An Auto-Calibrating Semi-Adiabatic Calorimetric Methodology for Strength Prediction and Quality Control of Ordinary and Ultra-High-Performance Concretes
Abstract
:1. Introduction
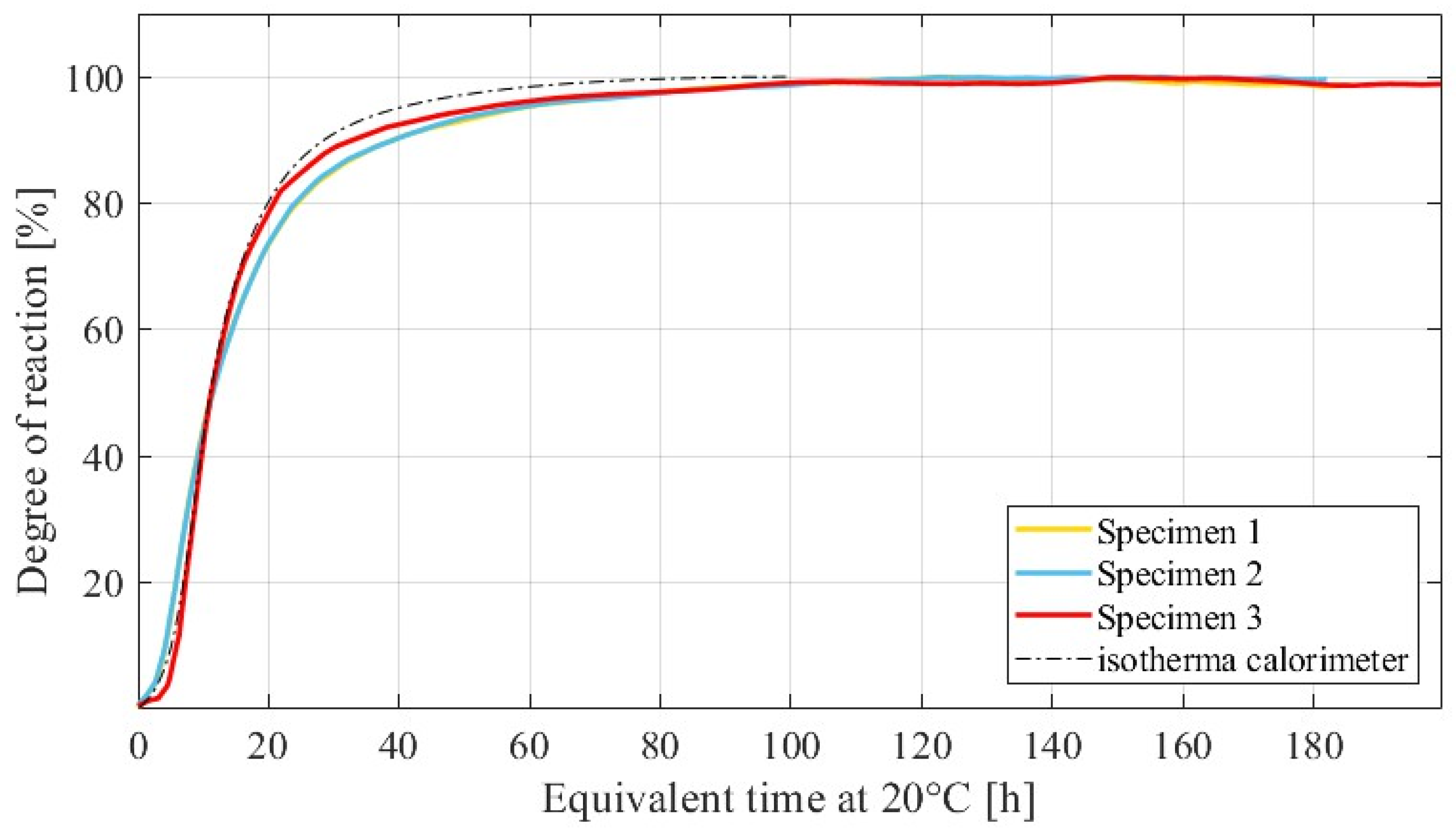
1.1. Adiabatic Technique to Calculate the Degree of Reaction
1.2. Semi-Adiabatic Calorimeter (SAC) to Calculate the Adiabatic Heat Release
1.3. Standard and Non-Standard Methods to Determine the Heat-Loss Factor λ
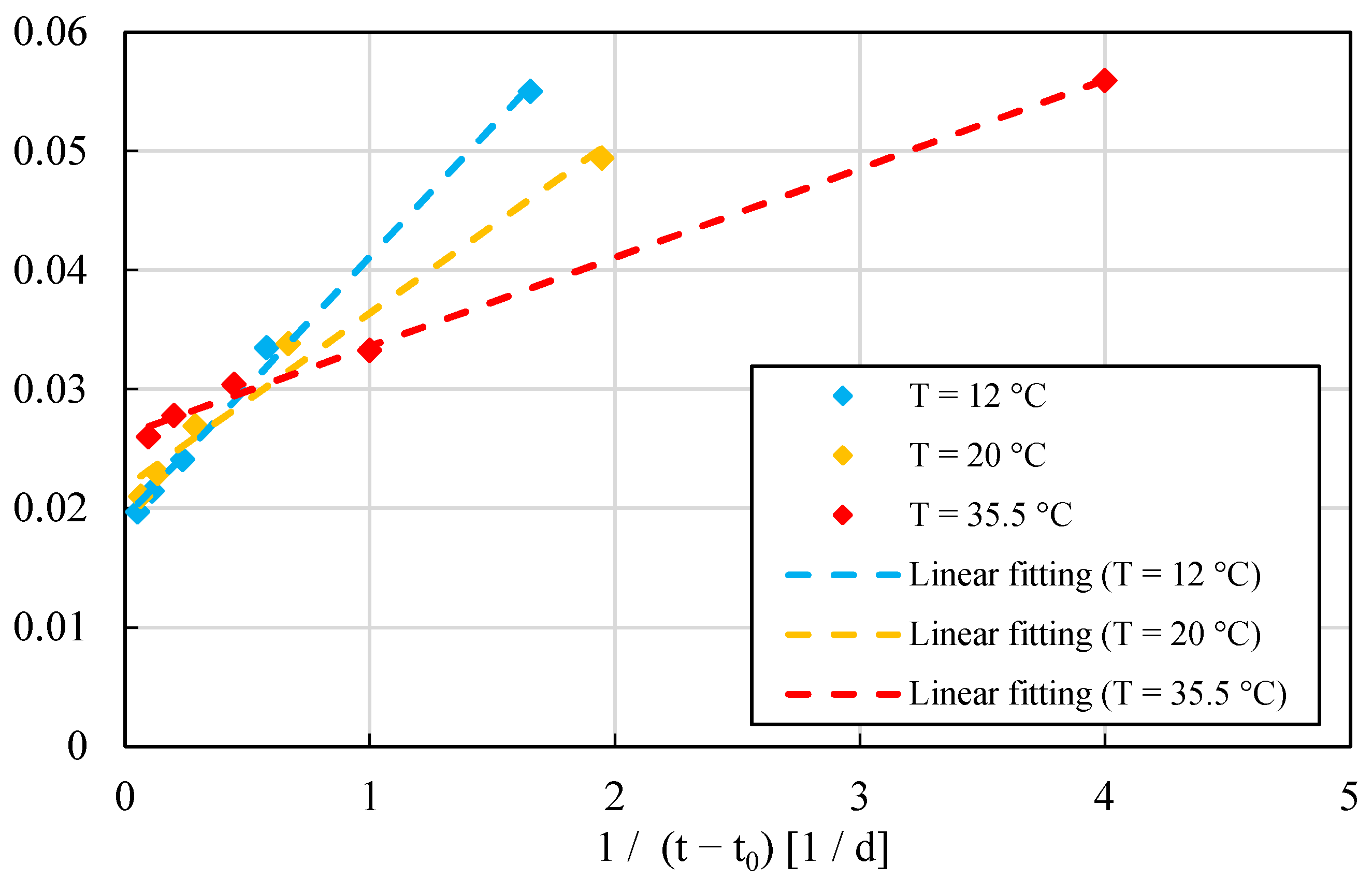
1.4. Determination of the Activation Energy by Using Adiabatic Heat Curves
- It must be possible to measure or derive the adiabatic heat of hydration of two specimens; see Section 1;
- The two specimens must not have the same initial temperature and temperature rise.
2. Materials and Methods
3. Results
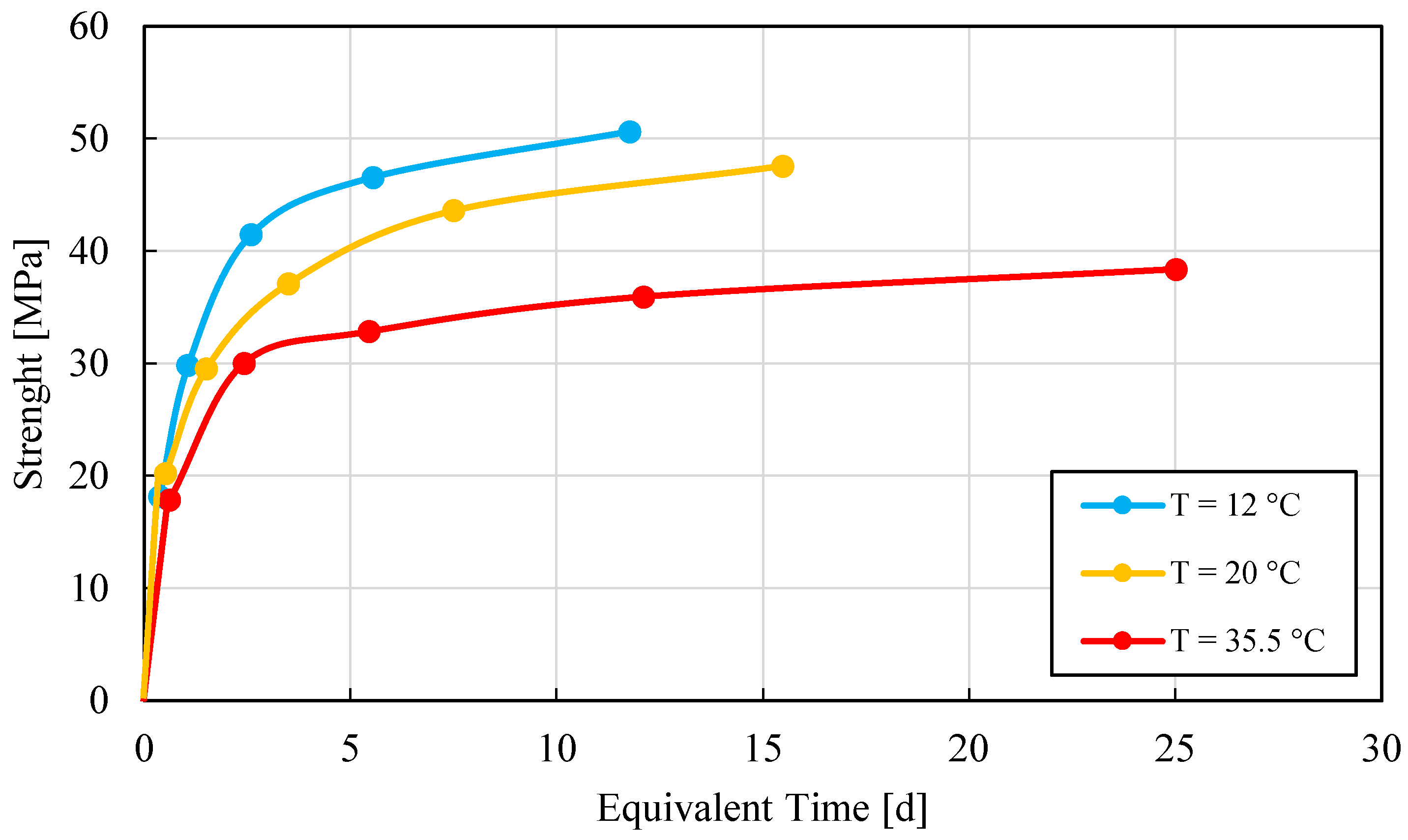
3.1. UHPC Series
3.2. OC-C30/37 Series
4. Discussion
5. Conclusions
- The SAC test is an accurate, inexpensive, fast and self-calibrating method to calculate the activation energy of cement-based materials;
- The quality of predictions of strength–maturity curves provided by the proposed method can be constantly improved as the specimens’ population increases;
- The SAC test is a good quality control tool since it can detect any variation of w/c ratio, change of quality or type of cement, compatibility problems with a new plasticizer and unexpected change of the mix proportions;
- This fast and inexpensive method to control quality of any concrete class is limited by the fact that values for the activation energy are obtained under specific temperature conditions and might not accurately represent the value of Ea under a much different temperature. A method which can overcome this limitation is the resistivity model based on Winner bounds, even though available data show that it is still not suitable for concrete due to the poor electrode contact and non-uniformity caused by the presence of coarse aggregate.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ASTM C1074-04; Standard Practice for Estimating Concrete Strength by the Maturity Method. ASTM: West Conshohocken, PA, USA, 2007.
- Hafiz, M.A.; Skibsted, J.; Denarié, E. Influence of low curing temperatures on the tensile response of low clinker strain hardening UHPC under full restraint. Cem. Concr. Res. 2020, 128, 105940. [Google Scholar] [CrossRef]
- Lu, X.; Tong, F.; Zha, X.; Liu, G. Equivalent method for obtaining concrete age on the basis of electrical resistivity. Sci. Rep. 2021, 11, 21720. [Google Scholar] [CrossRef] [PubMed]
- Nurse, R.W. Steam curing of concrete. Mag. Concr. Res. 1949, 1, 79–88. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.I.S.; Luxán, M.P.; Frías, M.; García, N. The influence of different additions on portland cement hydration heat. Cem. Concr. Res. 1993, 23, 46–54. [Google Scholar] [CrossRef]
- Bernhardt, C.J. Hardening of concrete at different temperatures. In Proceedings of the RILEM Symposium on Winter Concreting, Copenhagen, Denmark, 1 February 1956. Session BII. [Google Scholar]
- Hansen, P.F.; Pedersen, J. Maturity Computer for Controlled Curing and Hardening of Concrete. Nord. Betong. 1977, 1, 19–34. [Google Scholar]
- Hansen, J.F.; Pedersen, P. Curing of Concrete Structures. CEB Inf. Bull. 1985, 24, 21–28. [Google Scholar]
- McIntosh, J.D. Electrical curing of concrete. Mag. Concr. Res. 1949, 1, 21–28. [Google Scholar] [CrossRef]
- McIntosh, J.D. The Effects of Low Temperature Curing on Compressive Strenght of Concrete. In Proceedings of the RILEM Symposium on Winter Concreting, Copenhagen, Danemark, 1 February 1956. Session BII. [Google Scholar]
- Plowman, J.M. Curing temperature, age and strength of concrete. Mag. Concr. Res. 1953, 8, 13–22. [Google Scholar] [CrossRef]
- Saul, A.G.A. Principles underlying the steam curing of concrete at atmospheric pressure. Mag. Concr. Res. 1951, 2, 127–140. [Google Scholar] [CrossRef]
- Carino, N.; Lew, H. The maturity method: From theory to application. In Proceedings of the Structures 2001: A Structural Engineering Odyssey, Washington, DC, USA, 21–23 May 2001; pp. 1–19. [Google Scholar] [CrossRef]
- Copeland, L.E.; Kantro, D.L.; Verbeck, G. Chemistry of Hydration of Portland Cement. Int. Congr. Chem. Cem. 1960, 1, 429–468. [Google Scholar]
- De Schutter, G. Influence of hydration reaction on engineering properties of hardening concrete. Mater. Struct. 2002, 3, 447–452. [Google Scholar] [CrossRef]
- Schindler, A.K. Effect of temperature on hydration of cementitious materials. ACI Mater. J. 2004, 101, 72–81. [Google Scholar] [CrossRef]
- Arrhenius, S. On the reaction velocity of the inversion of cane sugar by acids. In Selected Readings in Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1967. [Google Scholar] [CrossRef]
- Viviani, M.; Glisic, B.; Smith, I.F.C. Three-day prediction of concrete compressive strength evolution. ACI Mater. J. 2005, 102, 231–236. [Google Scholar]
- Viviani, M. Monitoring and Modelling of Construction Materials during Hardening. PhD Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2005. [Google Scholar] [CrossRef]
- Viviani, M.; Glisic, B.; Smith, I.F.C. System for monitoring the evolution of the thermal expansion coefficient and autogenous deformation of hardening materials. Smart Mater. Struct. 2006, 15, 137–146. [Google Scholar] [CrossRef]
- Viviani, M.; Glisic, B.; Smith, I.F.C. Separation of thermal and autogenous deformation at varying temperatures using optical fiber sensors. Cem. Concr. Compos. 2007, 29, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Viviani, M.; Glisic, B.; Scrivener, K.L.; Smith, I.F.C. Equivalency points: Predicting concrete compressive strength evolution in three days. Cem. Concr. Res. 2008, 38, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Djamai, Z.I.; Salvatore, F.; Larbi, A.S.; Cai, G.; Mankibi, M.E. Multiphysics analysis of effects of encapsulated phase change materials (PCMs) in cement mortars. Cem. Concr. Res. 2019, 119, 51–63. [Google Scholar] [CrossRef]
- Ng, P.L.; Ng, I.Y.T.; Kwan, A.K.H. Heat Loss Compensation in Semi-Adiabatic Curing Test of Concrete. ACI Mater. J. 2008, 105, 52–61. [Google Scholar] [CrossRef]
- EN 196-1; Methods of Testing Cement—Part 9: Heat of Hydration, Semi-Adiabatic Method. SIST: Ljubljana, Slovenia, 2007.
- Attari, A.; McNally, C.; Richardson, M.G. A combined SEM–Calorimetric approach for assessing hydration and porosity development in GGBS concrete. Cem. Concr. Comp. 2016, 68, 46–56. [Google Scholar] [CrossRef]
- Brandštetr, J.; Polcer, J.; Krátký, J.; Holešinský, R.; Havlica, J. Possibilities of the use of Isoperibolic calorimetry for assessing the hydration behavior of cementitious systems. Cem. Concr. Res. 2001, 31, 941–947. [Google Scholar] [CrossRef]
- Gragg, F.M.; Skalny, J. A simple calorimeter for hydration studies. Cem. Concr. Res. 1972, 2, 745–748. [Google Scholar] [CrossRef]
- Gruyaert, E.; Robeyst, N.; de Belie, N. Study of the hydration of Portland cemente blended with blast-furnace slag by calorimetry and thermogravimetry. J. Therm. Anal. Calorim. 2010, 102, 941–951. [Google Scholar] [CrossRef]
- Hernandez-Bautista, E.; Bentz, D.P.; Sandoval-Torres, S.; de Cano-Barrita, P.F. Numerical simulation of heat and mass transport during hydration of Portland cement mortar in semi-adiabatic and steam curing conditions. Cem. Concr. Compos. 2016, 69, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Matthieu, B.; Farid, B.; Jean-Michel, T.; Georges, N. Analysis of semi-adiabiatic tests for the prediction of early-age behavior of massive concrete structures. Cem. Concr. Comp. 2012, 34, 634–641. [Google Scholar] [CrossRef]
- Riding, K.A.; Poole, J.L.; Folliard, K.J.; Juenger, M.C.G.; Schindler, A.K. Modeling hydration of cementitious systems. ACI Mater. J. 2012, 109, 225–234. [Google Scholar]
- Xu, Q.; Ruiz, J.M.; Hu, J.; Wang, K.; Rasmussen, R.O. Modeling hydration properties and temperature developments of early-age concrete pavement using calorimetry tests. Thermochim. Acta 2011, 512, 76–85. [Google Scholar] [CrossRef]
- Cement: Heat of Hydration. Report NT BUILD 480. 1997. Available online: www.nordtest.org (accessed on 19 December 2021).
- Savino, V.; Lanzoni, L.; Tarantino, A.M.; Viviani, M. Tensile constitutive behavior of high and ultra-high performance fibre-reinforced-concretes. Constr. Build. Mater. 2018, 186, 525–536. [Google Scholar] [CrossRef]
- Savino, V.; Lanzoni, L.; Tarantino, A.M.; Viviani, M. An extended model to predict the compressive, tensile and flexural strengths of HPFRCs and UHPCs: Definition and experimental validation. Compos. Part B Eng. 2019, 163, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Bétons Fibrés à Ultra-Hautes Performances (Ultra High Performance Fibre-Reinforced Concretes); Interim Recommendations; Comité Scientifique et Technique de l’AFGC: Paris, France, 2002.
- Béton Fibré Ultra-Performant (BFUP); Technical Report SIA 2052; Interim: Paris, France, 2015.
- Justo-Reinoso, I.; Hernandez, M.; Lucero, C.; Srubar, W. Dispersion and effects of metal impregnated granular activated carbon particles on the hydration of antimicrobial mortars. Cem. Concr. Comp. 2020, 110, 103588. [Google Scholar] [CrossRef]
- Li, Z.; Corr, D.; Han, B.; Shah, S.P. Investigating the effect of carbon nanotube on early age hydration of cementitious composites with isothermal calorimetry and Fourier transform infrared spectroscopy. Cem. Concr. Comp. 2020, 107, 103513. [Google Scholar] [CrossRef]
- Pang, X.; Bentz, D.; Meyer, C.; Funkhouser, G.; Darbe, R. A comparison study of Portland cement hydration kinetics as measured by chemical shrinkage and isothermal calorimetry. Cem. Concr. Comp. 2013, 39, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Suraneni, P.; Weiss, J. Examining the pozzolanicity of supplementary cementitious materials using isothermal calorimetry and thermogravimetric analysis. Cem. Concr. Comp. 2017, 83, 273–278. [Google Scholar] [CrossRef]
- Savino, V.; Lanzoni, L.; Tarantino, A.M.; Viviani, M. Modèle prédictif visant à optimiser les composants du BFUP en réponse aux exigences d’application. In Proceedings of the Troisième Journöe d’Étude BFUP—Béton Fibré Ultra-Performant: Concevoir, Dimensionner, Construire, Fribourg, Switzerland, 24 October 2019; pp. 101–111. [Google Scholar]
- Pelliciari, M.; Tarantino, A.M. Equilibrium and Stability of Anisotropic Hyperelastic Graphene Membranes. J. Elast. 2021, 144, 169–195. [Google Scholar] [CrossRef]
- Pelliciari, M.; Pasca, D.P.; Aloisio, A.; Tarantino, A.M. Size effect in single layer graphene sheets and transition from molecular mechanics to continuum theory. Int. J. Mech. Sci. 2022, 214, 106895. [Google Scholar] [CrossRef]
- EN 206-1; Concrete—Part 1: Specification, Performance, Production and Conformity. SIST: Ljubljana, Slovenia, 2000.
- Verbeck, G.J.; Helmuth, R.H. Structures and Physical Properties of Cement Paste. Int. Congr. Chem. Cem. 1968, 3, 1–44. [Google Scholar]
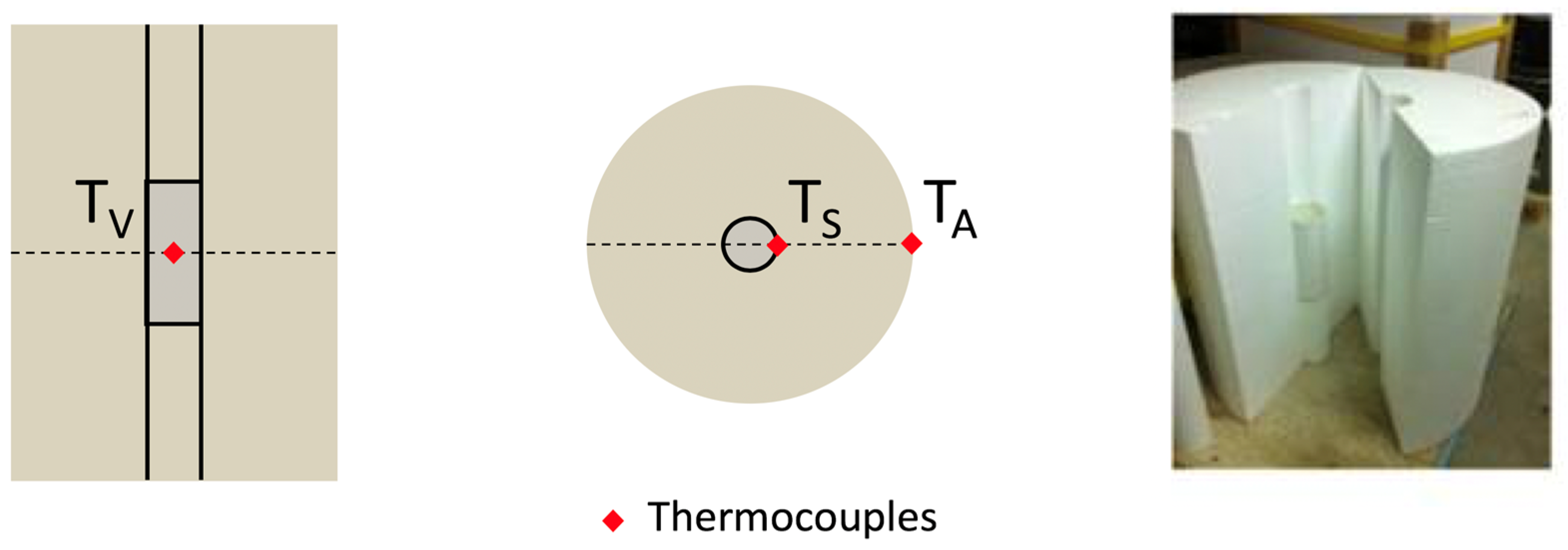
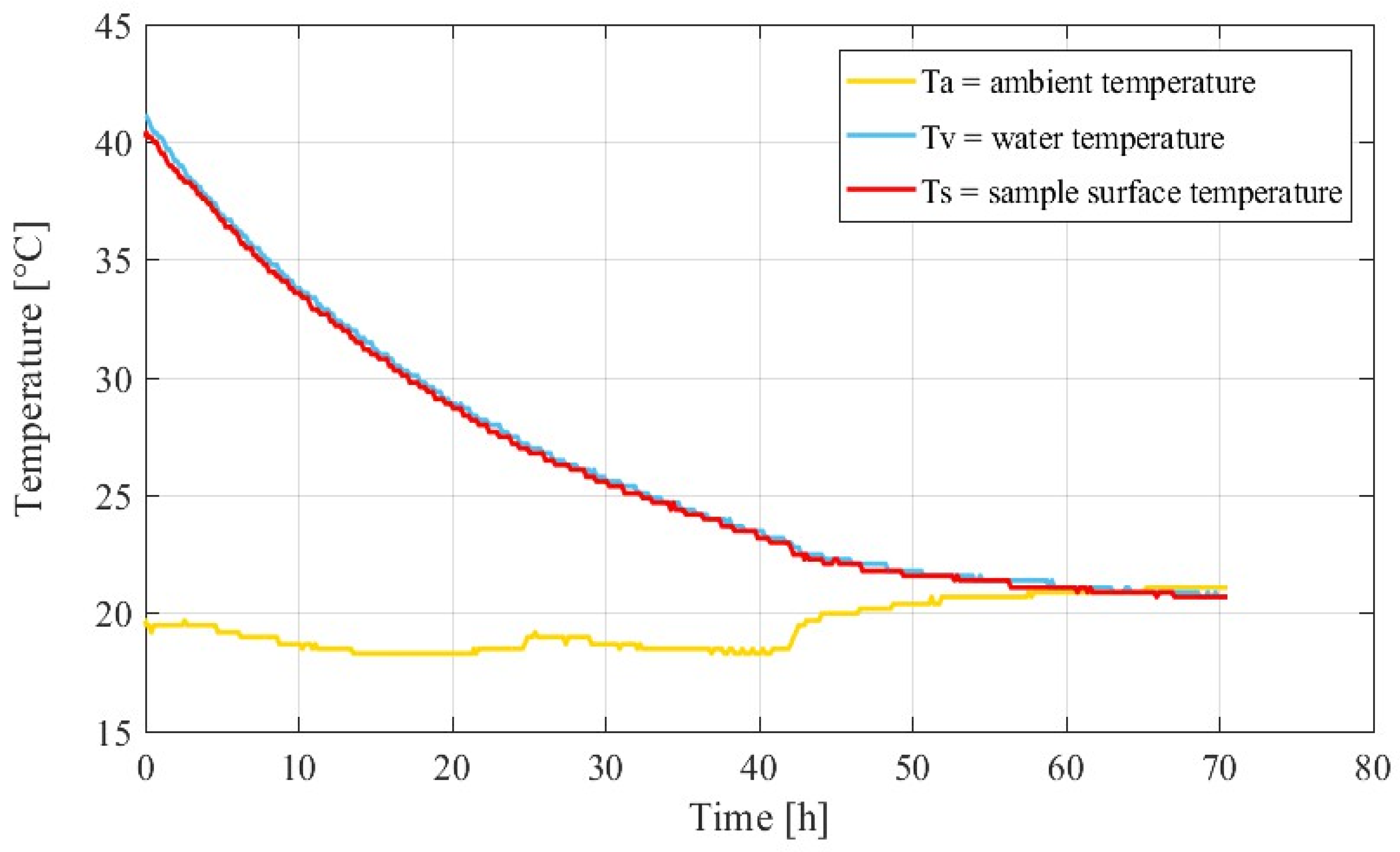
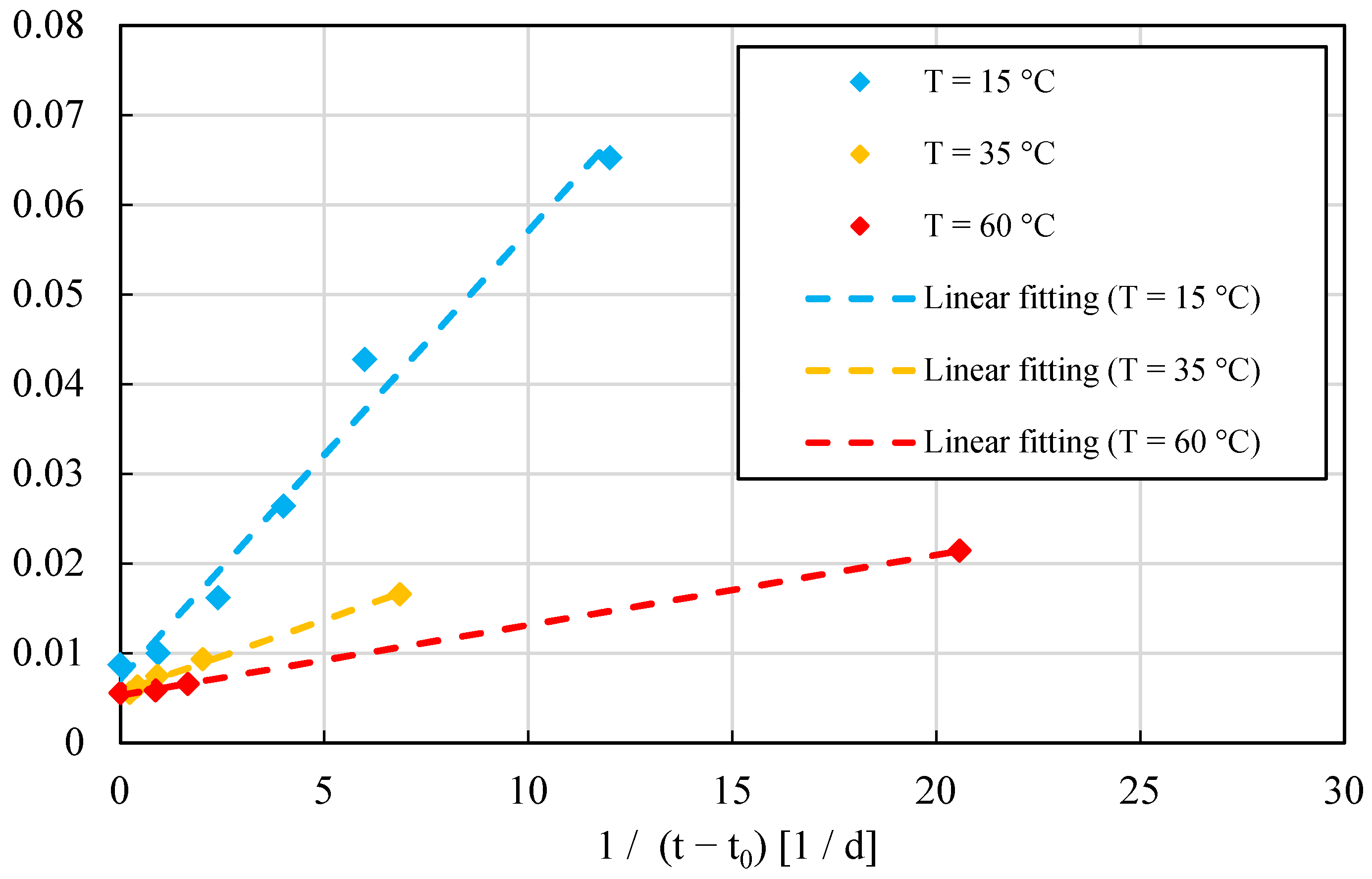
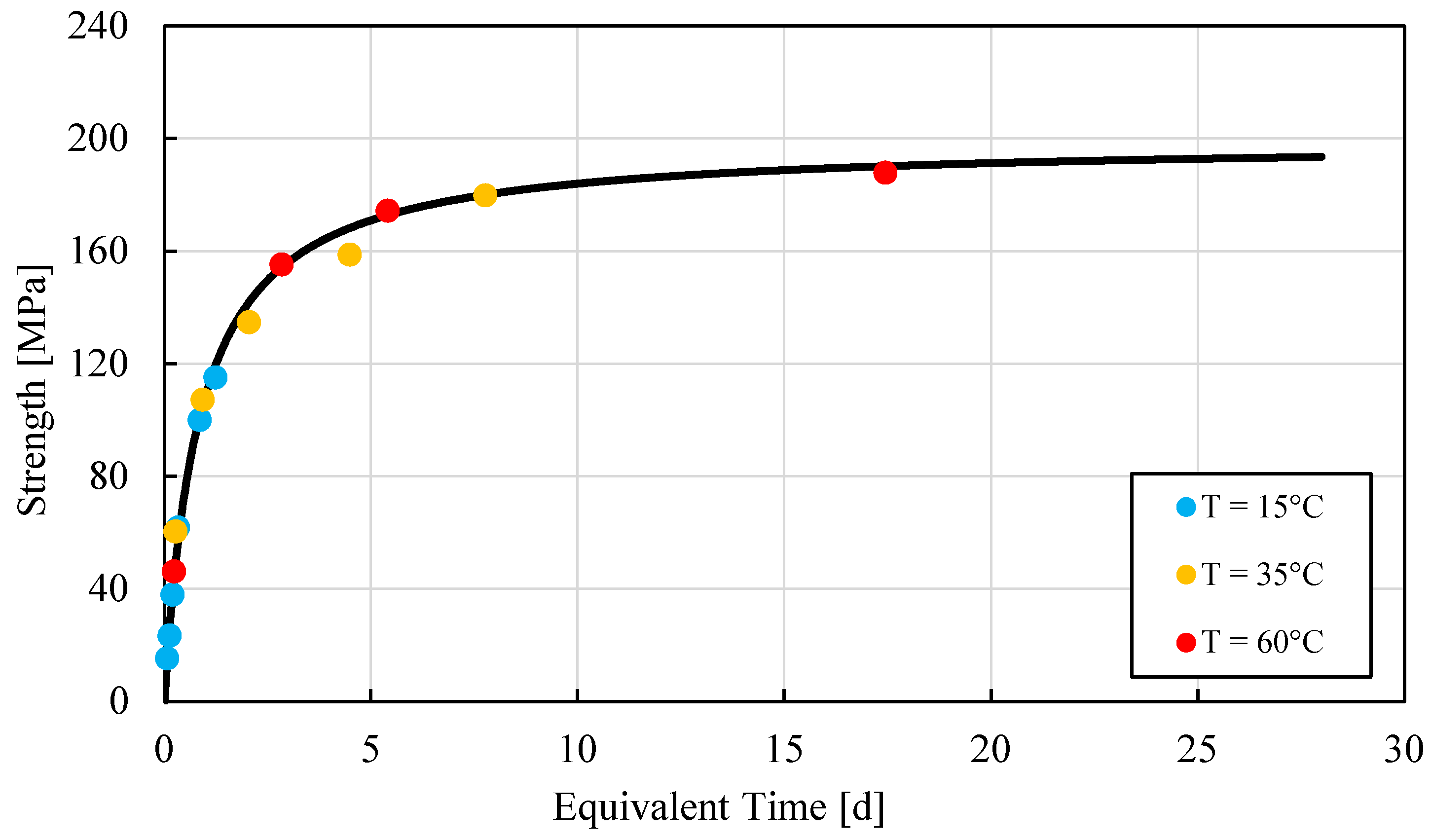

| Component | Kg/m3 |
|---|---|
| Premix 1 (cement, aggregate 0–6, silica fume) | 2296 |
| Water | 180 |
| Superplasticizer 1 | 40 |
| Set and hardening accelerator 1 | 20 |
| Steel fibers 2 (20/0.3 mm) | 195 |
| Component | Kg/m3 |
|---|---|
| Cement CEM II/A LL 425N | 340 |
| Water | 164 |
| Plasticizer | 0.015 |
| Rheology modifier | 0.005 |
| Aggregate (0–32) SSD | 1945 |
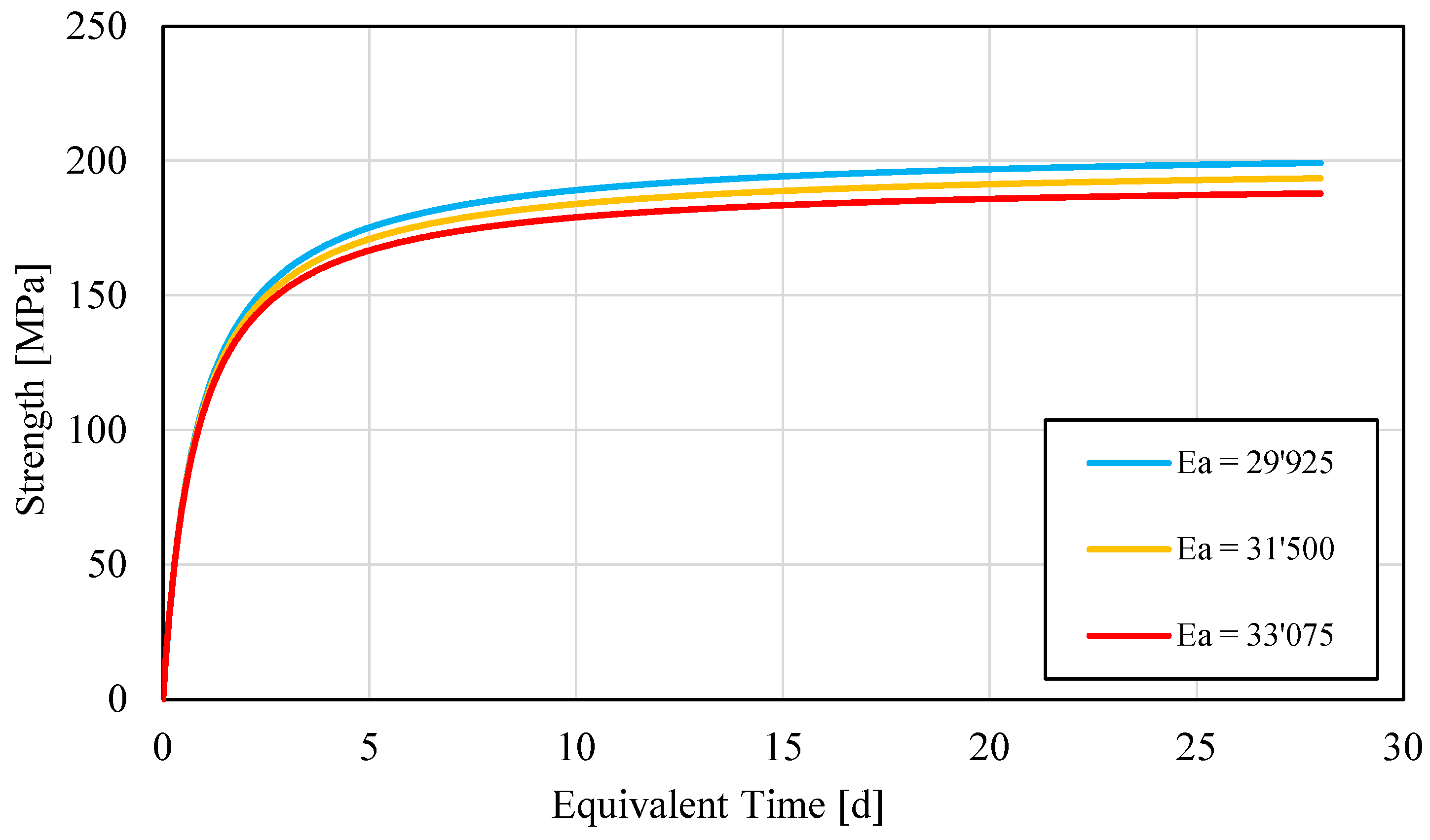
| Specimen Couple | Activation Energy Ea |
|---|---|
| S1–S2 | 31,161 |
| S1–S3 | 27,437 |
| S2–S3 | 32,772 |
| Series Couple | Activation Energy Ea |
|---|---|
| Couple 1 | 40,441 |
| Couple 2 | 44,595 |
| Couple 3 | 37,485 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viviani, M.; Lanzoni, L.; Savino, V.; Tarantino, A.M. An Auto-Calibrating Semi-Adiabatic Calorimetric Methodology for Strength Prediction and Quality Control of Ordinary and Ultra-High-Performance Concretes. Materials 2022, 15, 96. https://doi.org/10.3390/ma15010096
Viviani M, Lanzoni L, Savino V, Tarantino AM. An Auto-Calibrating Semi-Adiabatic Calorimetric Methodology for Strength Prediction and Quality Control of Ordinary and Ultra-High-Performance Concretes. Materials. 2022; 15(1):96. https://doi.org/10.3390/ma15010096
Chicago/Turabian StyleViviani, Marco, Luca Lanzoni, Vincenzo Savino, and Angelo Marcello Tarantino. 2022. "An Auto-Calibrating Semi-Adiabatic Calorimetric Methodology for Strength Prediction and Quality Control of Ordinary and Ultra-High-Performance Concretes" Materials 15, no. 1: 96. https://doi.org/10.3390/ma15010096
APA StyleViviani, M., Lanzoni, L., Savino, V., & Tarantino, A. M. (2022). An Auto-Calibrating Semi-Adiabatic Calorimetric Methodology for Strength Prediction and Quality Control of Ordinary and Ultra-High-Performance Concretes. Materials, 15(1), 96. https://doi.org/10.3390/ma15010096







