Removing Pollutants from Sewage Waters with Ground Apricot Kernel Shell Material
Abstract
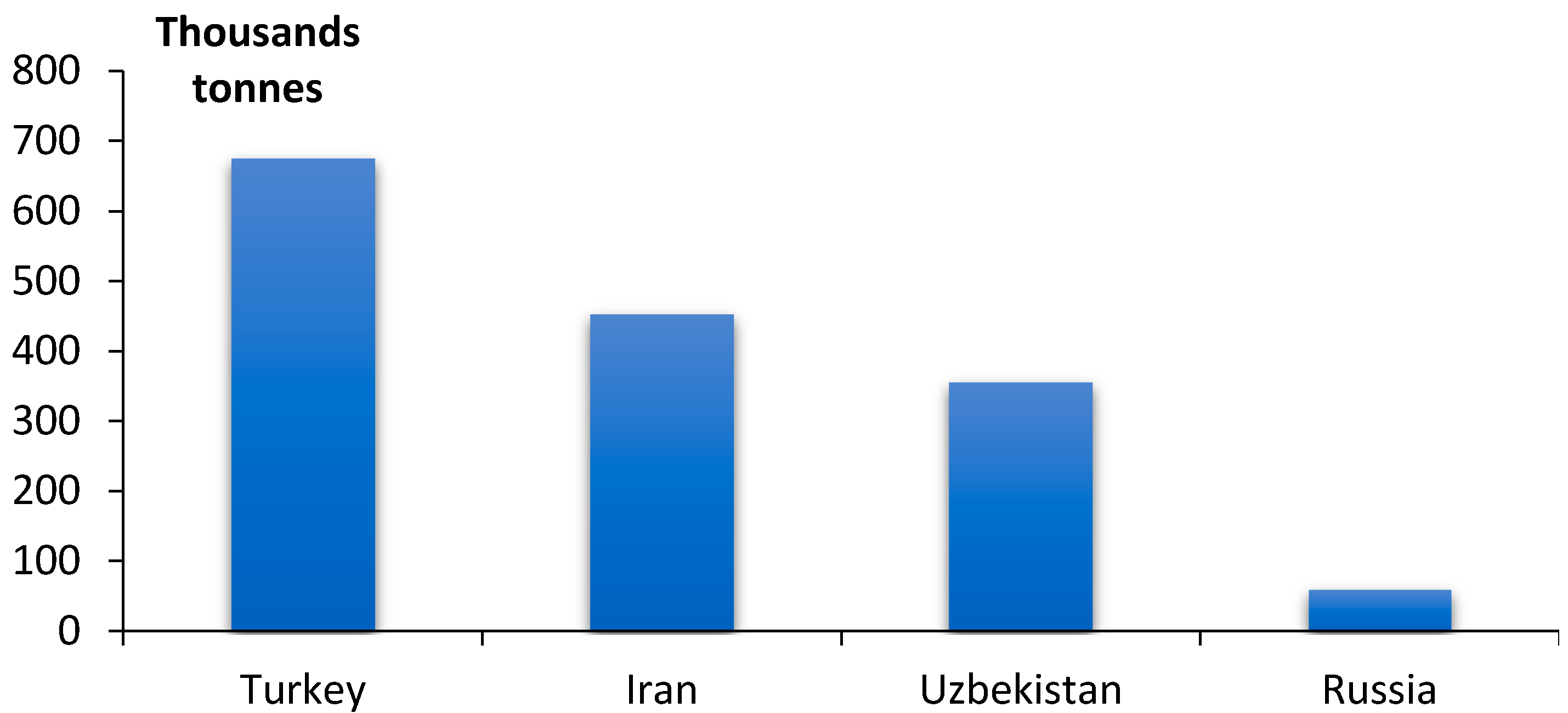
:1. Introduction
- -
- Analyze the ways to increase the sorption capacity of apricot kernel shell biomass;
- -
- Perform own microstructural and energy dispersive studies;
- -
- Improve the sorption properties of apricot kernel biomass;
- -
- Use the activated biomass of apricot kernel shells to remove heavy metal ions from aquatic environments;
- -
- Extract dyes from the aqueous media by the biomass of apricot the kernels;
- -
- Purify the aqueous media from pharmaceutical products and pesticides;
- -
- Extract the oil and oil products.
2. Materials and Methods
3. Results and Discussion
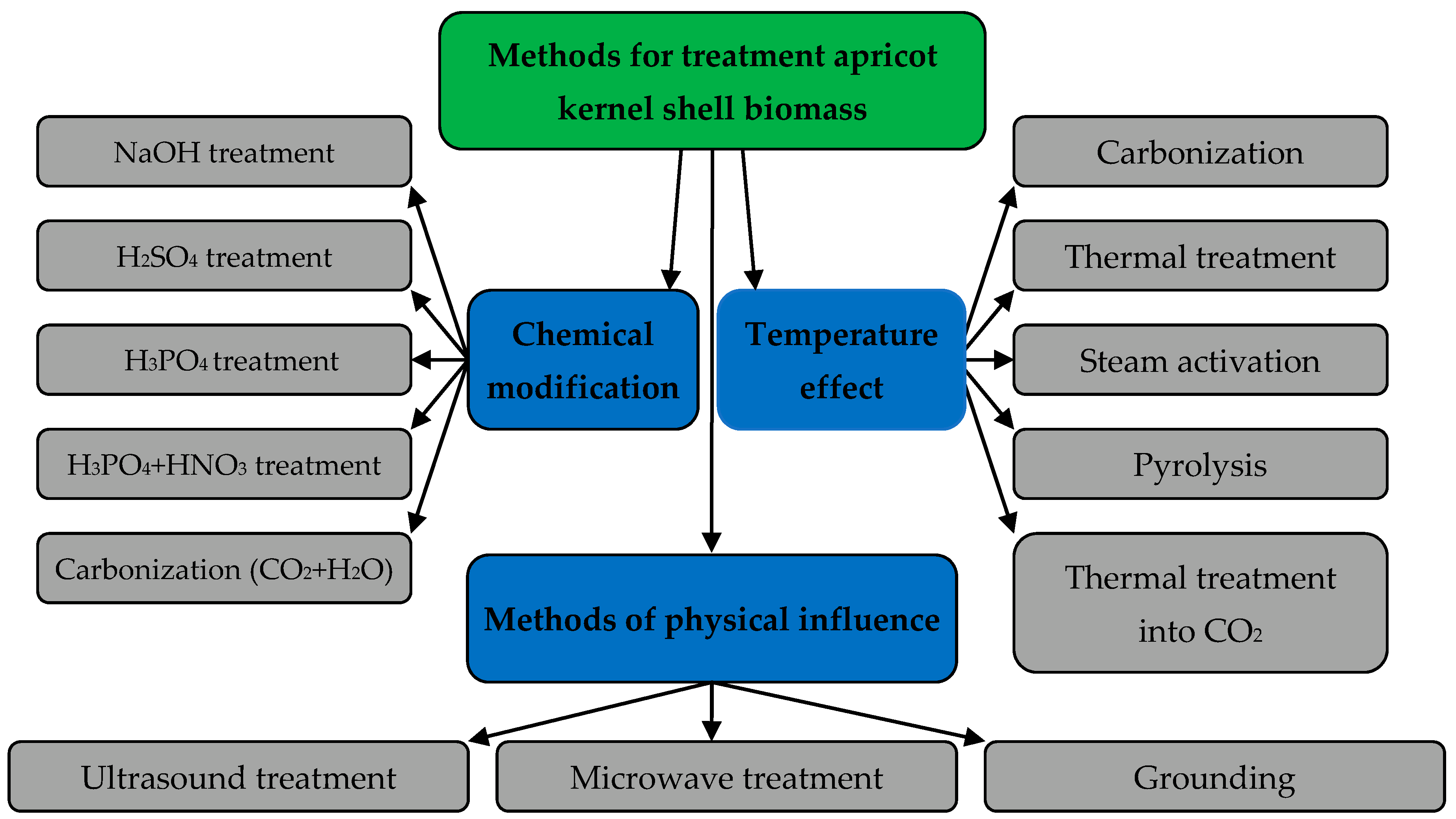
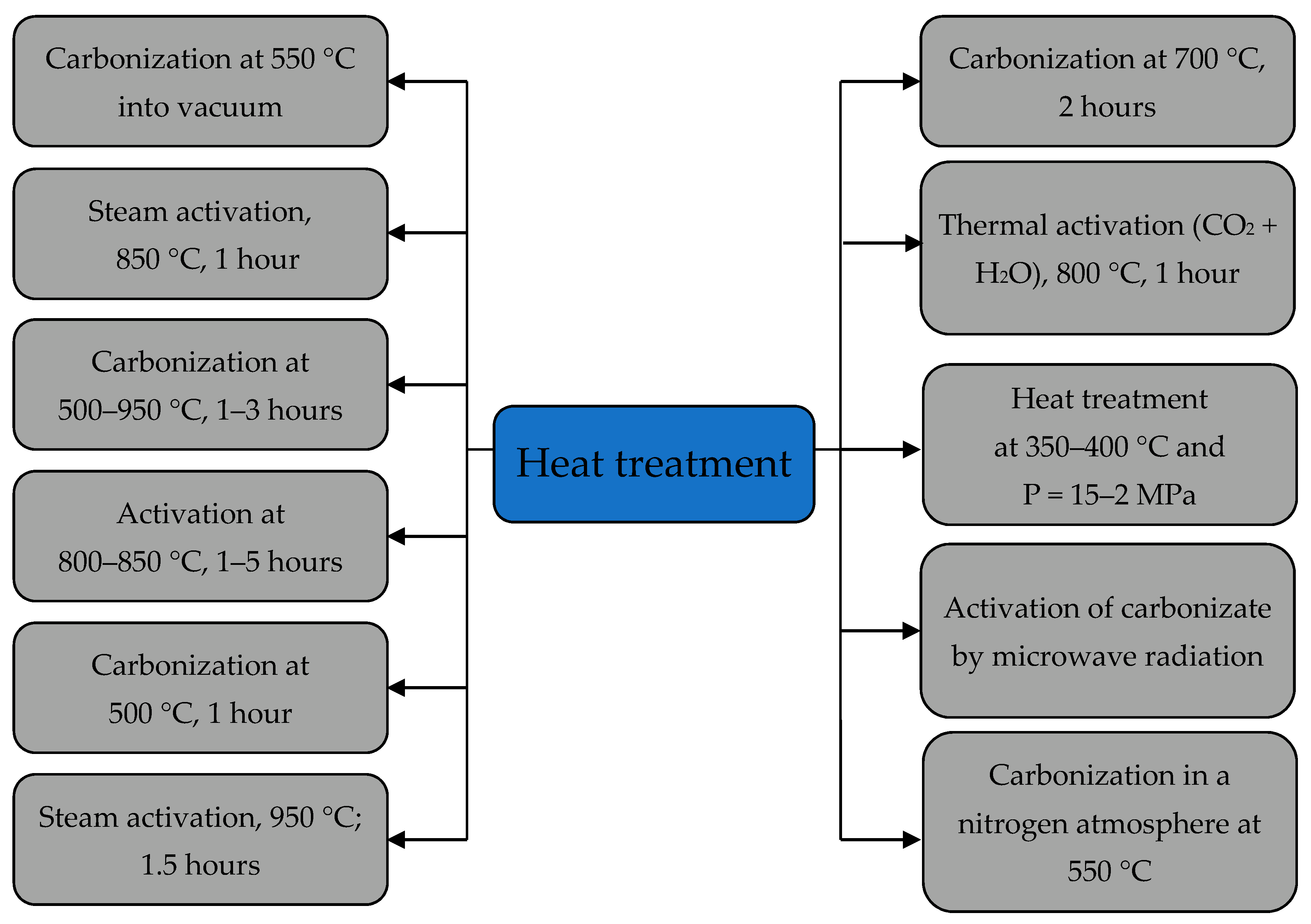
3.1. Ways to Increase the Sorption Capacity of the Apricot Kernel Shell Biomass
- -
- Thermal treatment at 250 °C;
- -
- Ultrasonic treatment of 200 W during 15 min with a frequency of 22 kHz;
- -
- Alkali treatment with NaOH solution on the sorption capacity of apricot kernel shells for Cu2+, Pb2+ and Zn2+ ions;
- -
- Treating the sorption material sample with microwave radiation of 300 W for 10 min.
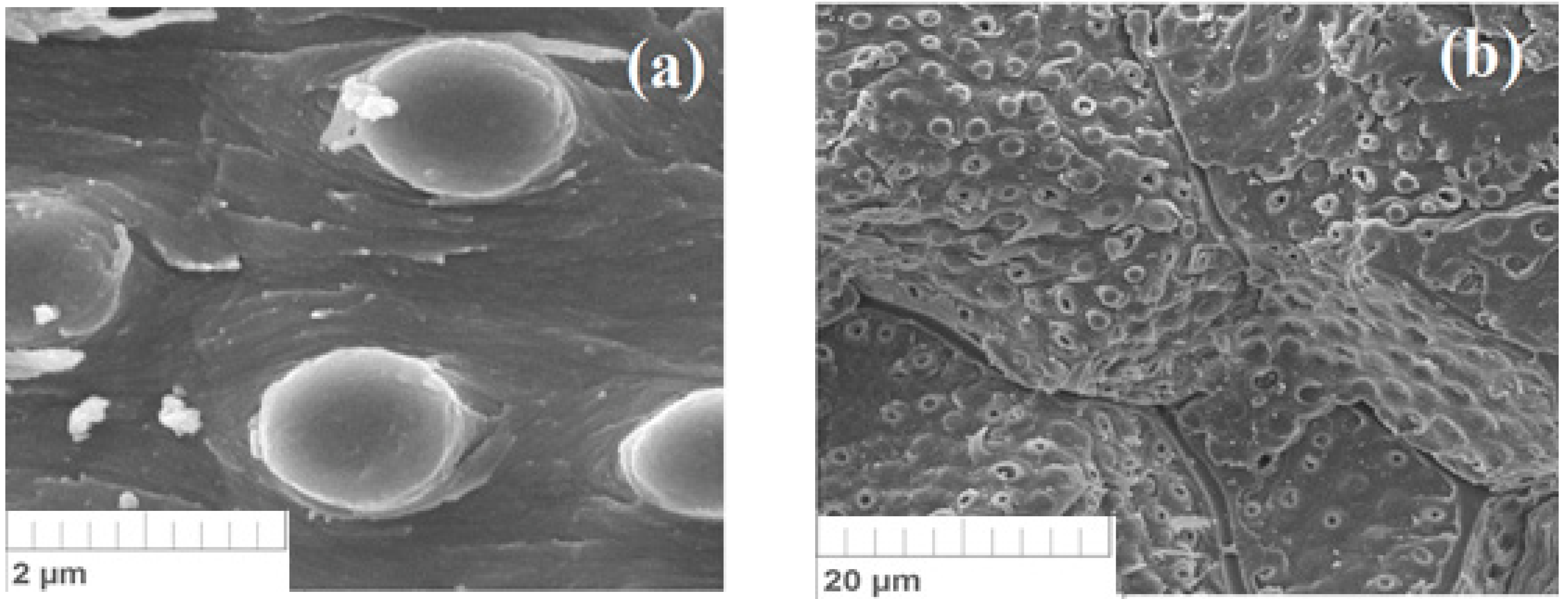
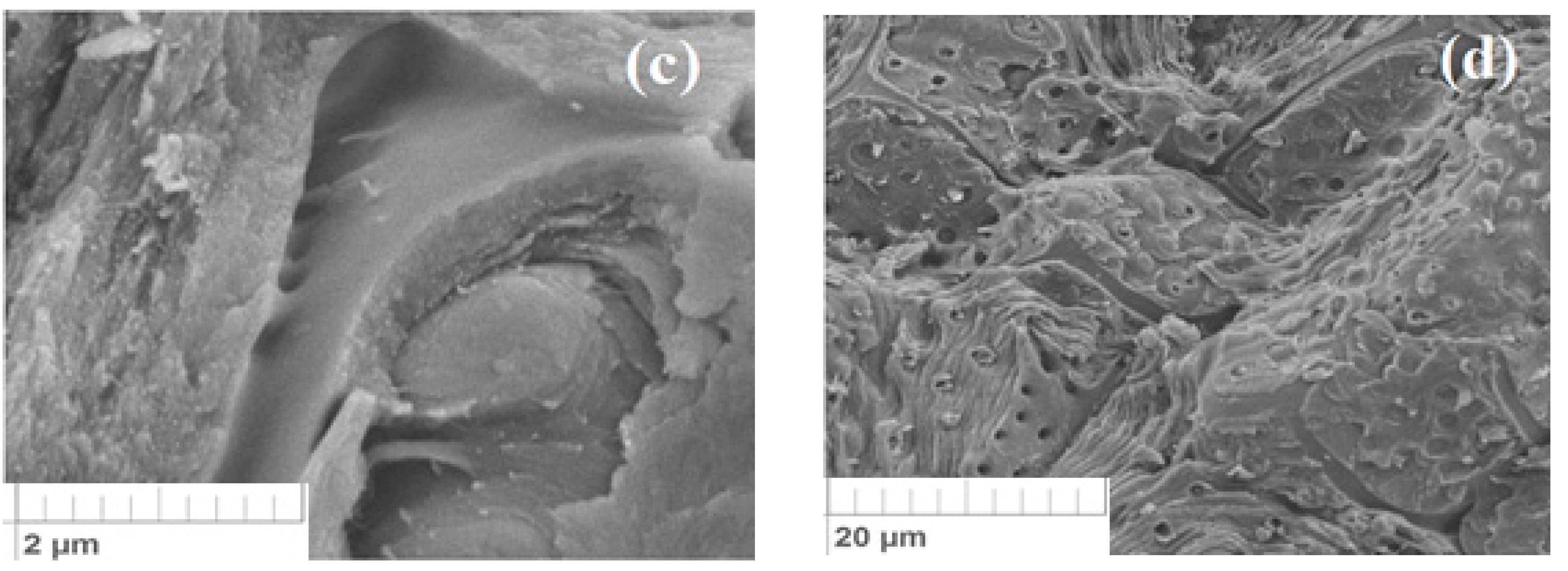
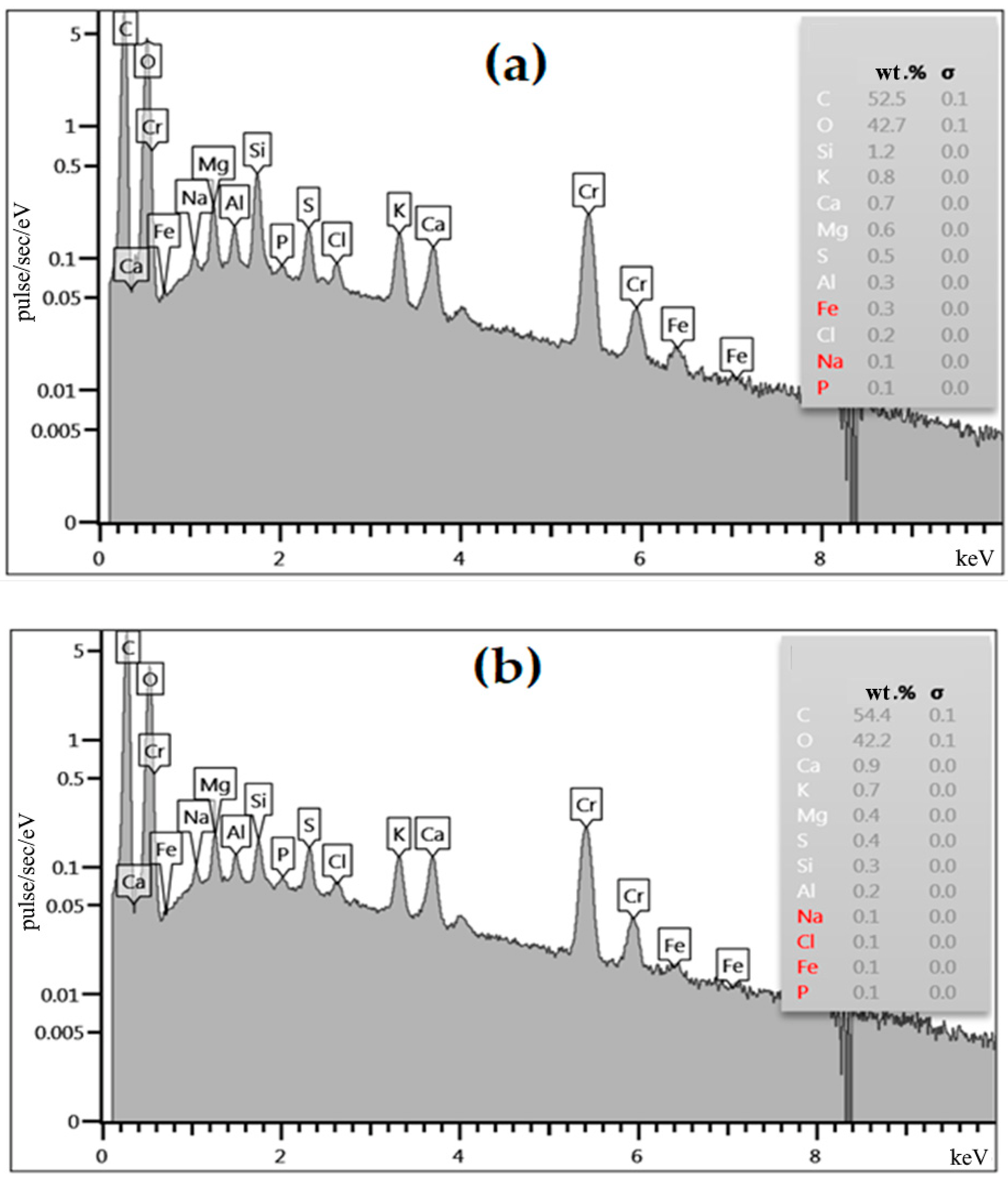
3.2. Own Microstructural Studies of Some Physicochemical Properties of the Apricot Kernel Shell Biomass
3.3. Improving the Sorption Properties of the Apricot Kernel Shell Biomass
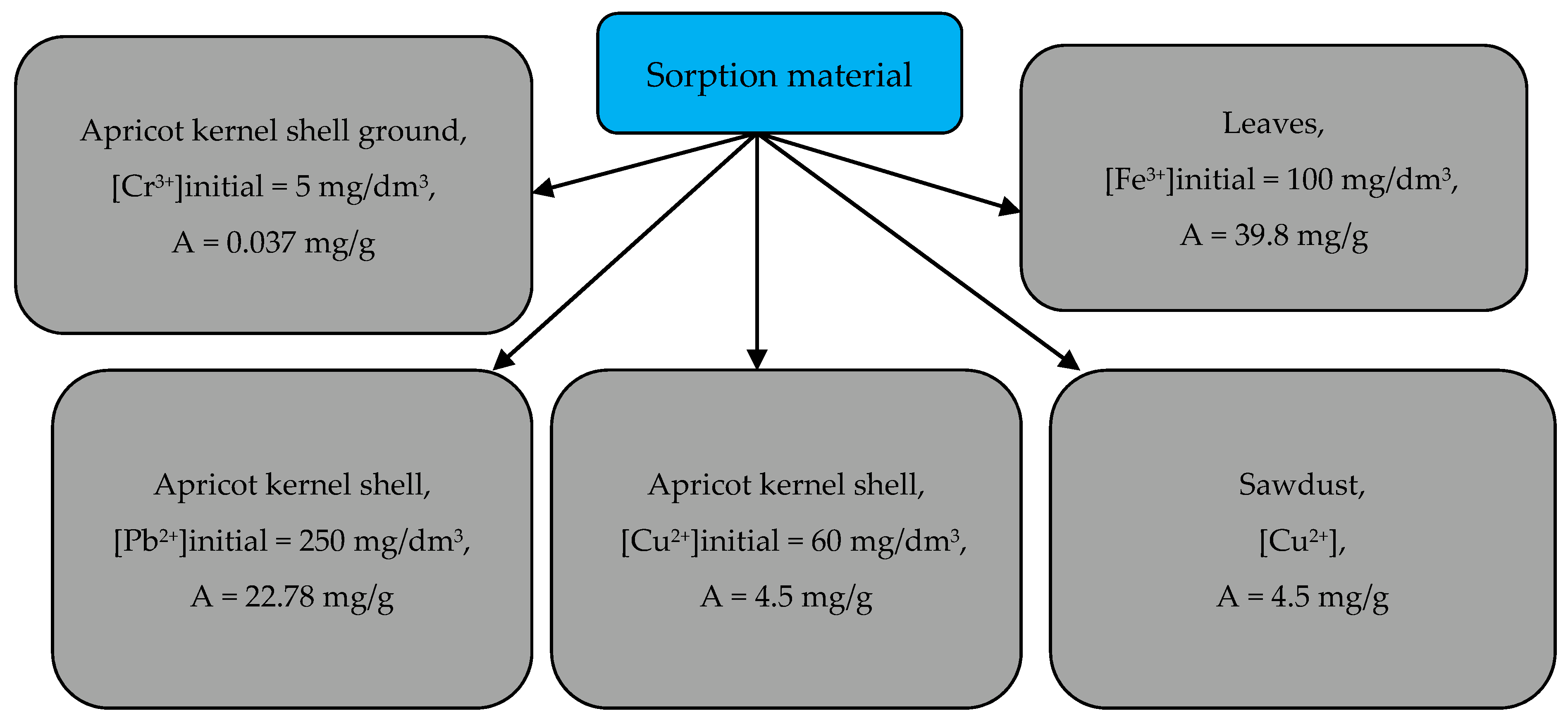
3.4. Removal of Heavy Metal Ions from Aqueous Media by Activated Biomass of Apricot Kernel Shells
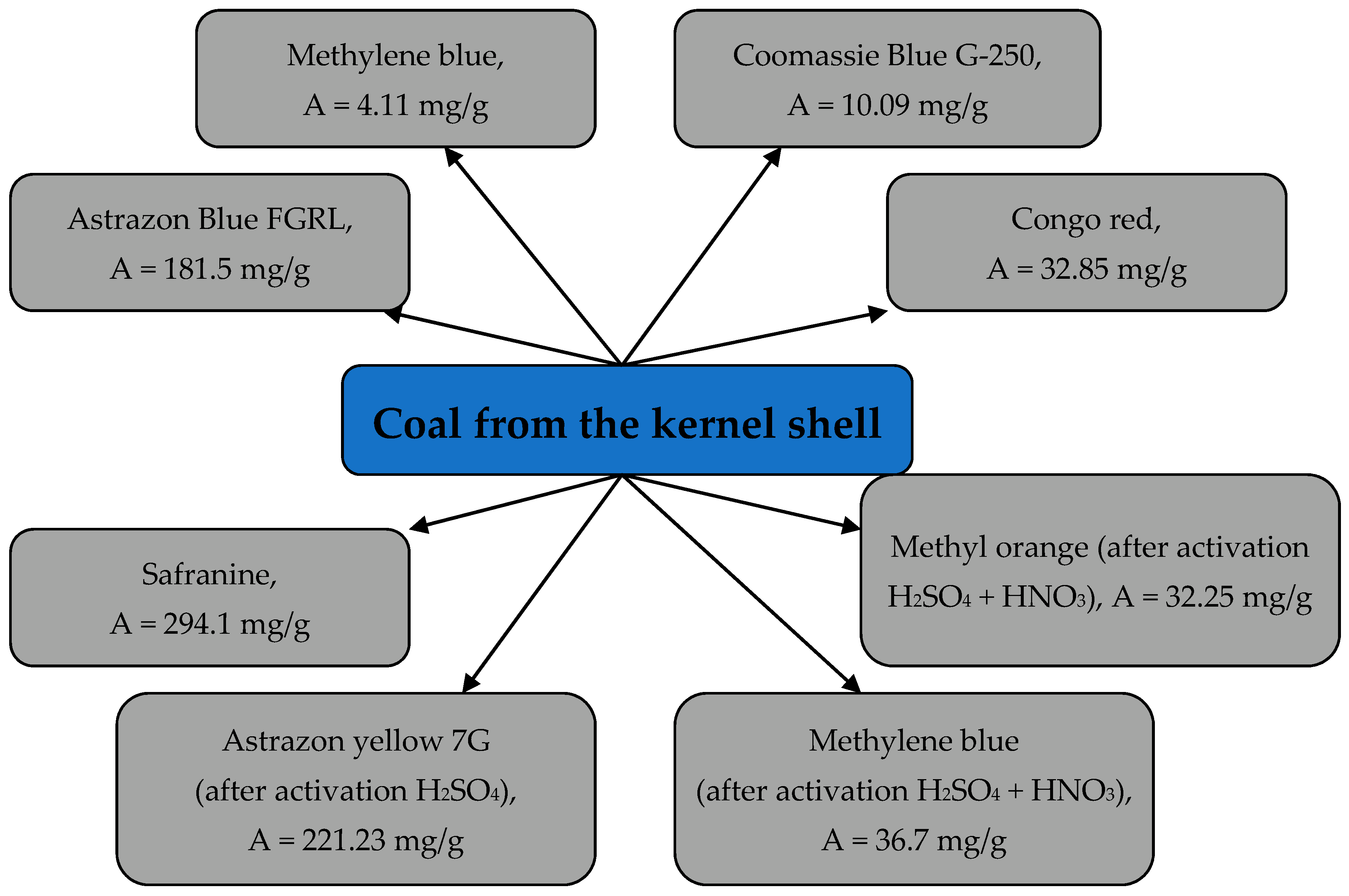
3.5. Extraction of Dyes from Aqueous Media by the Biomass of Apricot Kernel Shells
3.6. Purification of Aqueous Media from Pharmaceutical Products and Pesticides
3.7. Extraction of Petroleum and Its Products
3.8. Brief Comparison of the Treatment Methods
4. Conclusions
- -
- Various ways of increasing the sorption capacity of apricot kernel biomass was analyzed. It was established that heat treatment at 250 °C is the most effective method.
- -
- The author’s microstructural studies proved that the specific structure of the biomaterial allows the retention of molecules and ions of pollutants. In this case, the heat treatment of the material leads to an increase in the defectiveness of the surface, increasing the sorption properties for many substances.
- -
- Ground apricot kernels were tested for the removal of heavy metal ions and dyes from the aquatic environment. Sonication or chemicals can improve the sorption characteristics of crushed apricot kernel shells. The sorption characteristics of ground apricot kernels shells can be increased by ultrasonic treatment. Thus, the ultrasonic treatment of 200 W during 15 min with a frequency of 22 kHz helps to increase the maximum sorption capacity from 6.6 mg/g to 9.9 mg/g for Cr6+ ions.
- -
- The influence of alkali treatment with an NaOH solution on the sorption capacity of apricot kernel shells for Cu2+, Pb2+ and Zn2+ ions was studied. It was determined that the alkali treatment increases the maximum sorption capacity from 4.83, 24.53 and 5.42 mg/g to 12.25, 46.45 and 8.73 mg/g, respectively.
- -
- The main way to use apricot kernels is the production of activated carbons. The choice of this material is explained by the fact that the shell of an apricot kernels is a low-ash material, and the high true density determines the possibility of obtaining solid adsorbents on its basis. In addition, this raw material already has a natural system of pores and channels in its structure, which can be developed through various carbonization and subsequent activation methods. Depending on their carbonization and activation parameters, activated carbons can have various surface areas ranging from 25 m2/g to 1200 m2/g. It is necessary to research the other components and waste products of apricot biomass processing for usage as sorption materials. The possibility of improving the sorption characteristics of apricot biomass with chemical or physical-chemical treatment was demonstrated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krebsz, M.; Pasinszki, T.; Tung, T.T.; Nine, J.; Losic, D. Multiple applications of bio-graphene foam for efficient chromate ion removal and oil-water separation. Chemosphere 2021, 263, 127790. [Google Scholar] [CrossRef] [PubMed]
- Karić, N.; Maia, A.S.; Teodorović, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.; Đolić, M. Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in)organic pollutants in wastewater treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Alekseeva, A.A.; Shaikhiev, I.G.; Fazullin, D.D.; Kharlyamov, D.A.; Mavrin, G.V.; Stepanova, S.V.; Shaimardanova, A.S. The use of leaves of different tree species as a sorption material for extraction of heavy metal ions from aqueous media. Int. J. Pharm. Technol. 2016, 8, 14375–14391. [Google Scholar]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B.L. Application of Casuarina equisetifolia needle for the removal of methylene blue and malachite green dyes from aqueous solution. Alex. Eng. J. 2015, 54, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized biochar obtained from pine needles as a novel adsorbent to remove caffeine from aqueous solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Jeon, C.; Solis, K.L.; An, H.R.; Hong, Y.; Igalavithana, A.D.; Ok, Y.S. Sustainable removal of Hg(II) by sulfur-modified pine-needle biochar. J. Hazard. Mater. 2020, 388, 122048. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiate. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Değirmen, G.; Kılıç, M.; Çepelioğullar, Ö.; Pütün, A.E. Removal of copper(II) and cadmium(II) ions from aqueous solutions by biosorption onto pine cone. Water Sci. Technol. 2012, 66, 564–572. [Google Scholar] [CrossRef]
- Deniz, F. Color removal from aqueous solutions of metal-containing dye using pine cone. Desalination Water Treat. 2013, 51, 4573–4581. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit peel waste as a novel low-cost bio adsorbent. Rev. Chem. Eng. 2015, 31, 361–381. [Google Scholar] [CrossRef]
- Ay, Ç.Ö.; Özcan, A.S.; Erdoğan, Y.; Özcan, A. Characterization of Punica granatum L. peels and quantitatively determination of its biosorption behavior towards lead(II) ions and Acid Blue 40. Colloids Surf. B 2012, 100, 197–204. [Google Scholar]
- Mallampati, R.; Xuanjun, L.; Adin, A.; Valiyaveettil, S. Fruit peels as efficient renewable adsorbents for removal of dissolved heavy metals and dyes from water. ACS Sustain. Chem. Eng. 2015, 3, 1117–1124. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Yeddou, A.R. Potential of sawdust materials for the removal of dyes and heavy metals: Examination of isotherms and kinetics. Desalination Water Treat. 2016, 57, 24019–24034. [Google Scholar] [CrossRef]
- Bouaziz, I.; Hamza, M.; Sellami, A.; Abdelhedi, R.; Savaii, A.; Groenen Serrano, K. New hybrid process combining adsorption on sawdust and electroxidation using a BDD anode for the treatment of dilute wastewater. Sep. Purif. Technol. 2017, 175, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chouchene, A.; Jeguirim, M.; Trouvé, G.; Favre-Reguillon, A.; Le Buzit, G. Combined process for the treatment of olive oil mill wastewater: Absorption on sawdust and combustion of the impregnated sawdust. Bioresour. Technol. 2010, 101, 6962–6971. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Hernández-Montoya, V. Characterization of mango pit as raw material in the preparation of activated carbon for wastewater treatment. Biochem. Eng. J. 2007, 36, 230–238. [Google Scholar] [CrossRef]
- Eder, S.; Müller, K.; Azzari, P.; Arcifa, A.; Nyström, L. Mass Transfer Mechanism and Equilibrium Modelling of Hydroxytyrosol Adsorption on Olive Pit–Derived Activated Carbon. Chem. Eng. J. 2021, 404, 126519. [Google Scholar] [CrossRef]
- Youssef, A.M.; Radwan, N.R.E.; Abdel-Gawad, I.; Singer, G.A.A. Textural properties of activated carbons from apricot kernels. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 143–151. [Google Scholar] [CrossRef]
- Souza, I.P.A.F.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. Preparation of biosorbents from the Jatoba (Hymenaea courbaril) fruit shell for removal of Pb(II) and Cd(II) from aqueous solution. Environ. Monit. Assess. 2017, 189, 632–644. [Google Scholar] [CrossRef]
- Halysh, V.; Sevastyanova, O.; Riazanova, A.V.; Pasalskiy, B.; Budnyak, T.; Lindström, M.E.; Kartel, M. Walnut shells as a potential low-cost lignocellulosic sorbent for dyes and metal ions. Cellulose 2018, 25, 4729–4742. [Google Scholar] [CrossRef]
- Omar, E.A.S.; Neama, A.R.; Maha, M.E. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar]
- Hagen, L.S.; Khadari, B.; Lambert, P.; Audergon, J.-M. Genetic diversity in apricot revealed by AFLP markers: Species and cultivar comparisons. Theor. Appl. Genet. 2002, 105, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions: A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Zaurov, D.E.; Molnar, T.J.; Eisenman, S.W.; Ford, T.M.; Mavlyanova, R.F.; Capik, J.M.; Funk, C.R.; Goffreda, J.C. Genetic Resources of Apricots (Prunus armeniaca L.) in Central Asi. HortScience 2013, 48, 681–691. [Google Scholar] [CrossRef]
- Khazaei, I.; Aliabad, M.; Mosavian, H.T.H. Use of agricultural waste for removal of Cr(VI) from aqueous solution. Iran. J. Chem. Eng. 2011, 8, 11–23. [Google Scholar]
- Kalipci, E.; Namal, O.O. Removal of Cr(VI) using a novel adsorbent modification. Ultrasonic method with apricot kernel shells. Environ. Prot. Eng. 2018, 44, 79–93. [Google Scholar] [CrossRef]
- Šoštaric, T.; Petrovic, M.; Milojkovic, J.; Lacnjevac, C.; Cosovic, A.; Stanojevic, M.; Stojanovic, M. Application of apricot kernel waste from fruit processing industry in environmental cleanup: Copper biosorption study. Fruits 2015, 70, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Milojković, J.; Mihajlović, M.; Šoštarić, T.; Lopičić, Z.; Petrović, M.; Lačnjevac, Č.; Stojanović, M. Ispitivanje efikasnosti različitih sorpcionih materijala za uklanjanje Cu(II) jona iz vodenog rastvora. Zaštita Materijala 2014, 55, 25–31. [Google Scholar]
- Petrovic, M.S.; Šoštaric, T.D.; Pezo, L.L.; Stankovic, S.M.; Lačnjevac, Č.M.; Milojković, J.T.; Stojanović, M.D. Usefulness of ANN-based model for copper removal from aqueous solutions using agro industrial waste materials. Chem. Ind. Chem. Eng. Q. 2015, 21, 249–259. [Google Scholar] [CrossRef]
- Šoštaric, T.D.; Petrovic, M.S.; Petrovic, E.T.; Milojković, J.T.; Kragovic, M.M.; Lačnjevac, Č.M.; Stojanović, M.D. Uticaj hemijske modifikacije na adsorpcione karakteristike biosorbenta na bazi koštica kajsija. Zaštita Materijala 2015, 56, 321–328. [Google Scholar]
- Milojković, J.; Stojanović, M.; Mihajlović, M.; Lopičić, Z.; Šoštarić, T.; Petrović, M.; Petrovic, E. Pb (II) biosorption by selected waste biomass. Zaštita Materijala 2016, 57, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Kahraman, S.; Dogan, N.; Erdemoglu, S. Use of various agricultural wastes for the removal of heavy metal ions. Int. J. Environ. Pollut. 2008, 34, 275–284. [Google Scholar] [CrossRef]
- Šoštarić, T.D.; Petrović, M.S.; Pasto, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Namal, O.O.; Kalipci, E. Adsorption kinetics of methylene blue using alkali and microwave-modified apricot kernels. Sep. Sci. Technol. 2019, 54, 1722–1738. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sust. Energ. Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sust. Energ. Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Paraskeva, P.; Kalderis, D.; Diamadopoulos, E. Production of activated carbon from agricultural by-products. J. Chem. Technol. Biotechnol. 2008, 83, 581–592. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Oesterle, P.; Wurzer, C.; Mašek, O.; Jansson, S. Removal of contaminants of emerging concern from multicomponent systems using carbon dioxide activated biochar from lignocellulosic feedstocks. Bioresour. Technol. 2021, 340, 12556. [Google Scholar] [CrossRef]
- Jayakumar, A.; Wurzer, C.; Soldatou, S.; Edwards, C.; Mašek, O. New directions and challenges in engineering biologically-enhanced biochar for biological water treatment. Sci. Total Environ. 2021, 796, 148977. [Google Scholar] [CrossRef]
- Alslaibi, T.M.; Abustan, I.; Ahmad, M.A.; Foul, A.A. A review: Production of activated carbon from agricultural byproducts via conventional and microwave heating. J. Chem. Technol. Biotechnol. 2013, 88, 1183–1190. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit kernels and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Gergova, K.; Eser, S. Effects of activation method on the pore structure of activated carbons from apricot kernels. Carbon 1996, 34, 879–888. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot kernels and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- Usenkulova, S.Z.; Satayev, M.I.; Samonin, V.V. Production of active carbons from apricot pit shells by thermal activation in the mixture of carbon dioxide and water vapors. Biosci. Biotechnol. Res. Asia 2016, 13, 1319–1325. [Google Scholar]
- Philip, C.A.; Girgis, B.S. Adsorption characteristics of microporous carbons from apricot kernels activated by phosphoric acid. J. Chem. Technol. Biotechnol. 1996, 67, 248–254. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A.; Samra, S.E.; Girgis, B.S. Adsorption characteristics of activated carbons obtained from corncobs. Colloids Surf. A Physicochem. Eng. Asp. 2001, 180, 209–221. [Google Scholar] [CrossRef]
- Pap, S.; Knudsen, T.Š.; Radonić, J.; Maletić, S.; Igić, S.M.; Sekulić, M.T. Utilization of fruit processing industry waste as green activated carbon for the treatment of heavy metals and chlorophenols contaminated water. J. Clean. Prod. 2017, 162, 958–972. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K.; Patelski, P.; Dziekońska-Kubczak, U.; Jusel, T. Treatment with activated carbon and other adsorbents as an effective method for the removal of volatile compounds in agricultural distillates. Food. Addit. Contam. Part A 2017, 34, 714–727. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Abd Hamid, S.B.; Das, R.; Hasan, M.R.; Zain, S.M.; Khalid, K.; Uddin, M.N. Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. BioResources 2013, 8, 6523–6555. [Google Scholar] [CrossRef]
- Mechati, F.; Bouchelta, C.; Medjram, M.S.; Benrabaa, R.; Ammouchi, N. Effect of hard and soft structure of different biomasses on the porosity development of activated carbon prepared under N2/microwave radiations. J. Environ. Chem. Eng. 2015, 3, 1928–1938. [Google Scholar] [CrossRef]
- Khalil, L.B. Porosity characteristics of chars derived from different lignocellulosic materials. Adsorp. Sci. Technol. 1999, 17, 729–739. [Google Scholar] [CrossRef]
- Jankovic, B.; Manic, N.; Dodevski, V.; Radovic, I.; Pijovic, M.M.; Katnić, Đ.; Tasic, G.S. Physico-chemical characterization of carbonized apricot kernel shell as precursor for activated carbon preparation in clean technology utilization. J. Clean. Prod. 2019, 236, 117614. [Google Scholar] [CrossRef]
- Moo-Tun, N.M.; Valadez-González, A.; Uribe-Calderon, J.A. Thermo-oxidative aging of low density polyethylene blown films in presence of cellulose nanocrystals and a pro-oxidant additive. Polym. Bull. 2018, 75, 3149–3169. [Google Scholar] [CrossRef]
- Sentorun-Shalaby, C.; Ucak-Astarlioglu, M.G.; Artok, L.; Sarici, C. Preparation and characterization of activated carbons by one-step steam pyrolysis/activation from apricot kernels. Microporous Mesoporous Mater. 2006, 88, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Amran, M.; Murali, G.; Fediuk, R.; Vatin, N.; Vasilev, Y.; Abdelgader, H. Palm Oil Fuel Ash-Based Eco-Efficient Concrete: A Critical Review of the Short-Term Properties. Materials 2021, 14, 332. [Google Scholar] [CrossRef]
- Soleimani, M.; Kaghazchi, T. Adsorption of gold ions from industrial wastewater using activated carbon derived from hard shell of apricot kernels—An agricultural waste. Bioresour. Technol. 2008, 99, 5374–5383. [Google Scholar] [CrossRef]
- Soleimani, M.; Kaghazchi, T. Activated hard shell of apricot kernels: A promising adsorbent in gold recovery. Chin. J. Chem. Eng. 2008, 16, 112–118. [Google Scholar] [CrossRef]
- Abbas, M.; Kaddour, S.; Trari, M. Kinetic and equilibrium studies of cobalt adsorption on apricot kernel activated carbon. J. Ind. Eng. Chem. 2014, 20, 745–751. [Google Scholar] [CrossRef]
- Yüksel, S.; Orhan, R. The removal of Cr(VI) from aqueous solution by activated carbon prepared from apricot, peach kernel and almond shell mixture in a fixed-bed column. Arab. J. Sci. Eng. 2019, 44, 5345–5357. [Google Scholar] [CrossRef]
- Nikhil, N. Removal of Chromium Using Activated Almond and Apricot Shells. Master’s Thesis, Delhi Technological University, Delhi, India, 2017; p. 71. [Google Scholar]
- Özçimen, D.; Ersoy-Meriçboyu, A. Adsorption of Copper(II) Ions onto hazelnut shell and apricot kernel activated carbons. Adsorp. Sci. Technol. 2010, 28, 327–340. [Google Scholar] [CrossRef]
- Savova, D.; Petrov, N.; Yardim, M.F.; Ekinci, E.; Budinova, T.; Razvigorova, M.; Minkova, V. The influence of the texture and surface properties of carbon adsorbents obtained from biomass products on the adsorption of manganese ions from aqueous solution. Carbon 2003, 41, 1897–1903. [Google Scholar] [CrossRef]
- Mouni, L.; Merabet, D.; Bouzaza, A.; Belkhiri, L. Adsorption of Pb(II) from aqueous solutions using activated carbon developed from Apricot kernel. Desalination 2011, 276, 148–153. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Zouggaghe, F.; Tafer, M. Removal of Pb (II) from aqueous solution by adsorption using activated carbon developed from Apricot kernel: Equilibrium and kinetic. Desalination Water Treat. 2013, 8, 6412–6419. [Google Scholar]
- Mouni, L.; Belkhiri, L.; Tafer, M.; Zouggaghe, F.; Kadmi, Y. Studies on the removal of Pb(II) from wastewater by activated carbon developed from Apricot kernel activated with sulphuric acid. Mor. J. Chem. 2014, 2, 452–456. [Google Scholar]
- Sabermahani, F.; Mahani, N.M.; Noraldiny, M. Removal of thallium (I) by activated carbon prepared from apricot nucleus shell and modified with rhodamine B. Toxin Rev. 2017, 36, 154–160. [Google Scholar] [CrossRef]
- Vojoudi, H.; Badiei, A.; Banaei, A.; Bahar, S.; Karimi, S.; Ziarani, G.M.; Ganjali, M.R. Extraction of gold, palladium and silver ions using organically modified silica-coated magnetic nanoparticles and silica gel as a sorbent. Mikrochim. Acta 2017, 184, 3859–3866. [Google Scholar] [CrossRef]
- Kaušpėdienė, D.; Gefenienė, A.; Ragauskas, R.; Pakštas, V. Comparative investigation of plain and silver impregnated activated carbons for the removal of cyanide from basic aqueous solutions in the batch process. Chem. Eng. Commun. 2017, 204, 1258–1269. [Google Scholar] [CrossRef]
- El-Saharty, A.; Mahmoud, S.N.; Manjood, A.H.; Nassar, A.A.H.; Ahme, A.M. Effect of apricot kernel activated carbon adsorbent on the removal of toxic heavy metals ions from aqueous solutions. Int. J. Ecotoxicol. Ecobiol. 2018, 3, 51–62. [Google Scholar]
- Pap, S.; Bezanovic, V.; Radonic, J.; Babic, A.; Saric, S.; Adamovic, D.; Sekulic, M.T. Synthesis of highly-efficient functionalized biochars from fruit industry waste biomass for the removal of chromium and lead. J. Mol. Liq. 2018, 268, 315–325. [Google Scholar] [CrossRef]
- Sekulić, M.T.; Pap, S.; Stojanović, Z.; Bošković, N.; Radonić, J.; Knudsen, T.Š. Efficient removal of priority, hazardous priority and emerging pollutants with Prunus armeniaca functionalized biochar from aqueous wastes: Experimental optimization and modeling. Sci. Total Environ. 2018, 613–614, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Tsibranska, I.; Hristova, E. Use of activated carbons from apricot kernels for heavy metals removal. Comptes Rendus Acad. Bulg. Des Sci. 2011, 64, 831–838. [Google Scholar]
- Tsibranska, I.; Hristova, E. Comparison of different kinetic models for adsorption of heavy metals onto activated carbon from apricot kernels. Bulg. Chem. Commun. 2011, 43, 370–377. [Google Scholar]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot kernel. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Tsibranska, I.; Hristova, E. Modelling of heavy metal adsorption into activated carbon from apricot kernels in fluidized bed. Chem. Eng. Process. 2010, 49, 1122–1127. [Google Scholar] [CrossRef]
- Kobya, M.; Demirba, S.E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot kernel. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Nguyen, X.C.; Nguyen, T.T.H.; Nguyen, T.H.C.; Van Le, Q.; Vo, T.Y.B.; Tran, T.C.P.; La, D.D.; Gopalakrishnan, K.; Nguyen, V.K.; Chang, S.W.; et al. Sustainable carbonaceous biochar adsorbents derived from agro-wastes and invasive plants for cation dye adsorption from water. Chemosphere 2021, 282, 131009. [Google Scholar] [CrossRef]
- Gergova, K.; Petrov, N.; Eser, S. Adsorption properties and microstructure of activated carbons produced from agricultural by-products by steam pyrolysis. Carbon 1994, 1994, 693–702. [Google Scholar] [CrossRef]
- Adhami, S.; Ghorbanpoor, H.; Azak, B.; Kapucu, S.; Nurbas, M.; Avci, H. A novel approach for water treatment by using activated carbon: Apricot kernel shell. J. Eng. Archit. Fac. Eskiseh. Osman. Univ. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Jabir, H.A.; Abid, S.R.; Murali, G.; Ali, S.H.; Klyuev, S.; Fediuk, R.; Vatin, N.; Promakhov, V.; Vasilev, Y. Experimental Tests and Reliability Analysis of the Cracking Impact Resistance of UHPFRC. Fibers 2020, 8, 74. [Google Scholar] [CrossRef]
- Karagozoglu, B.; Tasdemir, M.; Demirbas, E.; Kobya, M. The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: Kinetic and equilibrium studies. J. Hazard. Mater. 2007, 147, 297–306. [Google Scholar] [CrossRef]
- Abbas, M.; Cherfi, A.; Kaddour, S.; Aksil, T.; Trari, M. Kinetic and Equilibrium Studies of Coomassie Blue G-250 Adsorption on Apricot Kernel Activated Carbon. J. Environ. Anal. Toxicol. 2015, 5, 8. [Google Scholar]
- Abbas, M.; Trari, M. Kinetic, equilibrium and thermodynamic study on the removal of Congo Red from aqueous solutions by adsorption on to apricot kernel. Process Saf. Environ. Prot. 2015, 98, 424–436. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Equilibrium and isotherm modeling of toxic dye adsorption onto modified apricot kernel. Algerian J. Res. Technol. 2017, 2, 39–49. [Google Scholar]
- Belaroui, K.; Seghier, A.; Hadjel, M. Synthesis of activated carbon based on apricot kernels for wastewater treatment. Desalination Water Treat. 2014, 52, 1422–1433. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Lesovik, V.S.; Mochalov, A.V.; Otsokov, K.A.; Lashina, I.V.; Timokhin, R.A. Composite binders for concrete of protective structures. Mag. Civ. Eng. 2018, 82, 208–218. [Google Scholar]
- Demirbas, E.; Kobya, M.; Sulak, M.T. Adsorption kinetics of a basic dye from aqueous solutions onto apricot kernel activated carbon. Bioresour. Technol. 2008, 99, 5368–5373. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Smoliakov, A.K.; Timokhin, R.A.; Batarshin, V.O.; Yevdokimova, Y.G. Using thermal power plants waste for building materials. IOP Conf. Ser. Earth Environ. Sci. 2018, 87, 092010. [Google Scholar] [CrossRef] [Green Version]
- Makul, N.; Fediuk, R.; Amran, M.; Zeyad, A.M.; Murali, G.; Vatin, N.; Klyuev, S.; Ozbakkaloglu, T.; Vasilev, Y. Use of Recycled Concrete Aggregates in Production of Green Cement-Based Concrete Composites: A Review. Crystals 2021, 11, 232. [Google Scholar] [CrossRef]
- Fediuk, R.; Smoliakov, A.; Muraviov, A. Mechanical properties of fiber-reinforced concrete using composite binders. Adv. Mater. Sci. Eng. 2017, 2017, 2316347. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wua, Z.; Wu, Z.; Ye, B. Adsorption behaviors of atrazine and Cr(III) onto different activated carbons in single and co-solute systems. Powder Technol. 2018, 329, 207–216. [Google Scholar] [CrossRef]
- Chernysheva, N.; Lesovik, V.; Fediuk, R.; Vatin, N. Improvement of Performances of the Gypsum-Cement Fiber Reinforced Composite (GCFRC). Materials 2020, 13, 3847. [Google Scholar] [CrossRef] [PubMed]
- Marzbali, M.H.; Esmaieli, M.; Abolghasemi, H.; Marzbalic, M.H. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nutshells: A batch study. Process Saf. Environ. Prot. 2016, 102, 700–709. [Google Scholar] [CrossRef]
- De Azevedo, A.R.G.; Klyuev, S.; Marvila, M.T.; Vatin, N.; Alfimova, N.; De Lima, T.E.S.; Fediuk, R.; Olisov, A. Investigation of the Potential Use of Curauá Fiber for Reinforcing Mortars. Fibers 2020, 8, 69. [Google Scholar] [CrossRef]
- Ani, J.U.; Akpomie, K.G.; Okoro, U.C.; Aneke, L.E.; Onukwuli, O.D.; Ujam, O.T. Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fediuk, R.; Yushin, A. Composite binders for concrete with reduced permeability. IOP Conf. Ser. -Mater. Sci. Eng. 2016, 116, 012021. [Google Scholar] [CrossRef]
- Abid, S.R.; Murali, G.; Amran, M.; Vatin, N.; Fediuk, R.; Karelina, M. Evaluation of Mode II Fracture Toughness of Hybrid Fibrous Geopolymer Composites. Materials 2021, 14, 349. [Google Scholar] [CrossRef]
- Volodchenko, A.A.; Lesovik, V.S.; Cherepanova, I.A. Peculiarities of non-autoclaved lime wall materials production using clays. In Proceedings of the International Conference on Mechanical Engineering, Automation and Control Systems, Tomsk, Russia, 4–6 December 2017; Volume 327. [Google Scholar]
- Rouissi, C. Valorisation des Coproduits d’Abricot (Prunus armeniaca L.) de la Wilaya de Batna. Master’s Thesis, Université Echahid Hamma Lakhdar, Echahid Hamma Lakhdar University, El Oued, People’s Democratic Republic of Algeria, 2018; p. 118. [Google Scholar]
- Fediuk, R.; Mosaberpanah, M.A.; Lesovik, V. Development of fiber reinforced self-compacting concrete (FRSCC): Towards an efficient utilization of quaternary composite binders and fibers. Adv. Concr. Constr. 2020, 9, 387–395. [Google Scholar]
- Petrova, B.; Budinova, T.; Tsyntsarski, B.; Kochkodan, V.; Shkavro, Z.; Petrov, N. Removal of aromatic hydrocarbons from water by activated carbon from apricot kernels. Chem. Eng. J. 2010, 165, 258–264. [Google Scholar] [CrossRef]
- Fediuk, R.; Smoliakov, A.; Stoyushko, N. Increase in composite binder activity. IOP Conf. Ser. Mater. Sci. Eng. 2016, 156, 012042. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S. Removal of some substituted phenols by activated carbon obtained from agricultural waste. Water Res. 1998, 32, 1169–1177. [Google Scholar] [CrossRef]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Gholampour, A. Fly Ash-Based Eco-Efficient Concretes: A Comprehensive Review of the Short-Term Properties. Materials 2021, 14, 4264. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Z.; Sun, Z.; Ye, J. Comparison study of naphthalene adsorption on activated carbons prepared from different raws. Korean J. Chem. Eng. 2018, 35, 2086–2096. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikhiev, I.; Shaykhieva, K.; Sverguzova, S.; Fomina, E.; Vinogradenko, Y.; Fediuk, R.; Amran, M.; Svintsov, A.P.; Azevedo, A.R.G.d.; Gunasekaran, M. Removing Pollutants from Sewage Waters with Ground Apricot Kernel Shell Material. Materials 2022, 15, 3428. https://doi.org/10.3390/ma15103428
Shaikhiev I, Shaykhieva K, Sverguzova S, Fomina E, Vinogradenko Y, Fediuk R, Amran M, Svintsov AP, Azevedo ARGd, Gunasekaran M. Removing Pollutants from Sewage Waters with Ground Apricot Kernel Shell Material. Materials. 2022; 15(10):3428. https://doi.org/10.3390/ma15103428
Chicago/Turabian StyleShaikhiev, Ildar, Karina Shaykhieva, Svetlana Sverguzova, Ekaterina Fomina, Yuriy Vinogradenko, Roman Fediuk, Mugahed Amran, Alexander P. Svintsov, Afonso Rangel Garcez de Azevedo, and Murali Gunasekaran. 2022. "Removing Pollutants from Sewage Waters with Ground Apricot Kernel Shell Material" Materials 15, no. 10: 3428. https://doi.org/10.3390/ma15103428
APA StyleShaikhiev, I., Shaykhieva, K., Sverguzova, S., Fomina, E., Vinogradenko, Y., Fediuk, R., Amran, M., Svintsov, A. P., Azevedo, A. R. G. d., & Gunasekaran, M. (2022). Removing Pollutants from Sewage Waters with Ground Apricot Kernel Shell Material. Materials, 15(10), 3428. https://doi.org/10.3390/ma15103428








