Investigation of Biocompatible PEO Coating Growth on cp-Ti with In Situ Spectroscopic Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Electrolytic Oxidation
2.2. In Situ Impedance Spectroscopy
2.3. In Situ Optical Emission Spectroscopy
2.4. Surface Characterization
3. Results
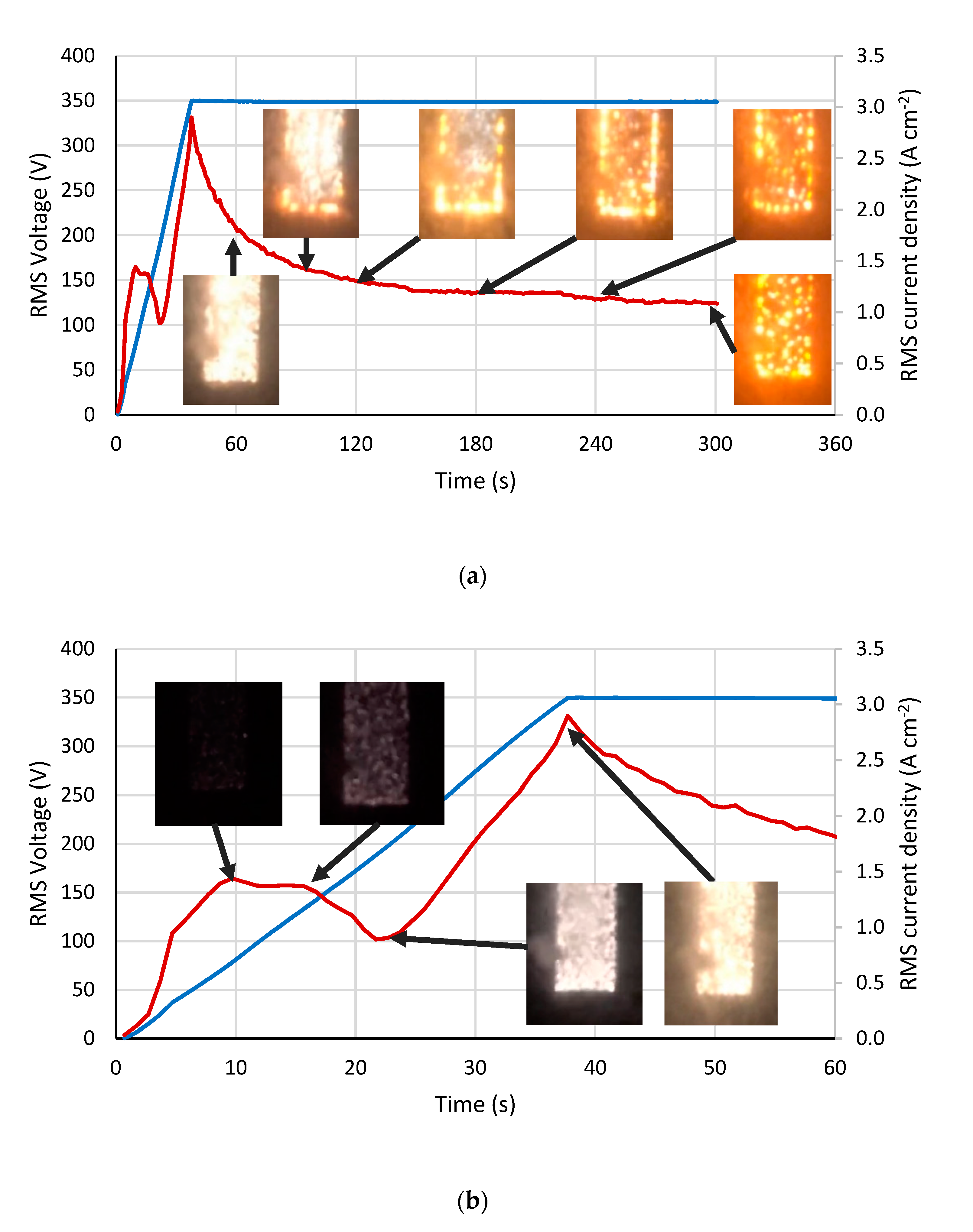
3.1. Electric Characteristics and Microdischarge Appearance during PEO of Ti
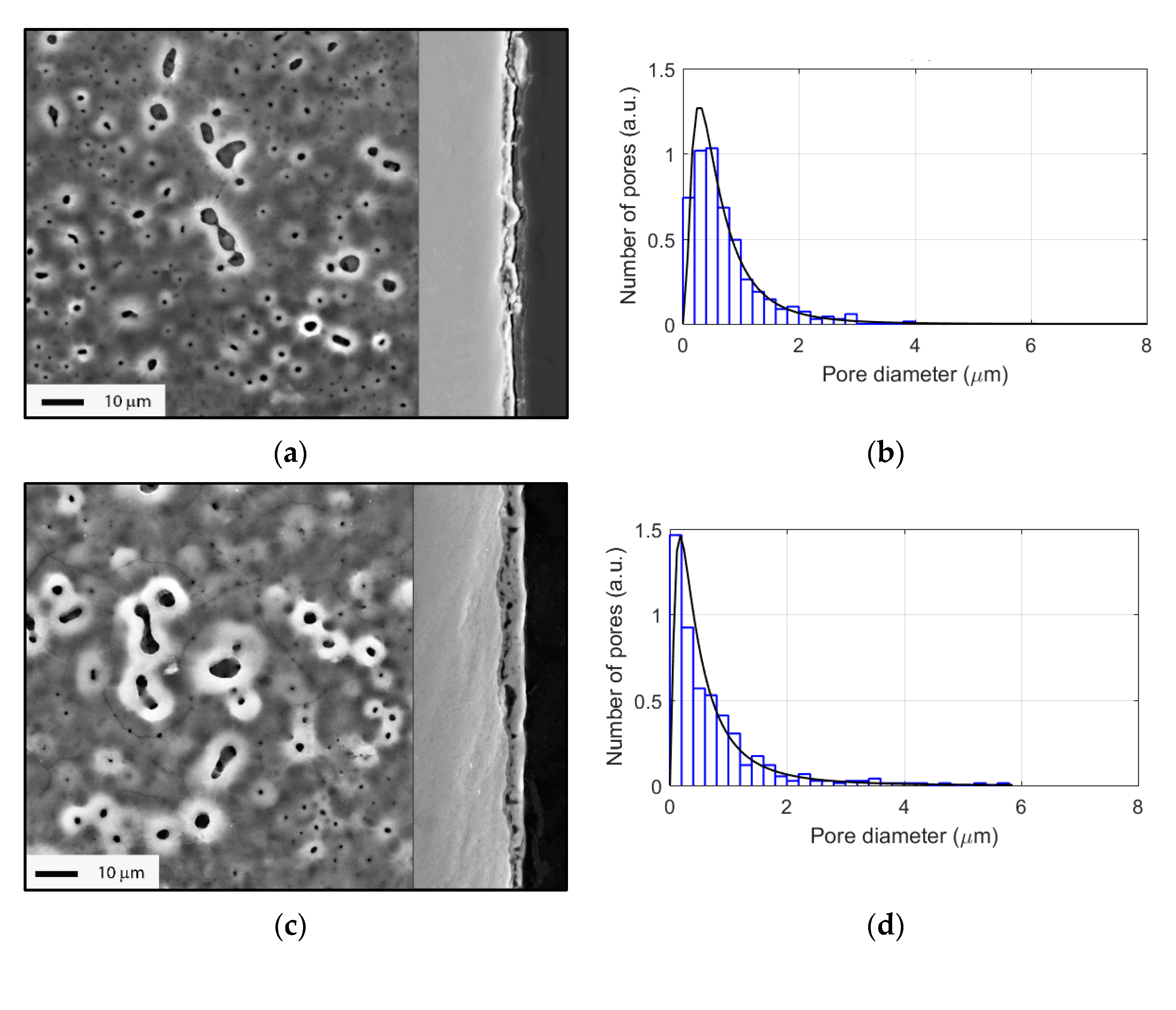
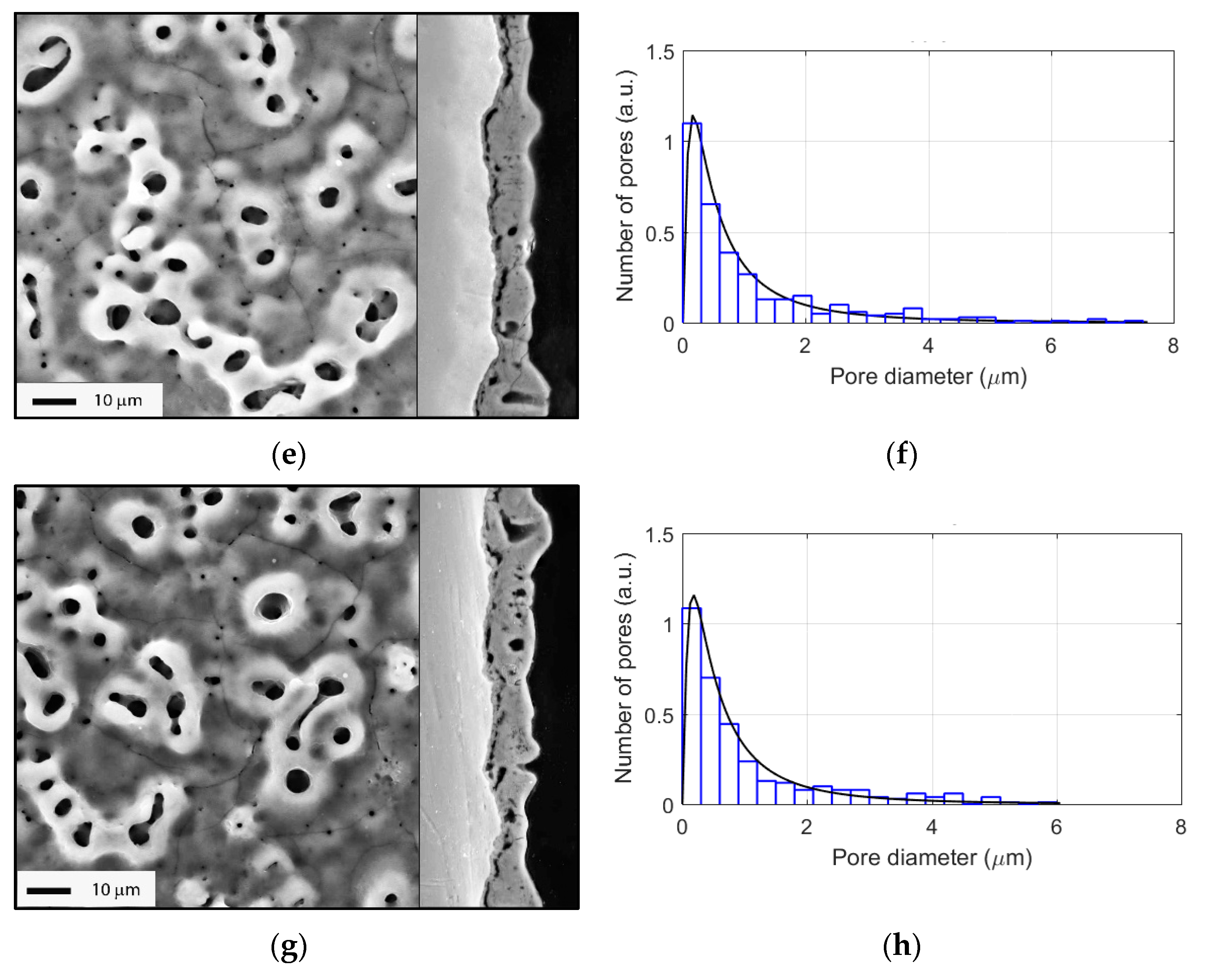
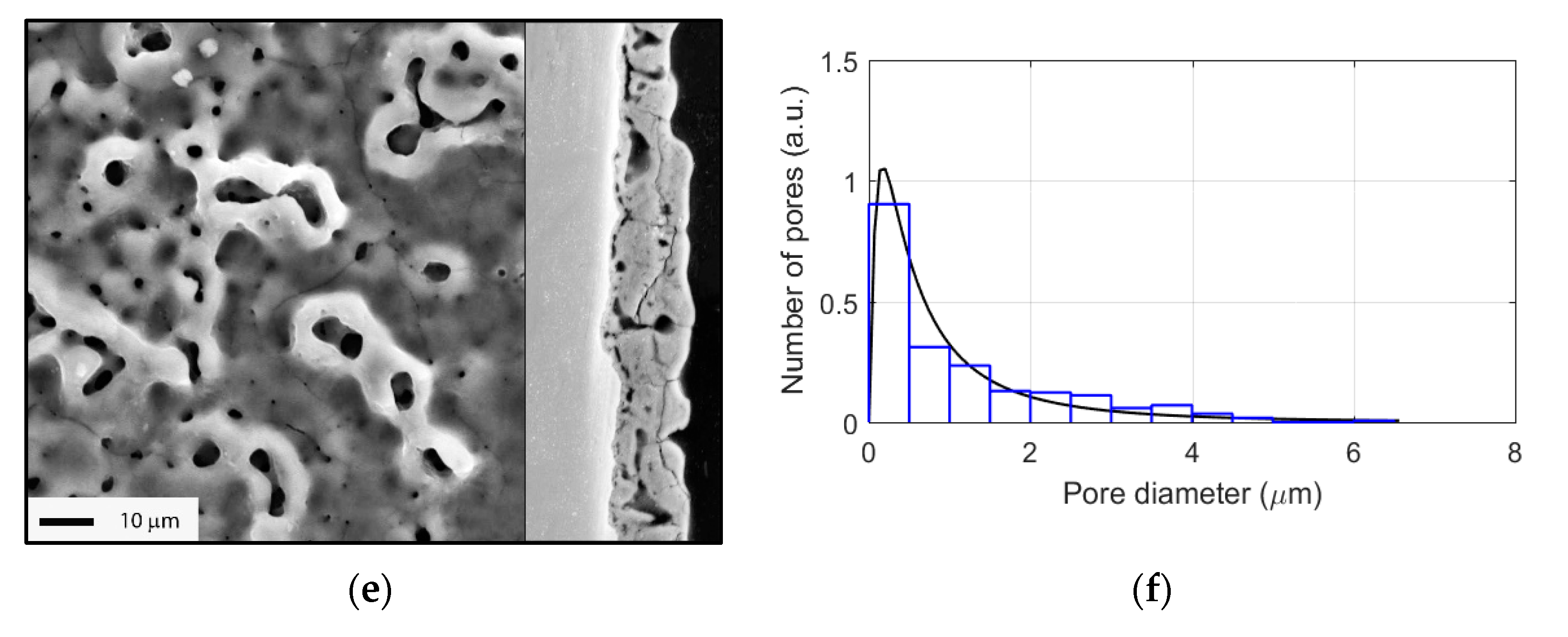
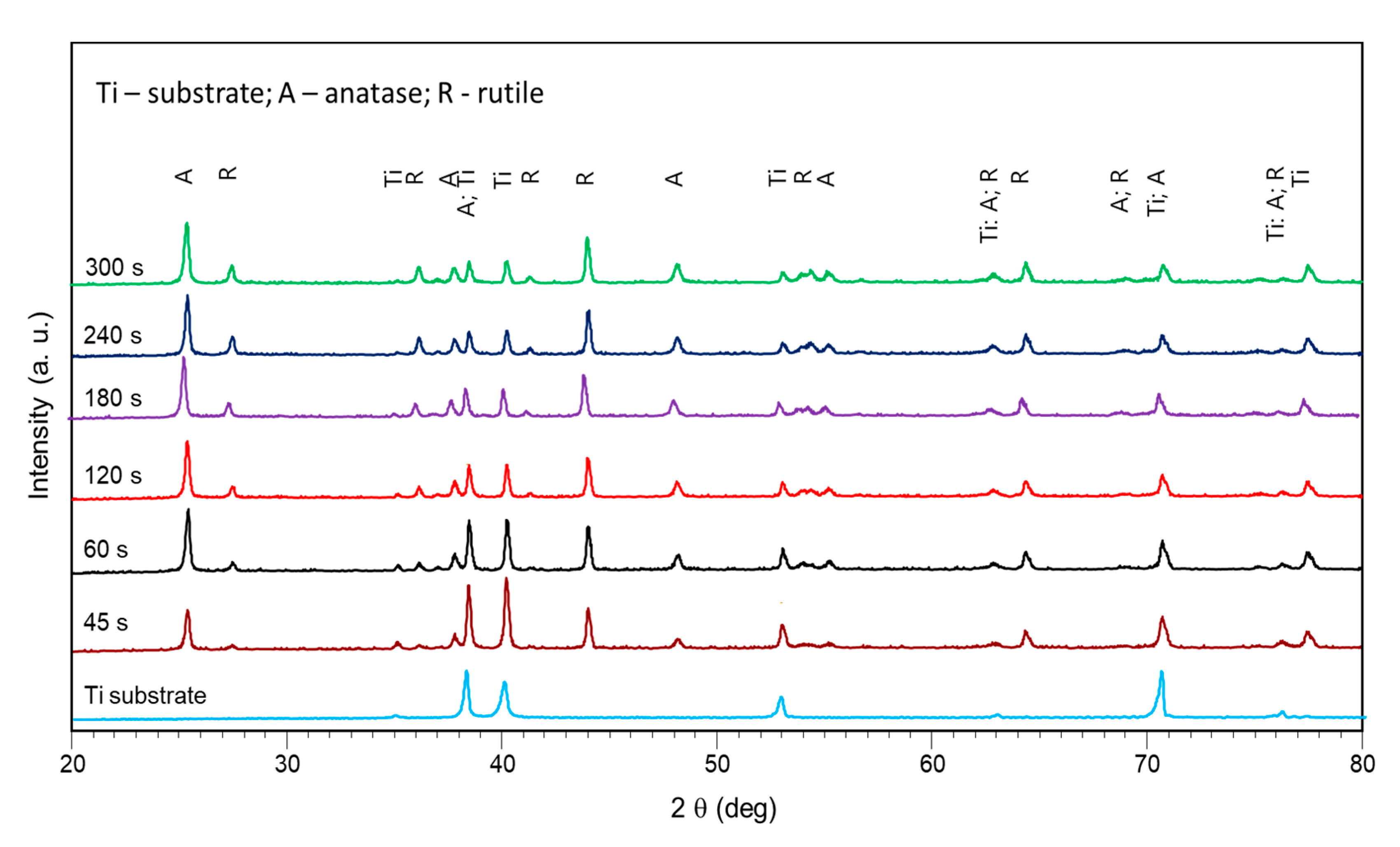
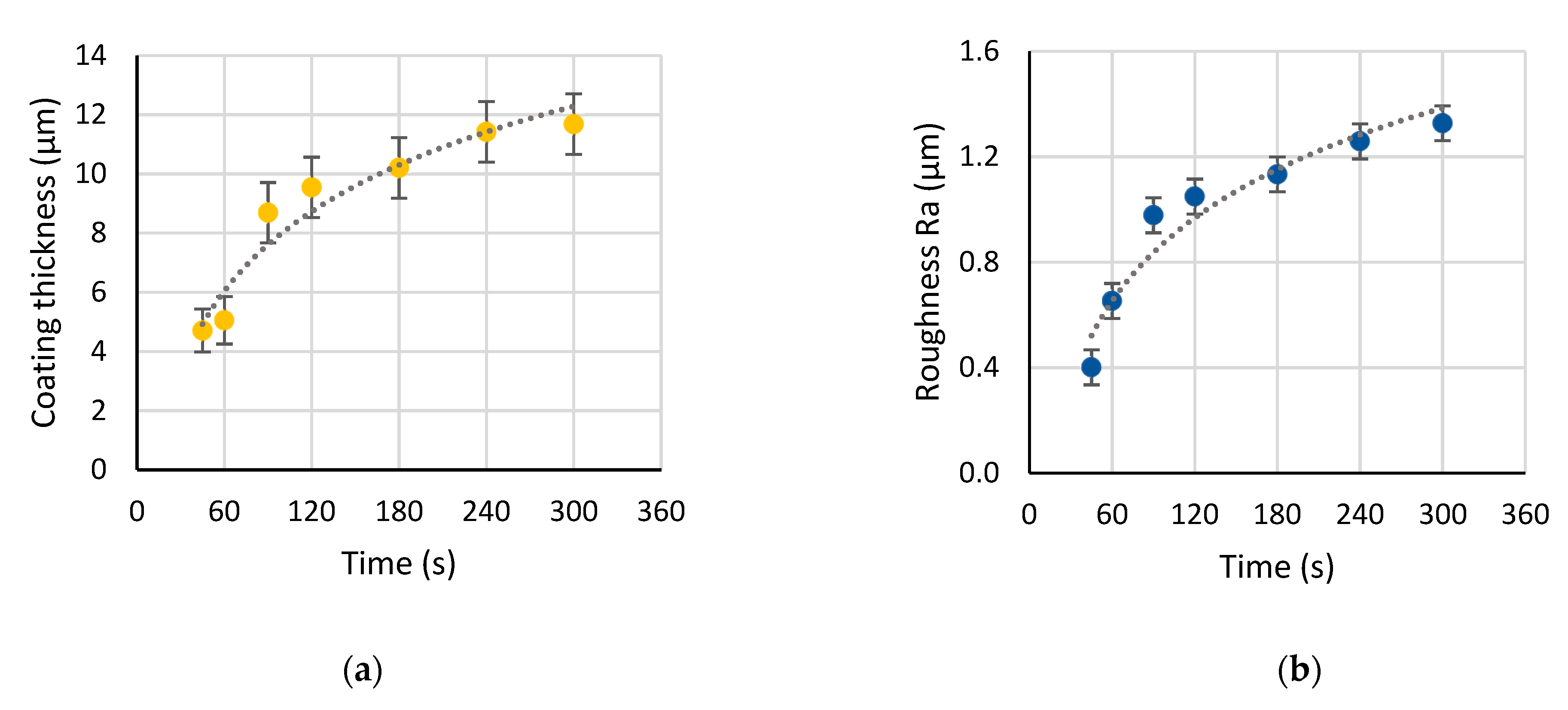
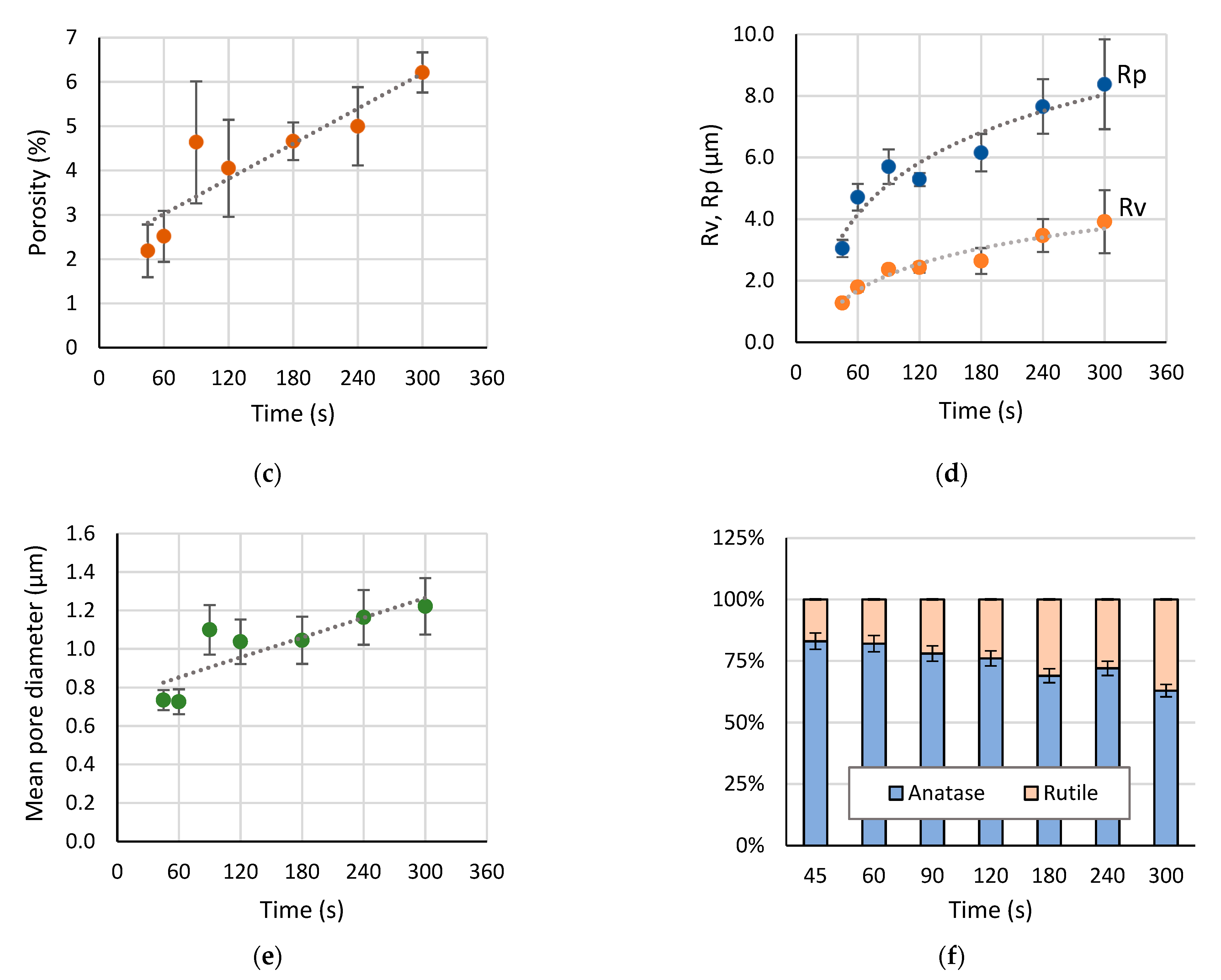
3.2. Evolution of Surface Morphology and Phase Composition during PEO of Ti
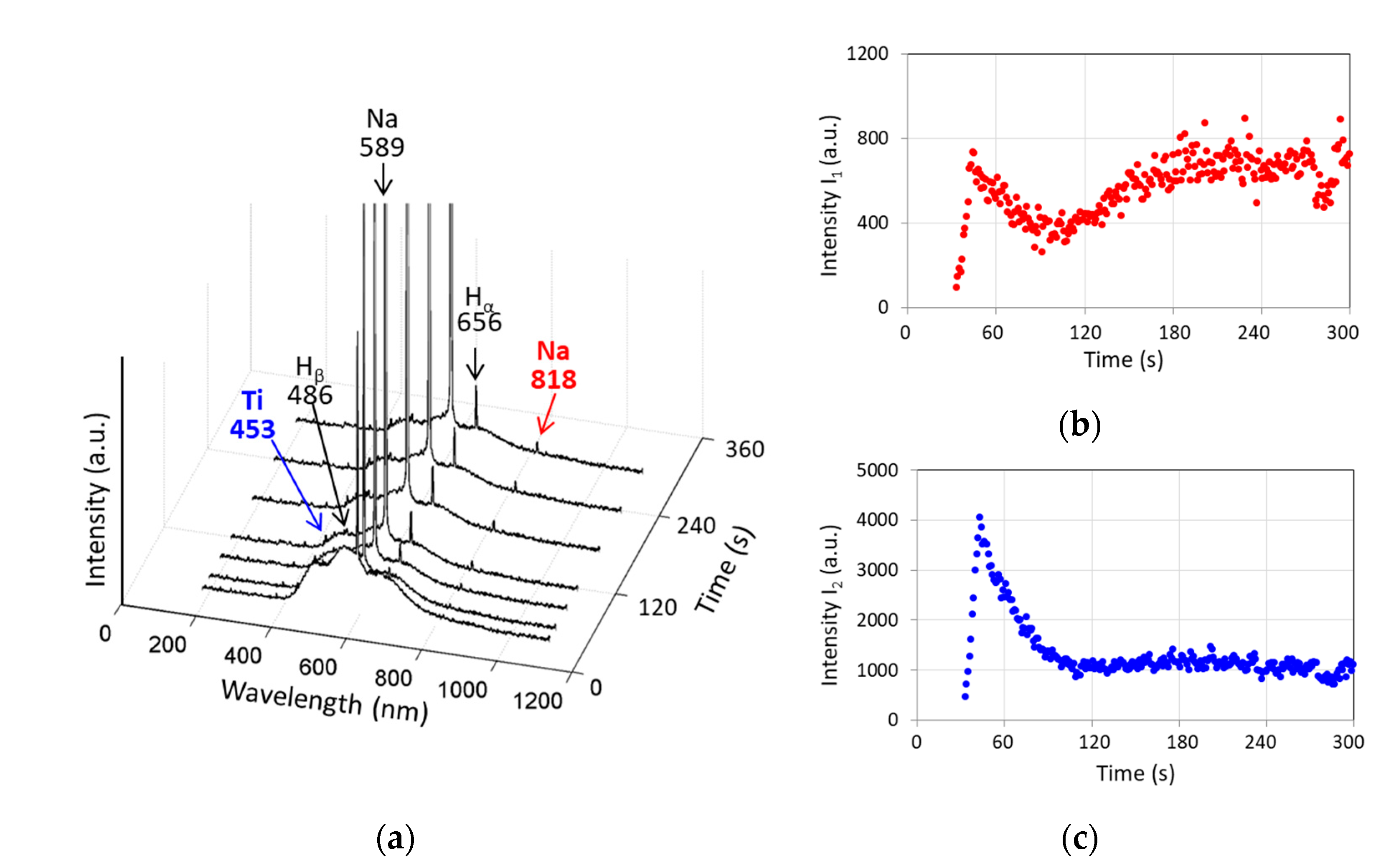
3.3. Evolution of Optical Emission Spectra during PEO of Ti
3.4. Evolution of In Situ Impedance Spectra during PEO of Ti
4. Discussion
5. Conclusions
- The plasma electrolytic oxidation in the pulsed bipolar mode under the voltage control, comprising a ramp for the soft start and a steady state regime, provided the active coating growth on cp-Ti for up to 120 s of treatment. After this time, the coating growth rate decreased 10-fold, and up to 300 s, the coating thickness, roughness, and morphology did not change significantly. In the time range from 120 to 300 s, a notable linear growth appeared in the rutile content due to an increase in the power of the microdischarges. Given that for the biocompatibility, the anatase should prevail in the coating composition, the increase in the treatment time is not necessary after 120 s. This coating is suitable for the realization of the biomimetic approach, and it can serve as an inorganic matrix for further introduction of bioactive organic molecules.
- The intensities of the spectral lines of the substrate material, and of the electrolyte species present in the emission generated by the microdischarges changed with the coating growth. Since the spectral line of the substrate material decreases, this supports the contribution to the PEO process mechanism understanding that the breakdown occurs in the barrier layer. Since the electrolyte species’ spectral lines grow, this justifies the ionization of the electrolyte vapor in the deepening coating pore.
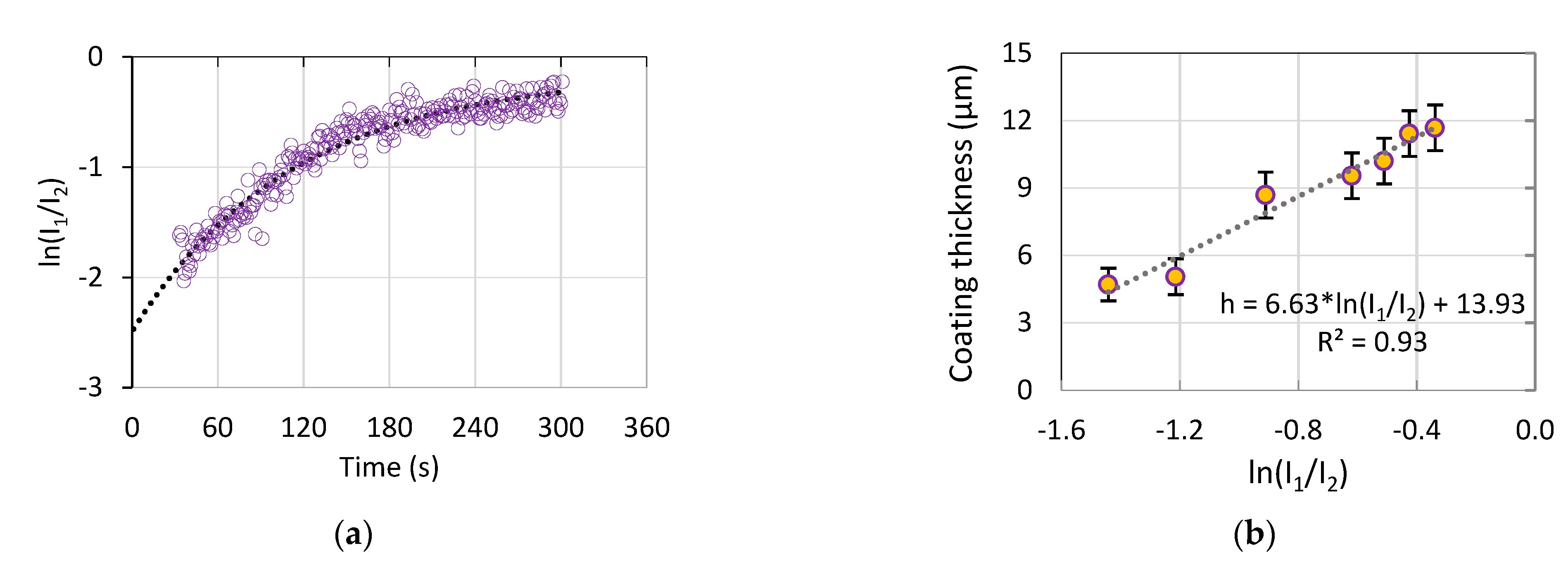
- As a result, an optical method was proposed and justified for the coating thickness estimation. The method comprises the log scale ratio of the electrolyte component emission intensity over the substrate material emission intensity; this ratio is highly correlated with the coating thickness and roughness (R2 > 0.92), therefore, the coating properties can be estimated during the PEO treatment.
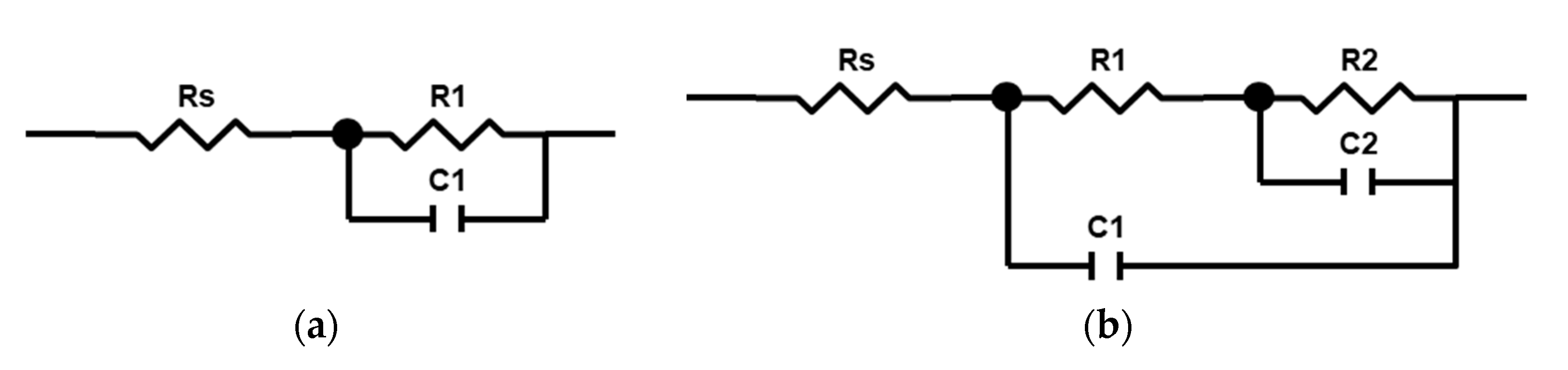
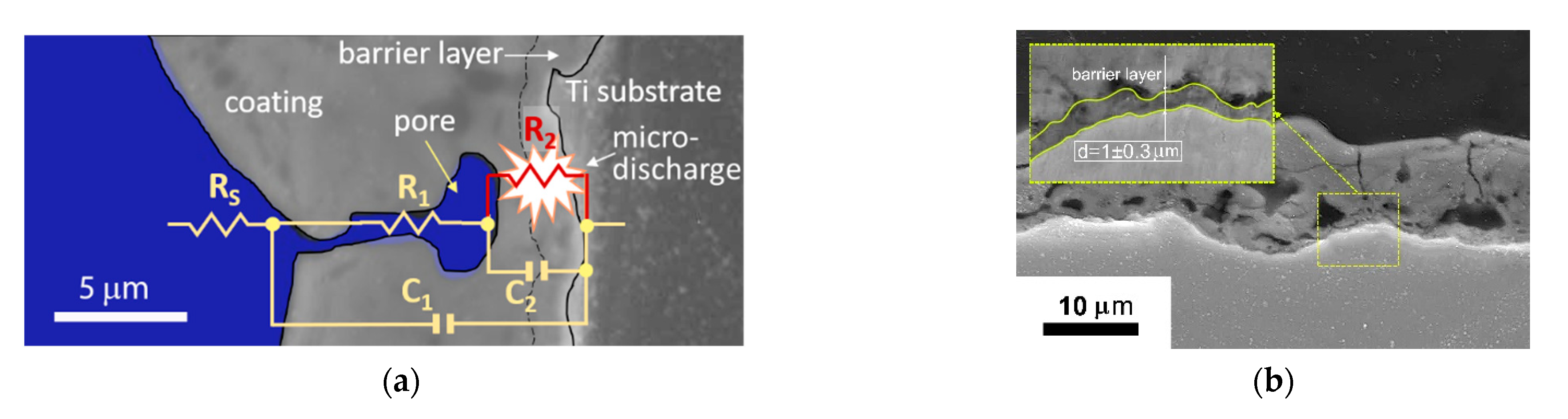
- The in situ impedance spectroscopy helped to evaluate the PEO process frequency response and to propose the underlying equivalent circuit. The microdischarge ignition changed the impedance spectra so that a negative time constant appeared in the system. The impedance spectra fitting with a ladder circuit showed that the negative differential resistance belongs to the inner barrier layer of the coating where the microdischarge starts the breakdown.
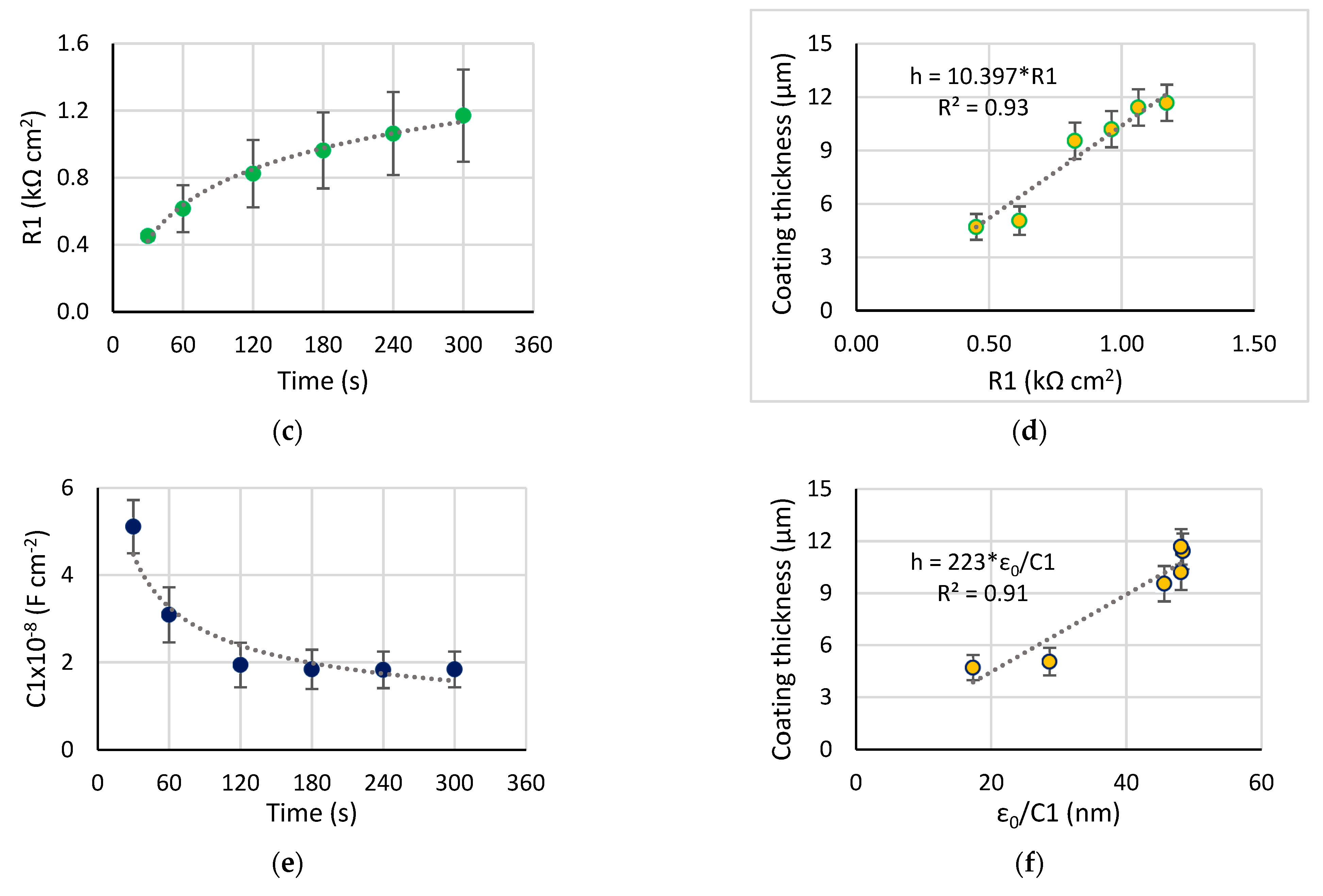
- The correlation was established between the resistance R1, 1/C1, and the coating thickness and roughness (R2 > 0.93), therefore, the coating properties can be estimated during the PEO treatment with this method. The evolution of the equivalent circuit parameters showed that after 120 s of the PEO, no significant changes appeared, therefore, this time can be recommended for the coating formation.
- Two analyzed and justified in situ spectroscopic methods help to correct the treatment time in the vicinity of 120 s when the coating precisely achieves the required properties by using an appropriately designed process control and diagnostic system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Sam Froes, F.H.; Qian, M. Titanium background, alloying behavior and advanced fabrication techniques-An overview. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 23–37. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Parfenova, L.V.; Dyakonov, G.S.; Danilko, K.V.; Mukaeva, V.R.; Farrakhov, R.G.; Lukina, E.S.; Valiev, R.Z. Surface functionalization via PEO coating and RGD peptide for nanostructured titanium implants and their in vitro assessment. Surf. Coat. Technol. 2019, 357, 669–683. [Google Scholar] [CrossRef]
- Echeverry-Rendón, M.; Galvis, O.; Aguirre, R.; Robledo, S.; Castaño, J.G.; Echeverría, F. Modification of titanium alloys surface properties by plasma electrolytic oxidation (PEO) and influence on biological response. J. Mater. Sci. Mater. Med. 2017, 28, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Lv, Y.; Yu, Y.; Dong, Z. Formation mechanism, corrosion behaviour and biological property of hydroxyapatite/TiO2 coatings fabricated by plasma electrolytic oxidation. Surf. Coat. Technol. 2020, 386, 125483. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021, 16, e2000116. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Nagay, B.E.; Ribeiro, A.L.R.; da Cruz, N.C.; Rangel, E.C.; Fais, L.M.G.; Vaz, L.G.; Barão, V.A.R. Functionalization of an experimental Ti-Nb-Zr-Ta alloy with a biomimetic coating produced by plasma electrolytic oxidation. J. Alloys Compd. 2019, 770, 1038–1048. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Mohedano, M.; Martínez-Corriá, R.; Ramos, V.; Arrabal, R.; Matykina, E. In vitro and in vivo evaluation of PEO-modified titanium for bone implant applications. Surf. Coat. Technol. 2018, 347, 358–368. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Chen, C.; Zhao, Z. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Sampatirao, H.; Radhakrishnapillai, S.; Dondapati, S.; Parfenov, E.; Nagumothu, R. Developments in plasma electrolytic oxidation (PEO) coatings for biodegradable magnesium alloys. Mater. Today Proc. 2021, 46, 1407–1415. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A.; Nevyantseva, R.R.; Gorbatkov, M.V.; Liang, C.J.; Matthews, A. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modelling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Tsai, D.S.; Chou, C.C. Review of the soft sparking issues in plasma electrolytic oxidation. Metals 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions–A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Pardo, A.; Matykina, E. Bioactive multi-elemental PEO-coatings on titanium for dental implant applications. Mater. Sci. Eng. C 2019, 97, 738–752. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.-C. Triggering the hydroxyapatite deposition on the surface of PEO-coated Ti–6Al–4V alloy via the dual incorporation of Zn and Mg ions. J. Alloys Compd. 2020, 819, 153038. [Google Scholar] [CrossRef]
- Ahounbar, E.; Mousavi Khoei, S.M.; Omidvar, H. Characteristics of in-situ synthesized Hydroxyapatite on TiO2 ceramic via plasma electrolytic oxidation. Ceram. Int. 2019, 45, 3118–3125. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Diodati, S.; Pigato, M.; Bonesso, M.; Dabalà, M. Microstructural and corrosion properties of hydroxyapatite containing PEO coating produced on AZ31 Mg alloy. Materials 2021, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, E.; Parfenova, L.; Mukaeva, V.; Farrakhov, R.; Stotskiy, A.; Raab, A.; Danilko, K.; Rameshbabu, N.; Valiev, R. Biofunctionalization of PEO coatings on titanium implants with inorganic and organic substances. Surf. Coat. Technol. 2020, 404, 126486. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Lukina, E.S.; Galimshina, Z.R.; Gil’fanova, G.U.; Mukaeva, V.R.; Farrakhov, R.G.; Danilko, K.V.; Dyakonov, G.S.; Parfenov, E.V. Biocompatible Organic Coatings Based on Bisphosphonic Acid RGD-Derivatives for PEO-Modified Titanium Implants. Molecules 2020, 25, 229. [Google Scholar] [CrossRef] [Green Version]
- Matykina, E.; Arrabal, R.; Pardo, A.; Mohedano, M.; Mingo, B.; Rodríguez, I.; González, J. Energy-efficient PEO process of aluminium alloys. Mater. Lett. 2014, 127, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Wang, Y.; Sun, Z.; Cui, Y.; Jin, T.; Wei, D.; Ouyang, J.; Jia, D.; Zhou, Y. Plasma electrolytic oxidation induced ‘local over-growth’ characteristic across substrate/coating interface: Effects and tailoring strategy of individual pulse energy. Surf. Coat. Technol. 2018, 342, 198–208. [Google Scholar] [CrossRef]
- Nadaraia, K.V.; Suchkov, S.N.; Imshinetskiy, I.M.; Mashtalyar, D.V.; Sinebrykhov, S.L.; Gnedenkov, S.V. Some new aspects of the study of dependence of properties of PEO coatings on the parameters of current in potentiodynamic mode. Surf. Coat. Technol. 2021, 426, 127744. [Google Scholar] [CrossRef]
- Mukaeva, V.R.; Melnichuk, O.V.; Vasilev, R.A.; Lutfrakhmanov, I.M.; Parfenov, E.V. Study into initial stages of plasma electrolytic oxidation of magnesium alloy. J. Phys. Conf. Ser. 2020, 1713, 012030. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, G.; Lv, Y.; Wu, Y.; Dong, Z. Growth mechanism of titania on titanium substrate during the early stage of plasma electrolytic oxidation. Surf. Coat. Technol. 2020, 400, 126202. [Google Scholar] [CrossRef]
- Casanova, L.; La Padula, M.; Pedeferri, M.; Diamanti, M.V.; Ormellese, M. An insight into the evolution of corrosion resistant coatings on titanium during bipolar plasma electrolytic oxidation in sulfuric acid. Electrochim. Acta 2021, 379, 138190. [Google Scholar] [CrossRef]
- Sowa, M.; Parafiniuk, M.; Mouzêlo, C.M.S.; Kazek-Kęsik, A.; Zhidkov, I.S.; Kukharenko, A.I.; Cholakh, S.O.; Kurmaev, E.Z.; Simka, W. DC plasma electrolytic oxidation treatment of gum metal for dental implants. Electrochim. Acta 2019, 302, 10–20. [Google Scholar] [CrossRef]
- Gowtham, S.; Arunnellaiappan, T.; Rameshbabu, N. An investigation on pulsed DC plasma electrolytic oxidation of cp-Ti and its corrosion behaviour in simulated body fluid. Surf. Coat. Technol. 2016, 301, 63–73. [Google Scholar] [CrossRef]
- Yeung, W.K.; Reilly, G.C.; Matthews, A.; Yerokhin, A. In vitro biological response of plasma electrolytically oxidized and plasma-sprayed hydroxyapatite coatings on Ti-6Al-4V alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Lederer, S.; Arat, S.; Fuerbeth, W. Influence of Process Parameters on the Tribological Behavior of PEO Coatings on CP-Titanium 4+ Alloys for Biomedical Applications. Materials 2021, 14, 5364. [Google Scholar] [CrossRef] [PubMed]
- Mashtalyar, D.V.; Nadaraia, K.V.; Gnedenkov, A.S.; Imshinetskiy, I.M.; Piatkova, M.A.; Pleshkova, A.I.; Belov, E.A.; Filonina, V.S.; Suchkov, S.N.; Sinebryukhov, S.L.; et al. Bioactive coatings formed on titanium by plasma electrolytic oxidation: Composition and properties. Materials 2020, 13, 4121. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.; Gao, Y.; Yeung, W.K.; Strumban, E.; Leshchinsky, V.; Chu, P.J.; Matthews, A.; Yerokhin, A.; Maev, R.G. Characterization and corrosion evaluation of TiO2:n-HA coatings on titanium alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 269, 258–265. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A.L.; Matthews, A. Frequency response studies for the plasma electrolytic oxidation process. Surf. Coat. Technol. 2007, 201, 8661–8670. [Google Scholar] [CrossRef]
- Fatkullin, A.R.; Parfenov, E.V.; Yerokhin, A.; Lazarev, D.M.; Matthews, A. Effect of positive and negative pulse voltages on surface properties and equivalent circuit of the plasma electrolytic oxidation process. Surf. Coat. Technol. 2015, 284, 427–437. [Google Scholar] [CrossRef]
- Mukaeva, V.R.; Kulyasova, O.B.; Farrakhov, R.G.; Parfenov, E.V.; Zheng, Y.F. Diagnostics of the thickness of a plasma electrolytic oxidation coating on a nanostructured Mg-Sr alloy. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012067. [Google Scholar] [CrossRef]
- Gorbatkov, M.V.; Parfenov, E.V.; Mukaeva, V.R.; Zhernakov, S.V.; Yerokhin, A.L. A coating thickness measuring technic during plasma electrolytic oxidation based on optical emission spectra. Datchiki I Sist. 2018, 1, 36–39. [Google Scholar]
- Rogov, A.B.; Huang, Y.; Shore, D.; Matthews, A.; Yerokhin, A. Toward rational design of ceramic coatings generated on valve metals by plasma electrolytic oxidation: The role of cathodic polarisation. Ceram. Int. 2021, 47, 34137–34158. [Google Scholar] [CrossRef]
- Yerokhin, A.; Parfenov, E.V.; Matthews, A. In situ impedance spectroscopy of the plasma electrolytic oxidation process for deposition of Ca- and P-containing coatings on Ti. Surf. Coat. Technol. 2016, 301, 54–62. [Google Scholar] [CrossRef]
- Gorbatkov, M.V.; Parfenov, E.V.; Yerokhin, A.L.; Mukaeva, V.R. Method for Coating Thickness Measurement during the Process of Plasma Electrolytic Oxidation, and a Device for Its Implementation. Russian Patent 2672036, 8 November 2018. [Google Scholar]
- Parfenov, E.V.; Yerokhin, A. Methodology of data acquisition and signal processing for frequency response evaluation during plasma electrolytic surface treatments. In Process Control: Problems, Techniques and Applications; Nova Science Publishers: New York, NY, USA, 2011; pp. 63–96. [Google Scholar]
- Yerokhin, A.; Parfenov, E.V.; Liang, C.J.; Mukaeva, V.R.; Matthews, A. System linearity quantification for in-situ impedance spectroscopy of plasma electrolytic oxidation. Electrochem. Commun. 2013, 27, 137–140. [Google Scholar] [CrossRef]
- Mukaeva, V.R.; Gorbatkov, M.V.; Farrakhov, R.G.; Lazarev, D.M.; Stotskiy, A.G.; Parfenov, E.V. Advanced plasma electrolysis research equipment with in-situ process diagnostics. In Proceedings of the 2020 International Conference on Electrotechnical Complexes and Systems (ICOECS), Ufa, Russia, 27–30 October 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Gardner, W.A. Statistical Spectral Analysis—A Nonprobabilistic Theory; Prentice Hall: Davis, CA, USA, 1987; p. 566. [Google Scholar]
- Shen, V.K.; Siderius, D.W.; Krekelberg, W.P.; Hatch, H.W. NIST Standard Reference Database 78; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021; p. 20899. [Google Scholar] [CrossRef]
- Yerokhin, A.; Mukaeva, V.R.; Parfenov, E.V.; Laugel, N.; Matthews, A. Charge transfer mechanisms underlying Contact Glow Discharge Electrolysis. Electrochim. Acta 2019, 312, 441–456. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D-Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Barsukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Willey & Sons, Inc.: Hoboken, NJ, USA, 2005; p. 616. [Google Scholar]
- Jung, O.; Smeets, R.; Kopp, A.; Porchetta, D.; Hiester, P.; Heiland, M.; Friedrich, R.E.; Precht, C.; Hanken, H.; Gröbe, A.; et al. PEO-generated Surfaces Support Attachment and Growth of Cells In Vitro with No Additional Benefit for Micro-roughness in Sa (0.2–4 μm). In Vivo 2016, 30, 27–33. [Google Scholar] [PubMed]
- Sul, Y.T.; Johansson, C.; Wennerberg, A.; Cho, L.R.; Chang, B.S.; Albrektsson, T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: Surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int. J. Oral Maxillofac. Implant. 2005, 20, 349–359. [Google Scholar]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Bonkerud, J.; Zimmermann, C.; Weiser, P.M.; Vines, L.; Monakhov, E.V. On the permittivity of titanium dioxide. Sci. Rep. 2021, 11, 12443. [Google Scholar] [CrossRef]
- Dervos, C. Permittivity properties of thermally treated TiO2. Mater. Lett. 2004, 58, 1502–1507. [Google Scholar] [CrossRef]
- Roh, B.; Macdonald, D.D. Effect of oxygen vacancies in anodic titanium oxide films on the kinetics of the oxygen electrode reaction. Russ. J. Electrochem. 2007, 43, 125–135. [Google Scholar] [CrossRef]


| Time (s) | R1 (Ω cm2) | C1 (F·cm−2·10−8) | R2 (Ω cm2) | C2 (F·cm−2·10−8) | Chi-Sqr |
|---|---|---|---|---|---|
| 30 | 452.1 ± 24.7 | 5.11 ± 0.61 | - | - | 0.399 |
| 60 | 614.9 ± 140.5 | 3.09 ± 0.63 | −260.0 ± 144.1 | 14.40 ± 18.79 | 0.333 |
| 120 | 824.0 ± 201.5 | 1.94 ± 0.51 | −427.2 ± 208.5 | 7.43 ± 8.54 | 0.381 |
| 180 | 962.1 ± 226.6 | 1.84 ± 0.45 | −496.6 ± 232.2 | 6.96 ± 7.67 | 0.366 |
| 240 | 1063.0 ± 247.7 | 1.83 ± 0.42 | −558.2 ± 252.1 | 6.52 ± 6.91 | 0.352 |
| 300 | 1170.0 ± 275.3 | 1.84 ± 0.41 | −626.6 ± 278.4 | 6.03 ± 6.26 | 0.345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubakirova, V.; Farrakhov, R.; Sharipov, A.; Polyakova, V.; Parfenova, L.; Parfenov, E. Investigation of Biocompatible PEO Coating Growth on cp-Ti with In Situ Spectroscopic Methods. Materials 2022, 15, 9. https://doi.org/10.3390/ma15010009
Aubakirova V, Farrakhov R, Sharipov A, Polyakova V, Parfenova L, Parfenov E. Investigation of Biocompatible PEO Coating Growth on cp-Ti with In Situ Spectroscopic Methods. Materials. 2022; 15(1):9. https://doi.org/10.3390/ma15010009
Chicago/Turabian StyleAubakirova, Veta, Ruzil Farrakhov, Arseniy Sharipov, Veronika Polyakova, Lyudmila Parfenova, and Evgeny Parfenov. 2022. "Investigation of Biocompatible PEO Coating Growth on cp-Ti with In Situ Spectroscopic Methods" Materials 15, no. 1: 9. https://doi.org/10.3390/ma15010009
APA StyleAubakirova, V., Farrakhov, R., Sharipov, A., Polyakova, V., Parfenova, L., & Parfenov, E. (2022). Investigation of Biocompatible PEO Coating Growth on cp-Ti with In Situ Spectroscopic Methods. Materials, 15(1), 9. https://doi.org/10.3390/ma15010009








