Metallic Implants Used in Lumbar Interbody Fusion
Abstract
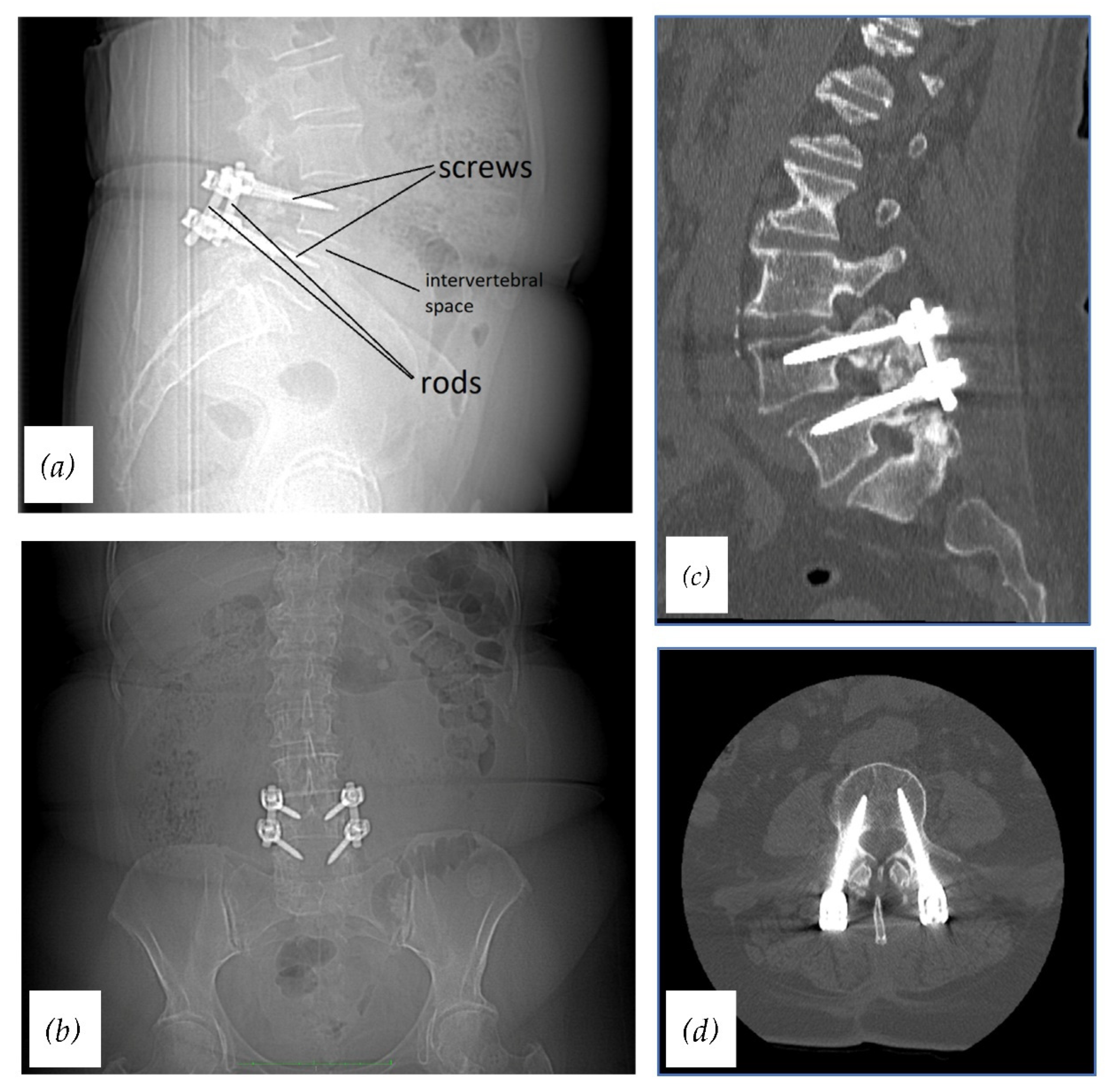
1. Introduction
2. Physical and Mechanical Properties of Implant Important in LIF
2.1. Fatigue Strength
2.2. Young’s Elastic Modulus
2.3. Corrosion Resistance
| Alloy | Ultimate Tensile Strength [MPa] | Yield Strength [MPa] | Fatigue Strength [MPa] | Young Modulus [GPa] | Corrosion Resistance (Breakdown Potential) [mV] | References |
|---|---|---|---|---|---|---|
| Commercial Pure Titanium (CP-Ti) | 240–550 | 170–480 | 430 | 115 | 9000 | [21,62] |
| Ti-6Al-4V | 930 | 860 | 500 | 110 | 25,000 | [21,62,63] |
| Ti-24Nb-4Zr-8Sn (Ti2448) | 665 ± 18 | 563 ± 38 | 375–500 | 53 ± 1 | nd | [21,64,65] |
| Cobalt–Chromium | 655 | 450 | 310 | 210 | 870 | [30,62,63,66] |
| Nickel–Titanium | 895 | 195–690 (austenitic phase) 70–140 (martensitic phase) | nd | 40–75 | >1000 | [63,65] |
| Nickel–Titanium (CS 64% porous) | nd | ~700 | nd | 1 | 772 | [67] |
| 316L Stainless Steel | 490–1350 | 190–690 | 146 | 210 | 400–600 | [21,47,62,67,68] |
3. Mechanical Characteristics of the Most Frequently Used Metal Alloys in LIF
3.1. Titanium
3.2. Cobalt–Chromium
3.3. Nitinol
3.4. Stainless Steel
4. Biological Response to Metal Implants Used in LIF
4.1. Wound Healing
4.2. Foreign Body Reaction
4.3. Response to Implant Wear Debris and Metal Ions
4.4. Innate Reaction
4.5. Adaptive Response
4.6. Biocompatibility of the Most Frequently Used Metal Alloys in LIF
4.6.1. Titanium
4.6.2. Titanium Alloys
4.6.3. Cobalt–Chromium
4.6.4. Nitinol
4.6.5. Stainless Steel
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kos, N.; Gradisnik, L.; Velnar, T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med. Arch. 2019, 73, 421. [Google Scholar] [CrossRef]
- Kalichman, L.; Kim, D.H.; Li, L.; Guermazi, A.; Hunter, D.J. Computed tomography–evaluated features of spinal degeneration: Prevalence, intercorrelation, and association with self-reported low back pain. Spine J. 2010, 10, 200. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef]
- Baliga, S.; Treon, K.; Craig, N.J.A. Low Back Pain: Current Surgical Approaches. Asian Spine J. 2015, 9, 645–657. [Google Scholar] [CrossRef]
- Provaggi, E.; Capelli, C.; Leong, J.J.H.; Kalaskar, D.M. A UK-based pilot study of current surgical practice and implant preferences in lumbar fusion surgery. Medicine 2018, 97, e11169. [Google Scholar] [CrossRef]
- Meng, B.; Bunch, J.; Burton, D.; Wang, J. Lumbar interbody fusion: Recent advances in surgical techniques and bone healing strategies. Eur. Spine J. 2020, 30, 22–33. [Google Scholar] [CrossRef]
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion—A literature review. J. Spine Surg. 2020, 6, 752–776. [Google Scholar] [CrossRef]
- Momin, A.A.; Steinmetz, M.P. Evolution of Minimally Invasive Lumbar Spine Surgery. World Neurosurg. 2020, 140, 622–626. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101. [Google Scholar] [CrossRef]
- Mesregah, M.K.; Yoshida, B.; Lashkari, N.; Abedi, A.; Meisel, H.-J.; Diwan, A.; Hsieh, P.; Wang, J.C.; Buser, Z.; Yoon, S.T. Demographic, clinical, and operative risk factors associated with postoperative adjacent segment disease in patients undergoing lumbar spine fusions: A systematic review and meta-analysis. Spine J. 2021. [Google Scholar] [CrossRef]
- Mannion, A.F.; Leivseth, G.; Brox, J.I.; Fritzell, P.; Hägg, O.; Fairbank, J.C.T. ISSLS Prize winner: Long-term follow-up suggests spinal fusion is associated with increased adjacent segment disc degeneration but without influence on clinical outcome: Results of a combined follow-up from 4 randomized controlled trials. Spine 2014, 39, 1373–1383. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Mo, Z.; Han, J.; Chen, Y.; Yu, H.; Wang, Q.; Liu, J.; Li, C.; Zhou, Y.; et al. Comparison of short-segment monoaxial and polyaxial pedicle screw fixation combined with intermediate screws in traumatic thoracolumbar fractures: A finite element study and clinical radiographic review. Clinics 2017, 72, 609–617. [Google Scholar] [CrossRef]
- Gholampour, S.; Shakouri, E.; Deh, H.H.H. Effect of drilling direction and depth on thermal necrosis during tibia drilling: An in vitro study. Technol. Health Care 2018, 26, 687–697. [Google Scholar] [CrossRef]
- Gholampour, S.; Deh, H.H.H. The effect of spatial distances between holes and time delays between bone drillings based on examination of heat accumulation and risk of bone thermal necrosis. Biomed. Eng. Online 2019, 18, 65. [Google Scholar] [CrossRef]
- Antunes, R.A.; De Oliveira, M.C.L. Corrosion fatigue of biomedical metallic alloys: Mechanisms and mitigation. Acta Biomater. 2012, 8, 937–962. [Google Scholar] [CrossRef]
- Lindsey, C.; Deviren, V.; Xu, Z.; Yeh, R.F.; Puttlitz, C.M. The effects of rod contouring on spinal construct fatigue strength. Spine 2006, 31, 1680–1687. [Google Scholar] [CrossRef]
- Yamanaka, K.; Mori, M.; Yamazaki, K.; Kumagai, R.; Doita, M.; Chiba, A. Analysis of the fracture mechanism of Ti-6Al-4V alloy rods that failed clinically after spinal instrumentation surgery. Spine 2015, 40, E767–E773. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Buckley, J.M.; Ames, C.; Deviren, V. The fatigue life of contoured cobalt chrome posterior spinal fusion rods. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 194–198. [Google Scholar] [CrossRef]
- Chan, K.S. Changes in fatigue life mechanism due to soft grains and hard particles. Int. J. Fatigue 2010, 32, 526–534. [Google Scholar] [CrossRef]
- Ghonem, H. Microstructure and fatigue crack growth mechanisms in high temperature titanium alloys. Int. J. Fatigue 2010, 32, 1448–1460. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Kyzioł, K.; Kaczmarek, Ł.; Brzezinka, G.; Kyzioł, A. Structure, characterization and cytotoxicity study on plasma surface modified Ti–6Al–4V and γ-TiAl alloys. Chem. Eng. J. 2014, 240, 516–526. [Google Scholar] [CrossRef]
- Slivka, M.A.; Fan, Y.K.; Eck, J.C. The Effect of Contouring on Fatigue Strength of Spinal Rods: Is it Okay to Re-bend and Which Materials Are Best? Spine Deform. 2013, 1, 395–400. [Google Scholar] [CrossRef]
- Tang, J.A.; Leasure, J.M.; Smith, J.S.; Buckley, J.M.; Kondrashov, D.; Ames, C.P. Effect of Severity of Rod Contour on Posterior Rod Failure in the Setting of Lumbar Pedicle Subtraction Osteotomy (PSO)A Biomechanical Study. Neurosurgery 2013, 72, 276–283. [Google Scholar] [CrossRef]
- Demura, S.; Murakami, H.; Hayashi, H.; Kato, S.; Yoshioka, K.; Yokogawa, N.; Ishii, T.; Igarashi, T.; Fang, X.; Tsuchiya, H. Influence of Rod Contouring on Rod Strength and Stiffness in Spine Surgery. Orthopedics 2015, 38, e520–e523. [Google Scholar] [CrossRef]
- Ohrt-Nissen, S.; Dahl, B.; Gehrchen, M. Choice of Rods in Surgical Treatment of Adolescent Idiopathic Scoliosis: What Are the Clinical Implications of Biomechanical Properties?—A Review of the Literature. Neurospine 2018, 15, 123–130. [Google Scholar] [CrossRef]
- Yoshihara, H. Rods in spinal surgery: A review of the literature. Spine J. 2013, 13, 1350–1358. [Google Scholar] [CrossRef]
- Yamada, K.; Sudo, H.; Iwasaki, N.; Chiba, A. Mechanical Analysis of Notch-Free Pre-Bent Rods for Spinal Deformity Surgery. Spine 2020, 45, E312–E318. [Google Scholar] [CrossRef]
- Kokabu, T.; Kanai, S.; Abe, Y.; Iwasaki, N.; Sudo, H. Identification of optimized rod shapes to guide anatomical spinal reconstruction for adolescent thoracic idiopathic scoliosis. J. Orthop. Res. 2018, 36, 3219–3224. [Google Scholar] [CrossRef]
- Almansour, H.; Sonntag, R.; Pepke, W.; Bruckner, T.; Kretzer, J.P.; Akbar, M. Impact of Electrocautery on Fatigue Life of Spinal Fusion Constructs-An In Vitro Biomechanical Study. Mater 2019, 12, 2471. [Google Scholar] [CrossRef]
- Zobel, S.M.; Morlock, M.M.; Huber, G. Fatigue strength reduction of Ti-6Al-4V titanium alloy after contact with high-frequency cauterising instruments. Med. Eng. Phys. 2020, 81, 58–67. [Google Scholar] [CrossRef]
- Sonntag, R.; Gibmeier, J.; Pulvermacher, S.; Mueller, U.; Eckert, J.; Braun, S.; Reichkendler, M.; Kretzer, J.P. Electrocautery Damage Can Reduce Implant Fatigue Strength. J. Bone Jt. Surg. 2019, 101, 868–878. [Google Scholar] [CrossRef]
- Huber, G.; Weik, T.; Morlock, M.M. Schädigung eines hüftendoprothesenschafts durch einsatz eines hochfrequenzmessers. Orthopade 2009, 38, 622–625. [Google Scholar] [CrossRef]
- Konrads, C.; Wente, M.N.; Plitz, W.; Rudert, M.; Hoberg, M. Damage to implants due to high-frequency electrocautery: Analysis of four fractured hip endoprostheses shafts. Orthopade 2014, 43, 1106–1111. [Google Scholar] [CrossRef]
- Rho, J.Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325–1330. [Google Scholar] [CrossRef]
- Teles, A.R.; Yavin, D.; Zafeiris, C.P.; Thomas, K.C.; Lewkonia, P.; Nicholls, F.H.; Swamy, G.; Jacobs, W.B. Fractures After Removal of Spinal Instrumentation: Revisiting the Stress-Shielding Effect of Instrumentation in Spine Fusion. World Neurosurg. 2018, 116, e1137–e1143. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.-E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 1–21. [Google Scholar] [CrossRef]
- Jha, N.; Mondal, D.P.; Dutta Majumdar, J.; Badkul, A.; Jha, A.K.; Khare, A.K. Highly porous open cell Ti-foam using NaCl as temporary space holder through powder metallurgy route. Mater. Des. 2013, 47, 810–819. [Google Scholar] [CrossRef]
- Hansen, D.C. Metal corrosion in the human body: The ultimate bio-corrosion scenario. Electrochem. Soc. Interface. 2008, 17, 31–34. [Google Scholar] [CrossRef]
- Kirkpatrick, J.S.; Venugopalan, R.; Beck, P.; Lemons, J. Corrosion on spinal implants. J. Spinal Disord. Tech. 2005, 18, 247–251. [Google Scholar] [CrossRef]
- Peterson, H.A. Metallic implant removal in children. J. Pediatr. Orthop. 2005, 25, 107–115. [Google Scholar] [CrossRef]
- Mali, S.A.; Singh, V.; Gilbert, J.L. Effect of mixed alloy combinations on fretting corrosion performance of spinal screw and rod implants. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1169–1177. [Google Scholar] [CrossRef]
- Cundy, T.P.; Delaney, C.L.; Rackham, M.D.; Antoniou, G.; Oakley, A.P.; Freeman, B.J.C.; Sutherland, L.M.; Cundy, P.J. Chromium Ion Release From Stainless Steel Pediatric Scoliosis Instrumentation. Spine 2010, 35, 967–974. [Google Scholar] [CrossRef]
- Del Rio, J.; Beguiristain, J.; Duart, J. Metal levels in corrosion of spinal implants. Eur. Spine J. 2007, 16, 1055–1061. [Google Scholar] [CrossRef]
- Singh, V.; Shorez, J.P.; Mali, S.A.; Hallab, N.J.; Gilbert, J.L. Material dependent fretting corrosion in spinal fusion devices: Evaluation of onset and long-term response. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2858–2868. [Google Scholar] [CrossRef]
- Panagiotopoulou, V.C.; Hothi, H.S.; Anwar, H.A.; Molloy, S.; Noordeen, H.; Rezajooi, K.; Sutcliffe, J.; Skinner, J.; Hart, A. Assessment of corrosion in retrieved spine implants. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 632–638. [Google Scholar] [CrossRef]
- Rosenbloom, S.N.; Corbett, R.A. An assessment of ASTMF 2129 electrochemical testing ofsmall medical implants—Lessons learned. In Proceedings of the CORROSION 2007, Nashville, Tennessee, 11–15 March 2007. [Google Scholar]
- Tahal, D.; Madhavan, K.; Chieng, L.O.; Ghobrial, G.M.; Wang, M.Y. Metals in Spine. World Neurosurg. 2017, 100, 619–627. [Google Scholar] [CrossRef]
- Garbacz, H.; Królikowski, A. Corrosion resistance of nanocrystalline titanium. Nanocryst. Titan. 2019, 145–173. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- MacDonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 4, 1761–1772. [Google Scholar] [CrossRef]
- Cundy, W.J.; Mascarenhas, A.R.; Antoniou, G.; Freeman, B.J.C.; Cundy, P.J. Local and systemic metal ion release occurs intraoperatively during correction and instrumented spinal fusion for scoliosis. J. Child. Orthop. 2015, 9, 39–43. [Google Scholar] [CrossRef]
- Sherman, B.; Crowell, T. Corrosion of Harrington rod in idiopathic scoliosis: Long-term effects. Eur. Spine J. 2018, 27, 298–302. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Joint Surg. Am. 2000, 82, 457–477. [Google Scholar] [CrossRef]
- Wang, J.C.; Yu, W.D.; Sandhu, H.S.; Betts, F.; Bhuta, S.; Delamarter, R.B. Metal debris from titanium spinal implants. Spine 1999, 24, 899–903. [Google Scholar] [CrossRef]
- Kumazawa, R.; Watari, F.; Takashi, N.; Tanimura, Y.; Uo, M.; Totsuka, Y. Effects of Ti ions and particles on neutrophil function and morphology. Biomaterials 2002, 23, 3757–3764. [Google Scholar] [CrossRef]
- Campbell, P.; Ebramzadeh, E.; Nelson, S.; Takamura, K.; De Smet, K.; Amstutz, H.C. Histological Features of Pseudotumor-like Tissues From Metal-on-Metal Hips. Clin. Orthop. Relat. Res. 2010, 468, 2321. [Google Scholar] [CrossRef]
- Maloney, W.J.; Smith, R.L. Periprosthetic osteolysis in total hip arthroplasty: The role of particulate wear debris. Medicine 1995, 77, 1448–1461. [Google Scholar] [CrossRef]
- Takahashi, S.; Delécrin, J.; Passuti, N. Intraspinal metallosis causing delayed neurologic symptoms after spinal instrumentation surgery. Spine 2001, 26, 1495–1498. [Google Scholar] [CrossRef]
- Tezer, M.; Kuzgun, U.; Hamzaoglu, A.; Ozturk, C.; Kabukcuoglu Sirvanci, M. Intraspinal metalloma resulting in late paraparesis. Arch. Orthop. Trauma Surg. 2005, 125, 417–421. [Google Scholar] [CrossRef]
- Beguiristain, J.; Del Río, J.; Duart, J.; Barroso, J.; Silva, A.; Villas, C. Corrosion and late infection causing delayed paraparesis after spinal instrumentation. J. Pediatr. Orthop. B 2006, 15, 320–323. [Google Scholar] [CrossRef]
- Gotman, I. Characteristics of Metals Used in Implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef]
- Lukina, E.; Kollerov, M.; Meswania, J.; Khon, A.; Panin, P.; Blunn, G.W. Fretting corrosion behavior of nitinol spinal rods in conjunction with titanium pedicle screws. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 601–610. [Google Scholar] [CrossRef]
- Zhang, L.; Klemm, D.; Eckert, J.; Hao, Y.; Sercombe, T. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti–24Nb–4Zr–8Sn alloy. Scr. Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Cui, C. Biocompatibility and fabrication of in situ bioceramic coating/titanium alloy biocomposites. Met. Biomed. Devices 2010, 202–232. [Google Scholar] [CrossRef]
- Park, J.; Lakes, R.S. Biomaterials: An Introduction. Available online: https://books.google.com/books/about/Biomaterials.html?id=bb68wb0R_EAC (accessed on 23 April 2022).
- Aihara, H.; Zider, J.; Fanton, G.; Duerig, T. Combustion Synthesis Porous Nitinol for Biomedical Applications. Int. J. Biomater. 2019, 2019, 4307461. [Google Scholar] [CrossRef]
- A Mohammad, K.; Ali, A.; Sahari, B.B.; Abdullah, S. Fatigue behavior of Austenitic Type 316L Stainless Steel. IOP Conf. Ser. Mater. Sci. Eng. 2012, 36, 012012. [Google Scholar] [CrossRef]
- Li, X.; Ye, S.; Yuan, X.P. Fabrication of biomedical Ti-24Nb-4Zr-8Sn alloy with high strength and low elastic modulus by powder metallurgy. J. Alloys Compd. 2019, 772, 968–977. [Google Scholar] [CrossRef]
- Etemadifar, M.R.; Andalib, A.; Rahimian, A.; Nodushan, S.M.H.T. Cobalt chromium-Titanium rods versus Titanium-Titanium rods for treatment of adolescent idiopathic scoliosis; which type of rod has better postoperative outcomes? Rev. Assoc. Med. Bras. 2018, 64, 1085–1090. [Google Scholar] [CrossRef]
- Gottstein, G.; Goerdeler, M.; Prasad, G.V.S.S. Encyclopedia of Condensed Matter Physics. Encycl. Condens. Matter Phys. 2005, 298–305. Available online: http://www.sciencedirect.com/science/article/pii/B0123694019005696 (accessed on 25 April 2022).
- Kafkas, F.; Ebel, T. Metallurgical and mechanical properties of Ti–24Nb–4Zr–8Sn alloy fabricated by metal injection molding. J. Alloys Compd. 2014, 617, 359–366. [Google Scholar] [CrossRef]
- Völker, B.; Jäger, N.; Calin, M.; Zehetbauer, M.; Eckert, J.; Hohenwarter, A. Influence of testing orientation on mechanical properties of Ti45Nb deformed by high pressure torsion. Mater. Des. 2017, 114, 40–46. [Google Scholar] [CrossRef]
- Delshadmanesh, M.; Khatibi, G.; Ghomsheh, M.Z.; Lederer, M.; Zehetbauer, M.; Danninger, H. Influence of microstructure on fatigue of biocompatible β-phase Ti-45Nb. Mater. Sci. Eng. A 2017, 706, 83–94. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sulkowski, B.; Waitz, T.; Ozaltin, K.; Chrominski, W.; Pukenas, A.; Horky, J.; Lewandowska, M.; Skrotzki, W.; Zehetbauer, M. Mechanical properties, structural and texture evolution of biocompatible Ti–45Nb alloy processed by severe plastic deformation. J. Mech. Behav. Biomed. Mater. 2016, 62, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Niinomi, M.; Nakai, M.; Hieda, J.; Ishimoto, T.; Nakano, T. Optimization of Cr content of metastable β-type Ti-Cr alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2012, 8, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Nune, K.C.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Cellular response of osteoblasts to low modulus Ti-24Nb-4Zr-8Sn alloy mesh structure. J. Biomed. Mater. Res. Part A 2017, 105, 859–870. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Chen, C.H.; Tsuang, F.Y.; Wu, L.C.; Lin, S.C.; Chiang, C.J. Removal of fixation construct could mitigate adjacent segment stress after lumbosacral fusion: A finite element analysis. Clin. Biomech. 2017, 43, 115–120. [Google Scholar] [CrossRef]
- Litak, J.; Czyzewski, W.; Szymoniuk, M.; Pastuszak, B.; Litak, J.; Litak, G.; Grochowski, C.; Rahnama-Hezavah, M.; Kamieniak, P. Hydroxyapatite Use in Spine Surgery—Molecular and Clinical Aspect. Materials 2022, 15, 2906. [Google Scholar] [CrossRef]
- Liu, G.-M.; Kong, N.; Zhang, X.-Y.; Bai, H.-T.; Yao, Y.; Han, H.-Z.; Luo, Y.-G. Extracellular matrix-coating pedicle screws conduct and induce osteogenesis. Eur. J. Orthop. Surg. Traumatol. 2013, 24, 173–182. [Google Scholar] [CrossRef]
- Shi, L.Y.; Wang, A.; Zang, F.Z.; Wang, J.X.; Pan, X.W.; Chen, H.J. Tantalum-coated pedicle screws enhance implant integration. Coll. Surf. B Biointerfaces 2017, 160, 22–32. [Google Scholar] [CrossRef]
- Yi, S.; Rim, D.C.; Park, S.W.; Murovic, J.A.; Lim, J.; Park, J. Biomechanical Comparisons of Pull Out Strengths After Pedicle Screw Augmentation with Hydroxyapatite, Calcium Phosphate, or Polymethylmethacrylate in the Cadaveric Spine. World Neurosurg. 2015, 83, 976–981. [Google Scholar] [CrossRef]
- Školáková, A.; Körberová, J.; Málek, J.; Rohanová, D.; Jablonská, E.; Pinc, J.; Salvetr, P.; Gregorová, E.; Novák, P. Microstructural, Mechanical, Corrosion and Cytotoxicity Characterization of Porous Ti-Si Alloys with Pore-Forming Agent. Materials 2020, 13, 5607. [Google Scholar] [CrossRef] [PubMed]
- Nune, K.C.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Osteoblast cellular activity on low elastic modulus Ti–24Nb–4Zr–8Sn alloy. Dent. Mater. 2017, 33, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zheng, S.; Dong, R.; Kang, M.; Zhou, H.; Zhao, D.; Zhao, J. Ti-24Nb-4Zr-8Sn Alloy Pedicle Screw Improves Internal Vertebral Fixation by Reducing Stress-Shielding Effects in a Porcine Model. BioMed Res. Int. 2018, 2018, 8639648. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Xue, P.; Li, Y.; Li, K.; Zhang, D.; Zhou, C. Superelasticity, corrosion resistance and biocompatibility of the Ti-19Zr-10Nb-1Fe alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 179–186. [Google Scholar] [CrossRef]
- Atapour, M.; Pilchak, A.L.; Frankel, G.S.; Williams, J.C. Corrosion behavior of β titanium alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 885–891. [Google Scholar] [CrossRef]
- Kilmametov, A.; Ivanisenko, Y.; Mazilkin, A.; Straumal, B.; Gornakova, A.; Fabrichnaya, O.; Kriegel, M.; Rafaja, D.; Hahn, H. The α→ω and β→ω phase transformations in Ti–Fe alloys under high-pressure torsion. Acta Mater. 2018, 144, 337–351. [Google Scholar] [CrossRef]
- Völker, B.; Maier-Kiener, V.; Werbach, K.; Müller, T.; Pilz, S.; Calin, M.; Eckert, J.; Hohenwarter, A. Influence of annealing on microstructure and mechanical properties of ultrafine-grained Ti45Nb. Mater. Des. 2019, 179, 107864. [Google Scholar] [CrossRef]
- Hu, N.; Xie, L.; Liao, Q.; Gao, A.; Zheng, Y.; Pan, H.; Tong, H.; Yang, D.; Gao, N.; Starink, M.J.; et al. A more defective substrate leads to a less defective passive layer: Enhancing the mechanical strength, corrosion resistance and anti-inflammatory response of the low-modulus Ti-45Nb alloy by grain refinement. Acta Biomater. 2021, 126, 524–536. [Google Scholar] [CrossRef]
- Ozaltin, K.; Chrominski, W.; Kulczyk, M.; Panigrahi, A.; Horky, J.; Zehetbauer, M.; Lewandowska, M. Enhancement of mechanical properties of biocompatible Ti–45Nb alloy by hydrostatic extrusion. J. Mater. Sci. 2014, 49, 6930–6936. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Qian, B.; Shen, Z.; Virtanen, S.; Odnevall Wallinder, I. In vitro biocompatibility of CoCrMo dental alloys fabricated by selective laser melting. Dent. Mater. 2014, 30, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.S.; Baas, J.; Jakobsen, T.; Soballe, K. Biomechanical implant fixation of CoCrMo coating inferior to titanium coating in a canine implant model. J. Biomed. Mater. Res. Part A 2010, 94, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Mathew, M.T.; Wimmer, M.A.; Lesuer, R.J. Effect of Tribolayer Formation on Corrosion of CoCrMo Alloys Investigated Using Scanning Electrochemical Microscopy. Anal. Chem. 2013, 85, 7159–7166. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, C.I.; Ames, C.P.; Demakakos, J.; Fu, K.-M.G.; Keshavarzi, S.; Li, C.M.Y.; Deviren, V.; Schwab, F.J.; Lafage, V.; et al. Assessment of Symptomatic Rod Fracture After Posterior Instrumented Fusion for Adult Spinal Deformity. Neurosurgery 2012, 71, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Takigawa, T.; Tanaka, M.; Sugimoto, Y.; Arataki, S.; Yamane, K.; Watanabe, N.; Ozaki, T.; Sarai, T. Implant Failure of Titanium Versus Cobalt-Chromium Growing Rods in Early-onset Scoliosis. Spine 2016, 41, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, E.; Klineberg, E.; Shaffrey, C.I.; Lafage, V.; Schwab, F.J.; Protopsaltis, T.; Scheer, J.K.; Mundis, G.M.; Fu, K.-M.G.; et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J. Neurosurg. Spine 2014, 21, 994–1003. [Google Scholar] [CrossRef]
- Serhan, H.; Mhatre, D.; Newton, P.; Giorgio, P.; Sturm, P. Would CoCr rods provide better correctional forces than stainless steel or titanium for rigid scoliosis curves? J. Spinal. Disord. Tech. 2013, 26, E70–E74. [Google Scholar] [CrossRef]
- Willson, R.; Zhou, H.; Fulzele, S.; Mitchell, S.M.; Chutkan, N. Shape Loss of Autoclaved, Machine-Bent Cobalt-Chrome and Titanium Spine Surgery Rods. Glob. Spine J. 2021, 11, 509–514. [Google Scholar] [CrossRef]
- Han, S.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Comparative Study Between Cobalt Chrome and Titanium Alloy Rods for Multilevel Spinal Fusion: Proximal Junctional Kyphosis More Frequently Occurred in Patients Having Cobalt Chrome Rods. World Neurosurg. 2017, 103, 404–409. [Google Scholar] [CrossRef]
- Han, S.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Lee, S.; Rhim, S.C. Rod stiffness as a risk factor of proximal junctional kyphosis after adult spinal deformity surgery: Comparative study between cobalt chrome multiple-rod constructs and titanium alloy two-rod constructs. Spine J. 2017, 17, 962–968. [Google Scholar] [CrossRef]
- Heneghan, C.; Langton, D.; Thompson, M. Ongoing problems with metal-on-metal hip implants. BMJ 2012, 344, e1349. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Posada, O.M.; Tate, R.J.; Dominic Meek, R.M.; Helen Grant, M. In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants. Lubricants 2015, 3, 539–568. [Google Scholar] [CrossRef]
- Ke, D.; Robertson, S.F.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. Effects of MgO and SiO2 on Plasma-Sprayed Hydroxyapatite Coating: An in Vivo Study in Rat Distal Femoral Defects. ACS Appl. Mater. Interfaces. 2017, 9, 25731–25737. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Shivaram, A.; Isik, M.; Avila, J.D.; Dernell, W.S.; Bose, S. Additively manufactured calcium phosphate reinforced CoCrMo alloy: Bio-tribological and biocompatibility evaluation for load-bearing implants. Addit. Manuf. 2019, 28, 312–324. [Google Scholar] [CrossRef]
- Kok, D.; Firkins, P.J.; Wapstra, F.H.; Veldhuizen, A.G. A new lumbar posterior fixation system, the memory metal spinal system: An in-vitro mechanical evaluation. BMC Musculoskelet. Disord. 2013, 14, 269. [Google Scholar] [CrossRef]
- Kassab, E.J.; Gomes, J.P. Assessment of nickel titanium and beta titanium corrosion resistance behavior in fluoride and chloride environments. Angle Orthod. 2013, 83, 864–869. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Oakes, R.S.; Froimchuk, E.; Jewell, C.M. Engineering Biomaterials to Direct Innate Immunity. Adv. Ther. 2019, 2, 1800157. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, Y.; Si, X.; Yao, H.; Ma, S.; Xu, Y.; Zhao, J.; Ma, C.; He, C.; Tang, Z.; et al. Biopolymer Immune Implants’ Sequential Activation of Innate and Adaptive Immunity for Colorectal Cancer Postoperative Immunotherapy. Adv. Mater. 2020, 33, e2004559. [Google Scholar] [CrossRef] [PubMed]
- Billing, F.; Jakobi, M.; Martin, D.; Gerlach, K.; Arefaine, E.; Weiss, M.; Schneiderhan-Marra, N.; Hartmann, H.; Shipp, C. The immune response to the SLActive titanium dental implant surface in vitro is predominantly driven by innate immune cells. J. Immunol. Regen. Med. 2021, 13, 100047. [Google Scholar] [CrossRef]
- Moran, M.M.; Sena, K.; A McNulty, M.; Sumner, D.; Virdi, A.S. Intramembranous bone regeneration and implant placement using mechanical femoral marrow ablation: Rodent models. BoneKEy Rep. 2016, 5, 837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical behaviours of the bone–implant interface: A review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef]
- Dewey, M.J.; Harley, B.A.C. Biomaterial design strategies to address obstacles in craniomaxillofacial bone repair. RSC Adv. 2021, 11, 17809–17827. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Anitua, E.; Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Influence of calcium ion-modified implant surfaces in protein adsorption and implant integration. Int. J. Implant Dent. 2021, 7, 32. [Google Scholar] [CrossRef]
- Smoljanovic, T.; Bojanic, I.; Cimic, M. Letters. Spine 2010, 35, E1010–E1011. [Google Scholar] [CrossRef]
- Dapunt, U.; Giese, T.; Lasitschka, F.; Reinders, J.; Lehner, B.; Kretzer, J.P.; Ewerbeck, V.; Hänsch, G.M. On the inflammatory response in metal-on-metal implants. J. Transl. Med. 2014, 12, 74. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Huang, Q.; Wang, L.Y.; Lei, Y.T.; Xu, H.; Shen, B.; Pei, F.X. Normal trajectory of Interleukin-6 and C-reactive protein in the perioperative period of total knee arthroplasty under an enhanced recovery after surgery scenario. BMC Musculoskelet. Disord. 2020, 21, 264. [Google Scholar] [CrossRef]
- Thelander, U.; Larsson, S. Quantitation of C-Reactive Protein Levels and Erythrocyte Sedimentation Rate After Spinal Surgery. Spine 1992, 17, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, G.; Piscopo, A.; Grubor, P.; Rollo, G.; Medici, A.; Pipola, V.; Bisaccia, M.; Caraffa, A.; Barron, E.M.; Nobile, F.; et al. Use of Common Inflammatory Markers in the Long-Term Screening of Total Hip Arthroprosthesis Infections: Our Experience. Adv. Orthop. 2017, 2017, 9679470. [Google Scholar] [CrossRef] [PubMed]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2021, 7, e10239. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2018, 84, 1–15. [Google Scholar] [CrossRef]
- Irandoust, S.; Müftü, S. The interplay between bone healing and remodeling around dental implants. Sci. Rep. 2020, 10, 4335. [Google Scholar] [CrossRef]
- Boden, S.D.; Zdeblick, T.A.; Sandhu, H.S.; Heim, S.E. The Use of rhBMP-2 in Interbody Fusion Cages. Spine 2000, 25, 376–381. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, L.; Tan, G. Fourth-generation biomedical materials. Mater. Today 2015, 19, 2–3. [Google Scholar] [CrossRef]
- Chen, W.; Xie, G.; Lu, Y.; Wang, J.; Feng, B.; Wang, Q.; Xu, K.; Bao, J. An improved osseointegration of metal implants by pitavastatin loaded multilayer films with osteogenic and angiogenic properties. Biomaterials 2021, 280, 121260. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; van Wijnen, A.J. Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef]
- Goto, M.; Matsumine, A.; Yamaguchi, S.; Takahashi, H.; Akeda, K.; Nakamura, T.; Asanuma, K.; Matsushita, T.; Kokubo, T.; Sudo, A. Osteoconductivity of bioactive Ti-6Al-4V implants with lattice-shaped interconnected large pores fabricated by electron beam melting. J. Biomater. Appl. 2020, 35, 1153–1167. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Eger, M.; Hiram-Bab, S.; Liron, T.; Sterer, N.; Carmi, Y.; Kohavi, D.; Gabet, Y. Mechanism and Prevention of Titanium Particle-Induced Inflammation and Osteolysis. Front. Immunol. 2018, 9, 2963. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Badylak, S.F.; Elisseeff, J. Immunomodulatory Biomaterials: Regulating the Immune Response with Biomaterials to Affect Clinical Outcome; Woodhead Publishing: Duxford, UK, 2021. [Google Scholar]
- Felgueiras, H.P.; Evans, M.D.; Migonney, V. Contribution of fibronectin and vitronectin to the adhesion and morphology of MC3T3-E1 osteoblastic cells to poly(NaSS) grafted Ti6Al4V. Acta Biomater. 2015, 28, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef]
- Saleh, L.S.; Bryant, S.J. In vitro and in vivo models for assessing the host response to biomaterials. Drug Discov. Today: Dis. Model. 2017, 24, 13–21. [Google Scholar] [CrossRef]
- Sun, H.; Zhi, K.; Hu, L.; Fan, Z. The Activation and Regulation of β2 Integrins in Phagocytes and Phagocytosis. Front. Immunol. 2021, 12, 978. [Google Scholar] [CrossRef]
- Zaveri, T.D.; Lewis, J.S.; Dolgova, N.V.; Clare-Salzler, M.J.; Keselowsky, B.G. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials 2014, 35, 3504–3515. [Google Scholar] [CrossRef]
- Kim, O.-H.; Kim, H.; Kang, J.; Yang, D.; Kang, Y.-H.; Lee, D.H.; Cheon, G.J.; Park, S.C.; Oh, B.-C. Impaired phagocytosis of apoptotic cells causes accumulation of bone marrow-derived macrophages in aged mice. BMB Rep. 2017, 50, 43–48. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; LaValle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Lucke, S.; Walschus, U.; Hoene, A.; Schnabelrauch, M.; Nebe, J.B.; Finke, B.; Schlosser, M. The in vivo inflammatory and foreign body giant cell response against different poly( l -lactide-co- d/l -lactide) implants is primarily determined by material morphology rather than surface chemistry. J. Biomed. Mater. Res. Part A 2018, 106, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Levey, R.E.; Coulter, F.B.; Scheiner, K.C.; Deotti, S.; Robinson, S.T.; McDonough, L.; Nguyen, T.T.; Steendam, R.; Canney, M.; Wylie, R.; et al. Assessing the Effects of VEGF Releasing Microspheres on the Angiogenic and Foreign Body Response to a 3D Printed Silicone-Based Macroencapsulation Device. Pharmaceutics 2021, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Chemokines Associated with Pathologic Responses to Orthopedic Implant Debris. Front. Endocrinol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Zhang, B.; Su, Y.; Zhou, J.; Zheng, Y.; Zhu, D. Toward a Better Regeneration through Implant-Mediated Immunomodulation: Harnessing the Immune Responses. Adv. Sci. 2021, 8, 2100446. [Google Scholar] [CrossRef]
- Zago, G.; Saavedra, P.H.V.; Keshari, K.R.; Perry, J.S.A. Immunometabolism of Tissue-Resident Macrophages—An Appraisal of the Current Knowledge and Cutting-Edge Methods and Technologies. Front. Immunol. 2021, 12, 665782. [Google Scholar] [CrossRef]
- Samelko, L.; Caicedo, M.; McAllister, K.; Jacobs, J.; Hallab, N.J. Metal-induced delayed type hypersensitivity responses potentiate particle induced osteolysis in a sex and age dependent manner. PLoS ONE 2021, 16, e0251885. [Google Scholar] [CrossRef]
- McKee, A.S.; Fontenot, A.P. Interplay of innate and adaptive immunity in metal-induced hypersensitivity. Curr. Opin. Immunol. 2016, 42, 25–30. [Google Scholar] [CrossRef]
- Couto, M.; Vasconcelos, D.; Sousa, D.M.; Sousa, B.; Conceição, F.; Neto, E.; Lamghari, M.; Alves, C.J. The Mechanisms Underlying the Biological Response to Wear Debris in Periprosthetic Inflammation. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Le Cann, S.; Tudisco, E.; Turunen, M.J.; Patera, A.; Mokso, R.; Tägil, M.; Belfrage, O.; Hall, S.A.; Isaksson, H. Investigating the Mechanical Characteristics of Bone-Metal Implant Interface Using in situ Synchrotron Tomographic Imaging. Front. Bioeng. Biotechnol. 2019, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Samelko, L.; Landgraeber, S.; McAllister, K.; Jacobs, J.; Hallab, N.J. Cobalt Alloy Implant Debris Induces Inflammation and Bone Loss Primarily through Danger Signaling, Not TLR4 Activation: Implications for DAMP-ening Implant Related Inflammation. PLoS ONE 2016, 11, e0160141. [Google Scholar] [CrossRef] [PubMed]
- Vinaik, R.; Abdullahi, A.; Barayan, D.; Jeschke, M.G. NLRP3 inflammasome activity is required for wound healing after burns. Transl. Res. 2019, 217, 47–60. [Google Scholar] [CrossRef]
- Baron, L.; Gombault, A.; Fanny, M.; Villeret, B.; Savigny, F.; Guillou, N.; Panek, C.; Le Bert, M.; Lagente, V.; Rassendren, F.; et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015, 6, e1629. [Google Scholar] [CrossRef]
- Boro, M.; Balaji, K.N. CXCL1 and CXCL2 Regulate NLRP3 Inflammasome Activation via G-Protein–Coupled Receptor CXCR2. J. Immunol. 2017, 199, 1660–1671. [Google Scholar] [CrossRef]
- Paniri, A.; Akhavan-Niaki, H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: Role of lncRNAs in cytokine storm modulation. Life Sci. 2020, 257, 118114. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.-R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Kapasa, E.R.; Giannoudis, P.V.; Jia, X.; Hatton, P.V.; Yang, X.B. The Effect of RANKL/OPG Balance on Reducing Implant Complications. J. Funct. Biomater. 2017, 8, 42. [Google Scholar] [CrossRef]
- Akyol, S.; Akgun, M.Y.; Yetmez, M.; Hanci, M.; Oktar, F.N.; Ben-Nissan, B. Comparative Analysis of NF-κB in the MyD88-Mediated Pathway After Implantation of Titanium Alloy and Stainless Steel and the Role of Regulatory T Cells. World Neurosurg. 2020, 144, e138–e148. [Google Scholar] [CrossRef]
- Sun, Z.; Zeng, J.; Wang, W.; Jia, X.; Wu, Q.; Yu, D.; Mao, Y. Magnoflorine Suppresses MAPK and NF-κB Signaling to Prevent Inflammatory Osteolysis Induced by Titanium Particles In Vivo and Osteoclastogenesis via RANKL In Vitro. Front. Pharmacol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N. Diagnosis of Metal Hypersensitivity in Orthopedics. Oper. Tech. Orthop. 2017, 27, 168–177. [Google Scholar] [CrossRef]
- Chaturvedi, T. Allergy related to dental implant and its clinical significance. Clin. Cosmet. Investig. Dent. 2013, 5, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.W.; Schalock, P.C. Metal Hypersensitivity Reactions to Orthopedic Implants. Dermatol. Ther. 2016, 7, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wawrzynski, J.; Gil, J.A.; Goodman, A.D.; Waryasz, G.R. Hypersensitivity to Orthopedic Implants: A Review of the Literature. Rheumatol. Ther. 2017, 4, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.A.; Crist, B.D. Nickel allergy to orthopaedic implants: A review and case series. J. Clin. Orthop. Trauma 2020, 11, S596–S603. [Google Scholar] [CrossRef] [PubMed]
- Adala, R.; Chakravarthy, M.; Srinivas, V.; Pai, S. Orthopaedic surgery in a patient with metal sensitivity. J. Cutan. Aesthetic Surg. 2011, 4, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef]
- Engelhart, S.; Segal, R.J. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis 2017, 99, 245–249. [Google Scholar]
- Hallab, N.J. A review of the biologic effects of spine implant debris: Fact from fiction. SAS J. 2009, 3, 143–160. [Google Scholar] [CrossRef]
- Hallab, N.J.; Caicedo, M.; McAllister, K.; Skipor, A.; Amstutz, H.; Jacobs, J. Asymptomatic prospective and retrospective cohorts with metal-on-metal hip arthroplasty indicate acquired lymphocyte reactivity varies with metal ion levels on a group basis. J. Orthop. Res. 2012, 31, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Hsueh-Chun, W.; Wang, H.-C.; Wu, M.-C.; Wu, S.-W.; Yeh, M.-L. Evaluation of osseous integration of titanium orthopedic screws with novel SLA treatment in porcine model. PLoS ONE 2017, 12, e0188364. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, Y.; Li, B.; Wang, X.; Li, H.; Liu, S.; Liang, C.; Wang, H. Biological and Mechanical Effects of Micro-Nanostructured Titanium Surface on an Osteoblastic Cell Line In vitro and Osteointegration In vivo. Appl. Biochem. Biotechnol. 2017, 183, 280–292. [Google Scholar] [CrossRef]
- Ota, T.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Shinmura, K.; Yokogawa, N.; Shirai, T.; Murakami, H.; et al. A comparison of bone conductivity on titanium screws inserted into the vertebra using different surface processing. J. Exp. Orthop. 2020, 7, 29. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef]
- Katsuura, Y.; Wright-Chisem, J.; Wright-Chisem, A.; Virk, S.; McAnany, S. The Importance of Surface Technology in Spinal Fusion. HSS J. Musculoskelet. J. Hosp. Spéc. Surg. 2020, 16, 113–116. [Google Scholar] [CrossRef]
- Li, Q.; Shen, A.; Wang, Z. Enhanced osteogenic differentiation of BMSCs and M2-phenotype polarization of macrophages on a titanium surface modified with graphene oxide for potential implant applications. RSC Adv. 2020, 10, 16537–16550. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jansen, J.A.; Yang, F.; Beucken, J.J.V.D. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C 2018, 95, 143–151. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2017, 20, 82–91. [Google Scholar] [CrossRef]
- Caneva, M.; Salata, L.A.; De Souza, S.S.; Bressan, E.; Botticelli, D.; Lang, N.P. Hard tissue formation adjacent to implants of various size and configuration immediately placed into extraction sockets: An experimental study in dogs. Clin. Oral Implant. Res. 2010, 21, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Khadija, G.; Saleem, A.; Akhtar, Z.; Naqvi, Z.; Gull, M.; Masood, M.; Mukhtar, S.; Batool, M.; Saleem, N.; Rasheed, T.; et al. Short term exposure to titanium, aluminum and vanadium (Ti 6Al 4V) alloy powder drastically affects behavior and antioxidant metabolites in vital organs of male albino mice. Toxicol. Rep. 2018, 5, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Doe, Y.; Ida, H.; Seiryu, M.; Deguchi, T.; Takeshita, N.; Sasaki, S.; Sasaki, S.; Irie, D.; Tsuru, K.; Ishikawa, K.; et al. Titanium surface treatment by calcium modification with acid-etching promotes osteogenic activity and stability of dental implants. Materialia 2020, 12, 100801. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Milheiro, A.; Nozaki, K.; Kleverlaan, C.J.; Muris, J.; Miura, H.; Feilzer, A.J. In vitro cytotoxicity of metallic ions released from dental alloys. Odontology 2016, 104, 136–142. [Google Scholar] [CrossRef]
- Brayda-Bruno, M.; Fini, M.; Pierini, G.; Giavaresi, G.; Rocca, M.; Giardino, R. Evaluation of Systemic Metal Diffusion after Spinal Pedicular Fixation with Titanium Alloy and Stainless Steel System: A 36-month Experimental Study in Sheep. Int. J. Artif. Organs 2001, 24, 41–49. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Choi, Y.-S.; Hwang, Y.-H.; Cho, H.-W.; Lee, D.-G. Biocompatibility and Biological Corrosion Resistance of Ti–39Nb–6Zr+0.45Al Implant Alloy. J. Funct. Biomater. 2020, 12, 2. [Google Scholar] [CrossRef]
- Maya, S.; Prakash, T.; Das Madhu, K.; Goli, D. Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed. Pharmacother. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Kretzer, J.P.; Mueller, U.; Streit, M.R.; Kiefer, H.; Sonntag, R.; Streicher, R.M.; Reinders, J. Ion release in ceramic bearings for total hip replacement: Results from an in vitro and an in vivo study. Int. Orthop. 2017, 42, 65–70. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Hamidi, M.F.F.A.; Harun, W.S.W.; Samykano, M.; Ghani, S.A.C.; Ghazalli, Z.; Ahmad, F.; Sulong, A.B. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Brewer, J.M.; Grant, M.H. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J. Immunotoxicol. 2011, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Posada, O.M.; Tate, R.J.; Grant, M.H. Toxicity of cobalt-chromium nanoparticles released from a resurfacing hip implant and cobalt ions on primary human lymphocytes in vitro. J. Appl. Toxicol. 2015, 35, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Hedbrant, A.; Eklund, D.; Andersson, L.; Bryngelsson, I.-L.; Persson, A.; Westberg, H.; Särndahl, E. Effects on white blood cell counts and the NLRP3 inflammasome due to dust and cobalt exposure in the hard metal industry. Biomarkers 2021, 27, 60–70. [Google Scholar] [CrossRef]
- Klasson, M.; Lindberg, M.; Westberg, H.; Bryngelsson, I.-L.; Tuerxun, K.; Persson, A.; Särndahl, E. Dermal exposure to cobalt studied in vitro in keratinocytes – effects of cobalt exposure on inflammasome activated cytokines, and mRNA response. Biomarkers 2021, 26, 674–684. [Google Scholar] [CrossRef]
- Wegienka, G.; Johnson, C.C.; Zoratti, E.; Havstad, S. Racial differences in allergic sensitization: Recent findings and future directions. Curr. Allergy Asthma Rep. 2013, 13, 255–261. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Ryhänen, J.; Niemi, E.; Serlo, W.; Niemelä, E.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of Nickel-Titanium Shape Memory Metal and Its Corrosion Behavior in Human Cell Cultures. J. Biomed. Mater. Res. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Haider, W.; Munroe, N.; Tek, V.; Gill, P.K.S.; Tang, Y.; McGoron, A.J. Cytotoxicity of Metal Ions Released from Nitinol Alloys on Endothelial Cells. J. Mater. Eng. Perform. 2011, 20, 816–818. [Google Scholar] [CrossRef]
- Bogdanski, D.; Koller, M.; Bram, M.; Stöver, D.; Buchkremer, H.; Choi, J.; Epple, M.; Muhr, G. Schnelle Analyse Der Biokompatibilität Mittels Gradierter Probekörper Am Beispiel Von Ni-NiTi-Ti. Biomed. Eng. Biomed. Tech. 2002, 47, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.J.L.; Dreher, M.L.; Zheng, J.; Chen, L.; Madamba, D.; Miyashiro, K.; Trépanier, C.; Nagaraja, S. Effects of Oxide Layer Composition and Radial Compression on Nickel Release in Nitinol Stents. Shape Mem. Superelast. 2015, 1, 319–327. [Google Scholar] [CrossRef]
- Nagaraja, S.; Sullivan, S.J.; Stafford, P.R.; Lucas, A.D.; Malkin, E. Impact of nitinol stent surface processing on in-vivo nickel release and biological response. Acta Biomater. 2018, 72, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, T.; Rokicki, R. Modification of Nitinol Biomaterial for Medical Applications. World Sci. News 2018, 96, 35–58. [Google Scholar]
- Lou, J.; Gao, Z.; Zhang, J.; He, H.; Wang, X. Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films. Coatings 2021, 11, 1543. [Google Scholar] [CrossRef]
- Pacheco, K.A. Allergy to Surgical Implants. Clin. Rev. Allergy Immunol. 2018, 56, 72–85. [Google Scholar] [CrossRef]
- Voggenreiter, G.; Leiting, S.; Brauer, H.; Leiting, P.; Majetschak, M.; Bardenheuer, M.; Obertacke, U. Immuno-inflammatory tissue reaction to stainless-steel and titanium plates used for internal fixation of long bones. Biomaterials 2002, 24, 247–254. [Google Scholar] [CrossRef]
- Mahendra, G.; Pandit, H.; Kliskey, K.; Murray, D.; Gill, H.S.; Athanasou, N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009, 80, 653–659. [Google Scholar] [CrossRef]
- Akyol, S.; Bozkus, H.; Cinar, S.A.; Hanci, M.M. Which is Better: Stainless Steel or Titanium Alloy? Turk. Neurosurg. 2017, 28, 4. [Google Scholar] [CrossRef]
- Niempoog, S.; Kukreja, S. Neuropathy Caused by Metal Hypersensitivity after Placement of Stainless Steel Plate. Case Rep. Orthop. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Thomas, P.; Von Der Helm, C.; Schopf, C.; Mazoochian, F.; Frommelt, L.; Gollwitzer, H.; Schneider, J.; Flaig, M.; Krenn, V.; Thomas, B.; et al. Patients with Intolerance Reactions to Total Knee Replacement: Combined Assessment of Allergy Diagnostics, Periprosthetic Histology, and Peri-implant Cytokine Expression Pattern. BioMed Res. Int. 2015, 2015, 910156. [Google Scholar] [CrossRef]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Merritt, K.; Brown, S.A. Release of hexavalent chromium from corrosion of stainless steel and cobalt?chromium alloys. J. Biomed. Mater. Res. 1995, 29, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Watchmaker, J.; Collins, R.; Chaney, K. Allergic Contact Dermatitis to Manganese in Metallic Implant. Dermatitis 2015, 26, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yin, Y.; Gong, L.; Liang, Z.; Zhu, C.; Ren, C.; Zheng, N.; Zhang, Q.; Liu, H.; Liu, W.; et al. Manganese nanodepot augments host immune response against coronavirus. Nano Res. 2020, 14, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, M.S.; Desai, R.; McAllister, K.; Reddy, A.; Jacobs, J.J.; Hallab, N.J. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. J. Orthop. Res. 2008, 27, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.I.P.; Gomes, A.M.; Rodrigues, M.F.; Pinto, T.S.; Fernandes, C.J.D.C.; Bezerra, F.J.; Zambuzzi, W.F. Cobalt-chromium-enriched medium ameliorates shear-stressed endothelial cell performance. J. Trace Elements Med. Biol. 2019, 54, 163–171. [Google Scholar] [CrossRef]
- Anderson, J.A.; Lamichhane, S.; Mani, G. Macrophage responses to 316L stainless steel and cobalt chromium alloys with different surface topographies. J. Biomed. Mater. Res. Part A 2016, 104, 2658–2672. [Google Scholar] [CrossRef]
- Safranski, D.; Dupont, K.; Gall, K. Pseudoelastic NiTiNOL in Orthopaedic Applications. Shape Mem Superelast. 2020, 6, 332–341. [Google Scholar] [CrossRef]
- Gholampour, S.; Gholampour, H. Correlation of a new hydrodynamic index with other effective indexes in Chiari I malformation patients with different associations. Sci. Rep. 2020, 10, 15907. [Google Scholar] [CrossRef]
| Titanium Alloy | Chemical Composition (%wt) | Phase Type | References |
|---|---|---|---|
| Commercial pure titanium (CP-Ti) | 99–99.5% Ti | α type | [71] |
| Ti-6Al-4V | 6.29% Al | α–β type | [31] |
| 4.02% V | |||
| <0.2% other elements | |||
| Ti balanced | |||
| Ti-24Nb-4Zr-8Sn | 24% Nb | α–β type | [72] |
| 4% Zr | |||
| 8% Sn | |||
| <0.3% other elements | |||
| Ti balanced | |||
| Ti-45Nb | 44.94% Ni | β type | [73,74,75] |
| <0.5% other elements | |||
| Ti balanced |
| Alloys | Foreign Body Reaction | Innate Reaction | Adaptive Response | Healing Process | References |
|---|---|---|---|---|---|
| Titanium | Formation of foreign body giant cells is common | Prolonged presence of neutrophils | Osteointegration | Enhanced osteogenic response | [182,183] |
| CoCr | Fewer instances of foreign body giant cell formation than in SS | Induction of IL-1B and T cell lymphocyte proliferation | Decrease in cytokine production over time | Enhanced angiogenesis | [197,198,199,220] |
| Nitinol | Inflammatory response due to Ni ions being released | Inflammation in presence of macrophages and lymphocytes | Rare cases of type IV delayed hypersensitivity response | Osteointegration higher than titanium | [221,222] |
| SS | Higher inflammatory response than in other analyzed materials | Inflammation in presence of macrophages and lymphocyte congregates | Buildup of lymphocytes, histiocytes, giant cells and inflammation | Increased inflammatory response slows down the healing process | [209,211,213,214] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litak, J.; Szymoniuk, M.; Czyżewski, W.; Hoffman, Z.; Litak, J.; Sakwa, L.; Kamieniak, P. Metallic Implants Used in Lumbar Interbody Fusion. Materials 2022, 15, 3650. https://doi.org/10.3390/ma15103650
Litak J, Szymoniuk M, Czyżewski W, Hoffman Z, Litak J, Sakwa L, Kamieniak P. Metallic Implants Used in Lumbar Interbody Fusion. Materials. 2022; 15(10):3650. https://doi.org/10.3390/ma15103650
Chicago/Turabian StyleLitak, Jakub, Michał Szymoniuk, Wojciech Czyżewski, Zofia Hoffman, Joanna Litak, Leon Sakwa, and Piotr Kamieniak. 2022. "Metallic Implants Used in Lumbar Interbody Fusion" Materials 15, no. 10: 3650. https://doi.org/10.3390/ma15103650
APA StyleLitak, J., Szymoniuk, M., Czyżewski, W., Hoffman, Z., Litak, J., Sakwa, L., & Kamieniak, P. (2022). Metallic Implants Used in Lumbar Interbody Fusion. Materials, 15(10), 3650. https://doi.org/10.3390/ma15103650







