Electrochemical Corrosion Behaviour of X70 Steel under the Action of Capillary Water in Saline Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. X70 Steel Specimens
2.1.2. Soil Specimens
2.2. Methods
3. Results and Discussion
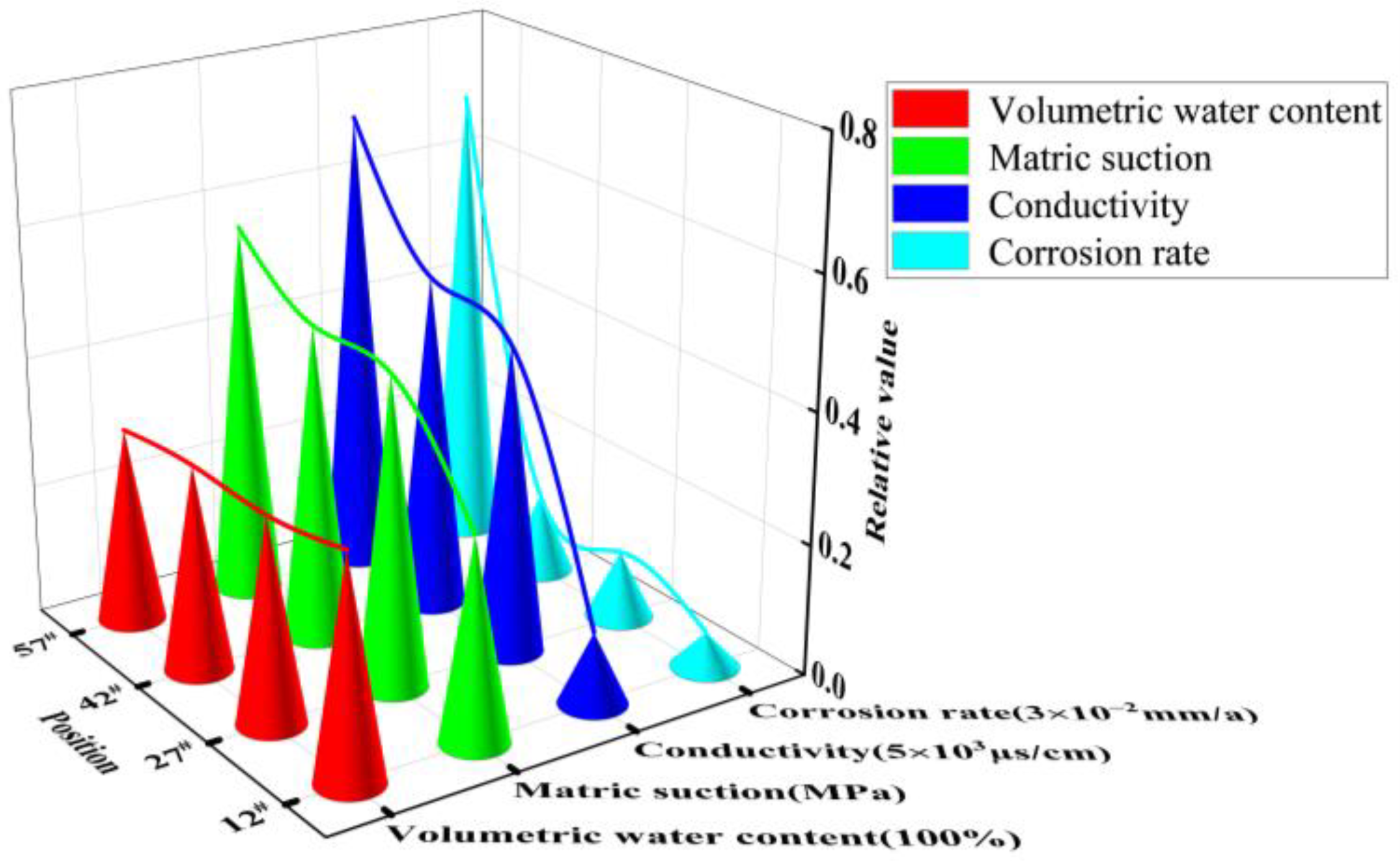
3.1. Water-Salt Migration Patterns under Capillary Water Action in Saline Soils
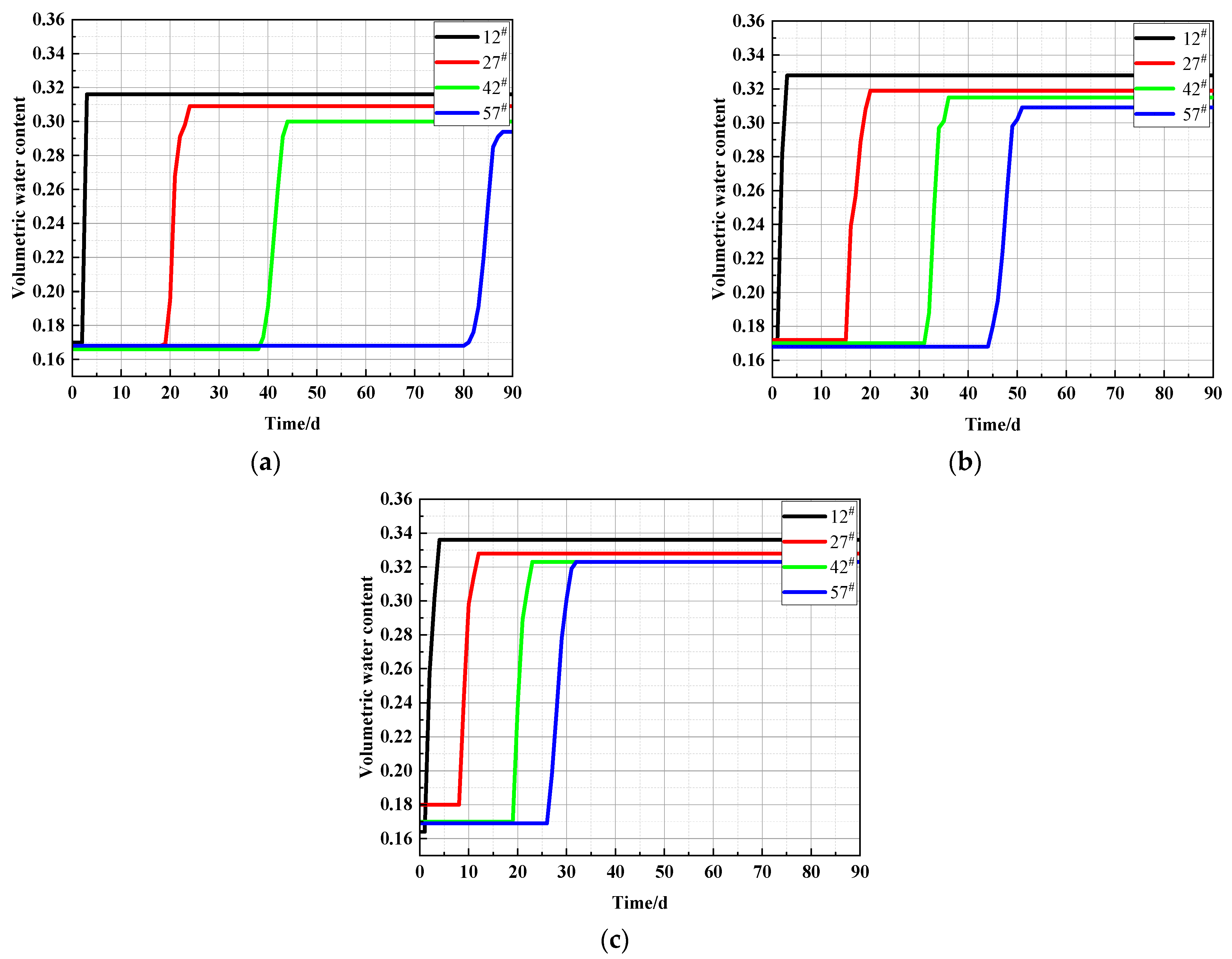
3.1.1. Variation of Volumetric Water Content at Different Locations in the Soil Column
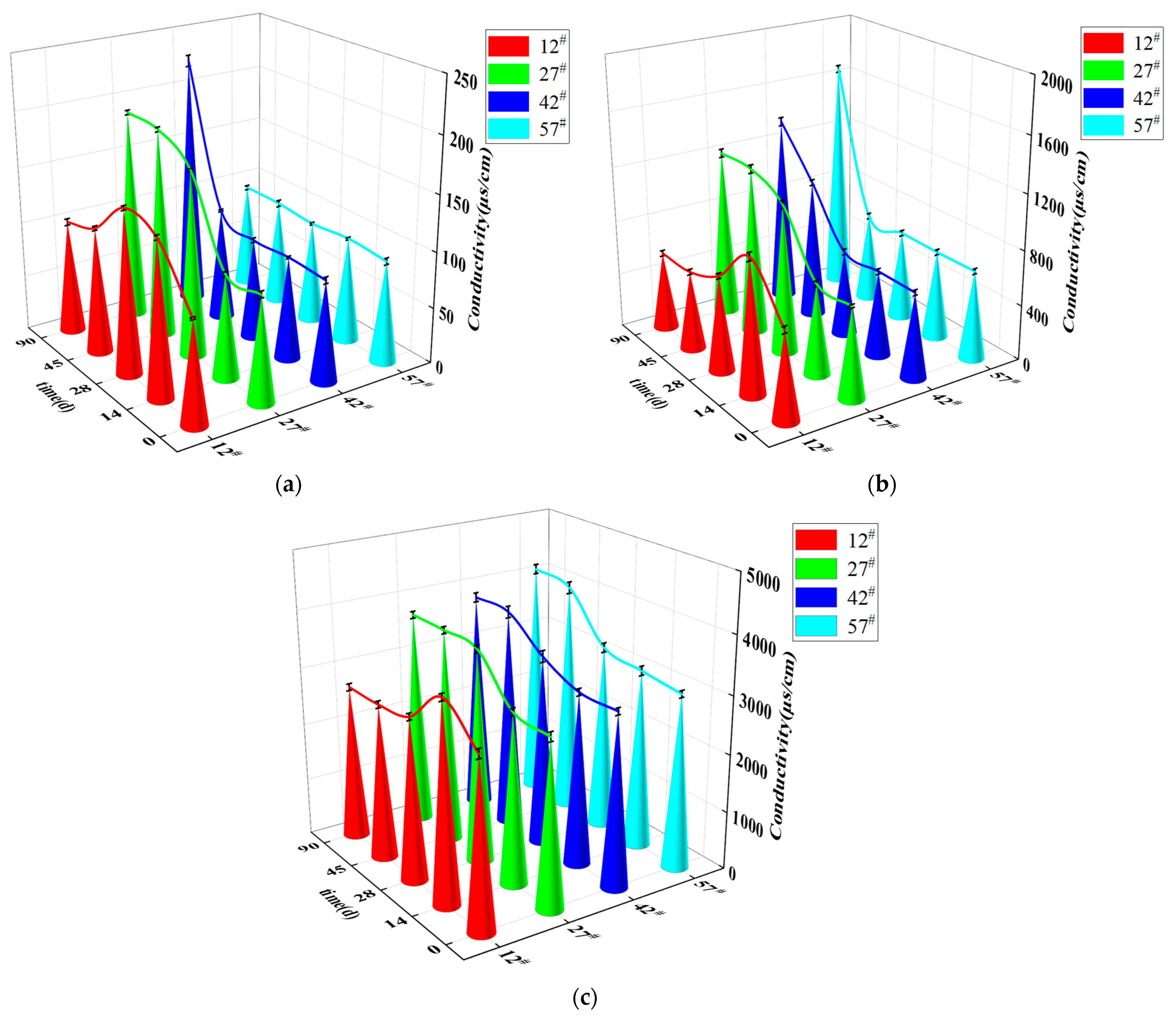
3.1.2. Variation of Conductivity at Different Locations in the Soil Column
3.2. Polarization Curves of X70 Steel in Saline Soils under Capillary Action
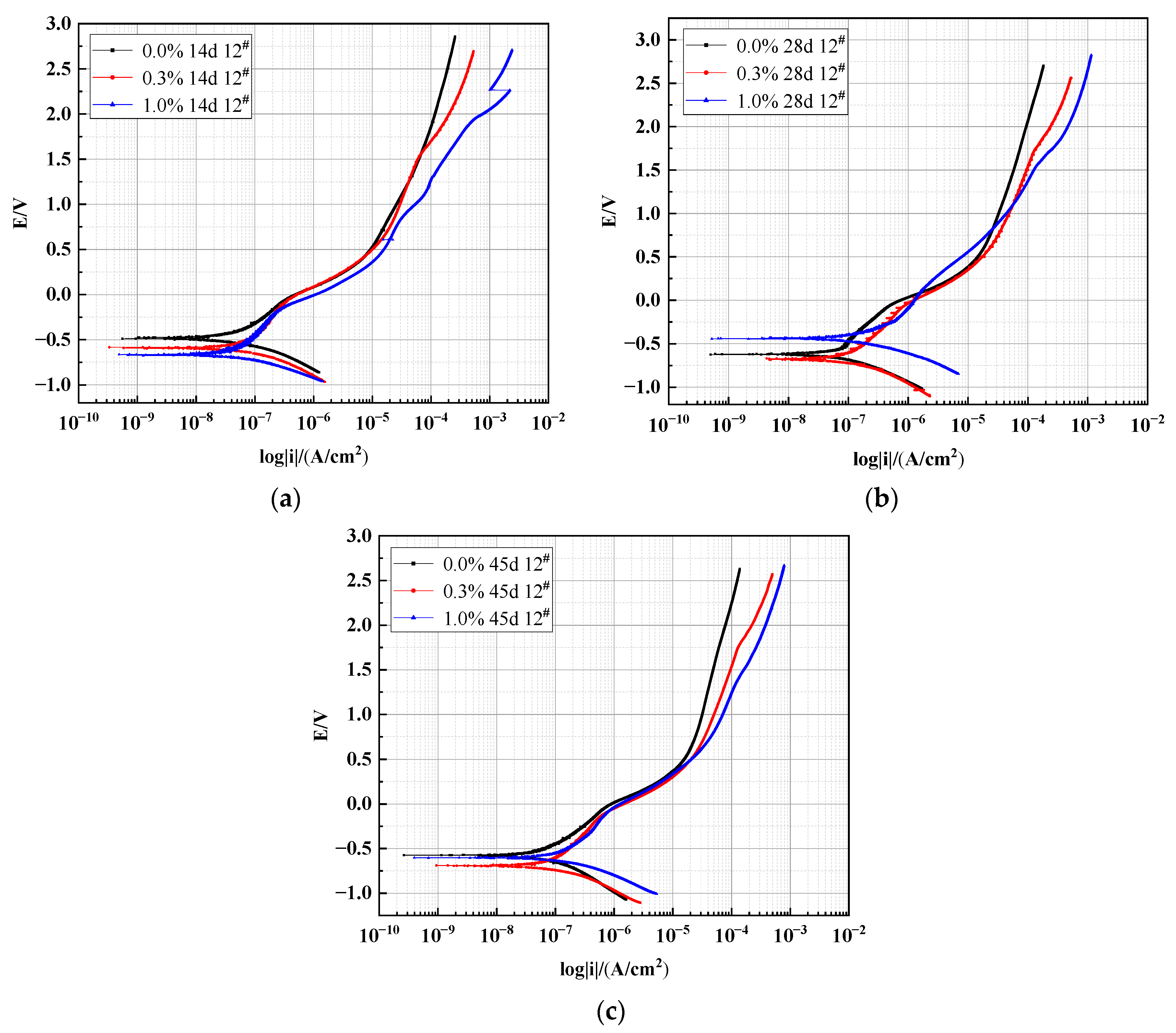
3.2.1. Effect of Different Salt Content on the Polarization Curve of X70 Steel under Capillary Action
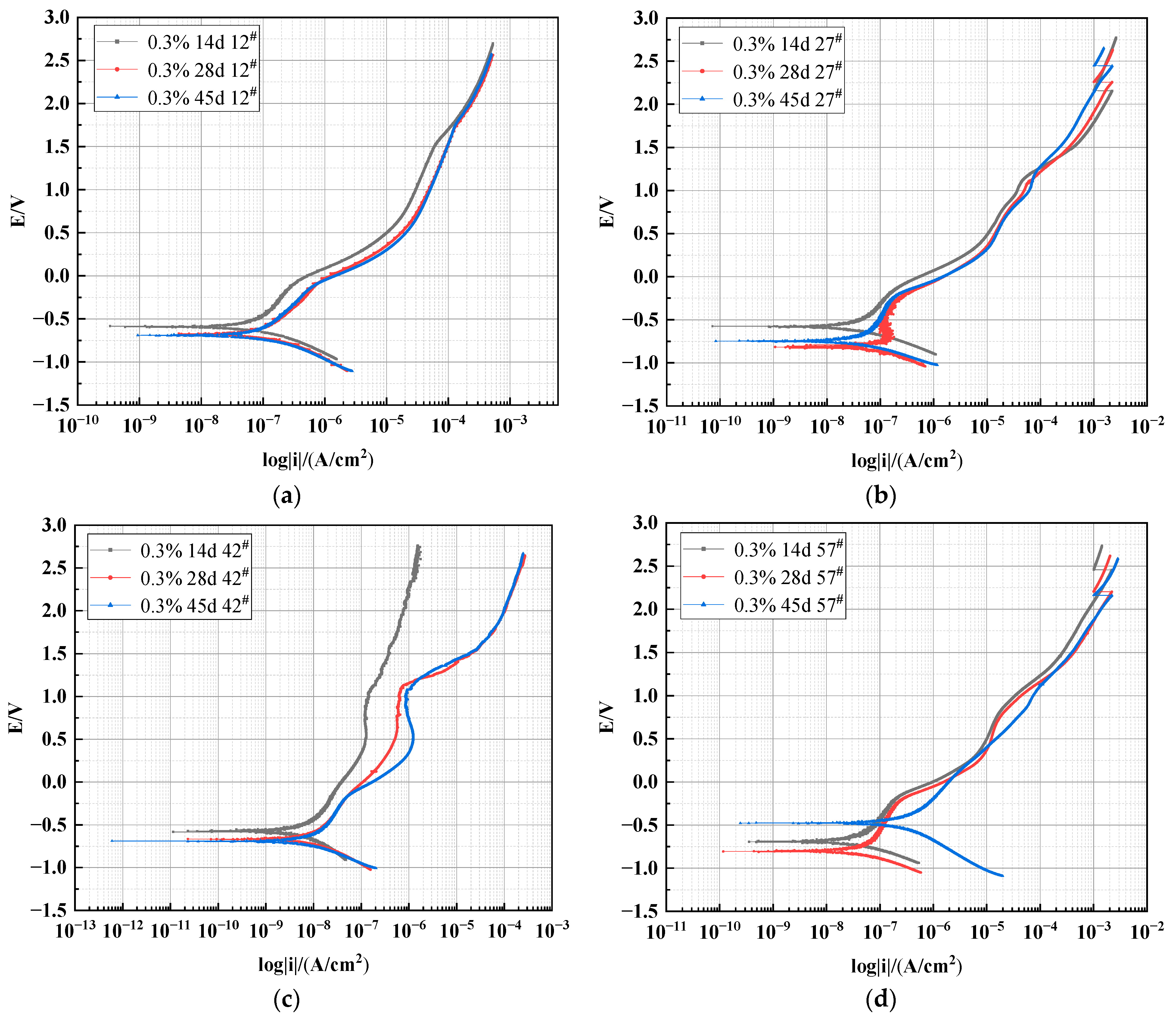
3.2.2. Effect of Different Heights on the Polarization Curve of X70 Steel under Capillary Action
3.3. Electrochemical Impedance Characteristics of X70 Steel in Saline Soils under Capillary Action
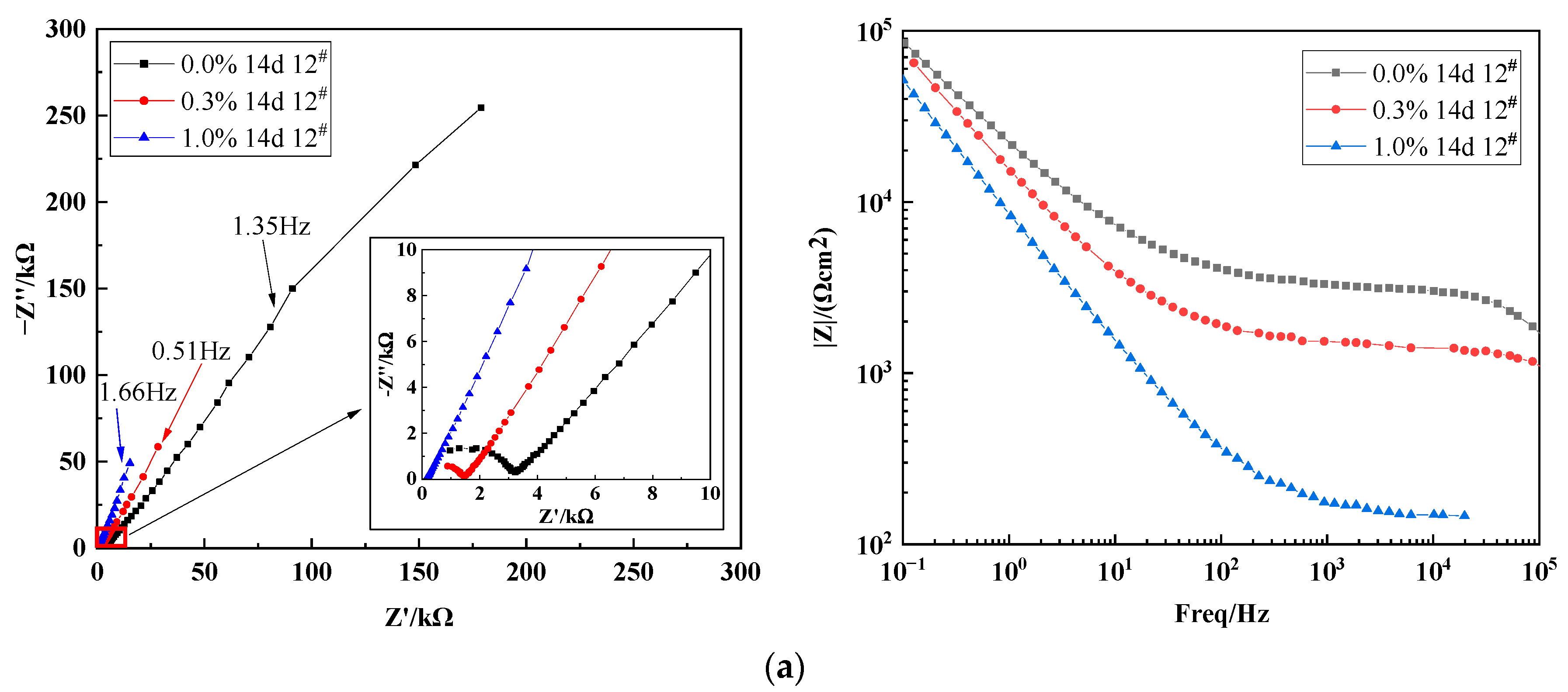
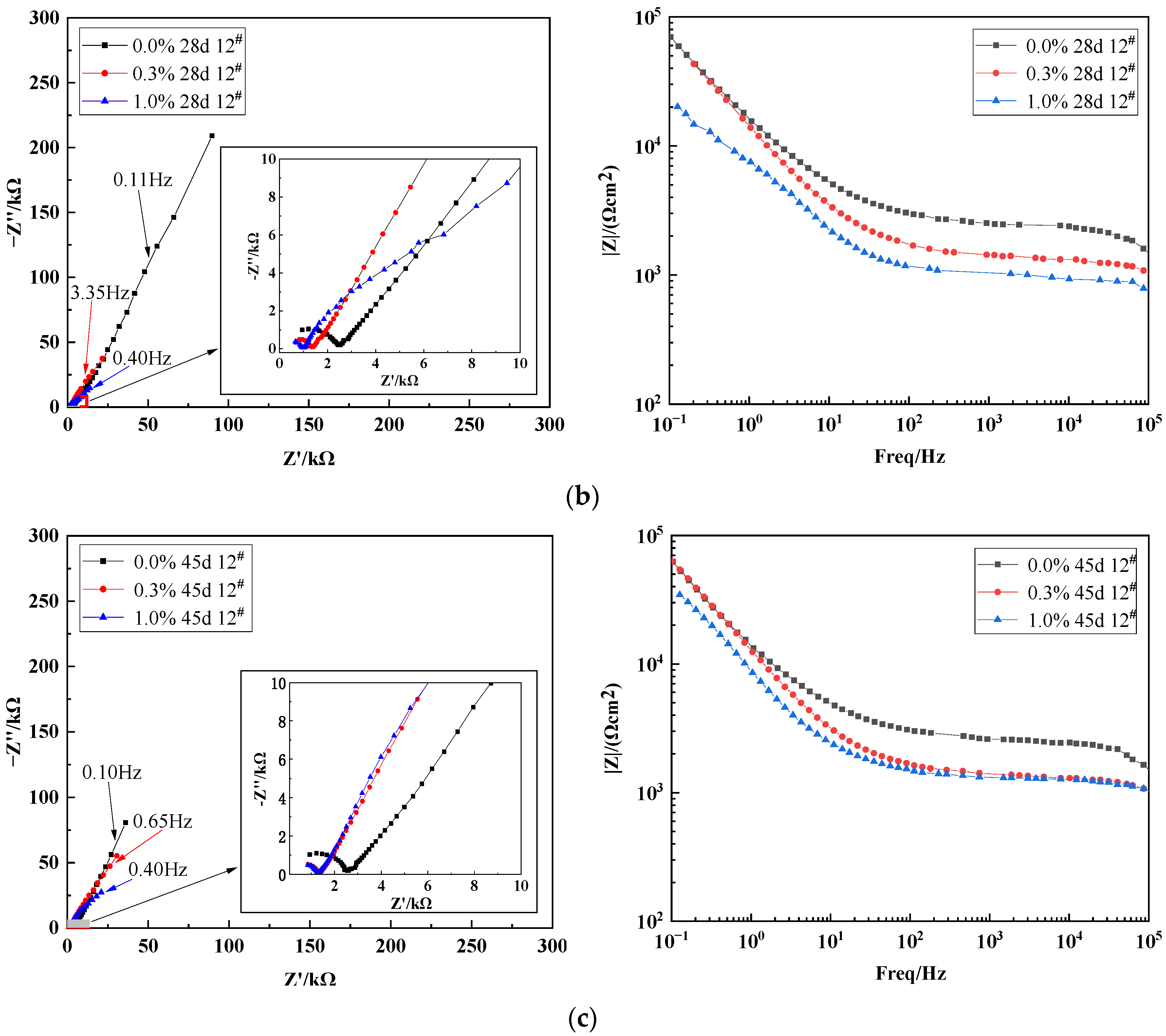
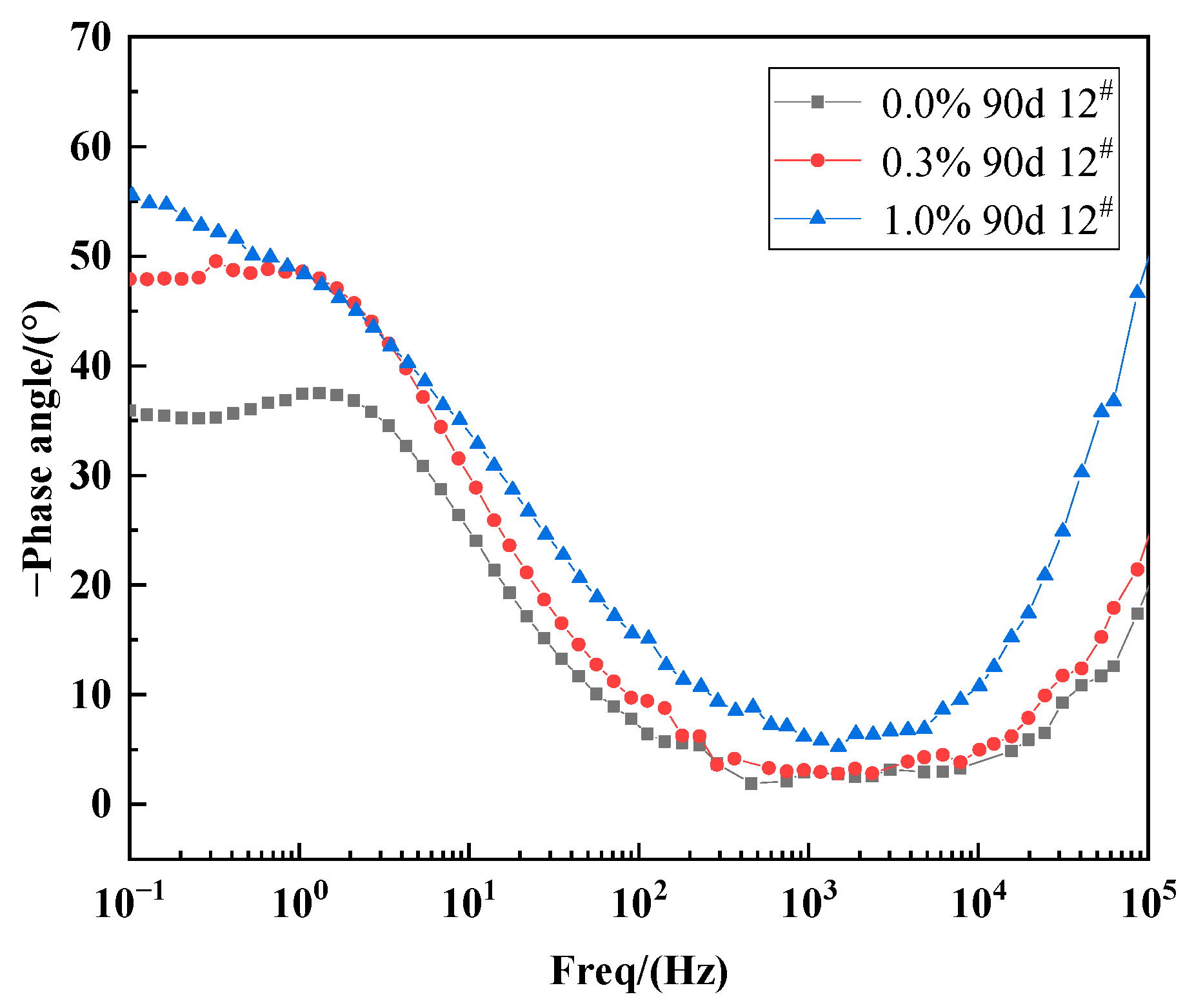
3.3.1. Effect of Varying Salt Content on the Impedance Characteristics of X70 Steel under Capillary Action
3.3.2. Effect of Height on the Impedance Characteristics of X70 Steel under Capillary Action
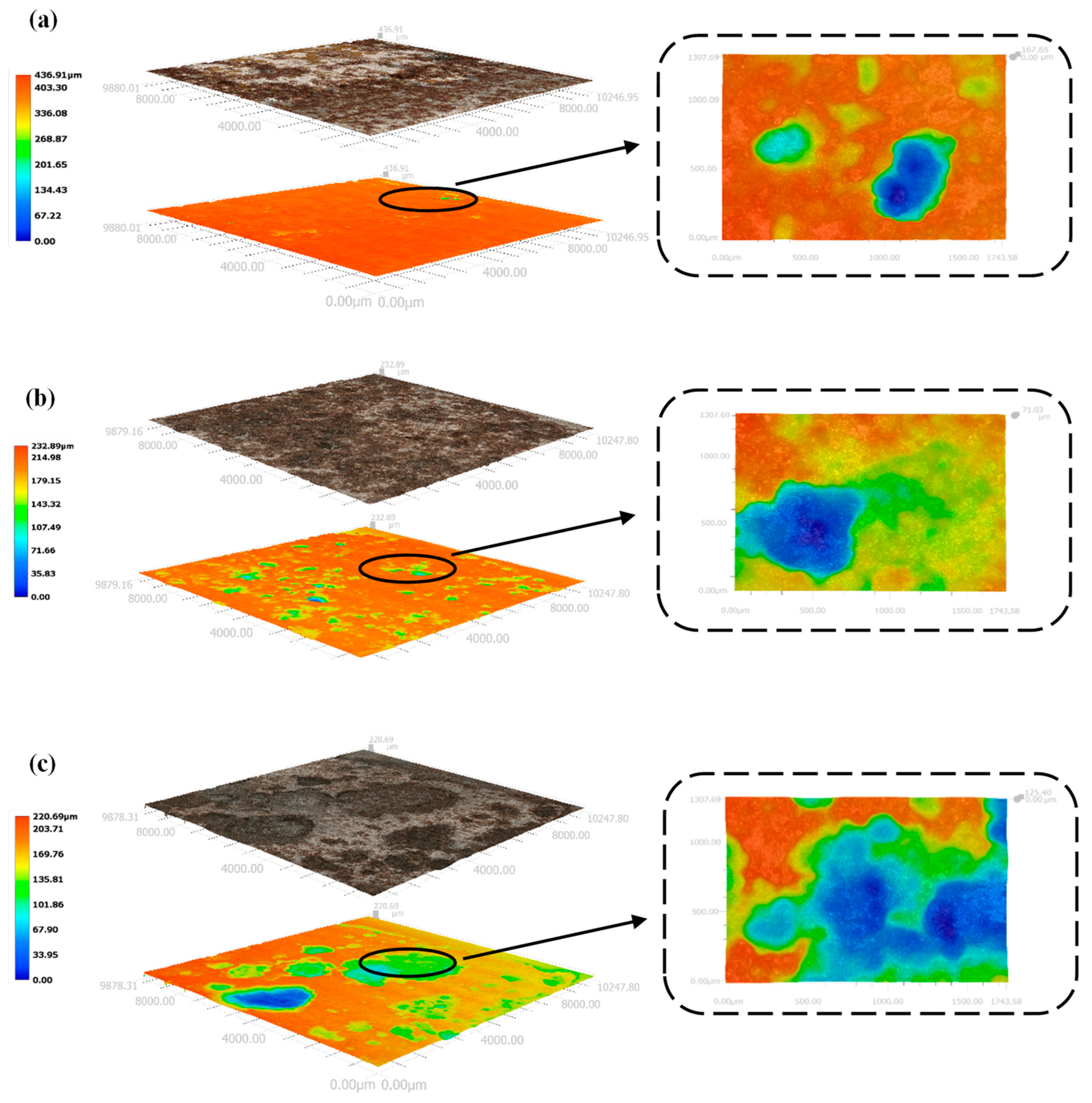
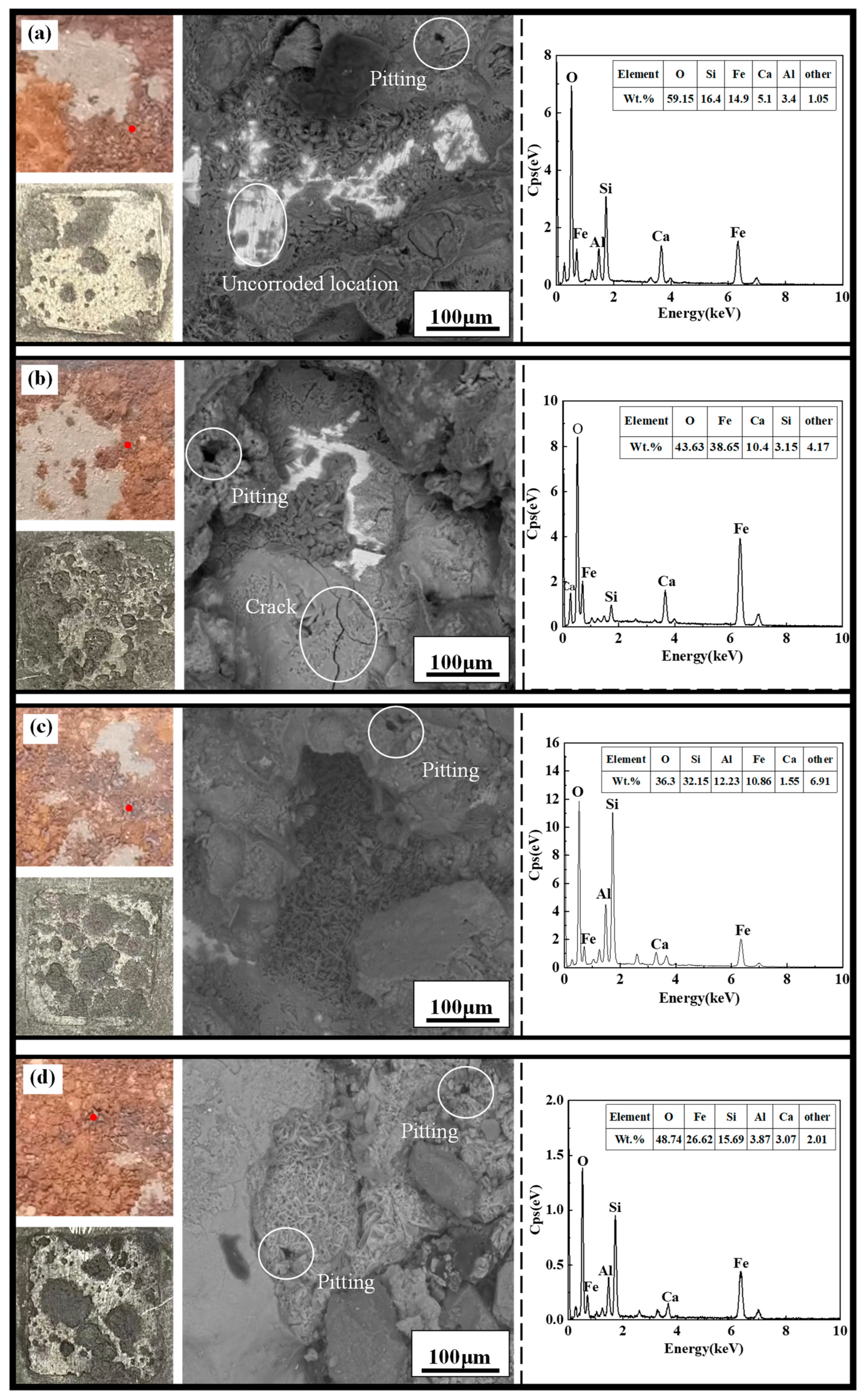
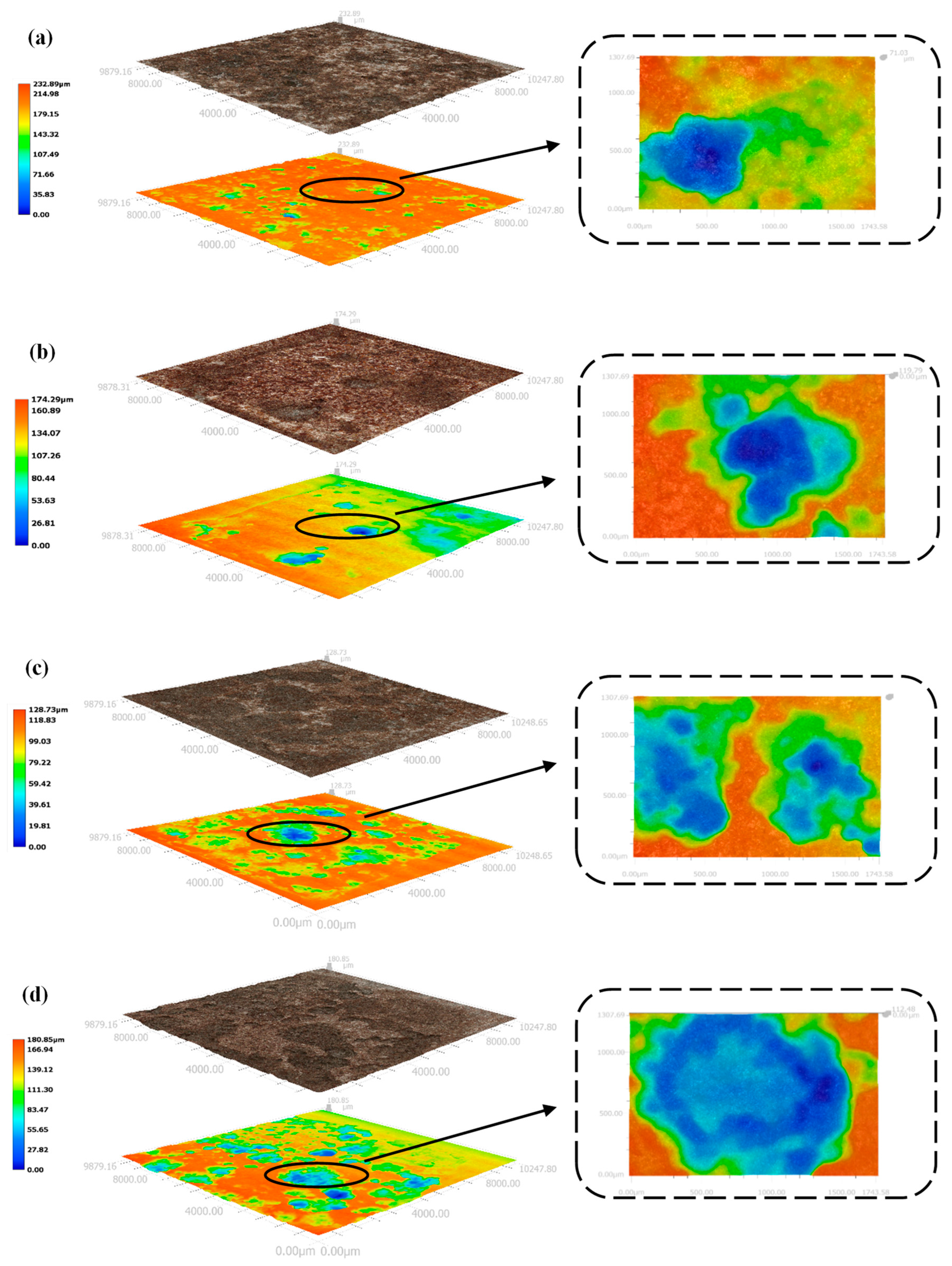
3.4. Macroscopic Corrosion Morphology of X70 Steel under Capillary Action in Saline Soils and the Corrosion Mechanism
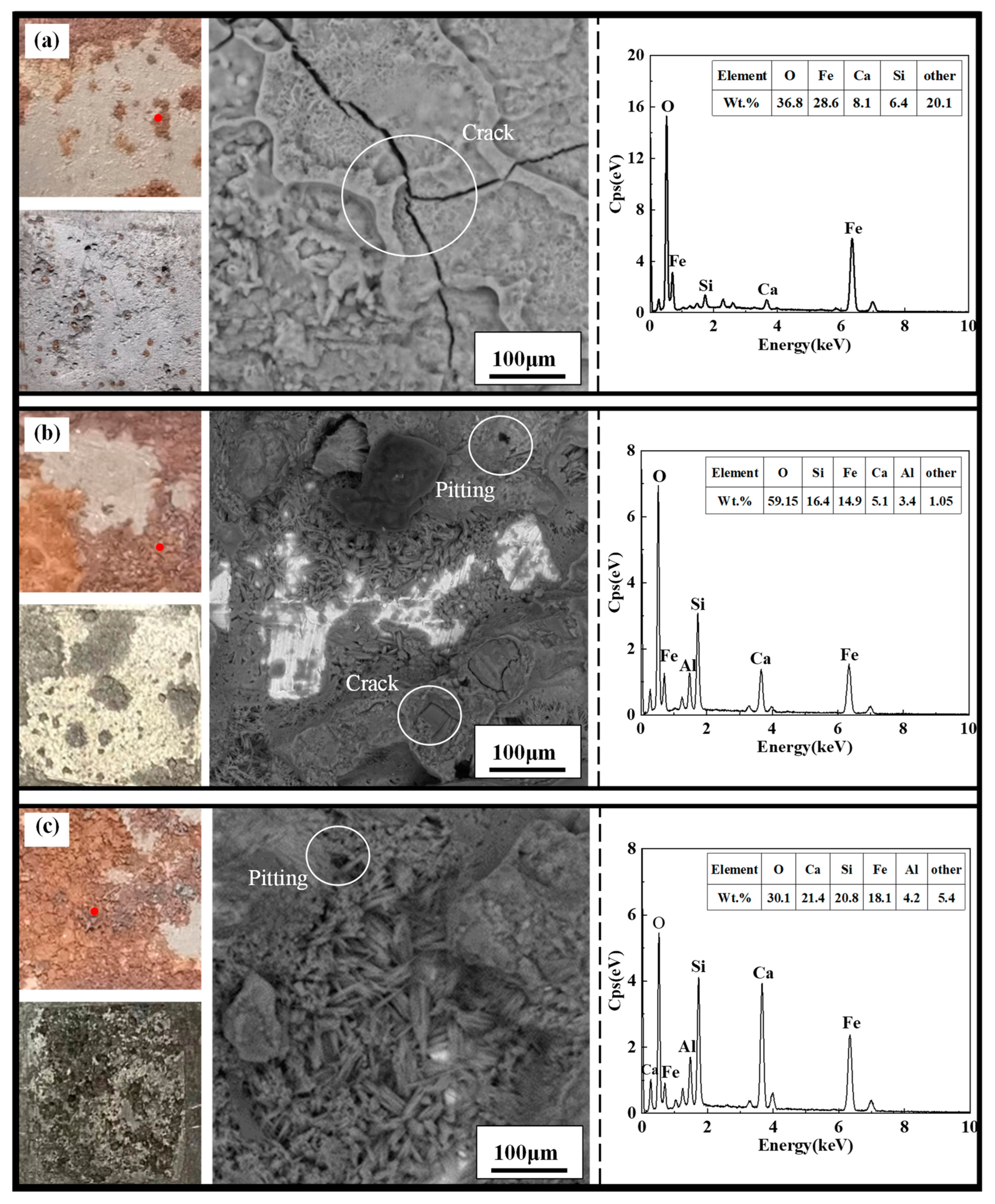
3.4.1. Macro and Micro Corrosion Profiles of X70 Steel
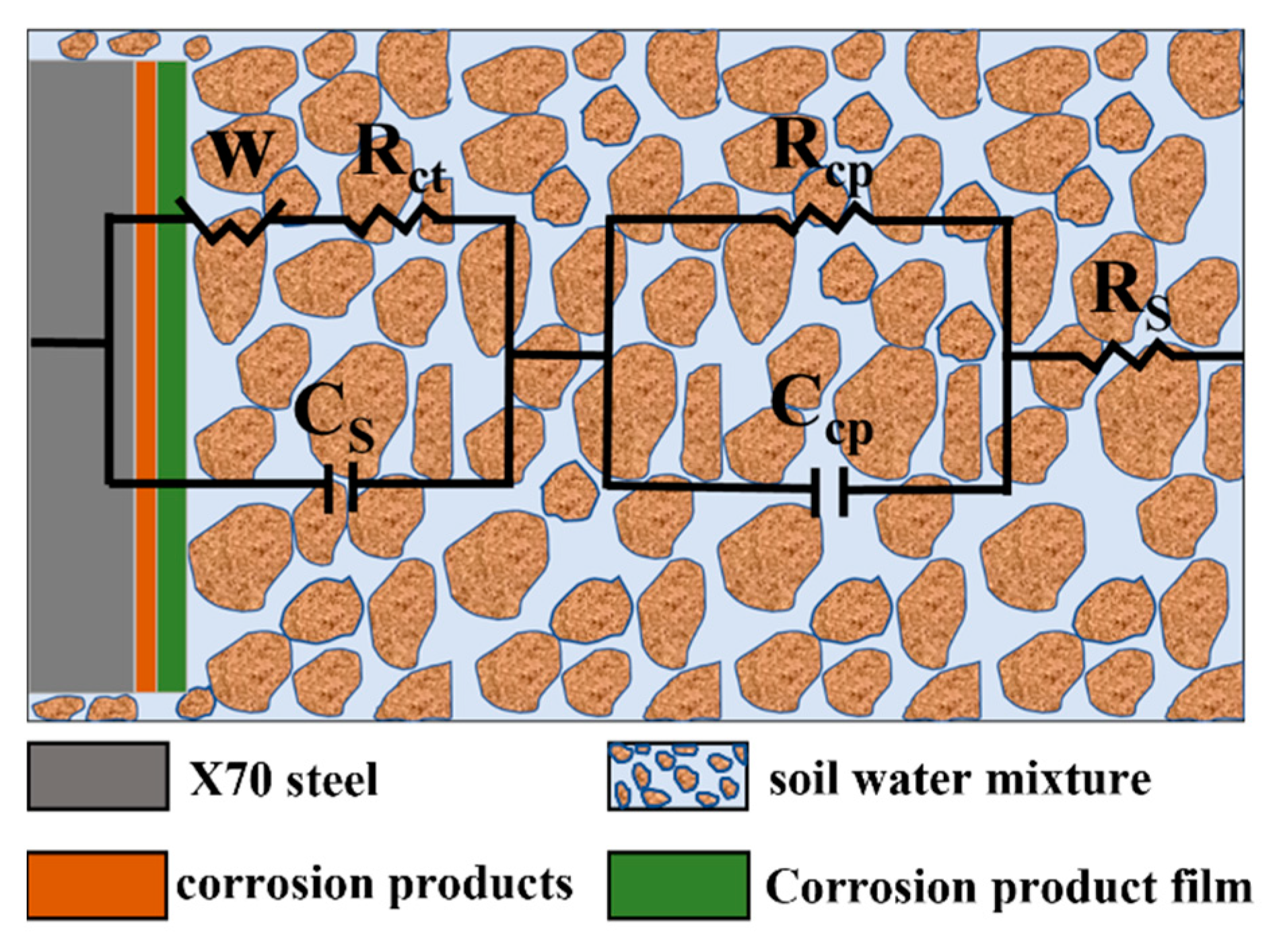
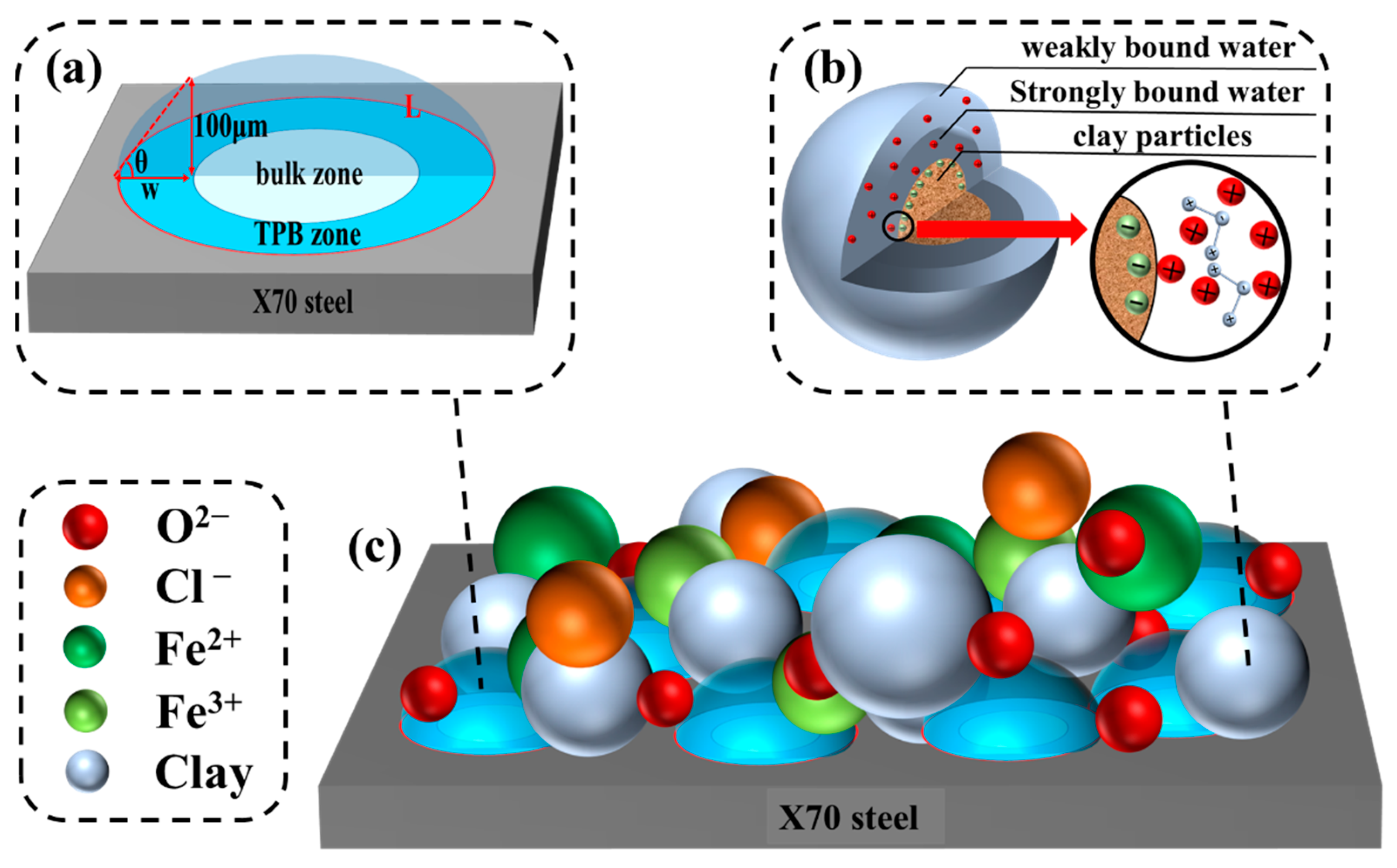
3.4.2. Corrosion Mechanism of X70 Steel under Capillary Action
4. Conclusions
- (1)
- The final stable water content of the soil column decreased with increasing height, and the rate of the capillary water rise was accelerated by the NaCl content of the soil, which promoted capillary action. The transport of salts in the soil lagged relative to the transport of water. Height position 12# in the soil column was used as a dividing interface. The soil conductivity above this position showed an increasing trend with rising capillary water.
- (2)
- The corrosion current density and corrosion rate of the X70 steel specimens at the same height positions in the soil column increased with increasing NaCl content. The corrosion behavior of X70 steel at different heights in the same soil column was significantly influenced by the transport of water and salt caused by the rise of the capillary water. The rise of the capillary wetting front position led to a solid/liquid/gas three-phase interface with the X70 steel specimens that enhanced the corrosion behavior. In addition, the accumulation of salts at a specific location also enhanced the X70 steel corrosion rate at that location.
- (3)
- The electrochemical impedance spectra of X70 steel in a capillary water environment exhibited a superposition of a bicircular arc with two time constants and a straight line representing mass transfer diffusion in the low-frequency region. The radius of the high-frequency capacitive arc resistance in the Nyquist plots decreased with increasing NaCl content in the soil column. Capillary pore channels below the capillary water wetting front were easily occupied by the solution. This led to an anoxic corrosion environment that reduced the X70 steel corrosion kinetics.
- (4)
- Higher NaCl content in the soil column led to a larger area covered by corrosion products, a greater number of corrosion pits, and deeper pits on the X70 steel specimen surfaces. After the capillary water was stabilized for a period of time, the area of these pits on the surface of the X70 steel specimen at height position 57# was significantly larger than that at other height positions. The pits at position 57# were also deeper. This was due to the combined effect of the changes to a number of factors caused by the rise of capillary water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, Y.S.; Kim, J.G.; Shin, D.H.; Kim, S.Y. A Corrosion Detection System for Buried Pipeline (II). Korean J. Met. Mater. 2005, 43, 306–317. [Google Scholar]
- Zhu, M.; Ma, J.; Yuan, Y.F.; Guo, S.Y.; Yin, S.M.; Du, C.W. The Effect of Annealing Time on Microstructure and AC Corrosion Behavior of X80 Steel in Simulated Solution of Alkaline Soil. J. Mater. Eng. Perform. 2019, 28, 6073–6080. [Google Scholar] [CrossRef]
- Zhang, Q.; Larson, S.L.; Ballard, J.H.; Zhu, X.; Knotek-Smith, H.M.; Han, F.X. Uranium metal corrosion in soils with different soil moisture regimes. Corros. Sci. 2021, 179, 109138. [Google Scholar] [CrossRef]
- Taghipour, M.; Lashkaripour, G.R.; Ghafoori, M.; Hafezimoghaddas, N. Evaluating the soil corrosion of Bushehr, Iran, based on a new classification system for corrosive soils. Anti-Corros. Methods Mater. 2016, 63, 347–354. [Google Scholar] [CrossRef]
- Cole, I.S.; Marney, D. The science of pipe corrosion: A review of the literature on the corrosion of ferrous metals in soils. Corros. Sci. 2021, 56, 5–16. [Google Scholar] [CrossRef]
- You, Z.; Lai, Y.; Zeng, H.; Yang, Y. Influence of water and sodium chloride content on corrosion behavior of cast iron in silty clay. Constr. Build. Mater. 2020, 238, 117762. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, G.; Chen, Y.; Zhao, P.; Tian, Y. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef]
- Li, M.; Shi, Z.-M.; Liu, Y.-B. Crevice Corrosion of K60 in Dry Desertification Saline Soil. J. Iron Steel Res. Int. 2013, 20, 76–80. [Google Scholar] [CrossRef]
- Ezuber, H.M.; Alshater, A.; Hossain, S.M.Z.; El-Basir, A. Impact of Soil Characteristics and Moisture Content on the Corrosion of Underground Steel Pipelines. Arab. J. Sci. Eng. 2021, 46, 6177–6188. [Google Scholar] [CrossRef]
- Wasim, M.; Shoaib, S.; Mubarak, N.M.; Inamuddin; Asiri, A.M. Factors influencing corrosion of metal pipes in soils. Environ. Chem. Lett. 2018, 16, 861–879. [Google Scholar] [CrossRef]
- Azoor, R.; Asselin, E.; Deo, R.; Kodikara, J.; Wijewickreme, D. Corrosion of cast iron pipelines buried in Fraser River silt subject to climate-induced moisture variations. Acta Geotech. 2020, 16, 873–884. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Le, Z.; Zhao, B.; Ma, J.; Yuan, Y.; Guo, S. Influence of alternating current on corrosion behavior of X100 steel in Golmud soil simulated solution with different pH. Int. J. Electrochem. Sci. 2020, 15, 10423–10431. [Google Scholar] [CrossRef]
- Wang, S.; Yin, X.; Zhang, H.; Liu, D.; Du, N. Coupling Effects of pH and Dissolved Oxygen on the Corrosion Behavior and Mechanism of X80 Steel in Acidic Soil Simulated Solution. Materials 2019, 12, 3175. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Hadjel, M.; Traisnel, M. Corrosion behavior of API 5L X-60 pipeline steel exposed to near-neutral pH soil simulating solution. Mater. Corros. 2006, 57, 771–777. [Google Scholar] [CrossRef]
- Xie, R.; Han, P.; Wang, X.; Li, B.; He, B.; Ma, F.; Bai, X. Effects of Pore Fluids and Sand Particles on Electrochemical Characteristics of Sandy Soil Containing Soluble Sodium Salt. Int. J. Electrochem. Sci. 2020, 15, 3543–3562. [Google Scholar] [CrossRef]
- Sun, F.; Xie, R.; He, B.; Chen, Z.; Bai, X.; Han, P. The Initial Stage Corrosion of X80 Steel in Saturated Sandy Soil Containing Cl- and SO42−. Int. J. Electrochem. Sci. 2021, 16, 150878. [Google Scholar] [CrossRef]
- Liu, X.; Tan, Y.; Zhang, X.; Liao, Q.; Wang, Z. Fabrication and performance evaluation of nickel-rich conductive coating for carbon steel grounding grids in saline-alkali soil solution. Corros. Rev. 2020, 38, 263–271. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, T.; Liu, M.; Wang, B.; Li, C.; Yin, F. Accelerating role of microbial film on soil corrosion of pipeline steel. Int. J. Press. Vessel. Pip. 2021, 192, 104395. [Google Scholar] [CrossRef]
- Suarez, E.M.; Lepkova, K.; Kinsella, B.; Machuca, L.L. Aggressive corrosion of steel by a thermophilic microbial consortium in the presence and absence of sand. Int. Biodeterior. Biodegrad. 2019, 137, 137–146. [Google Scholar] [CrossRef]
- Sachan, R.; Singh, A.K. Corrosion of steel due to iron oxidizing bacteria. Anti-Corrosion Methods Mater. 2019, 66, 19–26. [Google Scholar] [CrossRef]
- Procópio, L. The oil spill and the use of chemical surfactant reduce microbial corrosion on API 5L steel buried in saline soil. Environ. Sci. Pollut. Res. 2021, 28, 26975–26989. [Google Scholar] [CrossRef] [PubMed]
- Mand, J.; Enning, D. Oil field microorganisms cause highly localized corrosion on chemically inhibited carbon steel. Microb. Biotechnol. 2021, 14, 171–185. [Google Scholar] [CrossRef]
- Gadow, H.S.; Fakeeh, M. Green inhibitor of carbon steel corrosion in 1 M hydrochloric acid: Eruca sativa seed extract (experimental and theoretical studies). RSC Adv. 2022, 12, 8953–8986. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Han, P.; Xie, R.; He, B.; Bai, X. Study of the Electrochemical Corrosion Behaviour of X70 Steel in H2SO4 Contaminated Silty Soil. Int. J. Electrochem. Sci. 2018, 13, 8694–8710. [Google Scholar] [CrossRef]
- Wang, X.; Song, X.; Chen, Y.; Wang, Z.; Zhang, L. Corrosion Behavior of X70 and X80 Pipeline Steels in Simulated Soil Solution. Int. J. Electrochem. Sci. 2018, 13, 6436–6450. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.-G.; Du, C.-W. Effect of environmental factors on electrochemical behavior of X70 pipeline steel in simulated soil solution. J. Iron Steel Res. Int. 2009, 16, 52–57. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Khalaj, G.; Jandaghi, M.R.; Khalaj, M.J. Study on the correlation of toughness with chemical composition and tensile test results in microalloyed API pipeline steels. J. Min. Met. Sect. B Met. 2015, 51, 173–178. [Google Scholar] [CrossRef]
- Khalaj, G.; Pouraliakbar, H.; Arab, N.; Nazerfakhari, M. Correlation of passivation current density and potential by using chemical composition and corrosion cell characteristics in HSLA steels. Measurement 2015, 75, 5–11. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, M.; Liu, H. The Effects of Truncating the Capillary Fringe on Water-Table Dynamics During Periodic Forcing. Water Resour. Res. 2022, 58, e2021WR031112. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, S.; Dong, B.; Tang, L.; Lin, C.; Liu, Z.; Xing, F. Water distribution modelling of capillary absorption in cementitious materials. Constr. Build. Mater. 2019, 216, 468–475. [Google Scholar] [CrossRef]
- Yekta, A.E.; Manceau, J.-C.; Gaboreau, S.; Pichavant, M.; Audigane, P. Determination of Hydrogen–Water Relative Permeability and Capillary Pressure in Sandstone: Application to Underground Hydrogen Injection in Sedimentary Formations. Transp. Porous Media 2018, 122, 333–356. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Z.; Zheng, L.; Yang, Y.; Huang, D. An extended numerical manifold method for unsaturated soil-water interaction analysis at micro-scale. Int. J. Numer. Anal. Methods Géoméch. 2021, 45, 1500–1525. [Google Scholar] [CrossRef]
- Rudiyanto, R.; Sakai, M.; van Genuchten, M.T.; Alazba, A.A.; Setiawan, B.I.; Minasny, B. A complete soil hydraulic model accounting for capillary and adsorptive water retention, capillary and film conductivity, and hysteresis. Water Resour. Res. 2015, 51, 8757–8772. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, S. Effect on Silt Capillary Water Absorption upon Addition of Sodium Methyl Silicate (SMS) and Microscopic Mechanism Analysis. Coatings 2020, 10, 724. [Google Scholar] [CrossRef]
- Luo, Z.; Kong, J.; Ji, Z.; Shen, C.; Lu, C.; Xin, P.; Zhao, Z.; Li, L.; Barry, D.A. Watertable fluctuation-induced variability in the water retention curve: Sand column experiments. J. Hydrol. 2020, 589, 125125. [Google Scholar] [CrossRef]
- Li, K.; Horne, R. Steam-Water and Air-Water Capillary Pressures: Measurement and Comparison. J. Can. Pet. Technol. 2004, 43, 24–30. [Google Scholar] [CrossRef]
- Ince, I. Relationship Between Capillary Water Absorption Value, Capillary Water Absorption Speed, and Capillary Rise Height in Pyroclastic Rocks. Min. Met. Explor. 2021, 38, 841–853. [Google Scholar] [CrossRef]
- Iglauer, S.; Muggeridge, A. The Impact of Tides on the Capillary Transition Zone. Transp. Porous Media 2013, 97, 87–103. [Google Scholar] [CrossRef][Green Version]
- He, F.; Pan, Y.; Tan, L.; Zhang, Z.; Li, P.; Liu, J.; Ji, S.; Qin, Z.; Shao, H.; Song, X. Study of the water transportation characteristics of marsh saline soil in the Yellow River Delta. Sci. Total Environ. 2017, 574, 716–723. [Google Scholar] [CrossRef]
- Fang, M.W.; Wang, D.; Yin, L. Experimental study on water transfer in capillarity-thin film of frozen soil at high latitude. Fresen Environ. Bull. 2021, 30, 11271–11275. [Google Scholar]
- Rad, M.N.; Shokri, N. Nonlinear effects of salt concentrations on evaporation from porous media. Geophys. Res. Lett. 2012, 39, L04403. [Google Scholar] [CrossRef]
- Moreira, P.H.S.; van Genuchten, M.T.; Orlande, H.R.B.; Cotta, R.M. Bayesian estimation of the hydraulic and solute transport properties of a small-scale unsaturated soil column. J. Hydrol. Hydromech. 2016, 64, 30–44. [Google Scholar] [CrossRef]
- Henry, K.S.; Holtz, R.D. Geocomposite capillary barriers to reduce frost heave in soils. Can. Geotech. J. 2001, 38, 678–694. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Wang, Q.; Wang, G.; Liu, Y.; Peng, W.; Xu, X.; Liu, Y. Experimental Investigation of Water Migration Characteristics for Saline Soil. Pol. J. Environ. Stud. 2019, 28, 1495–1505. [Google Scholar] [CrossRef]
- Setia, R.; Marschner, P. Impact of total water potential and varying contribution of matric and osmotic potential on carbon mineralization in saline soils. Eur. J. Soil Biol. 2013, 56, 95–100. [Google Scholar] [CrossRef]
- Qian, Z.; Harianto, R.; Alfrendo, S.; Yiyao, Z.; Guoliang, D.; Xueliang, Z. Estimation of wetting hydraulic conductivity function for unsaturated sandy soil. Eng. Geol. 2021, 285, 106034. [Google Scholar] [CrossRef]
- Chen, P.; Wei, C.; Liu, J.; Ma, T.; Fu, P. Strength Theory Model of Unsaturated Soils with Suction Stress Concept. J. Appl. Math. 2013, 2013, 756854. [Google Scholar] [CrossRef]
- Cui, Z.; Jing, X.; Hao, J.; Doh, S. Analysis of Capillary Water Migration Law of Compacted Loess in Ningxia. Pol. J. Environ. Stud. 2021, 30, 61–70. [Google Scholar] [CrossRef]
- He, B.; Han, P.; Hou, L.; Zhang, D.; Bai, X. Understanding the effect of soil particle size on corrosion behavior of natural gas pipeline via modelling and corrosion micromorphology. Eng. Fail. Anal. 2017, 80, 325–340. [Google Scholar] [CrossRef]
- Khalaj, G.; Khalaj, M.-J. Investigating the corrosion of the Heat-Affected Zones (HAZs) of API-X70 pipeline steels in aerated carbonate solution by electrochemical methods. Int. J. Press. Vessel. Pip. 2016, 145, 155–163. [Google Scholar] [CrossRef]
- Qin, Q.; Wei, B.; Bai, Y.; Fu, Q.; Xu, J.; Sun, C.; Wang, C.; Wang, Z. Effect of alternating current frequency on corrosion behavior of X80 pipeline steel in coastal saline soil. Eng. Fail. Anal. 2021, 120, 105065. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Wang, Y. Measuring and modeling the dielectric constant of soil during freezing and thawing processes: An application on silty clay. Acta Geotech. 2022, 17, 1–20. [Google Scholar] [CrossRef]
- He, B.; Han, P.J.; Hou, L.F.; Lu, C.H.; Bai, X.H. Role of typical soil particle-size distributions on the long-term corrosion behavior of pipeline steel. Mater. Corros. 2016, 67, 471–483. [Google Scholar] [CrossRef]
- Mahlobo, M.G.R.; Olubambi, P.A.; Mjwana, P.; Jeannin, M.; Refait, P. Study of Overprotective-Polarization of Steel Subjected to Cathodic Protection in Unsaturated Soil. Materials 2021, 14, 4123. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Dam-Johansen, K.; Weinell, C.; Kiil, E.S. Cathodic delamination of seawater-immersed anticorrosive coatings: Mapping of parameters affecting the rate. Prog. Org. Coat. 2010, 68, 283–292. [Google Scholar] [CrossRef]
- He, B.; Han, P.; Lu, C.; Bai, X. Effect of soil particle size on the corrosion behavior of natural gas pipeline. Eng. Fail. Anal. 2015, 58, 19–30. [Google Scholar] [CrossRef]
- Bai, X.; He, B.; Han, P.; Xie, R.; Sun, F.; Chen, Z.; Liu, X.; Wang, Y. Effect of Temperature on the Electrochemical Corrosion Behavior of X80 Steel in Silty Soil Containing Sodium Chloride. J. Mater. Eng. Perform. 2022, 31, 968–983. [Google Scholar] [CrossRef]
- Rabin, N.N.; Islam, M.S.; Fukuda, M.; Yagyu, J.; Tagawa, R.; Sekine, Y.; Hayami, S. Enhanced mixed proton and electron conductor at room temperature from chemically modified single-wall carbon nanotubes. RSC Adv. 2022, 12, 8632–8636. [Google Scholar] [CrossRef]
- Mouri, E.; Kajiwara, K.; Kawasaki, S.; Shimizu, Y.; Bando, H.; Sakai, H.; Nakato, T. Impacts of negatively charged colloidal clay particles on photoisomerization of both anionic and cationic azobenzene molecules. RSC Adv. 2022, 12, 10855–10861. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.M.; Zhang, H. Role of secondary phase particles of 2519A aluminium alloy in localised corrosion. Mater. Res. Innov. 2013, 17, S83–S88. [Google Scholar] [CrossRef]
- Rangel, R.C.C.; Cruz, N.C.; Rangel, E.C. Role of the Plasma Activation Degree on Densification of Organosilicon Films. Materials 2019, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Rangel, R.C.; Cruz, N.C.; Milella, A.; Fracassi, F.; Rangel, E.C. Barrier and mechanical properties of carbon steel coated with SiOx/SiOxCyHz gradual films prepared by PECVD. Surf. Coat. Technol. 2019, 378, 12499. [Google Scholar] [CrossRef]
- Su, W.; Liu, Z. Numerical Simulation of Damage Evolution and Electrode Deformation of X100 Pipeline Steel during Crevice Corrosion. Materials 2022, 15, 2329. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; He, B.; Han, P.; Xie, R.; Sun, F.; Chen, Z.; Wang, Y.; Liu, X. Corrosion behavior and mechanism of X80 steel in silty soil under the combined effect of salt and temperature. RSC Adv. 2021, 12, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, J.; Lu, Y.-H.; Hu, J.-Z. Effect of length of gas/liquid/solid three-phase boundary zone on cathodic and corrosion behavior of metals. Electrochim. Acta 2009, 54, 1426–1435. [Google Scholar] [CrossRef]

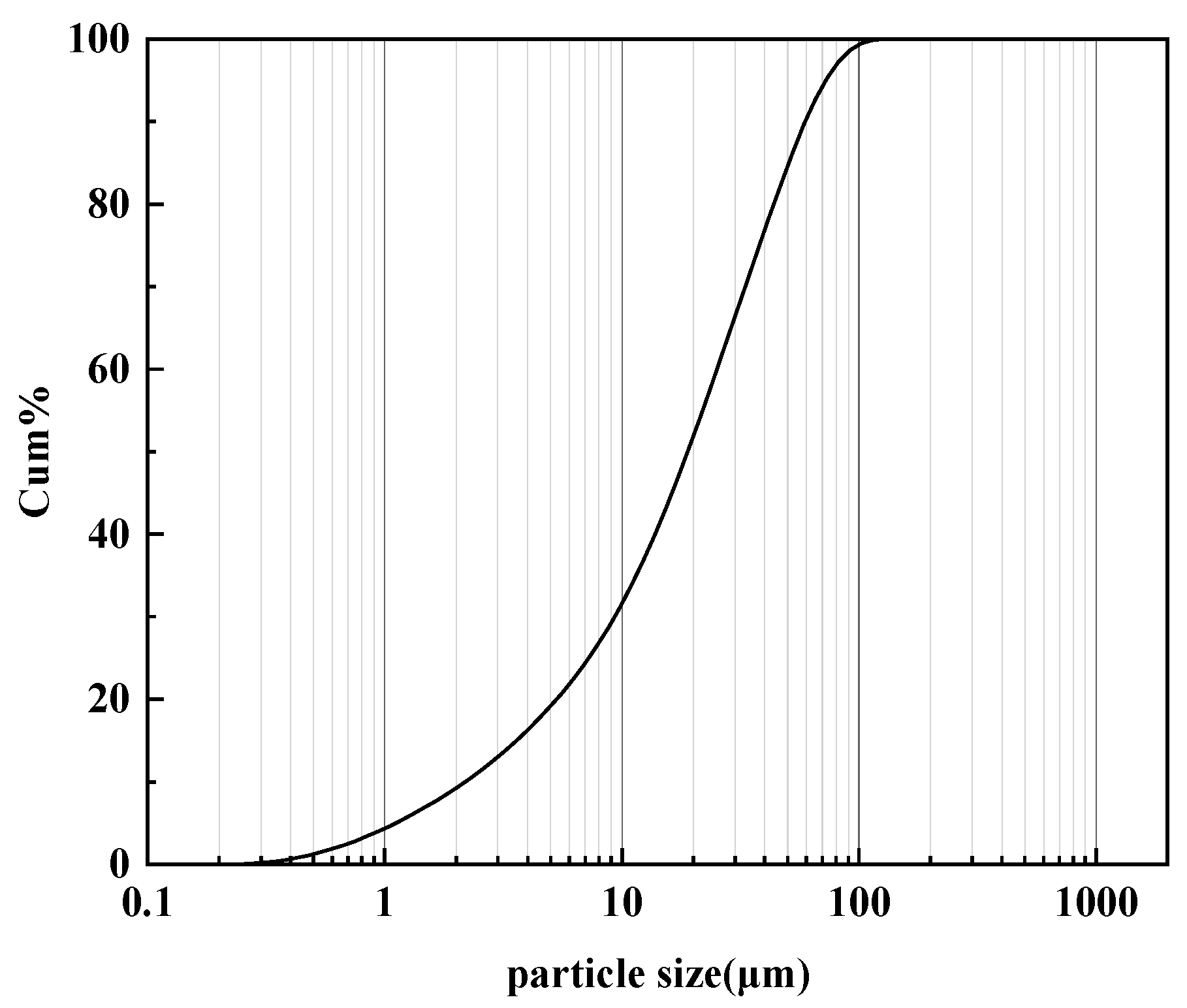

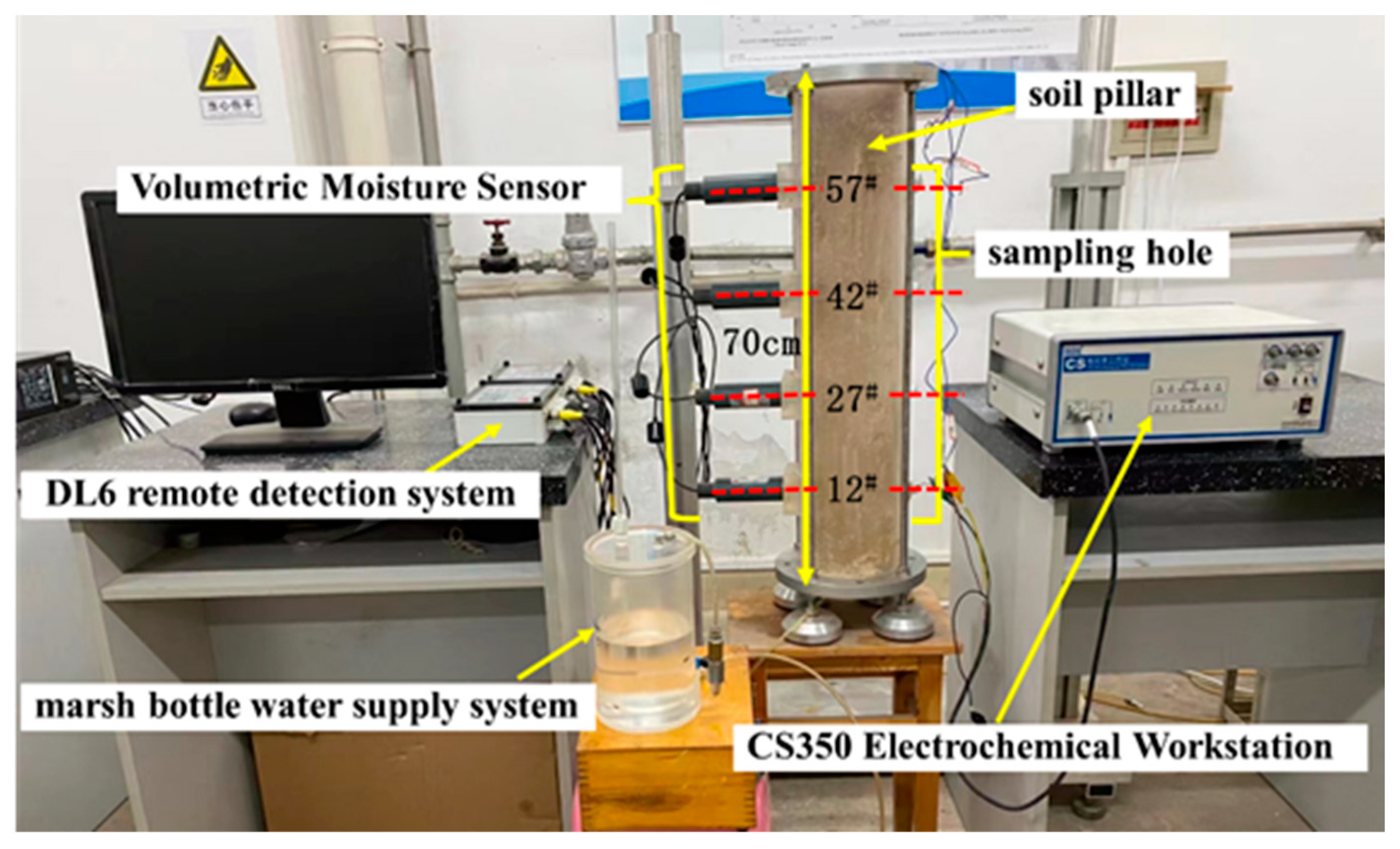
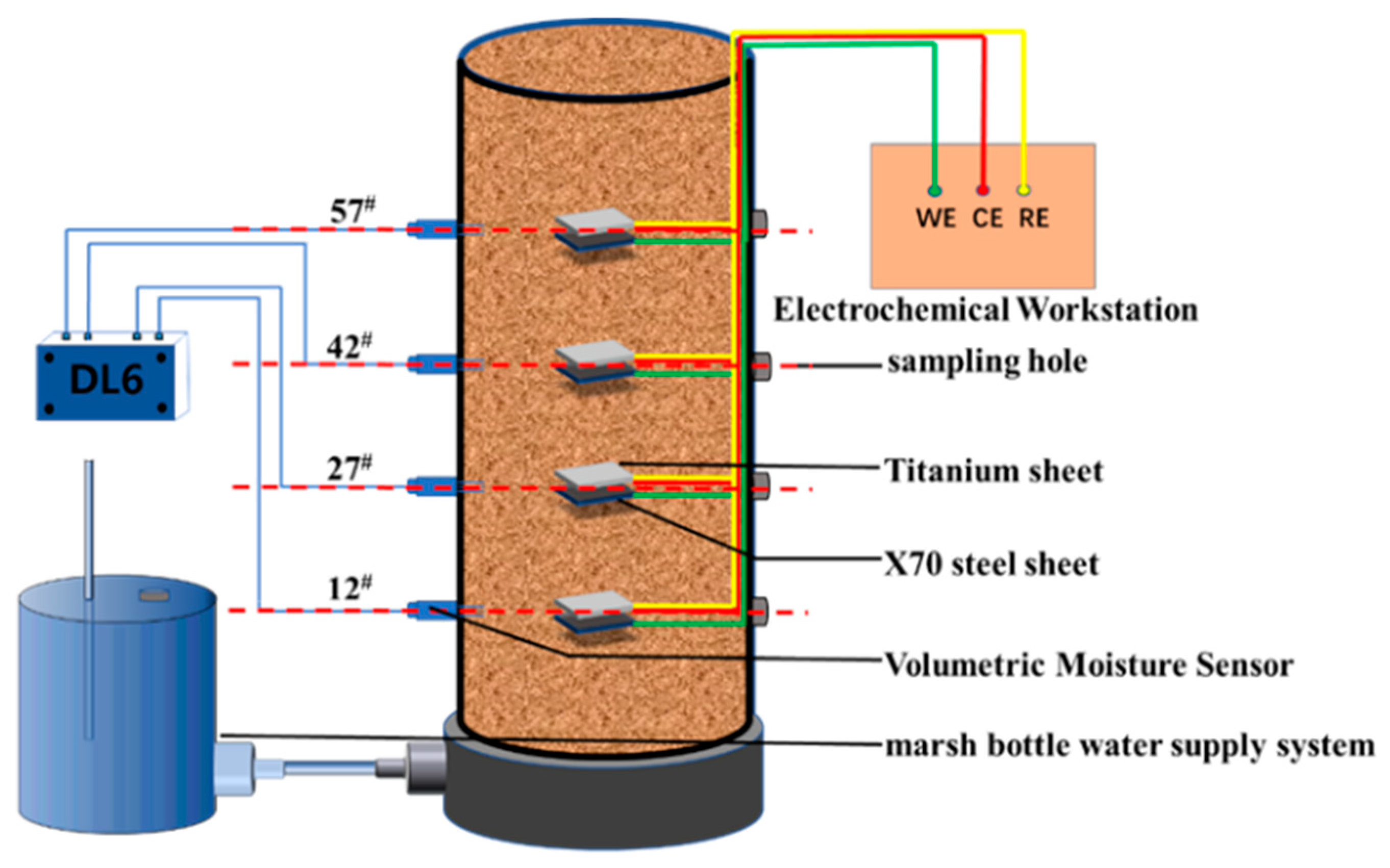



| Chemical Composition | C | Mn | Si | P | S | Nb | Cu | Cr | Ni | Mo | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Values/(%) | 0.050 | 1.540 | 0.190 | 0.009 | 0.001 | 0.070 | 0.230 | 0.200 | 0.200 | 0.190 | 0.02 |
| Parameters | Values |
|---|---|
| natural water content/(%) | 12.24 |
| original dry density/(g·cm−3) | 1.490 |
| maximum dry density/(g·cm−3) | 1.810 |
| optimum moisture content(%) | 20.80 |
| liquid limit/(%) | 30.30 |
| plastic limit/(%) | 18.80 |
| plastic index | 11.50 |
| Water Content/(%) | NaCl Content/(%) | Dry Soil Quality/(g) | NaCl Quality/(g) | Water Quality/(g) |
|---|---|---|---|---|
| 12.24 | 0 | 18,000 | 0 | 1689.3 |
| 0.3 | 18,000 | 54.0 | 1689.3 | |
| 1.0 | 18,000 | 180.0 | 1689.3 |
| Time/(Day) | NaCl Content/(%) | Ba/(mV/dec) | Bc/(mV/dec) | RP/(Ω/cm2) | Icorr/(A/cm2) | Ecorr/V | Corrosion Rate/(mm/a) |
|---|---|---|---|---|---|---|---|
| 14 | 0.0% | 295.61 | 168.37 | 9.64 × 105 | 3.3826 × 10−8 | −0.4848 | 0.3979 × 10−3 |
| 0.3% | 374.03 | 168.30 | 8.19 × 105 | 6.0887 × 10−8 | −0.5913 | 0.7162 × 10−3 | |
| 1.0% | 730.79 | 195.88 | 6.85 × 105 | 7.8136 × 10−8 | −0.6645 | 0.9191 × 10−3 | |
| 28 | 0.0% | 166.12 | 120.50 | 8.90 × 105 | 4.0271 × 10−8 | −0.6224 | 0.4737 × 10−3 |
| 0.3% | 329.91 | 330.07 | 8.36 × 105 | 6.0692 × 10−8 | −0.6614 | 0.7139 × 10−3 | |
| 1.0% | 238.77 | 180.14 | 6.11 × 105 | 1.1094 × 10−7 | −0.4396 | 1.3049 × 10−3 | |
| 45 | 0.0% | 158.14 | 114.31 | 9.92 × 105 | 2.8260 × 10−8 | −0.5743 | 0.3324 × 10−3 |
| 0.3% | 1731.4 | 263.48 | 5.26 × 105 | 1.7347 × 10−7 | −0.6943 | 2.0404 × 10−3 | |
| 1.0% | 742.85 | 217.10 | 4.80 × 105 | 1.8616 × 10−7 | −0.6034 | 2.1896 × 10−3 |
| High Position | Time/ (Day) | Ba/(mV/dec) | Bc/(mV/dec) | RP/(Ω/cm2) | Icorr/(A/cm2) | Ecorr/V | Corrosion Rate/(mm/a) |
|---|---|---|---|---|---|---|---|
| 12# | 14 | 374.03 | 168.30 | 8.19 × 105 | 6.0887 × 10−8 | −0.5913 | 0.7162 × 10−3 |
| 28 | 329.91 | 330.07 | 8.36 × 105 | 6.0692 × 10−8 | −0.6614 | 0.7139 × 10−3 | |
| 45 | 1731.4 | 263.48 | 5.26 × 105 | 1.7347 × 10−7 | −0.6942 | 2.0404 × 10−3 | |
| 27# | 14 | 184.58 | 139.67 | 1.33 × 106 | 1.9443 × 10−8 | −0.5745 | 0.2287 × 10−3 |
| 28 | 313.66 | 256.77 | 6.52 × 105 | 1.0495 × 10−7 | −0.8163 | 1.2344 × 10−3 | |
| 45 | 441.71 | 184.82 | 8.06 × 105 | 4.5026 × 10−8 | −0.7477 | 0.5296 × 10−3 | |
| 42# | 14 | 454.41 | 327.71 | 8.27 × 106 | 6.5365 × 10−9 | −0.5720 | 0.0769 × 10−3 |
| 28 | 446.85 | 247.65 | 2.72 × 106 | 9.2309 × 10−9 | −0.6677 | 0.1086 × 10−3 | |
| 45 | 403.99 | 222.72 | 6.32 × 106 | 8.1436 × 10−9 | −0.6921 | 0.0959 × 10−3 | |
| 57# | 14 | 296.26 | 232.05 | 7.81 × 105 | 4.5504 × 10−8 | −0.6889 | 0.5352 × 10−3 |
| 28 | 305.78 | 169.84 | 7.56 × 105 | 4.2272 × 10−8 | −0.8030 | 0.4972 × 10−3 | |
| 45 | 202.00 | 186.62 | 6.02 × 105 | 1.2525 × 10−7 | −0.4773 | 1.4732 × 10−3 |
| Time/ (Day) | NaCl Content/ (%) | Rs/ (Ω·cm2) | Ccp/ (F·cm−2) | Rcp/ (Ω·cm2) | Cs/ (F·cm−2) | Rct/ (Ω·cm2) | W1-R | W1-T | W1-P |
|---|---|---|---|---|---|---|---|---|---|
| 14 d | 0.0% | 203.8 | 8.45 × 10−10 | 2733 | 9.23 × 10−8 | 1225 | 1.66 × 106 | 191.9 | 0.6185 |
| 0.3% | 249.1 | 1.10 × 10−9 | 1118 | 2.57 × 10−7 | 586.6 | 3.68 × 105 | 15.84 | 0.6923 | |
| 1.0% | 148.6 | 3.59 × 10−6 | 54.56 | 5.58 × 10−6 | 478.5 | 3.38 × 105 | 16.54 | 0.7302 | |
| 28 d | 0.0% | 395.6 | 1.16 × 10−9 | 2035 | 1.28 × 10−7 | 1558 | 1.69 × 106 | 145.0 | 0.6878 |
| 0.3% | 291.2 | 1.31 × 10−9 | 992.0 | 2.44 × 10−7 | 568.4 | 1.96 × 105 | 6.941 | 0.7036 | |
| 1.0% | 271.2 | 2.86 × 10−9 | 615.4 | 4.61 × 10−6 | 386.8 | 2.09 × 104 | 1.303 | 0.4609 | |
| 45 d | 0.0% | 314.2 | 1.06 × 10−9 | 2209 | 1.26 × 10−7 | 1187 | 5.48 × 105 | 33.74 | 0.6918 |
| 0.3% | 252.5 | 1.20 × 10−9 | 1021 | 2.60 × 10−7 | 163.0 | 2.60 × 105 | 11.42 | 0.7139 | |
| 1.0% | 224.2 | 1.08 × 10−9 | 990.3 | 1.91 × 10−6 | 114.0 | 1.35 × 105 | 8.428 | 0.7065 |
| High Position | Time/ (Day) | Rs/ (Ω·cm2) | Ccp/ (F·cm−2) | Rcp/ (Ω·cm2) | Cs/ (F·cm−2) | Rct/ (Ω·cm2) | W1-R | W1-T | W1-P |
|---|---|---|---|---|---|---|---|---|---|
| 12# | 14 d | 249.1 | 1.10 × 10−9 | 1118 | 2.57 × 10−7 | 586.6 | 3.68 × 106 | 15.84 | 0.6923 |
| 28 d | 291.2 | 1.30 × 10−9 | 992.0 | 2.44 × 10−7 | 568.4 | 1.96 × 106 | 6.941 | 0.7036 | |
| 45 d | 252.5 | 1.20 × 10−9 | 1021 | 2.60 × 10−7 | 163.0 | 2.60 × 106 | 11.42 | 0.7139 | |
| 27# | 14 d | 175.6 | 1.19 × 10−6 | 29.77 | 1.81 × 10−7 | 493.9 | 2.95 × 106 | 19.39 | 0.6933 |
| 28 d | 169.2 | 4.30 × 10−6 | 30.14 | 2.58 × 10−6 | 473.8 | 1.99 × 106 | 4.939 | 0.7162 | |
| 45 d | 161.7 | 3.52 × 10−6 | 71.96 | 4.07 × 10−6 | 661.1 | 4.27 × 106 | 10.58 | 0.6986 | |
| 42# | 14 d | 414.5 | 4.22 × 10−9 | 2804 | 7.45 × 10−10 | 846.6 | 2.81 × 107 | 0.303 | 0.2811 |
| 28 d | 618.6 | 1.21 × 10−9 | 6170 | 2.85 × 10−9 | 598.3 | 3.79 × 107 | 98.42 | 0.3096 | |
| 45 d | 388.0 | 8.92 × 10−9 | 4455 | 1.27 × 10−8 | 631.2 | 1.32 × 107 | 7.561 | 0.5210 | |
| 57# | 14 d | 232.2 | 3.39 × 10−6 | 106.8 | 1.77 × 10−7 | 514.9 | 1.47 × 106 | 4.994 | 0.7032 |
| 28 d | 214.0 | 4.07 × 10−6 | 96.72 | 2.71 × 10−7 | 695.1 | 3.90 × 106 | 14.47 | 0.7535 | |
| 45 d | 224.4 | 4.12 × 10−6 | 64.16 | 6.03 × 10−6 | 1070 | 8.84 × 105 | 8.541 | 0.6606 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; He, B.; Feng, Y.; Hou, L.; Han, P.; Bai, X. Electrochemical Corrosion Behaviour of X70 Steel under the Action of Capillary Water in Saline Soils. Materials 2022, 15, 3426. https://doi.org/10.3390/ma15103426
Wei J, He B, Feng Y, Hou L, Han P, Bai X. Electrochemical Corrosion Behaviour of X70 Steel under the Action of Capillary Water in Saline Soils. Materials. 2022; 15(10):3426. https://doi.org/10.3390/ma15103426
Chicago/Turabian StyleWei, Jianjian, Bin He, Yongxiang Feng, Lifeng Hou, Pengju Han, and Xiaohong Bai. 2022. "Electrochemical Corrosion Behaviour of X70 Steel under the Action of Capillary Water in Saline Soils" Materials 15, no. 10: 3426. https://doi.org/10.3390/ma15103426
APA StyleWei, J., He, B., Feng, Y., Hou, L., Han, P., & Bai, X. (2022). Electrochemical Corrosion Behaviour of X70 Steel under the Action of Capillary Water in Saline Soils. Materials, 15(10), 3426. https://doi.org/10.3390/ma15103426





