pH and Redox-Dual Sensitive Chitosan Nanoparticles Having Methyl Ester and Disulfide Linkages for Drug Targeting against Cholangiocarcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
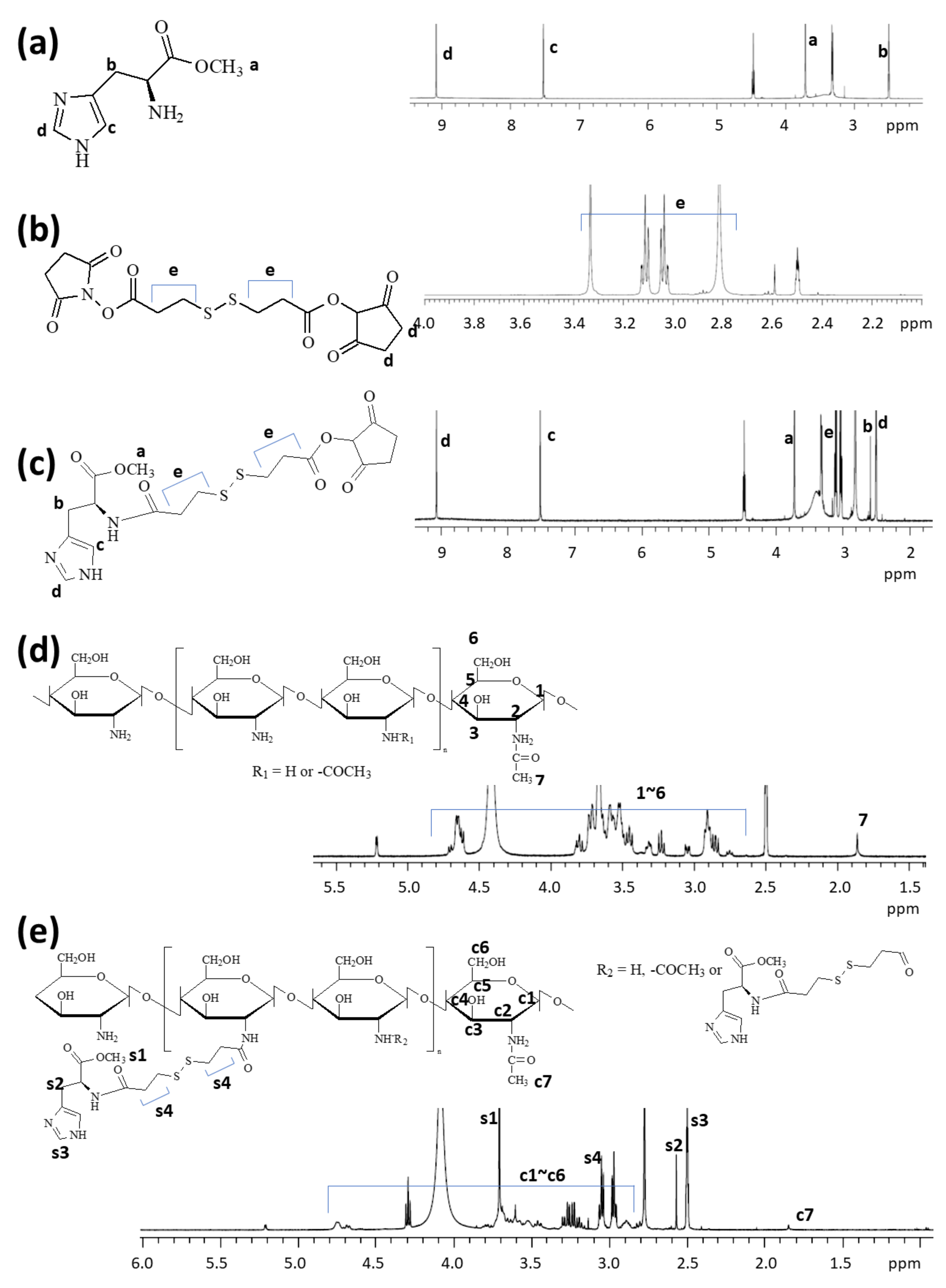
2.2. Synthesis of ChitoHISss Conjugates
2.3. H Nuclear Magnetic Resonance (NMR) Spectra
2.4. Preparation of DOX-Incorporated ChitoHISss Nanoparticles
2.5. Preparation of Ce6-Incorporated ChitoHISss Nanoparticles
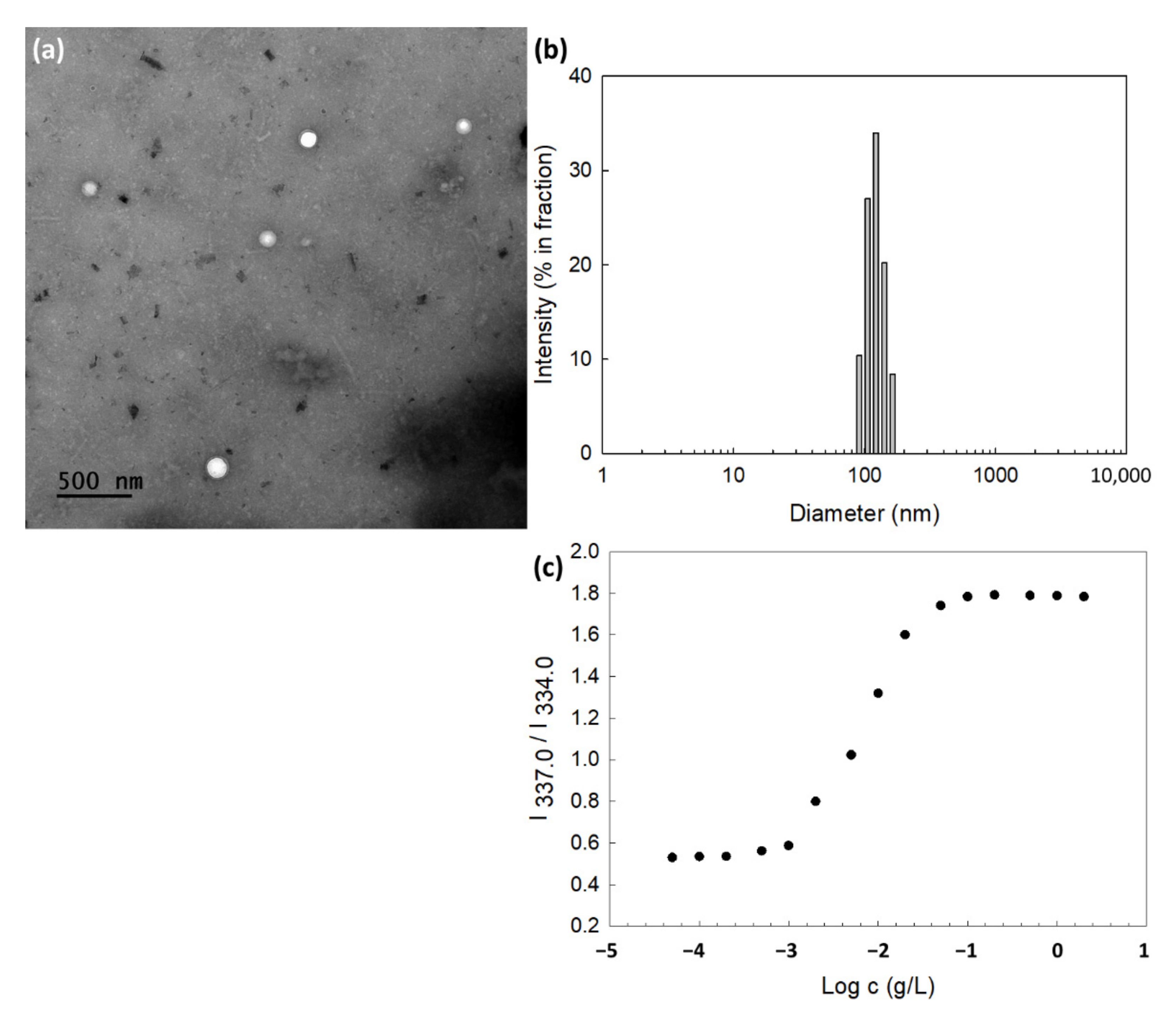
2.6. Transmission Electron Microscope (TEM)
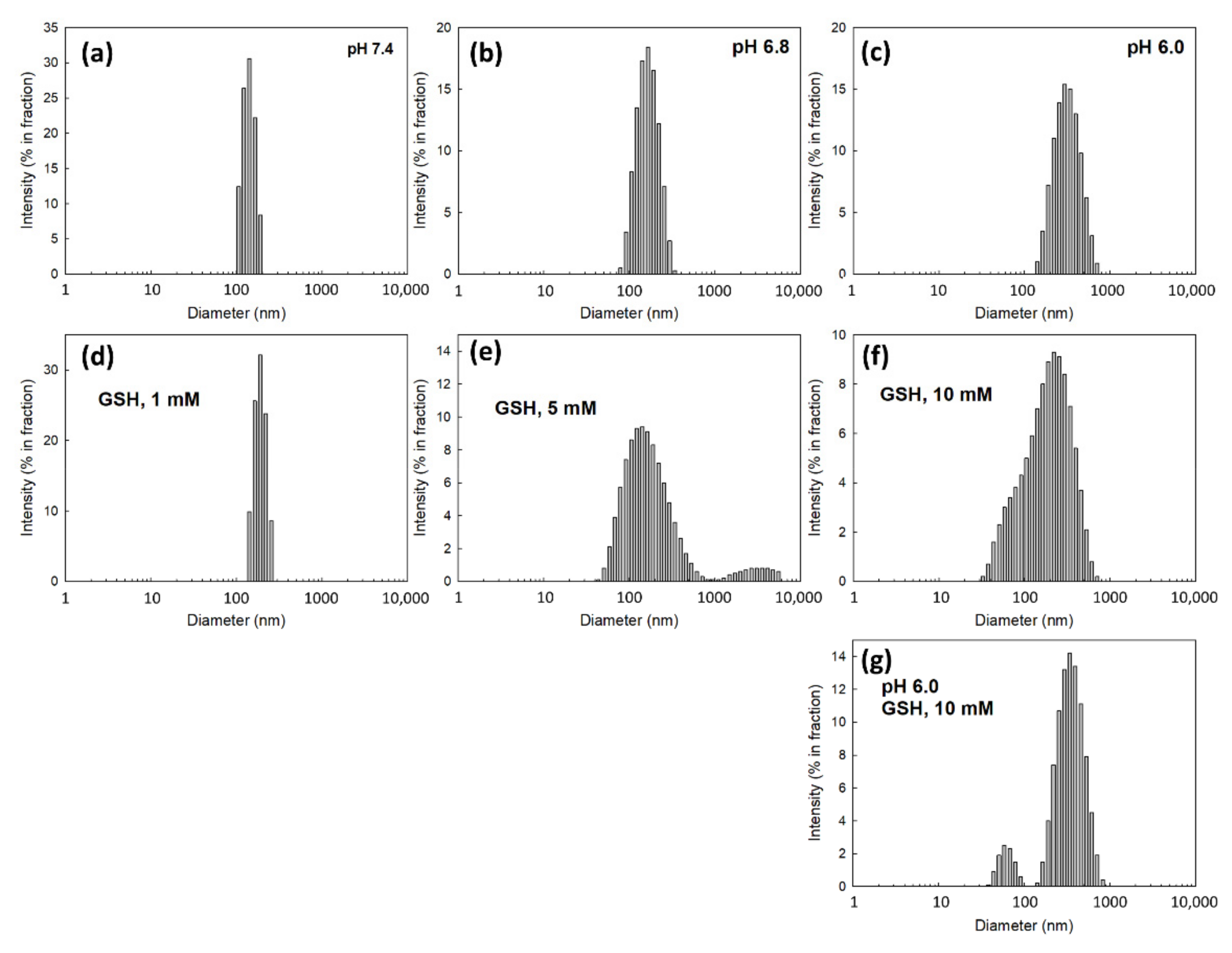
2.7. Analysis of Particle Size Distribution
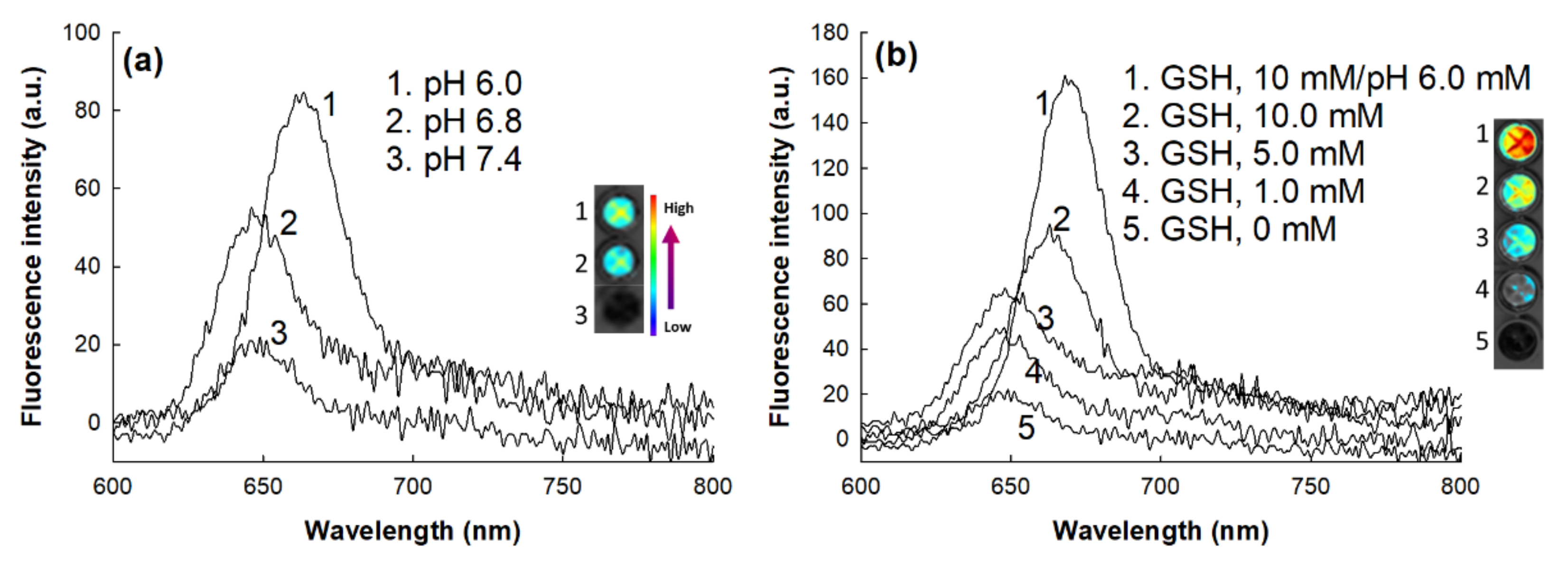
2.8. Fluorescence Spectrophotometer Measurement of Nanoparticle Solution
2.9. Drug Release from Nanoparticles
2.10. Cell Culture
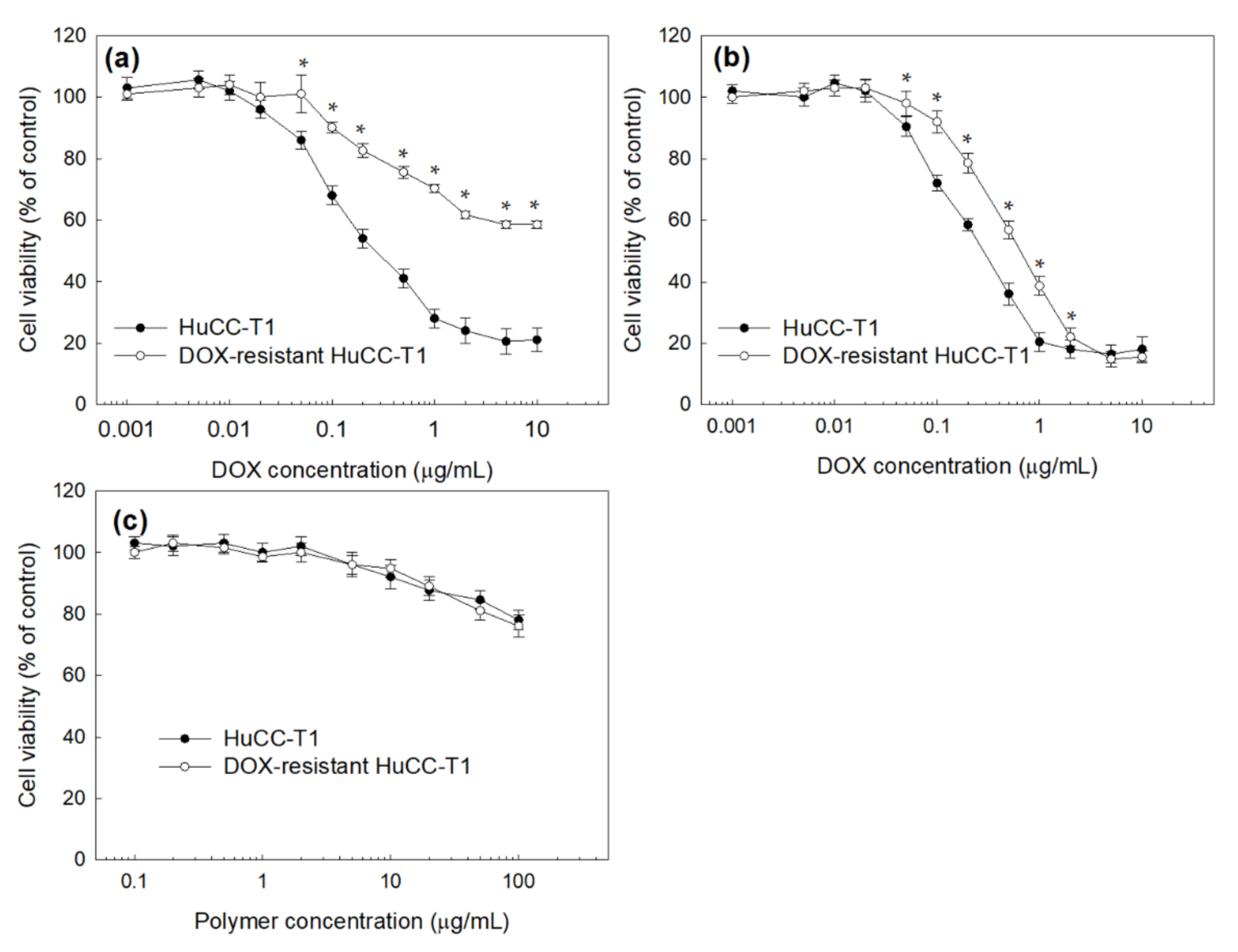
2.11. Anticancer Activity of DOX-Incorporated ChitoHISss Nanoparticles against DOX-Resistant HuCC-T1 Cells
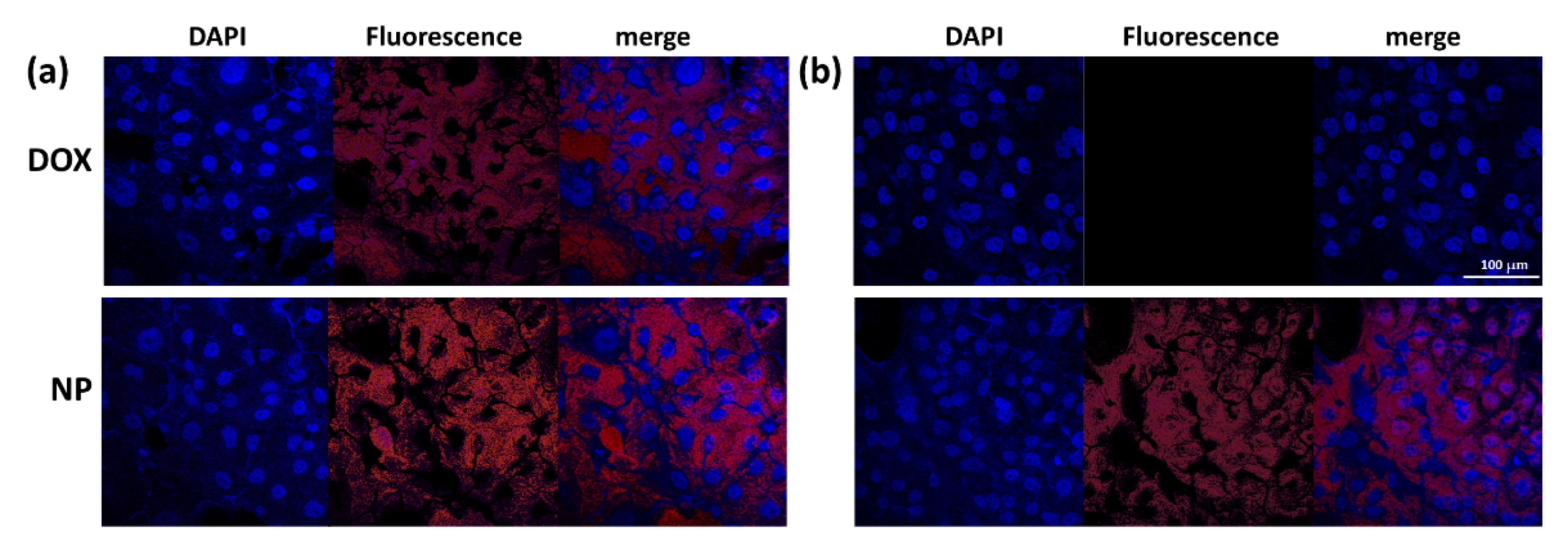
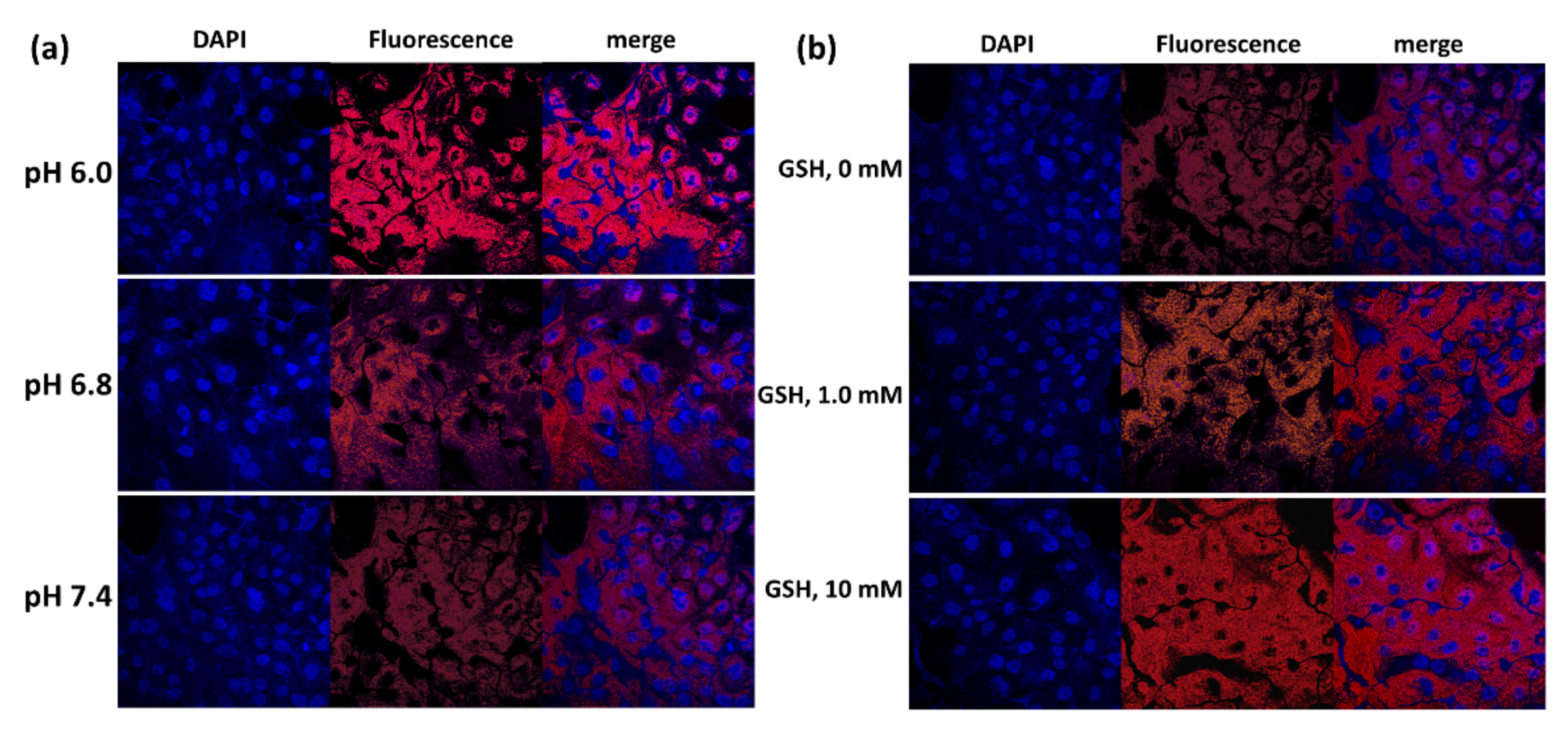
2.12. Observation of Cells with Fluorescence Microscope
2.13. In Vivo Animal Study
2.14. Statistical Analysis
3. Results
3.1. Synthesis of ChitoHISss Conjugates
3.2. Fabrication and Characterization of DOX-Incorporated ChitoHISss Nanoparticles
3.3. Anticancer Activity of ChitoHISss Nanoparticles In Vitro
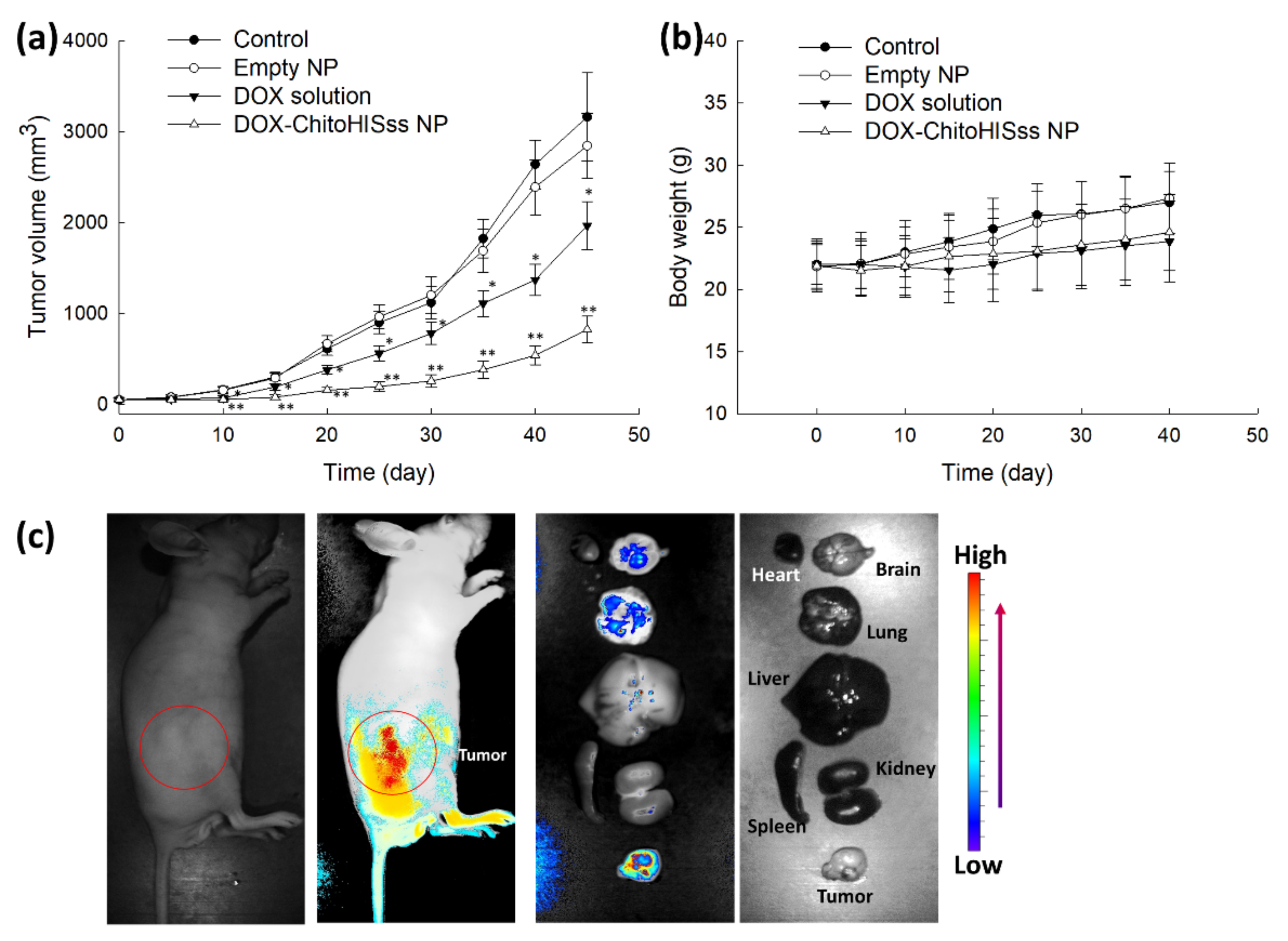
3.4. Antitumor Activity of ChitoHISss Nanoparticles In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lim, J.H. Cholangiocarcinoma: Morphologic classification according to growth pattern and imaging findings. Am. J. Roentgenol. 2003, 181, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Taylor-Robinson, S.D.; Toledano, M.B.; Beck, A.; Elliott, P.; Thomas, H.C. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J. Hepatol. 2002, 37, 806–813. [Google Scholar] [CrossRef]
- Sirica, A.E. Cholangiocarcinoma: Molecular targeting strategies for chemo- prevention and therapy. Hepatology 2005, 41, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, D.S.; Roberts, L.R. Diagnosis and management of cholangiocarcinoma. Curr. Gastroenterol. Rep. 2008, 10, 43–52. [Google Scholar] [CrossRef]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Goff, L.W. Advances in the management of biliary tract cancers. Clin. Adv. Hematol. Oncol. 2013, 11, 28–34. [Google Scholar]
- Brunner, T.B.; Eccles, C.L. Radiotherapy and chemotherapy as therapeutic strategies in extrahepatic biliary duct carcinoma. Strahlenther. Onkol. 2010, 186, 672–680. [Google Scholar] [CrossRef]
- Skipworth, J.R.; Olde Damink, S.W.; Imber, C.; Bridgewater, J.; Pereira, S.P.; Malagó, M. Surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment. Pharmacol. Ther. 2011, 34, 1063–1078. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Pathogenesis, diagnosis and management of cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef]
- Cereda, S.; Passoni, P.; Reni, M.; Viganò, M.G.; Aldrighetti, L.; Nicoletti, R.; Villa, E. The cisplatin, epirubicin, 5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary tract adenocarcinoma. Cancer 2010, 116, 2208–2214. [Google Scholar] [CrossRef]
- Kim, S.T.; Park, J.O.; Lee, J.; Lee, K.T.; Lee, J.K.; Choi, S.H.; Heo, J.S.; Park, Y.S.; Kang, W.K.; Park, K. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 2006, 106, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 36, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Cao, G.; Zhu, X.; Cui, Y.; Ji, X.; Li, X.; Yang, R.; Chen, H.; Xu, H.; et al. Phase II study of hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced perihilar cholangiocarcinoma. Radiology 2017, 283, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Baak, R.; Willemssen, F.E.J.A.; van Norden, Y.; Eskens, F.A.L.M.; Milder, M.T.W.; Heijmen, B.J.M.; Koerkamp, B.G.; Sprengers, D.; van Driel, L.M.J.W.; Klümpen, H.J.; et al. Stereotactic body radiation therapy after chemotherapy for unresectable perihilar cholangiocarcinoma: The STRONG trial, a Phase I safety and feasibility study. Cancers 2021, 13, 3991. [Google Scholar] [CrossRef]
- Leong, E.; Chen, W.W.; Ng, E.; Van Hazel, G.; Mitchell, A.; Spry, N. Outcomes from combined chemoradiotherapy in unresectable and locally advanced resected cholangiocarcinoma. J. Gastrointest. Cancer 2012, 43, 50–55. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted therapies in cholangiocarcinoma: Emerging evidence from clinical trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef]
- Uji, M.; Mizuno, T.; Ebata, T.; Sugawara, G.; Igami, T.; Uehara, K.; Nagino, M. A case of advanced intrahepatic cholangiocarcinoma accidentally, but successfully, treated with capecitabine plus oxaliplatin (CAPOX) therapy combined with bevacizumab: A case report. Surg. Case Rep. 2016, 2, 63. [Google Scholar] [CrossRef]
- Furuse, J.; Okusaka, T. Targeted therapy for biliary tract cancer. Cancers 2011, 3, 2243–2254. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Finnes, H.D.; Mahipal, A. Fibroblast growth factor receptor (FGFR) inhibitors in cholangiocarcinoma: Current status, insight on resistance mechanisms and toxicity management. Expert Opin. Drug Metab. Toxicol. 2022, 12, 85–98. [Google Scholar] [CrossRef]
- Mayr, C.; Kiesslich, T.; Modest, D.P.; Stintzing, S.; Ocker, M.; Neureiter, D. Chemoresistance and resistance to targeted therapies in biliary tract cancer: What have we learned? Expert Opin. Investig. Drugs 2022, 31, 221–233. [Google Scholar] [CrossRef]
- Massa, A.; Peraldo-Neia, C.; Vita, F.; Varamo, C.; Basiricò, M.; Raggi, C.; Bernabei, P.; Erriquez, J.; Sarotto, I.; Leone, F.; et al. Paclitaxel restores sensitivity to chemotherapy in preclinical models of multidrug-resistant intrahepatic cholangiocarcinoma. Front. Oncol. 2022, 12, 771418. [Google Scholar] [CrossRef] [PubMed]
- Sahai, V.; Catalano, P.J.; Zalupski, M.M.; Lubner, S.J.; Menge, M.R.; Nimeiri, H.S.; Munshi, H.G.; Benson, A.B., 3rd; O’Dwyer, P.J. Nab-paclitaxel and gemcitabine as first-line treatment of advanced or metastatic cholangiocarcinoma: A Phase 2 clinical trial. JAMA Oncol. 2018, 4, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.I.; Plengsuriyakarn, T.; Chittasupho, C.; Na-Bangchang, K. Enhanced oral bioavailability and biodistribution of atractylodin encapsulated in PLGA nanoparticle in cholangiocarcinoma. Clin. Exp. Pharmacol. Physiol. 2021, 48, 318–328. [Google Scholar] [CrossRef]
- Kwak, T.W.; Kim, D.H.; Jeong, Y.I.; Kang, D.H. Antitumor activity of vorinostat-incorporated nanoparticles against human cholangiocarcinoma cells. J. Nanobiotechnol. 2015, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Wan, Z.; Quan, Z.; Tan, Q. Investigation of thermo-sensitive amphiphilic micelles as drug carriers for chemotherapy in cholangiocarcinoma in vitro and in vivo. Int. J. Pharm. 2014, 463, 81–88. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Li, L.Z. Imaging mitochondrial redox potential and its possible link to tumor metastatic potential. J. Bioenerg. Biomembr. 2012, 44, 645–653. [Google Scholar] [CrossRef][Green Version]
- Jordan, B.F.; Sonveaux, P. Targeting tumor perfusion and oxygenation to improve the outcome of anticancer therapy. Front. Pharmacol. 2012, 3, 94. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Gilliesm, R.J. Tumor pH and its measurement. J. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef]
- Du, J.Z.; Li, H.J.; Wang, J. Tumor-acidity-cleavable maleic acid amide (TACMAA): A powerful tool for designing smart nanoparticles to overcome delivery barriers in cancer nanomedicine. Acc. Chem. Res. 2018, 51, 2848–2856. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, B.; Li, X.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Shell-sheddable micelles based on dextran-SS-poly(epsilon-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules 2010, 11, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Lee, S.J.; Oh, J.S.; Lee, S.G.; Jeong, Y.I.; Lee, H.C. Smart nanoparticles based on hyaluronic acid for redox-responsive and CD44 receptor-mediated targeting of tumor. Nanoscale Res. Lett. 2015, 10, 981. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef]
- Lee, H.M.; Jeong, Y.I.; Kim, D.H.; Kwak, T.W.; Chung, C.W.; Kim, C.H.; Kang, D.H. Ursodeoxycholic acid-conjugated chitosan for photodynamic treatment of HuCC-T1 human cholangiocarcinoma cells. Int. J. Pharm. 2013, 454, 74–81. [Google Scholar] [CrossRef]
- Lee, H.L.; Hwang, S.C.; Nah, J.W.; Kim, J.; Cha, B.; Kang, D.H.; Jeong, Y.I. Redox- and pH-responsive nanoparticles release piperlongumine in a stimuli-sensitive manner to inhibit pulmonary metastasis of colorectal carcinoma cells. J. Pharm. Sci. 2018, 107, 2702–2712. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, Y.I. Hybrid nanoparticles based on chlorin e6-conjugated hyaluronic acid/poly(l-histidine) copolymer for theranostic application to tumors. J. Mater. Chem. B 2018, 6, 2851–2859. [Google Scholar] [CrossRef]
- Curry, J.M.; Sprandio, J.; Cognetti, D. Tumor microenvironment in head and neck squamous cell carcinoma. Semin. Oncol. 2014, 41, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Feng, H.; Hu, B.; Deng, Z.; Wang, C.; Liu, B.; Luan, Y.; Ruan, Y.; Liu, X.; et al. Exploration of redox-related molecular patterns and the redox score for prostate cancer. Oxid. Med. Cell. Longev. 2021, 2021, 4548594. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, T.C.; Zhong, W.; Oberley, T.D. Redox imbalance and biochemical changes in cancer. Cancer Res. 2013, 73, 6118–6123. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.L.; Bissell, M.J. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist. Updates 2012, 15, 39–49. [Google Scholar] [CrossRef]
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469. [Google Scholar] [CrossRef]
- Erin, N.; Grahovac, J.; Brozovic, A.; Efferth, T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updates 2020, 53, 100715. [Google Scholar] [CrossRef]
- Wang, J.X.; Choi, S.Y.C.; Niu, X.; Kang, N.; Xue, H.; Killam, J.; Wang, Y. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int. J. Mol. Sci. 2020, 21, 8363. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, H.; Huang, C.; Lei, Y. Cancer drug resistance: Redox resetting renders a way. Oncotarget 2016, 7, 42740–42761. [Google Scholar] [CrossRef]
- Welters, M.J.; Fichtinger-Schepman, A.M.; Baan, R.A.; Flens, M.J.; Scheper, R.J.; Braakhuis, B.J. Role of glutathione, glutathione S-transferases and multidrug resistance-related proteins in cisplatin sensitivity of head and neck cancer cell lines. Br. J. Cancer 1998, 77, 556–561. [Google Scholar] [CrossRef][Green Version]
- Nunes, S.C.; Serpa, J. Glutathione in ovarian cancer: A double-edged sword. Int. J. Mol. Sci. 2018, 19, 1882. [Google Scholar] [CrossRef]
- Hwang, J.H.; Choi, C.W.; Kim, H.W.; Kim, D.H.; Kwak, T.W.; Lee, H.M.; Kim, C.H.; Chung, C.W.; Jeong, Y.I.; Kang, D.H. Dextran-b-poly(L-histidine) copolymer nanoparticles for pH-responsive drug delivery to tumor cells. Int. J. Nanomed. 2013, 8, 3197–3207. [Google Scholar]
- García, M.C.; Calderón-Montaño, J.M.; Rueda, M.; Longhi, M.; Rabasco, A.M.; López-Lázaro, M.; Prieto-Dapena, F.; González-Rodríguez, M.L. pH-temperature dual-sensitive nucleolipid-containing stealth liposomes anchored with PEGylated AuNPs for triggering delivery of doxorubicin. Int. J. Pharm. 2022, 619, 121691. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Tu, J.; Wang, J.; Shajii, A.; Kong, N.; Feng, C.; Zhang, Y.; Yu, M.; Xie, T.; Bharwani, Z.; et al. Glutathione-responsive prodrug nanoparticles for effective drug delivery and cancer therapy. ACS Nano 2019, 13, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Z.; Liu, X.; Lian, M.; Peng, H.; Zhang, C. Design and evaluation of glutathione responsive glycosylated camptothecin nanosupramolecular prodrug. Drug Deliv. 2021, 28, 1903–1914. [Google Scholar] [CrossRef]
- Chen, R.; Ma, Z.; Xiang, Z.; Xia, Y.; Shi, Q.; Wong, S.C.; Yin, J. Hydrogen peroxide and glutathione dual redox-responsive nanoparticles for controlled DOX release. Macromol. Biosci. 2020, 20, e1900331. [Google Scholar] [CrossRef]
- Yoon, H.M.; Kang, M.S.; Choi, G.E.; Kim, Y.J.; Bae, C.H.; Yu, Y.B.; Jeong, Y.I. Stimuli-responsive drug delivery of doxorubicin using magnetic nanoparticle conjugated poly(ethylene glycol)-g-chitosan copolymer. Int. J. Mol. Sci. 2021, 22, 13169. [Google Scholar] [CrossRef]
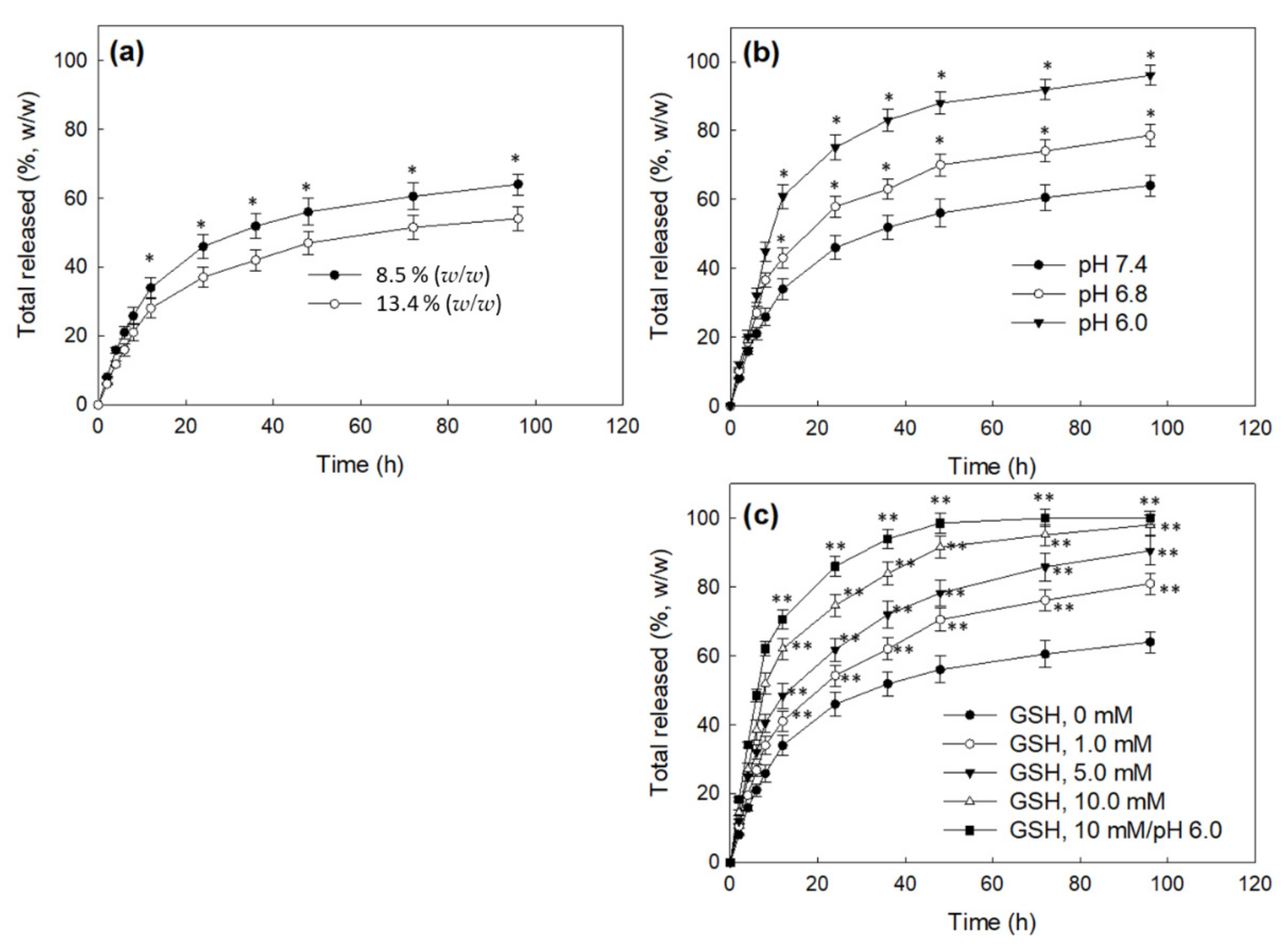

| Polymer/Drug Weight Ratio (mg/mg) | Drug Content (%, w/w) | Loading Efficiency (%, w/w) c | Particle Size (nm) | Polydispersity | |
|---|---|---|---|---|---|
| Theoretical a | Experimental b | ||||
| 40/0 | − | − | − | 120.5 ± 20.89 | 0.068 |
| 40/5 | 11.1 | 8.5 | 74.3 | 139.6 ± 24.94 | 0.075 |
| 40/10 | 20 | 13.4 | 61.9 | 160.5 ± 30.23 | 0.289 |
| IC50 (µg/mL) a | ||
|---|---|---|
| HuCC-T1 Cells | DOX-Resistant HuCC-T1 Cells | |
| DOX | 0.28 ± 0.013 | >10 |
| DOX-ChitoHISss NP | 0.32 ± 0.012 | 0.68 ± 0.024 |
| Emp NP | >100 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-I.; Lee, H.L.; Yun, J.-J.; Kim, J.; So, K.-H.; Jeong, Y.-I.; Kang, D.-H. pH and Redox-Dual Sensitive Chitosan Nanoparticles Having Methyl Ester and Disulfide Linkages for Drug Targeting against Cholangiocarcinoma Cells. Materials 2022, 15, 3795. https://doi.org/10.3390/ma15113795
Yang J-I, Lee HL, Yun J-J, Kim J, So K-H, Jeong Y-I, Kang D-H. pH and Redox-Dual Sensitive Chitosan Nanoparticles Having Methyl Ester and Disulfide Linkages for Drug Targeting against Cholangiocarcinoma Cells. Materials. 2022; 15(11):3795. https://doi.org/10.3390/ma15113795
Chicago/Turabian StyleYang, Ju-Il, Hye Lim Lee, Je-Jung Yun, Jungsoo Kim, Kyoung-Ha So, Young-IL Jeong, and Dae-Hwan Kang. 2022. "pH and Redox-Dual Sensitive Chitosan Nanoparticles Having Methyl Ester and Disulfide Linkages for Drug Targeting against Cholangiocarcinoma Cells" Materials 15, no. 11: 3795. https://doi.org/10.3390/ma15113795
APA StyleYang, J.-I., Lee, H. L., Yun, J.-J., Kim, J., So, K.-H., Jeong, Y.-I., & Kang, D.-H. (2022). pH and Redox-Dual Sensitive Chitosan Nanoparticles Having Methyl Ester and Disulfide Linkages for Drug Targeting against Cholangiocarcinoma Cells. Materials, 15(11), 3795. https://doi.org/10.3390/ma15113795






