Material Optimization Engineering toward xLiFePO4·yLi3V2(PO4)3 Composites in Application-Oriented Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
3. Electrochemical Characterization
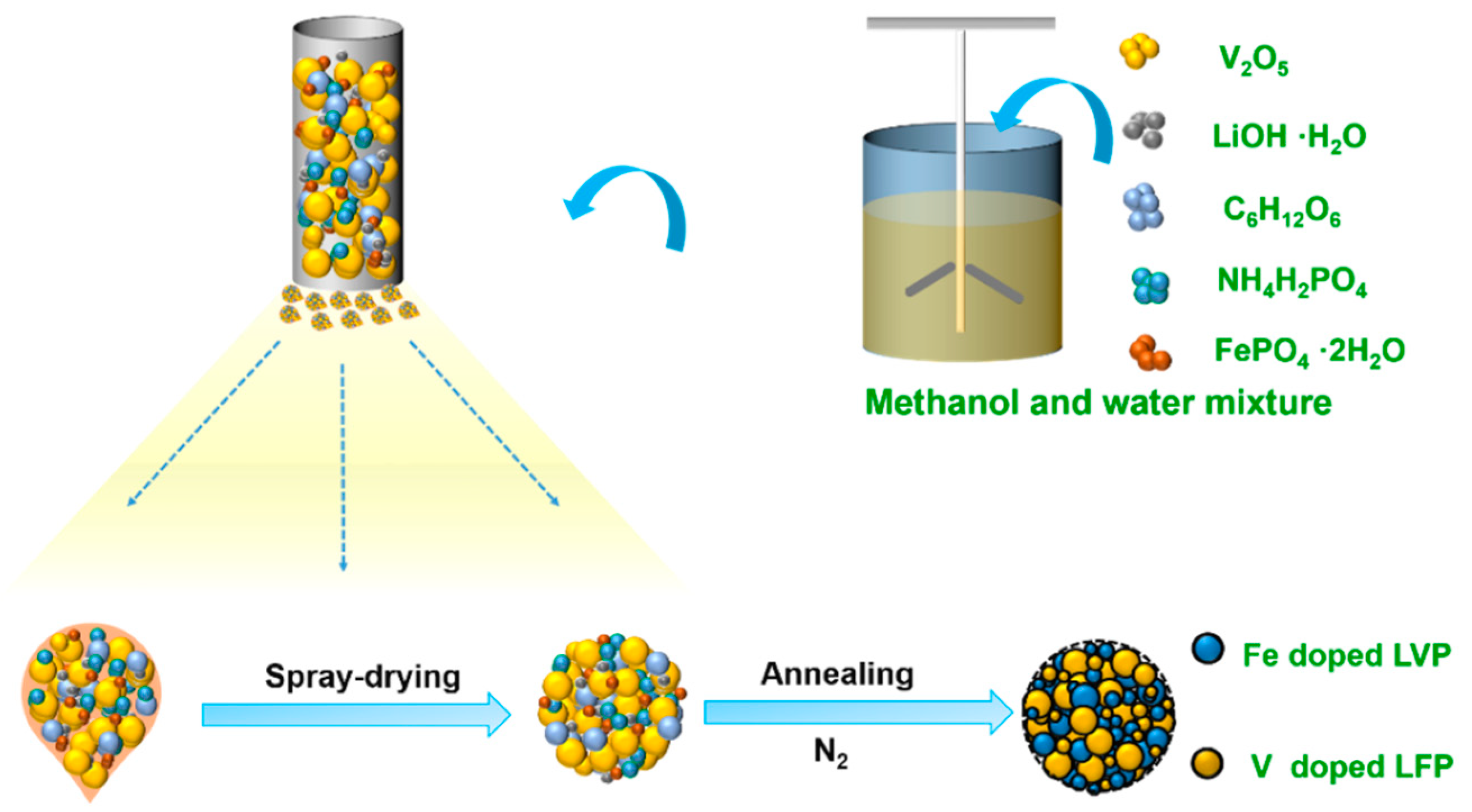
4. Synthetic Procedure
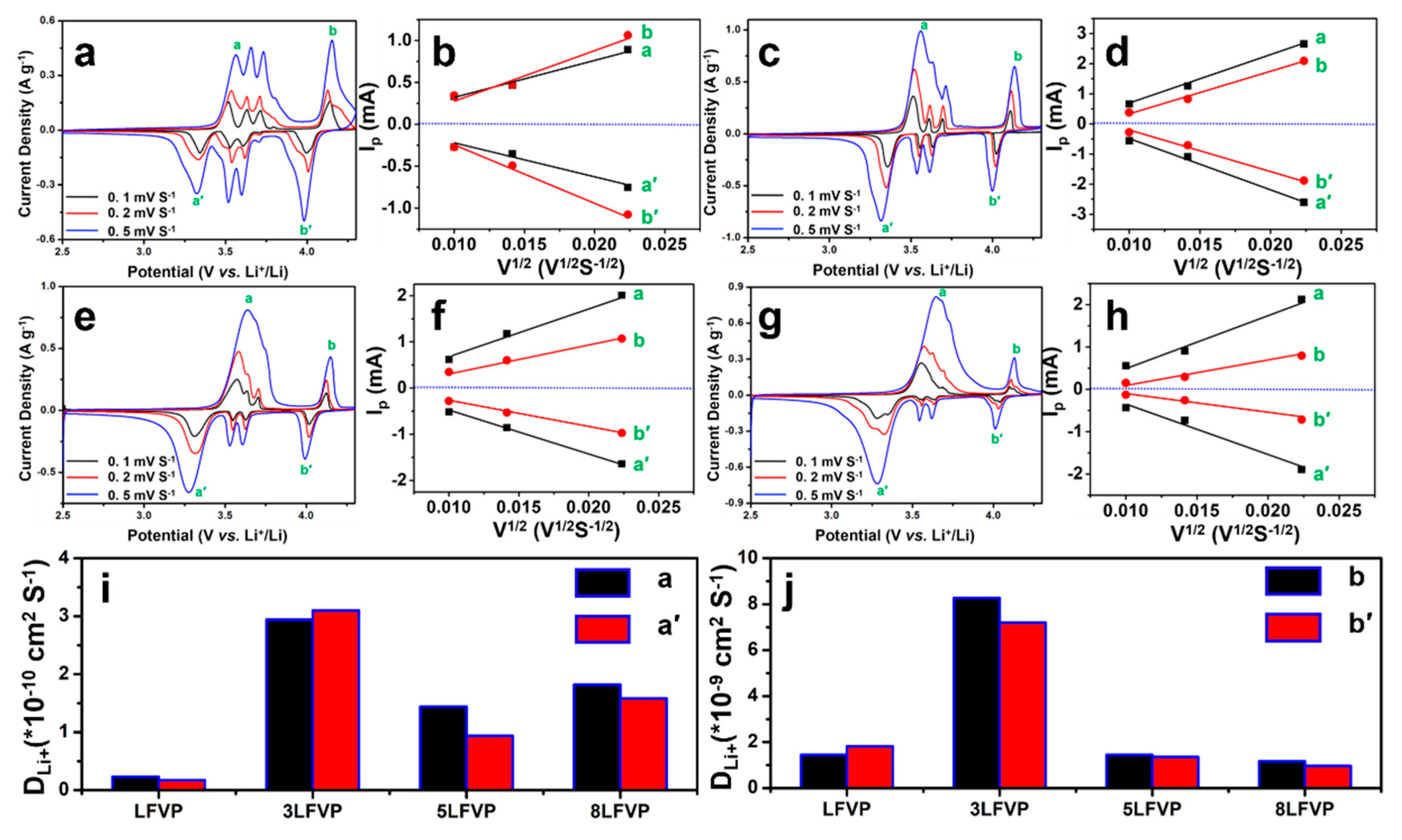
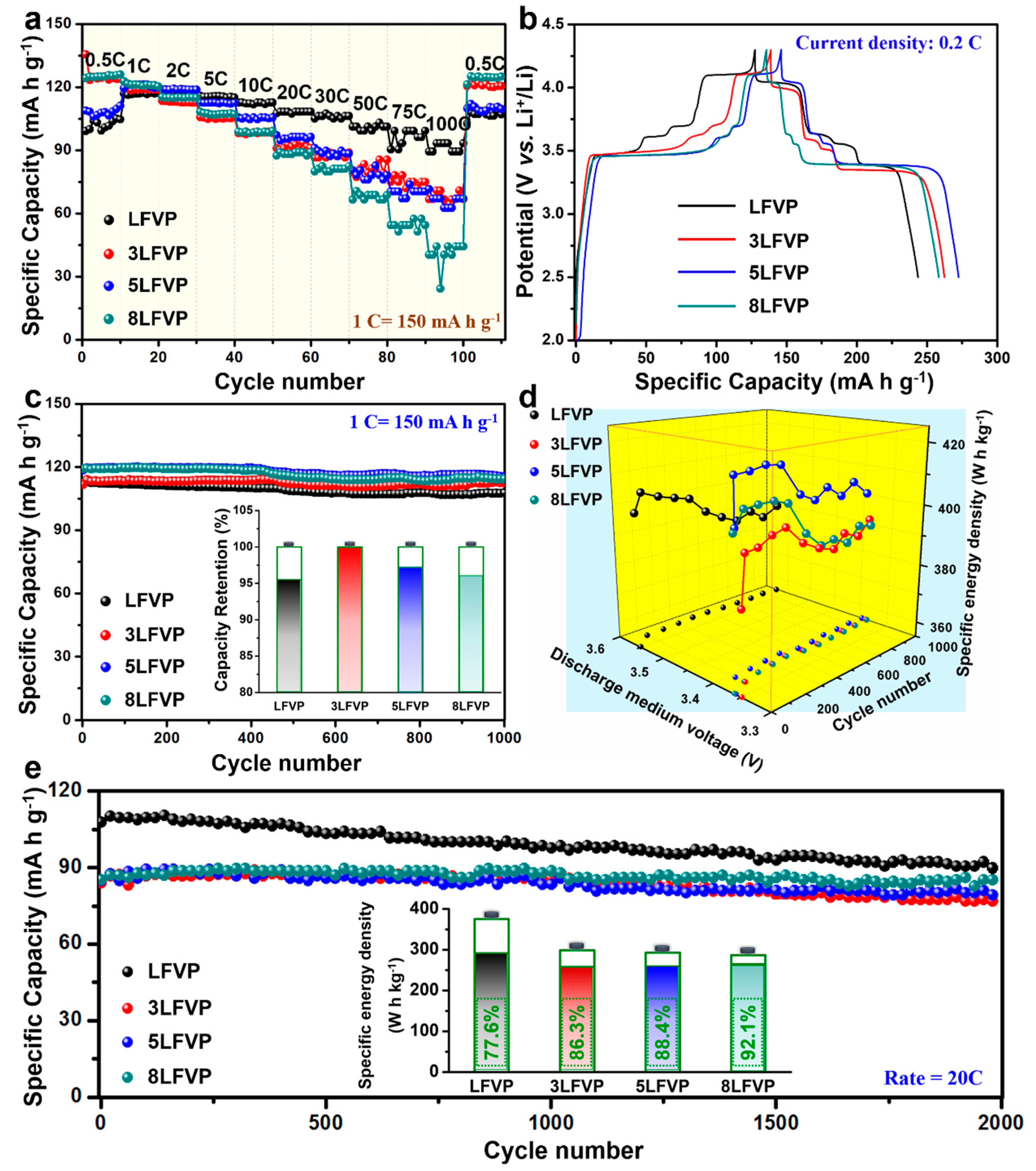
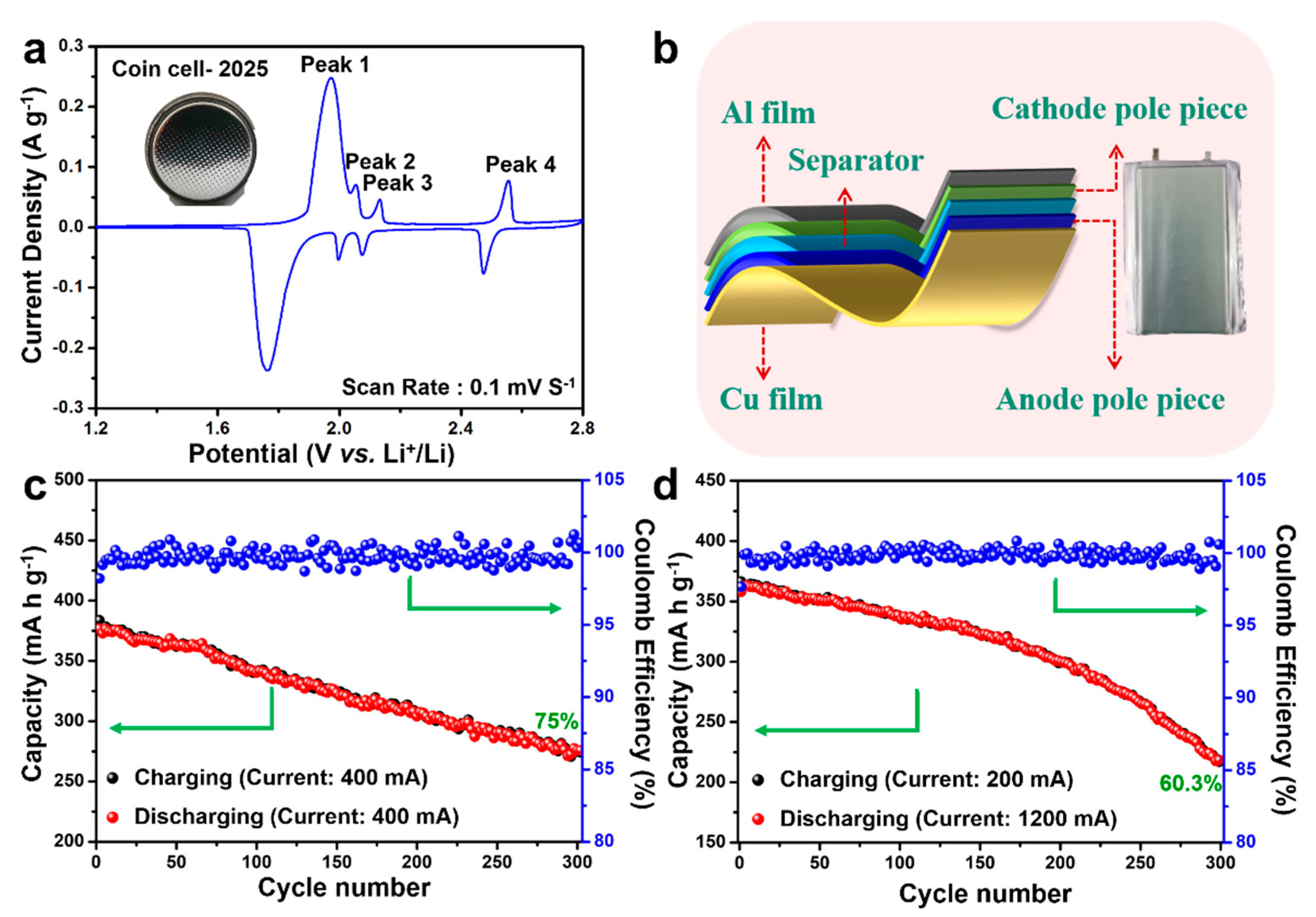
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broadstock, D.; Ji, Q.; Managi, S.; Zhang, D.Y. Pathways to carbon neutrality: Challenges and opportunities. Resour. Conserv. Recycl. 2021, 169, 105472. [Google Scholar] [CrossRef]
- Chen, S.Q.; Fang, K.; Dhakal, S.; Kharrazi, A.; Tong, K.K.; Ramaswami, A. Reshaping urban infrastructure for a carbon-neutral and sustainable future. Resour. Conserv. Recycl. 2021, 174, 105765. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhang, J.L.; Kintner-Meyer, M.C.W.; Lu, X.C.; Choi, D.W.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Xiong, F.Y.; Jiang, Y.L.; Cheng, L.; Yu, R.H.; Tan, S.S.; Tang, C.; Zuo, C.L.; An, Q.Y.; Zhao, Y.L.; Aaumet, J.-J.; et al. Low-strain TiP2O7 with three-dimensional ion channels as long-life and high-rate anode material for Mg-ion batteries. Interdiscipl. Mater. 2022, 1, 140–147. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Tang, Y.X.; Zhang, Y.Y.; Li, W.L.; Ma, B.; Chen, X.D. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Tan, H.T.; Xu, L.H.; Geng, H.B.; Rui, X.H.; Li, C.C.; Huang, S.M. Nanostructured Li3V2(PO4)3 Cathodes. Small 2018, 14, 1800567. [Google Scholar] [CrossRef]
- Ling, J.; Karuppiah, C.; Krishnan, S.G.; Reddy, M.V.; Misnon, I.I.; Ab Rahim, M.H.; Yang, C.C.; Jose, R. Phosphate Polyanion Materials as High-Voltage Lithium-Ion Battery Cathode: A Review. Energy Fuel 2021, 35, 10428–10450. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.H.; Tang, Y.K.; Jia, D.Z.; Huang, Y.D.; Pang, W.K.; Guo, Z.P.; Zhou, Z. LiFePO4 Particles Embedded in Fast Bifunctional Conductor rGO&C@Li3V2(PO4)3 Nanosheets as Cathodes for High-Performance Li-Ion Hybrid Capacitors. Adv. Funct. Mater. 2019, 29, 1807895. [Google Scholar]
- Hu, J.T.; Huang, W.Y.; Yang, L.Y.; Pan, F. Structure and performance of the LiFePO4 cathode material: From the bulk to the surface. Nanoscale 2020, 12, 15036–15044. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Sun, X.L. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Wang, X.F.; Feng, Z.J.; Hou, X.L.; Liu, L.L.; He, M.; He, X.S.; Huang, J.T.; Wen, Z.H. Fluorine doped carbon coating of LiFePO4 as a cathode material for lithium-ion batteries. Chem. Eng. J. 2020, 379, 122371. [Google Scholar] [CrossRef]
- Rui, X.H.; Yan, Q.Y.; Skyllas-Kazacos, M.; Lim, T.M. Li3V2(PO4)3 cathode materials for lithium-ion batteries: A review. J. Power Sources 2014, 258, 19–38. [Google Scholar] [CrossRef]
- Zhang, X.F.; Kuhnel, R.S.; Hu, H.T.; Eder, D.; Balducci, A. Going nano with protic ionic liquids-the synthesis of carbon coated Li3V2(PO4)3 nanoparticles encapsulated in a carbon matrix for high power lithium-ion batteries. Nano Energy 2015, 12, 207–214. [Google Scholar] [CrossRef]
- Liang, S.Q.; Cao, X.X.; Wang, Y.P.; Hu, Y.; Pan, A.Q.; Cao, G.Z. Uniform 8LiFePO4·Li3V2(PO4)3/C nanoflakes for high-performance Li-ion batteries. Nano Energy 2016, 22, 48–58. [Google Scholar] [CrossRef]
- Zhang, J.F.; Shen, C.; Zhang, B.; Zheng, J.C.; Peng, C.L.; Wang, X.W.; Yuan, X.B.; Li, H.; Chen, G.M. Synthesis and performances of 2LiFePO4·Li3V2(PO4)3/C cathode materials via spray drying method with double carbon sources. J. Power Sources 2014, 267, 227–234. [Google Scholar] [CrossRef]
- Jo, J.; Gim, J.; Song, J.; Kim, Y.; Mathew, V.; Kim, S.; Kim, S.; Park, S.; Baboo, J.P.; Kim, J. One-pot pyro-synthesis of a high energy density LiFePO4-Li3V2(PO4)3 nanocomposite cathode for lithium-ion battery applications. Ceram. Int. 2017, 43, 4288–4294. [Google Scholar] [CrossRef]
- He, W.; Wei, C.L.; Zhang, X.D.; Wang, Y.Y.; Liu, Q.Z.; Shen, J.X.; Wang, L.Z.; Yue, Y.Z. Li3V2(PO4)3/LiFePO4 composite hollow microspheres for wide voltage lithium ion batteries. Electrochim. Acta 2016, 219, 682–692. [Google Scholar] [CrossRef][Green Version]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Z.Y.; Liu, S.S.; Gao, B.F.; Wang, J.W. Effects of Particle Size Distribution on Compacted Density of Lithium Iron Phosphate 18650 Battery. J. Electrochem. Energy 2018, 15, 41011. [Google Scholar] [CrossRef]
- Fey, G.T.K.; Lin, Y.C.; Kao, H.M. Characterization and electrochemical properties of high tap-density LiFePO4/C cathode materials by a combination of carbothermal reduction and molten salt methods. Electrochim. Acta 2012, 80, 41–49. [Google Scholar] [CrossRef]
- Wang, J.S.; Shen, Z.G.; Yi, M. Hydraulic Compaction on Electrode to Improve the Volumetric Energy Density of LiFePO4/Graphite Batteries. Ind. Eng. Chem. Res. 2019, 58, 15407–15415. [Google Scholar] [CrossRef]
- Yi, D.W.; Cui, X.M.; Li, N.L.; Zhang, L.; Yang, D.Y. Enhancement of Electrochemical Performance of LiFePO4@C by Ga Coating. ACS Omega 2020, 5, 9752–9758. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.Z.; Jin, J.; Liu, J.; Li, Y.; Wang, H.E.; Chen, L.H.; Wang, B.J.; Su, B.L. Engineering 3D bicontinuous hierarchically macro-mesoporous LiFePO4/C nanocomposite for lithium storage with high rate capability and long cycle stability. Sci. Rep. 2016, 6, 25942. [Google Scholar] [CrossRef]
- Luo, Y.Z.; Xu, X.; Zhang, Y.X.; Pi, Y.Q.; Zhao, Y.L.; Tian, X.C.; An, Q.Y.; Wei, Q.L.; Mai, L.Q. Hierarchical Carbon Decorated Li3V2(PO4)3 as a Bicontinuous Cathode with High-Rate Capability and Broad Temperature Adaptability. Adv. Energy Mater. 2014, 4, 1400107. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, K.; Wang, H.Q.; Zhang, H.Z.; Li, X.F.; Zhang, H.M. “Three-in-One”: A New 3D Hybrid Structure of Li3V2(PO4)3 @ Biomorphic Carbon for High-Rate and Low-Temperature Lithium Ion Batteries. Adv. Mater. Interfaces 2017, 4, 1700686. [Google Scholar] [CrossRef]
- Wu, X.L.; Jiang, L.Y.; Cao, F.F.; Guo, Y.G.; Wan, L.J. LiFePO4 Nanoparticles Embedded in a Nanoporous Carbon Matrix: Superior Cathode Material for Electrochemical Energy-Storage Devices. Adv. Mater. 2009, 21, 2710–2714. [Google Scholar] [CrossRef]
- Li, Z.; He, L.H.; Zhu, Y.F.; Yang, C. A Green and Cost-Effective Method for Production of LiOH from Spent LiFePO4. ACS Sustain. Chem. Eng. 2020, 8, 15915–15926. [Google Scholar] [CrossRef]
- Cui, X.M.; Yi, D.W.; Li, N.L.; Zhang, L.; Zhang, X.F.; Yang, D.Y. Novel LaFeO3 Coating Modification for a LiFePO4 Cathode. Energy Fuel 2020, 34, 7600–7606. [Google Scholar] [CrossRef]
- Peng, Y.; Tan, R.; Ma, J.M.; Li, Q.H.; Wang, T.H.; Duan, X.C. Electrospun Li3V2(PO4)3 nanocubes/carbon nanofibers as free-standing cathodes for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 14681–14688. [Google Scholar] [CrossRef]
- Ding, X.K.; Zhang, L.L.; Yang, X.L.; Fang, H.; Zhou, Y.X.; Wang, J.Q.; Ma, D. Anthracite-Derived Dual-Phase Carbon-Coated Li3V2(PO4)3 as High-Performance Cathode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42788–42796. [Google Scholar] [CrossRef]
- Sun, H.X.; Du, H.R.; Yu, M.K.; Huang, K.F.; Yu, N.; Geng, B.Y. Vesicular Li3V2(PO4)3/C hollow mesoporous microspheres as an efficient cathode material for lithium-ion batteries. Nano Res. 2019, 12, 1937–1942. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.K.; Ning, Y.; Li, X.K.; Ruan, T.T.; Wang, F.; Wang, D.L.; Zhou, Y. Construction of Dual-Carbon Co-Modified LiFePO4 Nanocrystals via Microreactor Strategy for High-Performance Lithium Ion Batteries. Energy Technol. 2020, 8, 2000171. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.L.; Liu, B.R.; Yu, G.H. Single-Crystalline LiFePO4 Nanosheets for High-Rate Li-Ion Batteries. Nano Lett. 2014, 14, 2849–2853. [Google Scholar] [CrossRef]
- Ding, M.L.; Cheng, C.; Wei, Q.L.; Hu, Y.; Yan, Y.Y.; Dai, K.H.; Mao, J.; Guo, J.H.; Zhang, L.; Mai, L.Q. Carbon decorated Li3V2(PO4)3 for high-rate lithium-ion batteries: Electrochemical performance and charge compensation mechanism. J. Energy Chem. 2021, 53, 124–131. [Google Scholar] [CrossRef]
- Pan, A.Q.; Choi, D.W.; Zhang, J.G.; Liang, S.Q.; Cao, G.Z.; Nie, Z.M.; Arey, B.W.; Liu, J. High-rate cathodes based on Li3V2(PO4)3 nanobelts prepared via surfactant-assisted fabrication. J. Power Sources 2011, 196, 3646–3649. [Google Scholar] [CrossRef]
- Patil, V.; Oh, W.; Yoo, J.W.; Pu, L.; Park, J.H.; Yoon, W.S.; Yi, G.R. Carbon-Coated Supraballs of Randomly Packed LiFePO4 Nanoplates for High Rate and Stable Cycling of Li-Ion Batteries. Part. Part. Syst. Charact. 2019, 36, 1900149. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.Y.; Sun, J.K.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Watermelon-Inspired Si/C Microspheres with Hierarchical Buffer Structures for Densely Compacted Lithium-Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601481. [Google Scholar] [CrossRef]
- Xu, H.H.; Hu, X.L.; Sun, Y.M.; Luo, W.; Chen, C.J.; Liu, Y.; Huang, Y.H. Highly porous Li4Ti5O12/C nanofibers for ultrafast electrochemical energy storage. Nano Energy 2014, 10, 163–171. [Google Scholar] [CrossRef]
- Yu, L.; Wu, H.B.; Lou, X.W. Mesoporous Li4Ti5O12 Hollow Spheres with Enhanced Lithium Storage Capability. Adv. Mater. 2013, 25, 2296–2300. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, Y.; Luo, G.; Wang, P.; Xu, W.; Yu, J.; Zhang, X.; Fu, Z.; Yang, X.; Wang, L.; Ding, Y.; et al. Material Optimization Engineering toward xLiFePO4·yLi3V2(PO4)3 Composites in Application-Oriented Li-Ion Batteries. Materials 2022, 15, 3668. https://doi.org/10.3390/ma15103668
Pi Y, Luo G, Wang P, Xu W, Yu J, Zhang X, Fu Z, Yang X, Wang L, Ding Y, et al. Material Optimization Engineering toward xLiFePO4·yLi3V2(PO4)3 Composites in Application-Oriented Li-Ion Batteries. Materials. 2022; 15(10):3668. https://doi.org/10.3390/ma15103668
Chicago/Turabian StylePi, Yuqiang, Gangwei Luo, Peiyao Wang, Wangwang Xu, Jiage Yu, Xian Zhang, Zhengbing Fu, Xiong Yang, Li Wang, Yu Ding, and et al. 2022. "Material Optimization Engineering toward xLiFePO4·yLi3V2(PO4)3 Composites in Application-Oriented Li-Ion Batteries" Materials 15, no. 10: 3668. https://doi.org/10.3390/ma15103668
APA StylePi, Y., Luo, G., Wang, P., Xu, W., Yu, J., Zhang, X., Fu, Z., Yang, X., Wang, L., Ding, Y., & Wang, F. (2022). Material Optimization Engineering toward xLiFePO4·yLi3V2(PO4)3 Composites in Application-Oriented Li-Ion Batteries. Materials, 15(10), 3668. https://doi.org/10.3390/ma15103668





