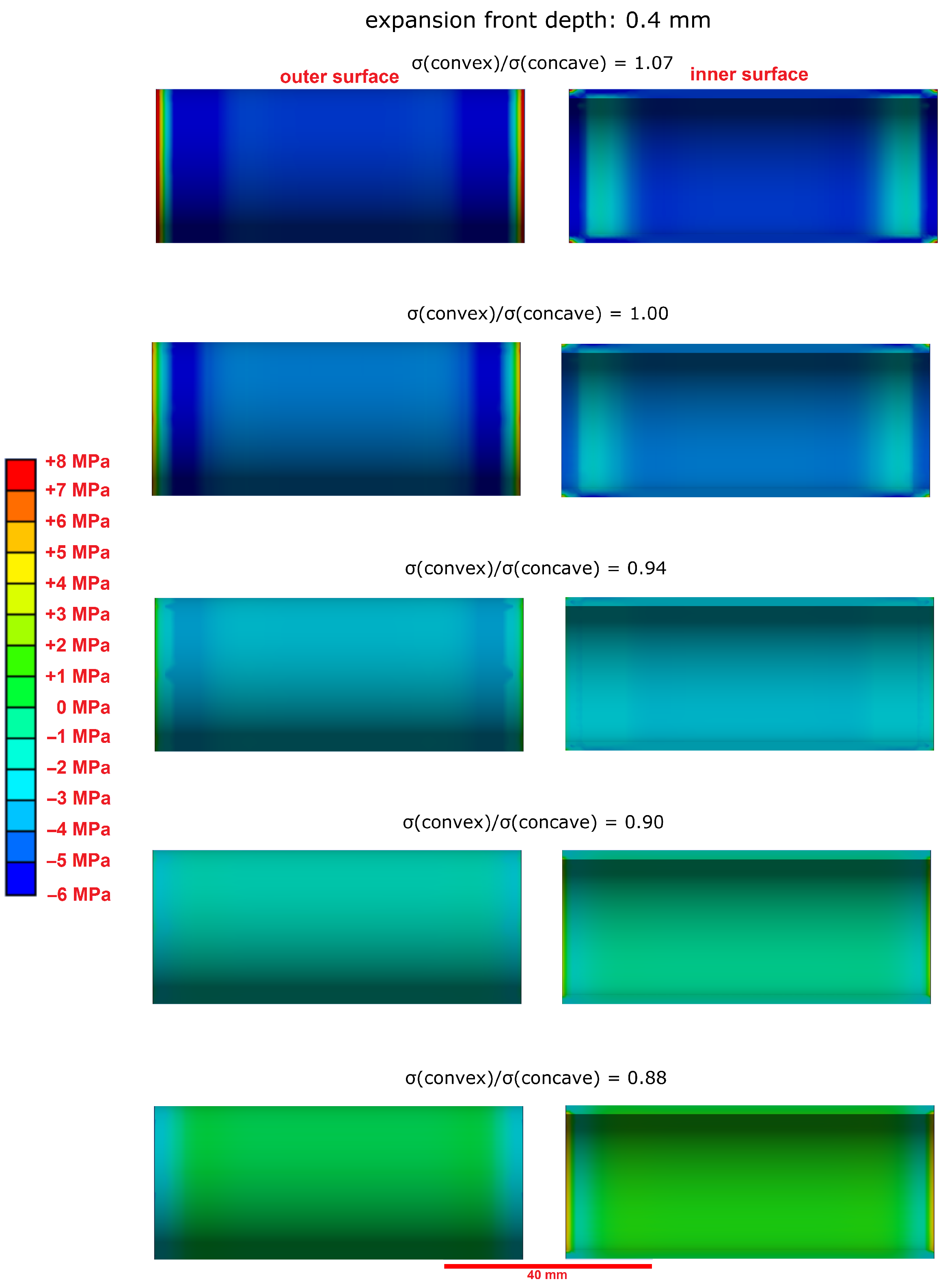
3.3. Effect of the Hollow Cylinders Curvature on the Stress Distribution
4. Conclusions
- Thermodynamically estimated crystallisation pressures for the formation of ettringite are much larger than the expansion stress observed experimentally in constrained hollow cylinders using stress cells. The stress produced by the crystallisation of ettringite or gypsum is affected by sample geometry, degree of restraint, pore sizes in which crystallisation occurs and relaxation processes.
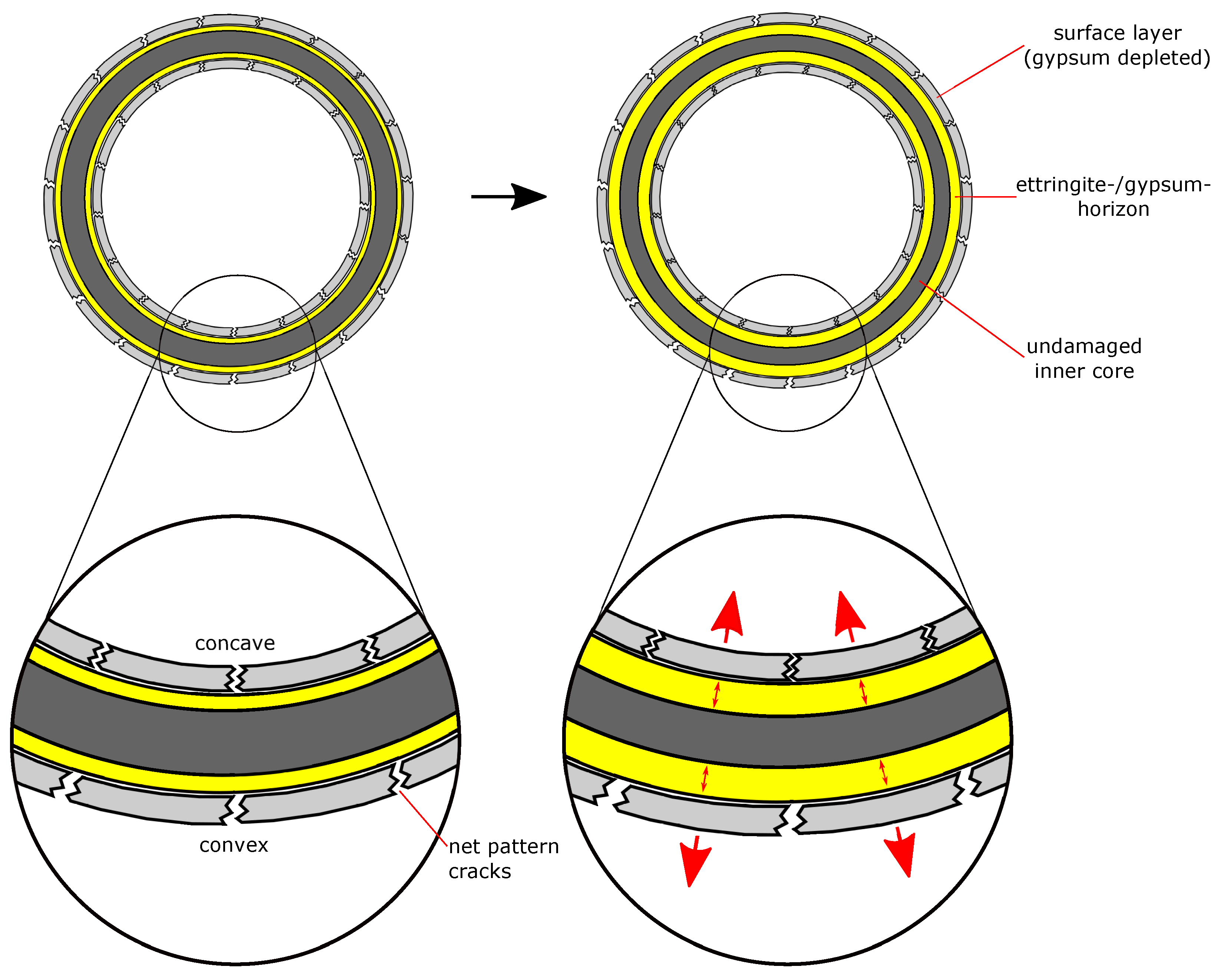
- The microstructural analysis and the macroscopic crack patterns of failed hollow cylinders indicate a higher degree of self-constraint beneath the concave inner surface compared to the convex outer surface when ettringite and/or gypsum are formed following the ingress of sulphate. Finite element modelling results indicate that this asymmetric self-constraint leads to tensile stresses in the inner core of the hollow cylinder and thus longitudinal cracks and failure. Hardened cement paste with lower tensile strength can therefore possibly support smaller maximum expansion stresses compared to samples with larger tensile strength when using the hollow cylinder method.
- Real building structures are likely to accommodate larger expansion stresses during an external sulphate attack than observed with the hollow cylinder method due to additional self-constraint in heavy structural components and the higher tensile strength of reinforced concrete. Like in the experiments used in this study, the maximum expansion stress in real building structures is most likely smaller than the thermodynamically predicted maximum crystallisation pressure, but this requires further verification. New long-term studies to quantify the expansion stress in concrete under field conditions would be required to elucidate this parameter. These studies would require a new experimental setup since the hollow cylinder method cannot be adapted for concrete due to the small wall thickness and the effect of asymmetrical self-constraint below the cylinder surfaces described in this study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, M.; Black, L. Current knowledge of external sulfate attack. Adv. Cem. Res. 2015, 27, 532–545. [Google Scholar] [CrossRef] [Green Version]
- Lothenbach, B.; Bary, B.; Bescop, B.L.; Schmidt, T.; Leterrier, N. Sulfate ingress in Portland cement. Cem. Concr. Res. 2010, 40, 1211–1225. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solutions. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Scrivener, K.L. On the relevance of volume increase for the length changes of mortar bars in sulfate solutions. Cem. Concr. Res. 2013, 46, 23–29. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Does gypsum formation during sulfate attack on concrete lead to expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Bellmann, F.; Möser, B.; Stark, J. Influence of sulfate solution concentration on the formation of gypsum in sulfate resistance test specimen. Cem. Concr. Res. 2006, 36, 358–363. [Google Scholar] [CrossRef]
- Schmidt, T.; Lothenbach, B.; Romer, M.; Neuenschwander, J.; Scrivener, K. Physical and microstructural aspects of sulfate attack on ordinary and limestone blended Portland cements. Cem. Concr. Res. 2009, 39, 1111–1121. [Google Scholar] [CrossRef]
- Wagner, M.; Decker, M.; Kunther, W.; Machner, A.; Beddoe, R.E.; Heisig, A.; Heinz, D. Gypsum formation mechanisms and their contribution to crystallisation pressure in sulfate resistant hardened cement pastes during early external sulfate attack at low sulfate concentrations. Cem. Concr. Res. 2022; currently under review. [Google Scholar]
- Correns, C.W. Growth and Dissolution of Crystals under Linear Pressure. Discuss. Faraday Soc. 1949, 5, 267–271. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Lippok, R. Hygral stress in hardened cement paste. Mater. Struct. 1999, 32, 627–634. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I.; Cavalaro, S.H.P. Effects of biaxial confinement in mortars exposed to external sulfate attack. Cem. Concr. Compos. 2019, 95, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Copuroglu, O.; Schlangen, E.; Han, N.; Xing, F. Expansion and degradation of cement paste in sodium sulfate solutions. Constr. Build. Mater. 2018, 158, 410–422. [Google Scholar] [CrossRef]
- El-Hachem, R.; Roziere, E.; Grondin, F.; Loukili, A. Multi-criteria analysis of the mechanism of degradation of Portland cement based mortars exposed to external sulphate attack. Cem. Concr. Res. 2012, 42, 1327–1335. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Stroh, J.; Meng, B.; Emmerling, F. Monitoring of sulphate attack on hardened cement paste studied by synchrotron XRD. Solid State Sci. 2015, 48, 278–285. [Google Scholar] [CrossRef]
- EAD 150009-00-0301; Blast Furnace Cement CEM III/A with Assessment of Sulfate Resistance (SR) and Optional with Low Effective Alkali Content (LA) and/or Low Heat of Hydration (LH). European Organisation for Technical Assessment (EOTA): Brussels, Belgium, 2017.
- CEN/TC 104-EN 206:2013; Concrete—Specification, Performance, Production and Conformity. European Committee for Standardization (CEN): Brussels, Belgium, 2013.
- Galan, I.; Beltagui, H.; García-Maté, M.; Glasser, F.P.; Imbabi, M.S. Impact of drying on pore structures in ettringite-rich cements. Cem. Concr. Res. 2016, 84, 85–94. [Google Scholar] [CrossRef]
- Decker, M.; Siegel, J.; Hilbig, H.; Heinz, D. LA-ICP-MS on hardened cement paste: Laser-material interaction, signal formation and optimization of laser fluence. Mater. Struct. 2021, 54, 144. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—I: The crystallization pressure of large crystals. J. Cryst. Growth 2005, 282, 455–469. [Google Scholar] [CrossRef]
- Maruyama, I. Origin of Drying Shrinkage of Hardened Cement Paste: Hydration Pressure. J. Adv. Concr. Technol. 2010, 8, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wagner, T.; Kulik, D.A.; Hingerl, F.F.; Dmytrieva, S.V. GEM-Selektor geochemical modeling package: TSolMod library and data interface for multicomponent phase models. Can. Mineral. 2012, 50, 1173–1195. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.Z.; Miron, D.G.; Myers, R. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef] [Green Version]
- Pipilikaki, P.; Papageorgiou, D.; Teas, C.; Chaniotakis, E.; Katsioti, M. The effect of temperature on thaumasite formation. Cem. Concr. Compos. 2008, 30, 964–969. [Google Scholar] [CrossRef]
- Cole, W.F.; Lancucki, C.J. A Refinement of the Crystal Structure of Gypsum CaSO4.2H2O. Acta Crystallogr. Sect. B 1974, B30, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Ikumi, T.; Cavalaro, S.H.P.; Segura, I.; de la Fuente, A.; Aguado, A. Simplified methodology to evaluate the external sulfate attack in concrete structures. Mater. Des. 2016, 89, 1147–1160. [Google Scholar] [CrossRef]
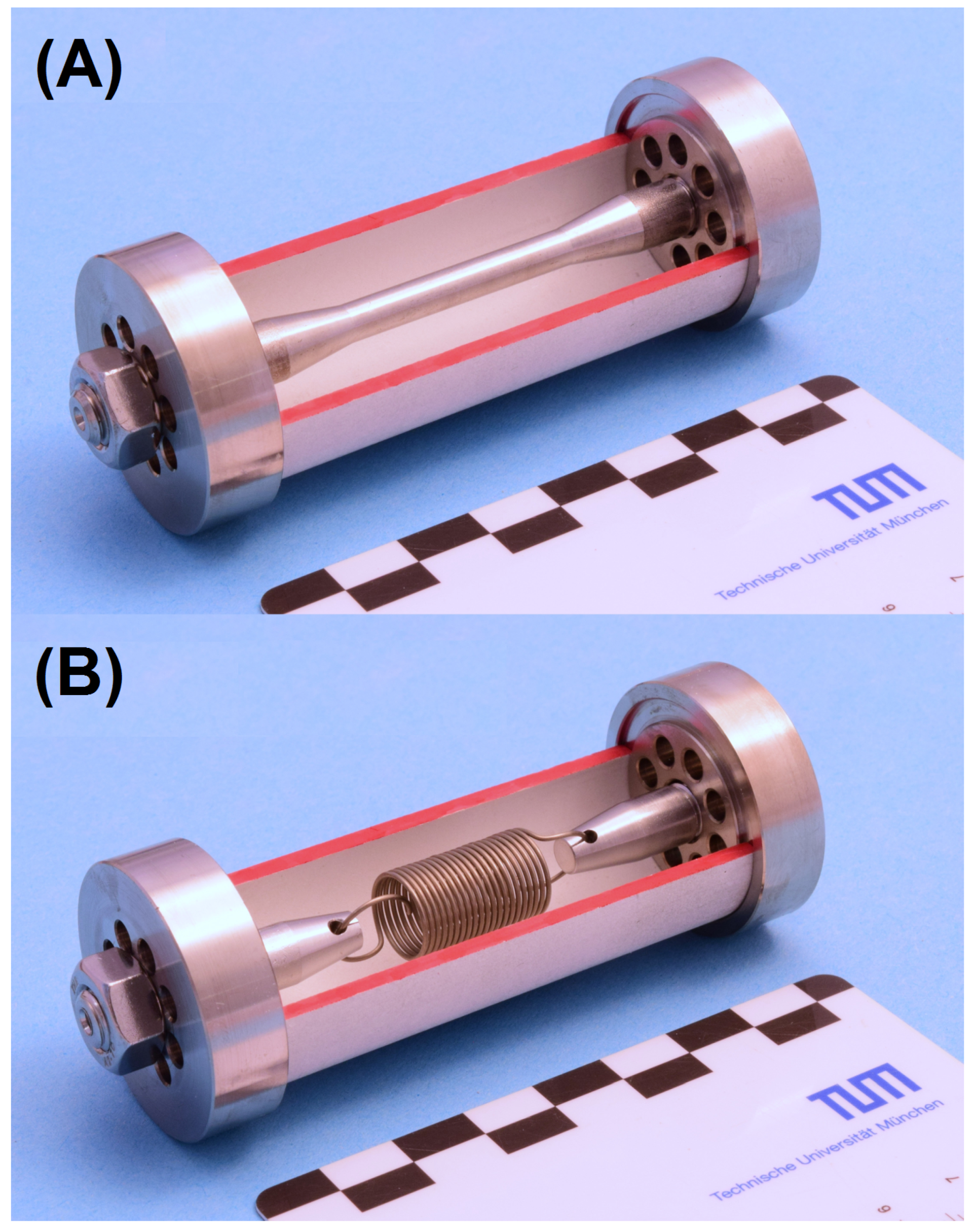
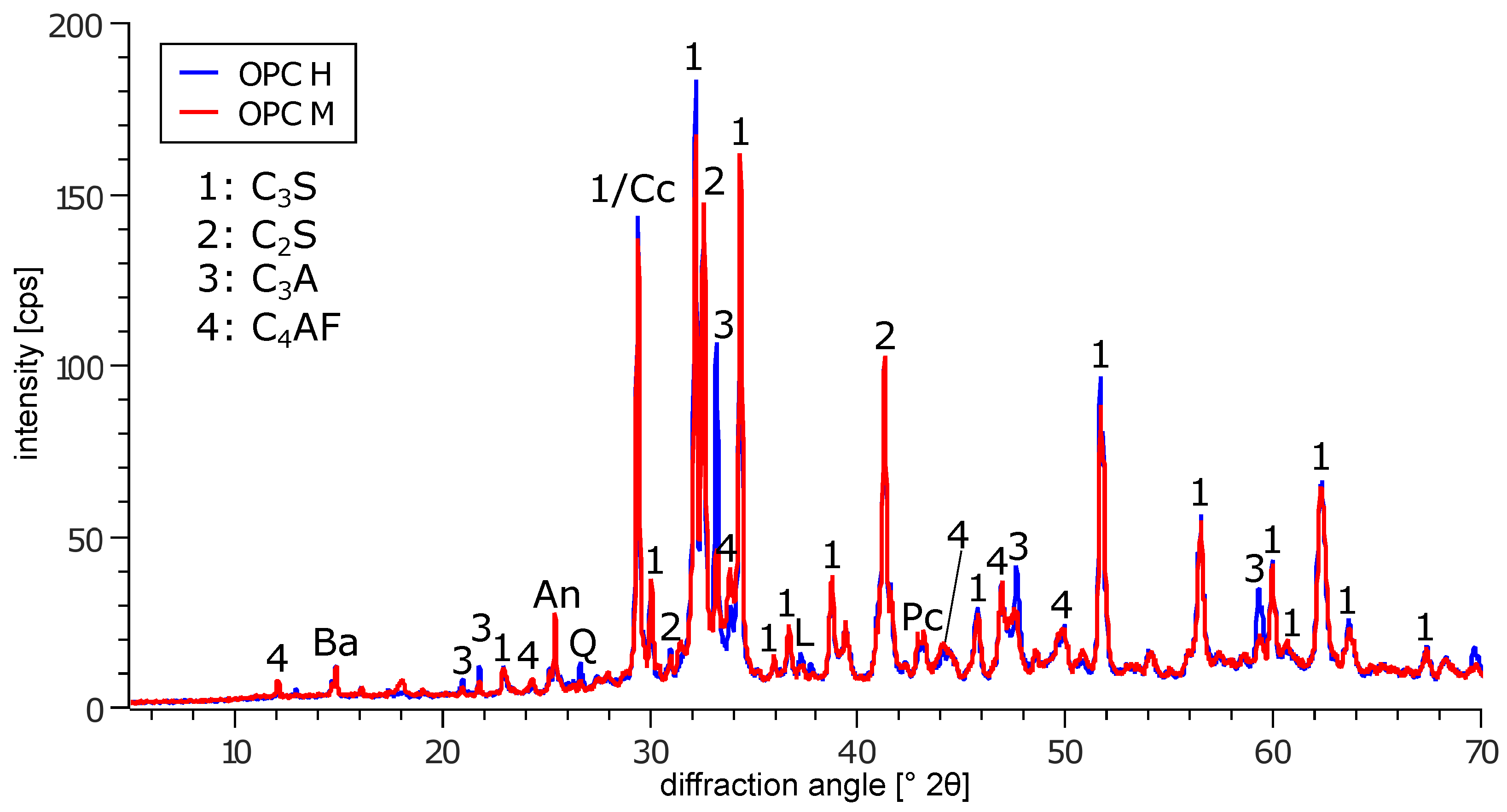



| Element | OPC H | OPC M | Phase | OPC H | OPC M | |
|---|---|---|---|---|---|---|
| LOI | 1.90 | 2.96 | C3S | 64 | 64 | |
| 0.25 | 0.49 | C2S | 12 | 12 | ||
| 0.84 | 1.14 | C3A | 12 | 6.5 | ||
| 63.70 | 63.16 | C4AF | 4 | 8.5 | ||
| 1.38 | 2.12 | Gypsum | n.d. | n.d. | ||
| 2.27 | 2.67 | Bassanite | 3 | n.d. | ||
| 5.25 | 4.50 | Lime | n.d. | 1 | ||
| 20.80 | 19.72 | Periclase | n.d. | 2 | ||
| 0.26 | 0.29 | Quartz | <1 | n.d. | ||
| 2.78 | 3.46 | Calcite | 3 | 3 | ||
| 0.28 | 0.26 | Arcanite | n.d. | 1 | ||
| 0.06 | 0.03 | Anhydrite | n.d. | 2 | ||
| ∑ elements | 99.77 | 100.80 | ∑ phases | 99 | 100 |
| Ettringite | Gypsum | |||||||
|---|---|---|---|---|---|---|---|---|
| Sulfate Concentration | OPC H | OPC M | OPC H | OPC M | ||||
| g L | 53.4 | 53.4 | 5.3 | 4.7 | ||||
| g L | 54.1 | 54.1 | 0.0 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, M.; Heisig, A.; Machner, A.; Beddoe, R.; Heinz, D. External Sulfate Attack on Cementitious Binders: Limitations and Effects of Sample Geometry on the Quantification of Expansion Stress. Materials 2022, 15, 3677. https://doi.org/10.3390/ma15103677
Wagner M, Heisig A, Machner A, Beddoe R, Heinz D. External Sulfate Attack on Cementitious Binders: Limitations and Effects of Sample Geometry on the Quantification of Expansion Stress. Materials. 2022; 15(10):3677. https://doi.org/10.3390/ma15103677
Chicago/Turabian StyleWagner, Matthias, Anne Heisig, Alisa Machner, Robin Beddoe, and Detlef Heinz. 2022. "External Sulfate Attack on Cementitious Binders: Limitations and Effects of Sample Geometry on the Quantification of Expansion Stress" Materials 15, no. 10: 3677. https://doi.org/10.3390/ma15103677
APA StyleWagner, M., Heisig, A., Machner, A., Beddoe, R., & Heinz, D. (2022). External Sulfate Attack on Cementitious Binders: Limitations and Effects of Sample Geometry on the Quantification of Expansion Stress. Materials, 15(10), 3677. https://doi.org/10.3390/ma15103677






