Electrocatalytic Properties of Mixed-Oxide-Containing Composite-Supported Platinum for Polymer Electrolyte Membrane (PEM) Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Type Supports and Electrocatalysts
2.3. Physicochemical Characterization of the Composite Supports and the Electrocatalysts
2.4. Electrochemical Characterization of Composite-Supported Electrocatalysts
3. Results and Discussion
3.1. Physicochemical Characterization of the Composite Supports and the Related Pt Electrocatalysts
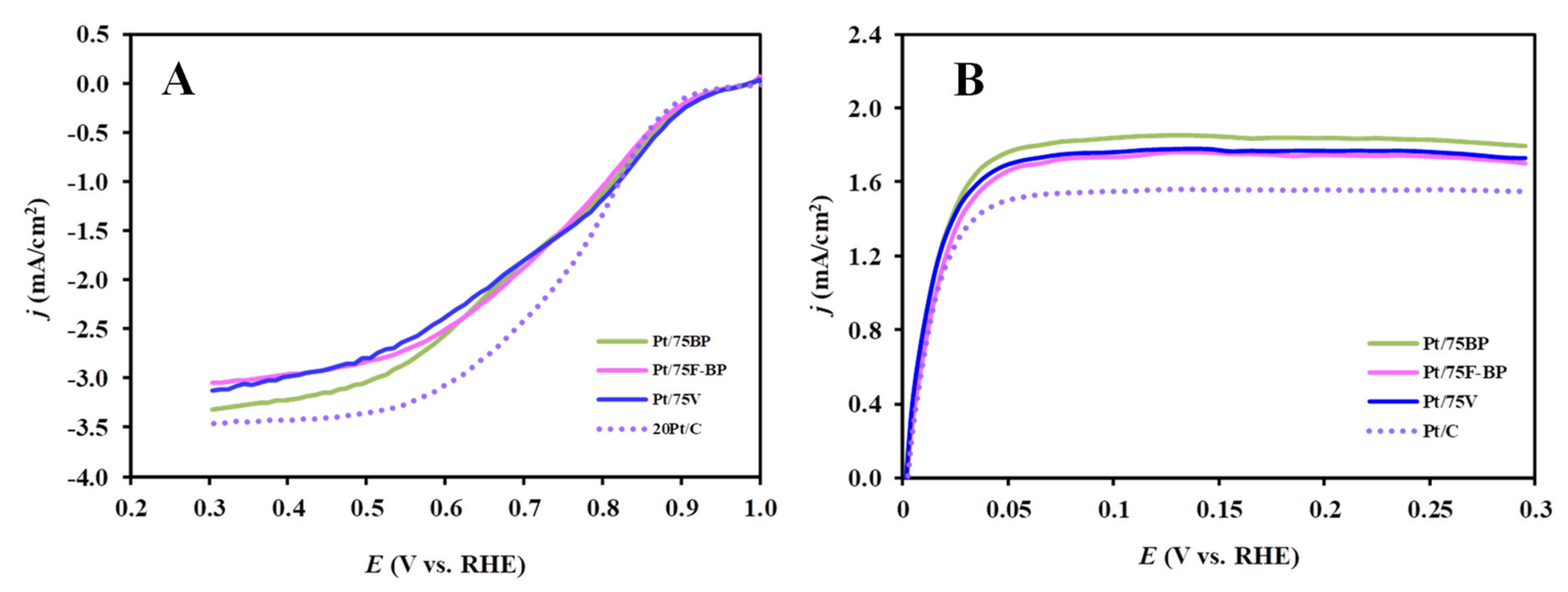
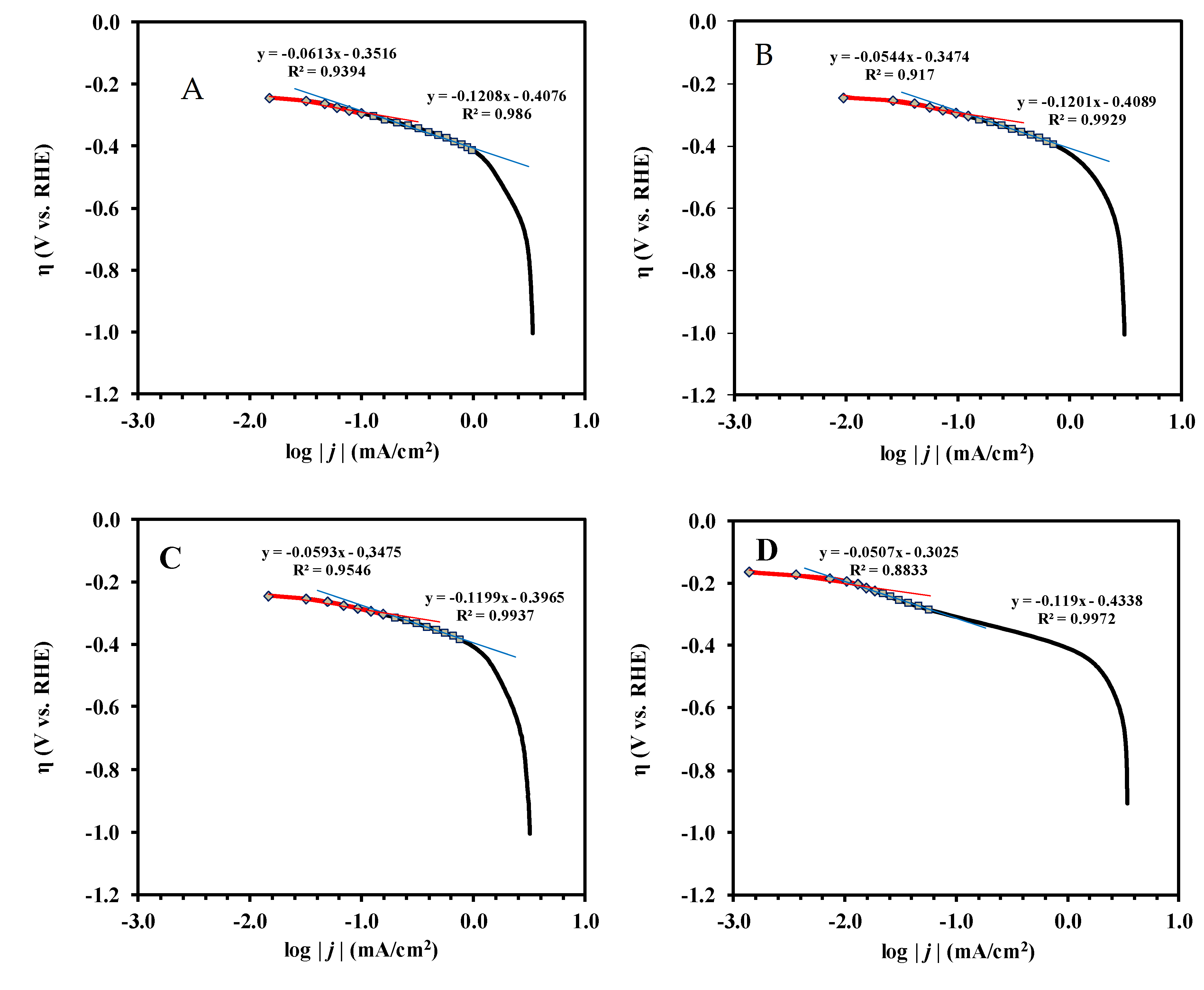
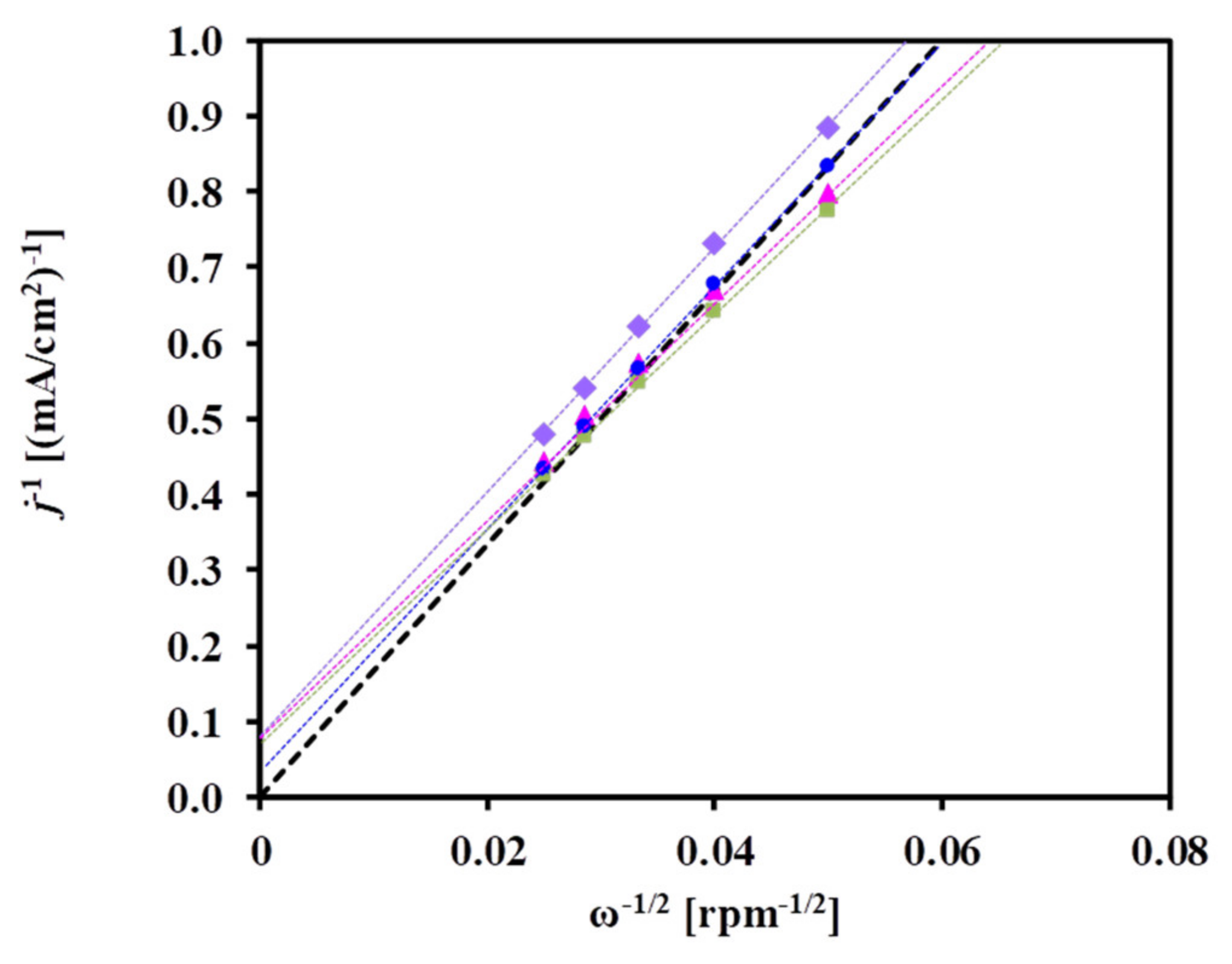
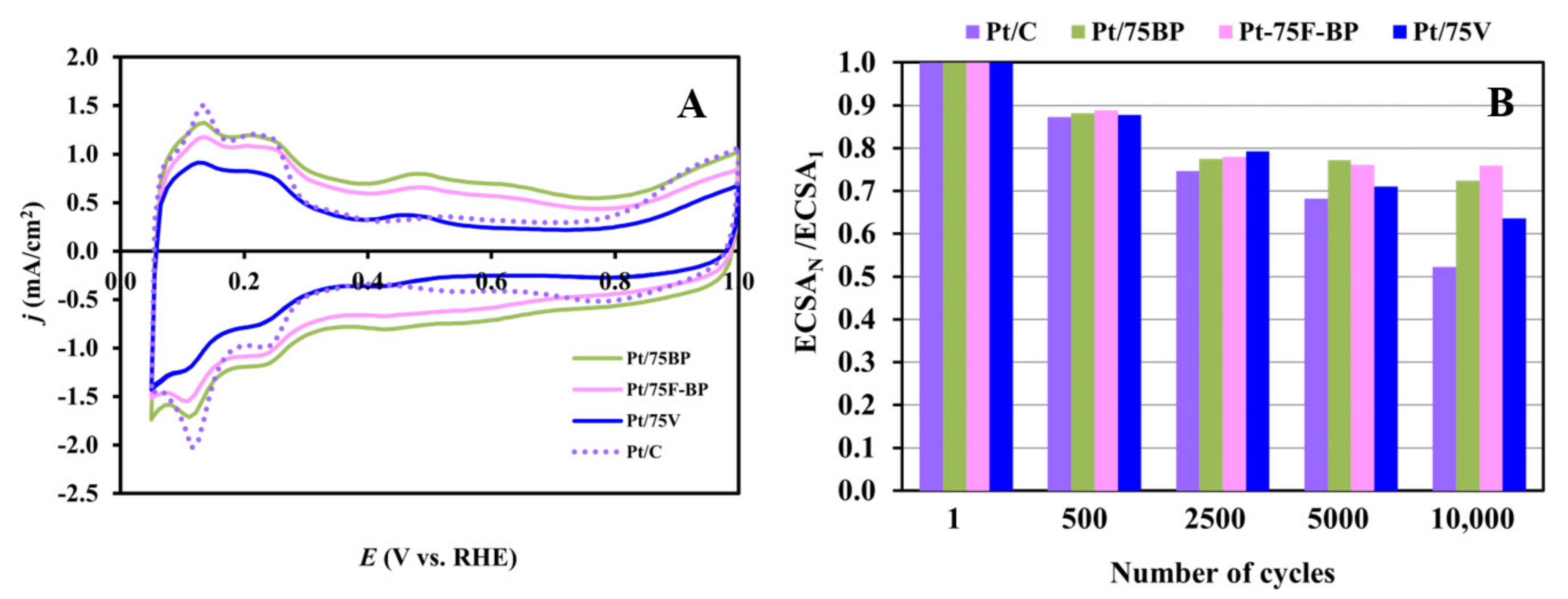
3.2. Electrochemical Characterization of the Pt/Ti0.8Mo0.2O2-C Electrocatalysts
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Sun, C. Cost-Effective Iron-Based Aqueous Redox Flow Batteries for Large-Scale Energy Storage Application: A Review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Fan, Y. Techno-Economic Challenges of Fuel Cell Commercialization. Engineering 2018, 4, 352–360. [Google Scholar] [CrossRef]
- Leader, A.; Gaustad, G.; Babbitt, C. The Effect of Critical Material Prices on the Competitiveness of Clean Energy Technologies. Mater. Renew. Sustain. Energy 2019, 8, 8. [Google Scholar] [CrossRef]
- Depcik, C.; Cassady, T.; Collicott, B.; Burugupally, S.P.; Li, X.; Alam, S.S.; Arandia, J.R.; Hobeck, J. Comparison of Lithium Ion Batteries, Hydrogen Fueled Combustion Engines, and a Hydrogen Fuel Cell in Powering a Small Unmanned Aerial Vehicle. Energy Convers. Manag. 2020, 207, 112514. [Google Scholar] [CrossRef]
- Andaloro, L.; Arista, A.; Agnello, G.; Napoli, G.; Sergi, F.; Antonucci, V. Study and Design of a Hybrid Electric Vehicle (Lithium Batteries-PEM FC). Int. J. Hydrogen Energy 2017, 42, 3166–3184. [Google Scholar] [CrossRef]
- Sarma, U.; Ganguly, S. Determination of the Component Sizing for the PEM Fuel Cell-Battery Hybrid Energy System for Locomotive Application Using Particle Swarm Optimization. J. Energy Storage 2018, 19, 247–259. [Google Scholar] [CrossRef]
- Pivetta, D.; Dall’Armi, C.; Taccani, R. Multi-Objective Optimization of Hybrid PEMFC/Li-Ion Battery Propulsion Systems for Small and Medium Size Ferries. Int. J. Hydrogen Energy 2021, 46, 35949–35960. [Google Scholar] [CrossRef]
- Arsalis, A.; Papanastasiou, P.; Georghiou, G.E. A Comparative Review of Lithium-Ion Battery and Regenerative Hydrogen Fuel Cell Technologies for Integration with Photovoltaic Applications. Renew. Energy 2022, 191, 943–960. [Google Scholar] [CrossRef]
- Xueqin, L.; Wu, Y.; Lian, J.; Zhang, Y. Energy Management and Optimization of PEMFC/Battery Mobile Robot Based on Hybrid Rule Strategy and AMPSO. Renew. Energy 2021, 171, 881–901. [Google Scholar] [CrossRef]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Unifying the 2e− and 4e− Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. Lett. 2012, 3, 2948–2951. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- She, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Dou, S.; Li, X.; Wang, J.; Wang, X. Strategies to Break the Scaling Relation toward Enhanced Oxygen Electrocatalysis. Matter 2019, 1, 1494–1518. [Google Scholar] [CrossRef]
- Sanij, F.D.; Balakrishnan, P.; Leung, P.; Shah, A.; Su, H.; Xu, Q. Advanced Pd-Based Nanomaterials for Electro-Catalytic Oxygen Reduction in Fuel Cells: A Review. Int. J. Hydrogen Energy 2021, 46, 14596–14627. [Google Scholar] [CrossRef]
- Ahn, C.Y.; Park, J.E.; Kim, S.; Kim, O.H.; Hwang, W.; Her, M.; Kang, S.Y.; Park, S.; Kwon, O.J.; Park, H.S.; et al. Differences in the Electrochemical Performance of Pt-Based Catalysts Used for Polymer Electrolyte Membrane Fuel Cells in Liquid Half-and Full-Cells. Chem. Rev. 2021, 121, 15075–15140. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Wei, L.; Li, J.; Zhao, X. Catalytic Performance and Synthesis of a Pt/Graphene-TiO2 Catalyst Using an Environmentally Friendly Microwave-Assisted Solvothermal Method. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 1680–1687. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current Status of Automotive Fuel Cells for Sustainable Transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Madheswaran, D.K.; Jayakumar, A. Recent Advancements on Non-Platinum Based Catalyst Electrode Material for Polymer Electrolyte Membrane Fuel Cells: A Mini Techno-Economic Review. Bull. Mater. Sci. 2021, 44, 287. [Google Scholar] [CrossRef]
- Sajid, A.; Pervaiz, E.; Ali, H.; Noor, T.; Baig, M.M. A Perspective on Development of Fuel Cell Materials: Electrodes and Electrolyte. Int. J. Energy Res. 2022, 46, 6953–6988. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, S.; Chen, S. Pt Utilization in Proton Exchange Membrane Fuel Cells: Structure Impacting Factors and Mechanistic Insights. Chem. Soc. Rev. 2022, 51, 1529–1546. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent Advances in Activity and Durability Enhancement of Pt/C Catalytic Cathode in PEMFC. Part II: Degradation Mechanism and Durability Enhancement of Carbon Supported Platinum Catalyst. J. Power Sources 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ige, O.O.; Barhoumi, E.M.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum Degradation Mechanisms in Proton Exchange Membrane Fuel Cell (PEMFC) System: A Review. Int. J. Hydrogen Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, J.; Luo, X.; Tu, Z. Comparison of the Performance and Degradation Mechanism of PEMFC with Pt/C and Pt Black Catalyst. Int. J. Hydrogen Energy 2022, 47, 5418–5428. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon Corrosion Mechanism and Mitigation Strategies in a Proton Exchange Membrane Fuel Cell (PEMFC): A Review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Sharma, R.; Andersen, S.M. Circular Use of Pt/C through Pt Dissolution from Spent PEMFC Cathode and Direct Reproduction of New Catalyst with Microwave Synthesis. Mater. Chem. Phys. 2021, 265, 124472. [Google Scholar] [CrossRef]
- Chourashiya, M.; Sharma, R.; Gyergyek, S.; Andersen, S.M. Gram-Size Pt/C Catalyst Synthesized Using Pt Compound Directly Recovered from an End-of-Life PEM Fuel Cell Stack. Mater. Chem. Phys. 2022, 276, 125439. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J.J. Degradation Mechanisms of Pt/C Fuel Cell Catalysts under Simulated Start-Stop Conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A Review of Polymer Electrolyte Membrane Fuel Cell Durability for Vehicular Applications: Degradation Modes and Experimental Techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An Overview of Metal Oxide Materials as Electrocatalysts and Supports for Polymer Electrolyte Fuel Cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Ghasemi, M.; Choi, J.; Ju, H. Performance Analysis of Pt/TiO2/C Catalyst Using a Multi-Scale and Two-Phase Proton Exchange Membrane Fuel Cell Model. Electrochim. Acta 2021, 366, 137484. [Google Scholar] [CrossRef]
- Subban, C.V.; Zhou, Q.; Hu, A.; Moylan, T.E.; Wagner, F.T.; Disalvo, F.J. Sol-Gel Synthesis, Electrochemical Characterization, and Stability Testing of Ti0.7W0.3O2 Nanoparticles for Catalyst Support Applications in Proton-Exchange Membrane Fuel Cells. J. Am. Chem. Soc. 2010, 132, 17531–17536. [Google Scholar] [CrossRef]
- Wang, D.; Subban, C.V.; Wang, H.; Rus, E.; Disalvo, F.J.; Abruña, H.D. Highly Stable and CO-Tolerant Pt/Ti0.7W0.3O2 Electrocatalyst for Proton-Exchange Membrane Fuel Cells. J. Am. Chem. Soc. 2010, 132, 10218–10220. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Pan, C.J.; Rick, J.; Su, W.N.; Hwang, B.J. Nanostructured Ti0.7Mo0.3O2 Support Enhances Electron Transfer to Pt: High-Performance Catalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2011, 133, 11716–11724. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ho, V.T.T.; Pan, C.J.; Liu, J.Y.; Chou, H.L.; Rick, J.; Su, W.N.; Hwang, B.J. Synthesis of Ti0.7Mo0.3O2Supported-Pt Nanodendrites and Their Catalytic Activity and Stability for Oxygen Reduction Reaction. Appl. Catal. B Environ. 2014, 154–155, 183–189. [Google Scholar] [CrossRef]
- Park, K.W.; Seol, K.S. Nb-TiO2 Supported Pt Cathode Catalyst for Polymer Electrolyte Membrane Fuel Cells. Electrochem. Commun. 2007, 9, 2256–2260. [Google Scholar] [CrossRef]
- Huang, S.Y.; Ganesan, P.; Popov, B.N. Electrocatalytic Activity and Stability of Niobium-Doped Titanium Oxide Supported Platinum Catalyst for Polymer Electrolyte Membrane Fuel Cells. Appl. Catal. B Environ. 2010, 96, 224–231. [Google Scholar] [CrossRef]
- Kumar, A.; Ramani, V. Ta0.3Ti0.7O2 Electrocatalyst Supports Exhibit Exceptional Electrochemical Stability. J. Electrochem. Soc. 2013, 160, F1207–F1215. [Google Scholar] [CrossRef]
- Anwar, M.T.; Yan, X.; Shen, S.; Husnain, N.; Zhu, F.; Luo, L.; Zhang, J. Enhanced Durability of Pt Electrocatalyst with Tantalum Doped Titania as Catalyst Support. Int. J. Hydrogen Energy 2017, 42, 30750–30759. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, M.; Shao, Z.; Zhang, C.; Qin, X.; Yi, B. Preparation and Characterization of Ti0.7Sn0.3O2 as Catalyst Support for Oxygen Reduction Reaction. J. Energy Chem. 2014, 23, 331–337. [Google Scholar] [CrossRef]
- Eckardt, M.; Gebauer, C.; Jusys, Z.; Wassner, M.; Hüsing, N.; Behm, R.J. Oxygen Reduction Reaction Activity and Long-Term Stability of Platinum Nanoparticles Supported on Titania and Titania-Carbon Nanotube Composites. J. Power Sources 2018, 400, 580–591. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern Trend of Anodes in Microbial Fuel Cells (MFCs): An Overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Gubán, D.; Borbáth, I.; Pászti, Z.; Sajó, I.; Drotár, E.; Hegedus, M.; Tompos, A. Preparation and Characterization of Novel Ti0.7W0.3O2-C Composite Materials for Pt-Based Anode Electrocatalysts with Enhanced CO Tolerance. Appl. Catal. B Environ. 2015, 174–175, 455–470. [Google Scholar] [CrossRef]
- Vass, Á.; Borbáth, I.; Pászti, Z.; Bakos, I.; Sajó, I.E.; Németh, P.; Tompos, A. Effect of Mo Incorporation on the Electrocatalytic Performance of Ti–Mo Mixed Oxide–Carbon Composite Supported Pt Electrocatalysts. React. Kinet. Mech. Catal. 2017, 121, 141–160. [Google Scholar] [CrossRef]
- Gubán, D.; Pászti, Z.; Borbáth, I.; Bakos, I.; Drotár, E.; Sajó, I.; Tompos, A. Design and Preparation of CO Tolerant Anode Electrocatalysts for PEM Fuel Cells. Period. Polytech. Chem. Eng. 2016, 60, 29–39. [Google Scholar] [CrossRef]
- Vass, Á.; Borbáth, I.; Bakos, I.; Pászti, Z.; Sáfrán, G.; Tompos, A. Stability Issues of CO Tolerant Pt-Based Electrocatalysts for Polymer Electrolyte Membrane Fuel Cells: Comparison of Pt/Ti0.8Mo0.2O2–C with PtRu/C. React. Kinet. Mech. Catal. 2019, 126, 679–699. [Google Scholar] [CrossRef]
- Vass, Á.; Borbáth, I.; Bakos, I.; Pászti, Z.; Sajó, I.E.; Tompos, A. Novel Pt Electrocatalysts: Multifunctional Composite Supports for Enhanced Corrosion Resistance and Improved CO Tolerance. Top. Catal. 2018, 61, 1300–1312. [Google Scholar] [CrossRef]
- Borbáth, I.; Zelenka, K.; Vass, Á.; Pászti, Z.; Szijjártó, G.P.; Sebestyén, Z.; Sáfrán, G.; Tompos, A. CO Tolerant Pt Electrocatalysts for PEM Fuel Cells with Enhanced Stability against Electrocorrosion. Int. J. Hydrogen Energy 2021, 46, 13534–13547. [Google Scholar] [CrossRef]
- Yazici, M.S.; Dursun, S.; Borbáth, I.; Tompos, A. Reformate Gas Composition and Pressure Effect on CO Tolerant Pt/Ti0.8Mo0.2O2–C Electrocatalyst for PEM Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 13524–13533. [Google Scholar] [CrossRef]
- Gubán, D.; Tompos, A.; Bakos, I.; Vass; Pászti, Z.; Szabó, E.G.; Sajó, I.E.; Borbáth, I. Preparation of CO-Tolerant Anode Electrocatalysts for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 13741–13753. [Google Scholar] [CrossRef]
- Borbáth, I.; Tálas, E.; Pászti, Z.; Zelenka, K.; Ayyubov, I.; Salmanzade, K.; Sajó, I.E.; Sáfrán, G.; Tompos, A. Investigation of Ti-Mo Mixed Oxide-Carbon Composite Supported Pt Electrocatalysts: Effect of the Type of Carbonaceous Materials. Appl. Catal. A Gen. 2021, 620, 118155. [Google Scholar] [CrossRef]
- Gubán, D.; Tompos, A.; Bakos, I.; Pászti, Z.; Gajdos, G.; Sajó, I.; Borbáth, I. CO Oxidation and Oxygen Reduction Activity of Bimetallic Sn–Pt Electrocatalysts on Carbon: Effect of the Microstructure and the Exclusive Formation of the Pt3Sn Alloy. React. Kinet. Mech. Catal. 2017, 121, 43–67. [Google Scholar] [CrossRef][Green Version]
- Fairely, N. CasaXPS Manual 2.3. 15; Casa Software Ltd.: Teignmouth, Devon, UK, 2009; pp. 1–177. [Google Scholar]
- Mohai, M. XPS MultiQuant: Multimodel XPS Quantification Software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Woods, R. Electroanalytical Chemistry: A Series of Advances; Bard, A.J., Ed.; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 1976; Volume 9, pp. 1–162. [Google Scholar]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Electrical Conductivity of Conductive Carbon Blacks: Influence of Surface Chemistry and Topology. Appl. Surf. Sci. 2003, 217, 181–193. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Fleury, P.A.; Damen, T.C. Raman Spectra of TiO2, MgF2, ZnF2, FeF2, and MnF2. Phys. Rev. 1967, 154, 522–526. [Google Scholar] [CrossRef]
- Gotić, M.; Ivanda, M.; Popović, S.; Musić, S.; Sekulić, A.; Turkovic, A.; Furić, K. Raman Investigation of Nanosized TiO2. J. Raman Spectrosc. 1997, 28, 555–558. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, C.; Li, Q.; Hou, J.; Li, Y.; Ai, S. Nitrogen- and Oxygen-Functionalized Carbon Nanotubes Supported Pt-Based Catalyst for the Selective Hydrogenation of Cinnamaldehyde. Appl. Catal. A Gen. 2015, 506, 134–142. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Diczházi, D.; Borbáth, I.; Bakos, I.; Szijjártó, G.P.; Tompos, A.; Pászti, Z. Design of Mo-Doped Mixed Oxide–Carbon Composite Supports for Pt-Based Electrocatalysts: The Nature of the Mo-Pt Interaction. Catal. Today 2021, 366, 31–40. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Mendoza-Sanchez, B.; Fernandez, V.; Veenstra, R.; Dukstiene, N.; Roberts, A.; Fairley, N. Generalized Molybdenum Oxide Surface Chemical State XPS Determination via Informed Amorphous Sample Model. Appl. Surf. Sci. 2015, 326, 151–161. [Google Scholar] [CrossRef]
- Brox, B.; Olefjord, I. ESCA Studies of MoO2 and MoO3. Surf. Interface Anal. 1988, 13, 3–6. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Watson, G.W.; Payne, D.J.; Atkinson, G.R.; Egdell, R.G.; Law, D.S.L. Theoretical and Experimental Study of the Electronic Structures of MoO3 and MoO2. J. Phys. Chem. C 2010, 114, 4636–4645. [Google Scholar] [CrossRef]
- Alkan, G.; Košević, M.; Mihailović, M.; Stopic, S.; Friedrich, B.; Stevanović, J.; Panić, V. Characterization of Defined Pt Particles Prepared by Ultrasonic Spray Pyrolysis for One-Step Synthesis of Supported ORR Composite Catalysts. Metals 2022, 12, 290. [Google Scholar] [CrossRef]
- Geppert, T.N.; Bosund, M.; Putkonen, M.; Stühmeier, B.M.; Pasanen, A.T.; Heikkilä, P.; Gasteiger, H.A.; El-Sayed, H.A. HOR Activity of Pt-TiO2-Y at Unconventionally High Potentials Explained: The Influence of SMSI on the Electrochemical Behavior of Pt. J. Electrochem. Soc. 2020, 167, 084517. [Google Scholar] [CrossRef]
- Sonkar, P.K.; Prakash, K.; Yadav, M.; Ganesan, V.; Sankar, M.; Gupta, R.; Yadav, D.K. Co(II)-Porphyrin-Decorated Carbon Nanotubes as Catalysts for Oxygen Reduction Reactions: An Approach for Fuel Cell Improvement. J. Mater. Chem. A 2017, 5, 6263–6276. [Google Scholar] [CrossRef]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-Performance Platinum-Free Oxygen Reduction Reaction and Hydrogen Oxidation Reaction Catalyst in Polymer Electrolyte Membrane Fuel Cell. Sci. Rep. 2018, 8, 3591. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; López de la Fuente, D.; Arriaga, L.G.; Vargas Gutiérrez, G.; Rodríguez Varela, F.J. Electrochemical Investigation of Pt-Co/MWCNT as an Alcohol-Tolerant ORR Catalyst for Direct Oxidation Fuel Cells. Int. J. Electrochem. Sci. 2011, 6, 1835–1854. [Google Scholar]
- Gochi-Ponce, Y.; Alonso-Nuñez, G.; Alonso-Vante, N. Synthesis and Electrochemical Characterization of a Novel Platinum Chalcogenide Electrocatalyst with an Enhanced Tolerance to Methanol in the Oxygen Reduction Reaction. Electrochem. Commun. 2006, 8, 1487–1491. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, C.; Kim, J.K.; Kim, J.H.; Chun, H.H.; Lee, H.; Kim, Y.T. Enhanced Electrocatalytic Performance Due to Anomalous Compressive Strain and Superior Electron Retention Properties of Highly Porous Pt Nanoparticles. J. Catal. 2012, 291, 69–78. [Google Scholar] [CrossRef]
- Varela, F.J.R.; Luna, S.F.; Savadogo, O. Synthesis and Evaluation of Highly Tolerant Pd Electrocatalysts as Cathodes in Direct Ethylene Glycol Fuel Cells (DEGFC). Energies 2009, 2, 944–956. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Rotating Disk Electrode Measurements on the CO Tolerance of a High-Surface Area Pt/Vulcan Carbon Fuel Cell Catalyst. J. Electrochem. Soc. 1999, 146, 1296–1304. [Google Scholar] [CrossRef]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky-Levich Analysis Applied to Nanoparticle Modified Rotating Disk Electrodes: Electrocatalysis or Misinterpretation. Nano Res. 2014, 7, 71–78. [Google Scholar] [CrossRef]
- Batchelor-Mcauley, C.; Compton, R.G. Thin-Film Modified Rotating Disk Electrodes: Models of Electron-Transfer Kinetics for Passive and Electroactive Films. J. Phys. Chem. C 2014, 118, 30034–30038. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Wang, J.X.; Markovic, N.M.; Adzic, R.R. Kinetic Analysis of Oxygen Reduction on Pt(111) in Acid Solutions: Intrinsic Kinetic Parameters and Anion Adsorption Effects. J. Phys. Chem. B 2004, 108, 4127–4133. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods—Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2000; ISBN 0471043729. [Google Scholar]
- Blizanac, B.B.; Ross, P.N.; Marković, N.M. Oxygen Reduction on Silver Low-Index Single-Crystal Surfaces in Alkaline Solution: Rotating Ring DiskAg(hkl) Studies. J. Phys. Chem. B 2006, 110, 4735–4741. [Google Scholar] [CrossRef]
- Holewinski, A.; Linic, S. Elementary Mechanisms in Electrocatalysis: Revisiting the ORR Tafel Slope. J. Electrochem. Soc. 2012, 159, H864–H870. [Google Scholar] [CrossRef]
- Chen, W.; Xiang, Q.; Peng, T.; Song, C.; Shang, W.; Deng, T.; Wu, J. Reconsidering the Benchmarking Evaluation of Catalytic Activity in Oxygen Reduction Reaction. iScience 2020, 23, 101532. [Google Scholar] [CrossRef]
- Hsueh, K.L.; Gonzalez, E.R.; Srinivasan, S. Electrolyte Effects on Oxygen Reduction Kinetics at Platinum: A Rotating Ring-Disc Electrode Analysis. Electrochim. Acta 1983, 28, 691–697. [Google Scholar] [CrossRef]
- Agbo, P.; Danilovic, N. An Algorithm for the Extraction of Tafel Slopes. J. Phys. Chem. C 2019, 123, 30252–30264. [Google Scholar] [CrossRef]
- Stassi, A.; D’Urso, C.; Baglio, V.; Di Blasi, A.; Antonucci, V.; Arico, A.S.; Castro Luna, A.M.; Bonesi, A.; Triaca, W.E. Electrocatalytic Behaviour for Oxygen Reduction Reaction of Small Nanostructured Crystalline Bimetallic Pt-M Supported Catalysts. J. Appl. Electrochem. 2006, 36, 1143–1149. [Google Scholar] [CrossRef]
- Meng, H.; Shen, P.K. Tungsten Carbide Nanocrystal Promoted Pt/C Electrocatalysts for Oxygen Reduction. J. Phys. Chem. B 2005, 109, 22705–22709. [Google Scholar] [CrossRef] [PubMed]
- Neyerlin, K.C.; Gu, W.; Jorne, J.; Gasteiger, H.A. Study of the Exchange Current Density for the Hydrogen Oxidation and Evolution Reactions. J. Electrochem. Soc. 2007, 154, B631–B635. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Zalitis, C.M.; Sharman, J.; Wright, E.; Kucernak, A.R. Properties of the Hydrogen Oxidation Reaction on Pt/C Catalysts at Optimised High Mass Transport Conditions and Its Relevance to the Anode Reaction in PEFCs and Cathode Reactions in Electrolysers. Electrochim. Acta 2015, 176, 763–776. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Gasteiger, H.A.; Stäb, G.D.; Urban, P.M.; Kolb, D.M.; Behm, R.J. Characterization of High-Surface-Area Electrocatalysts Using a Rotating Disk Electrode Configuration. J. Electrochem. Soc. 1998, 145, 2354–2358. [Google Scholar] [CrossRef]
- Maiorova, N.A.; Mikhailova, A.A.; Khazova, O.A.; Grinberg, V.A. Thin-Film Rotating Disk Electrode as a Tool for Comparing the Activity of Catalysts in the Hydrogen Oxidation Reaction. Russ. J. Electrochem. 2006, 42, 331–338. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Jusys, Z.; Gasteiger, H.A.; Behm, R.J.; Endruschat, U.; Boennemann, H. On the CO Tolerance of Novel Colloidal PdAu/Carbon Electrocatalysts. J. Electroanal. Chem. 2001, 501, 132–140. [Google Scholar] [CrossRef]
- Guillén-Villafuerte, O.; García, G.; Rodríguez, J.L.; Pastor, E.; Guil-López, R.; Nieto, E.; Fierro, J.L.G. Preliminary Studies of the Electrochemical Performance of Pt/X@MoO3/C (X = Mo2C, MoO2, Mo0) Catalysts for the Anode of a DMFC: Influence of the Pt Loading and Mo-Phase. Int. J. Hydrogen Energy 2013, 38, 7811–7821. [Google Scholar] [CrossRef]
- Justin, P.; Ranga Rao, G. Methanol Oxidation on MoO3 Promoted Pt/C Electrocatalyst. Int. J. Hydrogen Energy 2011, 36, 5875–5884. [Google Scholar] [CrossRef]
- Sheng, W.; Chen, S.; Vescovo, E.; Shao-Horn, Y. Size Influence on the Oxygen Reduction Reaction Activity and Instability of Supported Pt Nanoparticles. J. Electrochem. Soc. 2011, 159, B96–B103. [Google Scholar] [CrossRef]
- Martins, P.F.B.D.; Ticianelli, E.A. Electrocatalytic Activity and Stability of Platinum Nanoparticles Supported on Carbon-Molybdenum Oxides for the Oxygen Reduction Reaction. ChemElectroChem 2015, 2, 1298–1306. [Google Scholar] [CrossRef]
- Zana, A.; Rüdiger, C.; Kunze-Liebhäuser, J.; Granozzi, G.; Reeler, N.E.A.; Vosch, T.; Kirkensgaard, J.J.K.; Arenz, M. Core-Shell TiO2@C: Towards Alternative Supports as Replacement for High Surface Area Carbon for PEMFC Catalysts. Electrochim. Acta 2014, 139, 21–28. [Google Scholar] [CrossRef]
- Silva, C.; Borbáth, I.; Zelenka, K.; Sajó, I.E.; Sáfrán, G.; Tompos, A.; Pászti, Z. Effect of the Reductive Treatment on the State and Electrocatalytic Behavior of Pt in Catalysts Supported on Ti0.8Mo0.2O2-C Composite. React. Kinet. Mech. Catal. 2022, 135, 29–47. [Google Scholar] [CrossRef]
- Micoud, F.; Maillard, F.; Gourgaud, A.; Chatenet, M. Unique CO-Tolerance of Pt-WOx Materials. Electrochem. Commun. 2009, 11, 651–654. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Vinod Selvaganesh, S.; Bhat, S.D. Nitrogen and Carbon Doped Titanium Oxide as an Alternative and Durable Electrocatalyst Support in Polymer Electrolyte Fuel Cells. J. Power Sources 2016, 304, 360–372. [Google Scholar] [CrossRef]

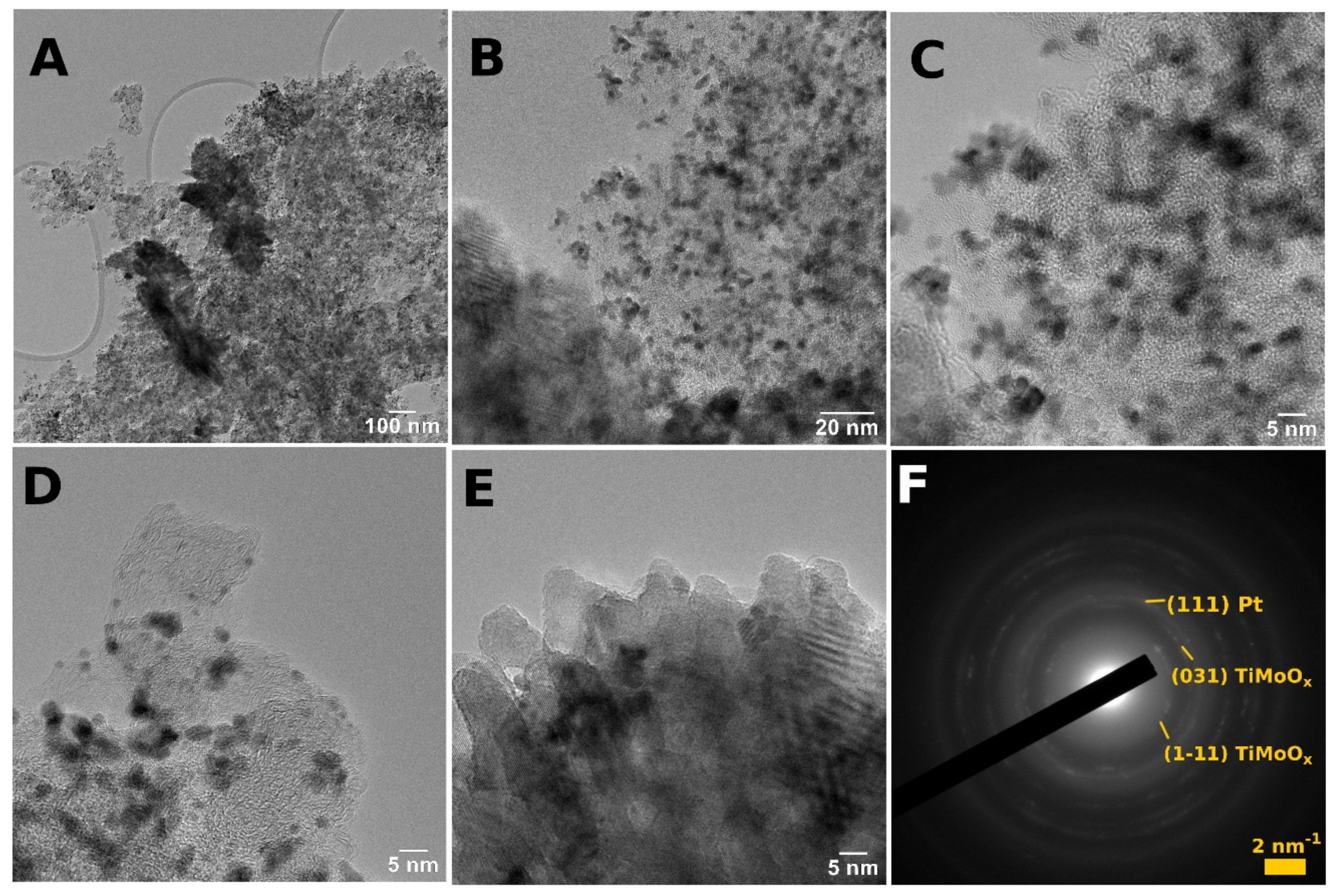
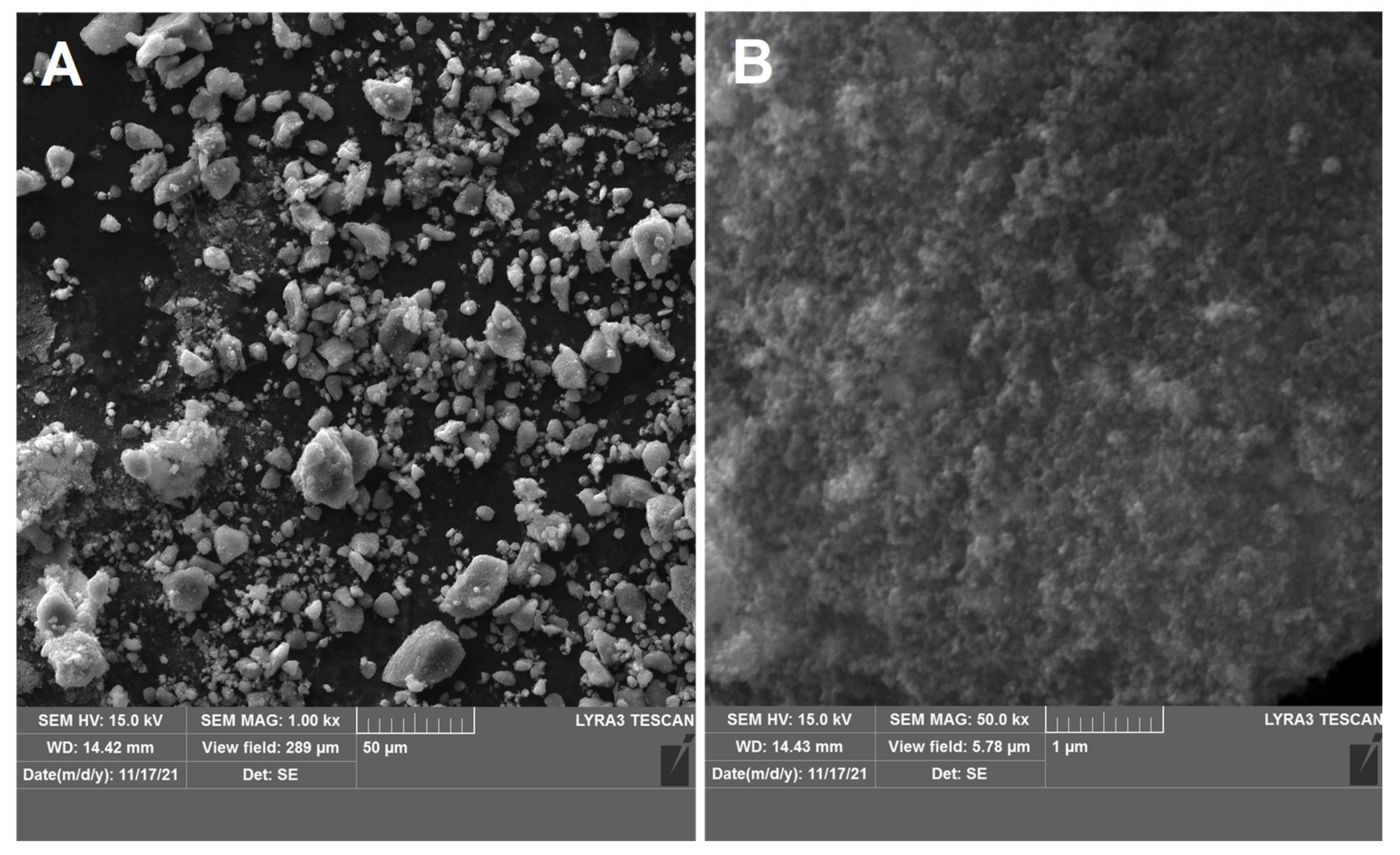
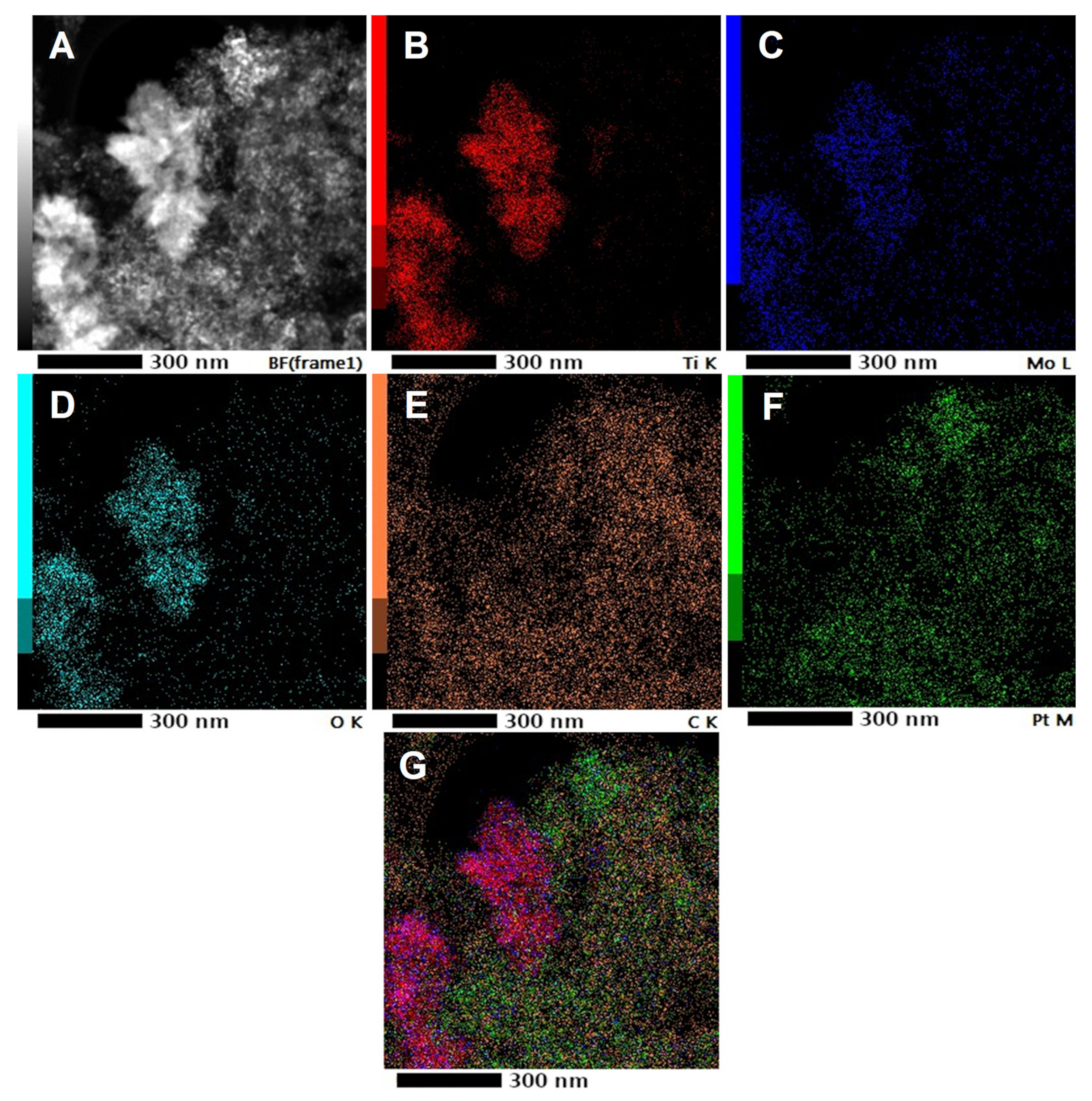
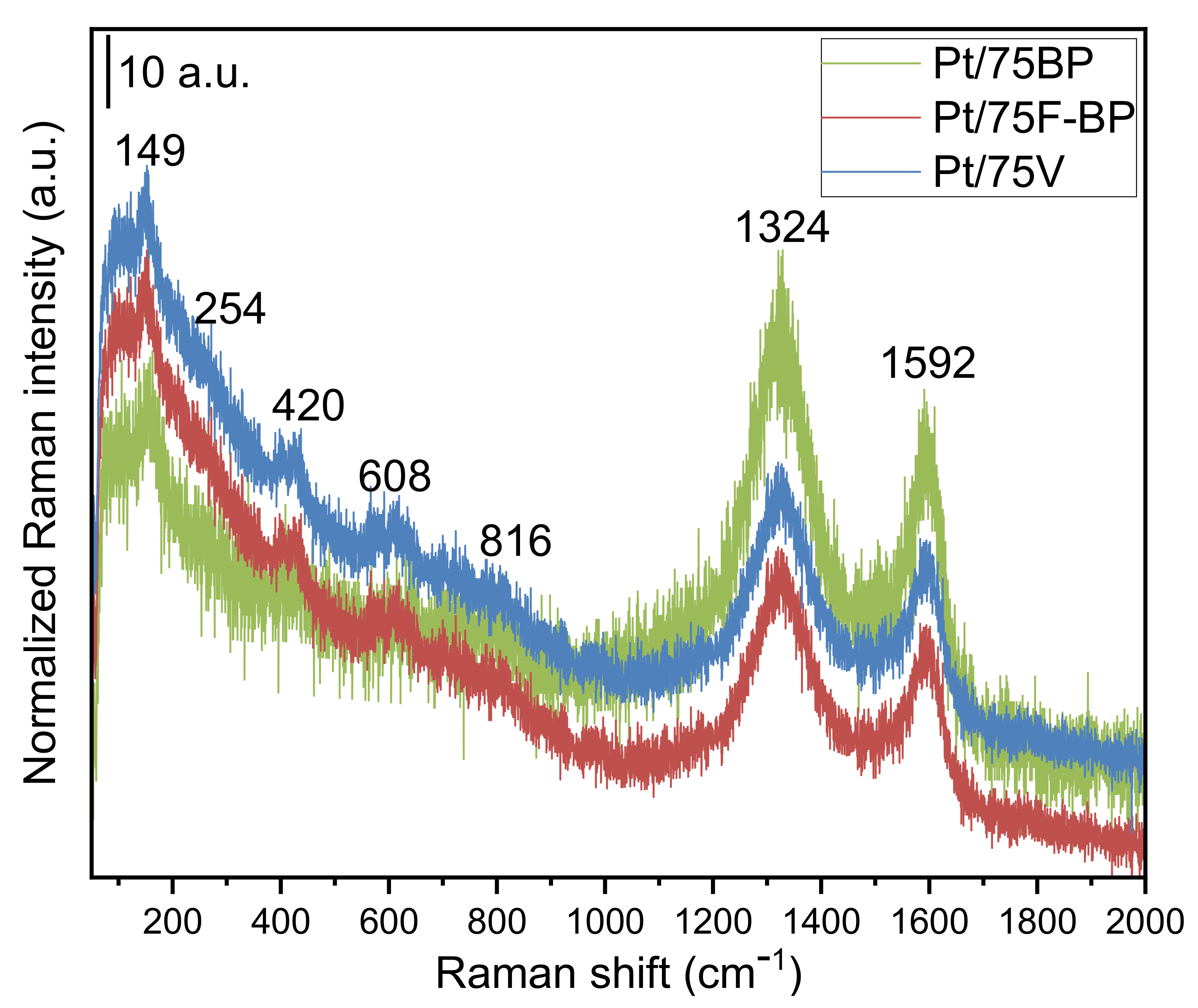

| Sample ID (a) | Nominal Composition of the Support | BET Surface Area, m2g−1 (b) | Pore Volume, cm3g−1 | Rutile Lattice Parameters, Å (c) | Pt Size, nm (XRD) |
|---|---|---|---|---|---|
| Pt/75BP | 25 wt.% Ti0.8Mo0.2O2-75 wt.% BP | 1120 | 2.01 | a = 4.630, c = 2.940 | 2.68 |
| Pt/75F-BP | 25 wt.% Ti0.8Mo0.2O2-75 wt.% F-BP | 726 | 1.32 | a = 4.630, c = 2.940 | 2.75 |
| Pt/75V | 25 wt.% Ti0.8Mo0.2O2-75 wt.% V | 175 | 0.48 | a = 4.630, c = 2.940 | 2.08 |
| Method/Value | Ti/Mo (at/at) (a) | TiMoOx/C (wt.%/wt.%) | Pt (wt.%) |
|---|---|---|---|
| Nominal | 80/20 | 25/75 | 20.0 |
| EDX (b) | 82.3/17.7 | 25.2/74.8 | 3.1 |
| EDX (c) | 82.1/17.9 | 45.8/54.2 | 20.6 |
| ICP-OES | 83.8/16.2 | 18.7/81.3 | 19.2 |
| Sample ID (a) | Ti/Mo (at/at) | Oxide/C (wt.%/wt.%) | Pt (wt.%) | |||
|---|---|---|---|---|---|---|
| Nominal | XPS | Nominal | XPS | Nominal | XPS | |
| Pt/75BP | 80/20 | 79.2/20.8 | 25/75 | 15/85 | 20 | 15 |
| Pt/75F-BP | 80/20 | 83.8/16.2 | 25/75 | 20/80 | 20 | 33 |
| Pt/75V | 80/20 | 80.5/19.5 | 25/75 | 19/81 | 20 | 42 |
| Catalyst | ECSA1, (a) m2/gPt | ECSA10,000, (b) m2/gPt | ΔECSA10,000, (c) % (a) |
|---|---|---|---|
| Pt/75BP | 69.7 ± 2.6 | 50.1 | 27.6 |
| Pt/75F-BP | 70.9 ± 1.6 | 53.5 | 24.1 |
| Pt/75V | 78.3 ± 2.6 | 50.5 | 36.4 |
| Pt/C | 87.2 ± 2.3 (d) | 46.7 | 47.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyubov, I.; Tálas, E.; Salmanzade, K.; Kuncser, A.; Pászti, Z.; Neațu, Ș.; Mirea, A.G.; Florea, M.; Tompos, A.; Borbáth, I. Electrocatalytic Properties of Mixed-Oxide-Containing Composite-Supported Platinum for Polymer Electrolyte Membrane (PEM) Fuel Cells. Materials 2022, 15, 3671. https://doi.org/10.3390/ma15103671
Ayyubov I, Tálas E, Salmanzade K, Kuncser A, Pászti Z, Neațu Ș, Mirea AG, Florea M, Tompos A, Borbáth I. Electrocatalytic Properties of Mixed-Oxide-Containing Composite-Supported Platinum for Polymer Electrolyte Membrane (PEM) Fuel Cells. Materials. 2022; 15(10):3671. https://doi.org/10.3390/ma15103671
Chicago/Turabian StyleAyyubov, Ilgar, Emília Tálas, Khirdakhanim Salmanzade, Andrei Kuncser, Zoltán Pászti, Ștefan Neațu, Anca G. Mirea, Mihaela Florea, András Tompos, and Irina Borbáth. 2022. "Electrocatalytic Properties of Mixed-Oxide-Containing Composite-Supported Platinum for Polymer Electrolyte Membrane (PEM) Fuel Cells" Materials 15, no. 10: 3671. https://doi.org/10.3390/ma15103671
APA StyleAyyubov, I., Tálas, E., Salmanzade, K., Kuncser, A., Pászti, Z., Neațu, Ș., Mirea, A. G., Florea, M., Tompos, A., & Borbáth, I. (2022). Electrocatalytic Properties of Mixed-Oxide-Containing Composite-Supported Platinum for Polymer Electrolyte Membrane (PEM) Fuel Cells. Materials, 15(10), 3671. https://doi.org/10.3390/ma15103671









