Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel
Abstract
:1. Introduction
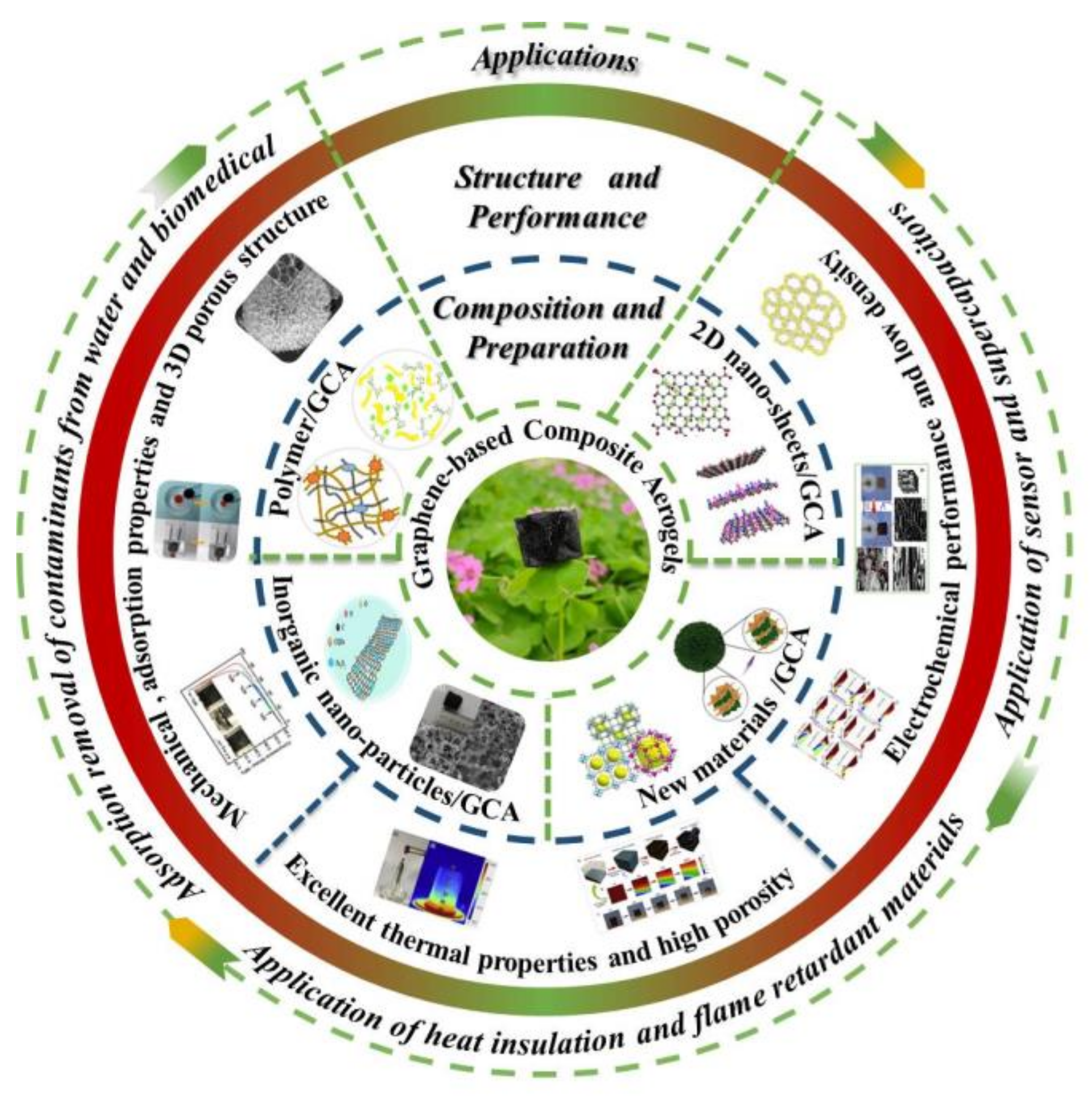
1.1. Graphene-Based Composite Aerogel
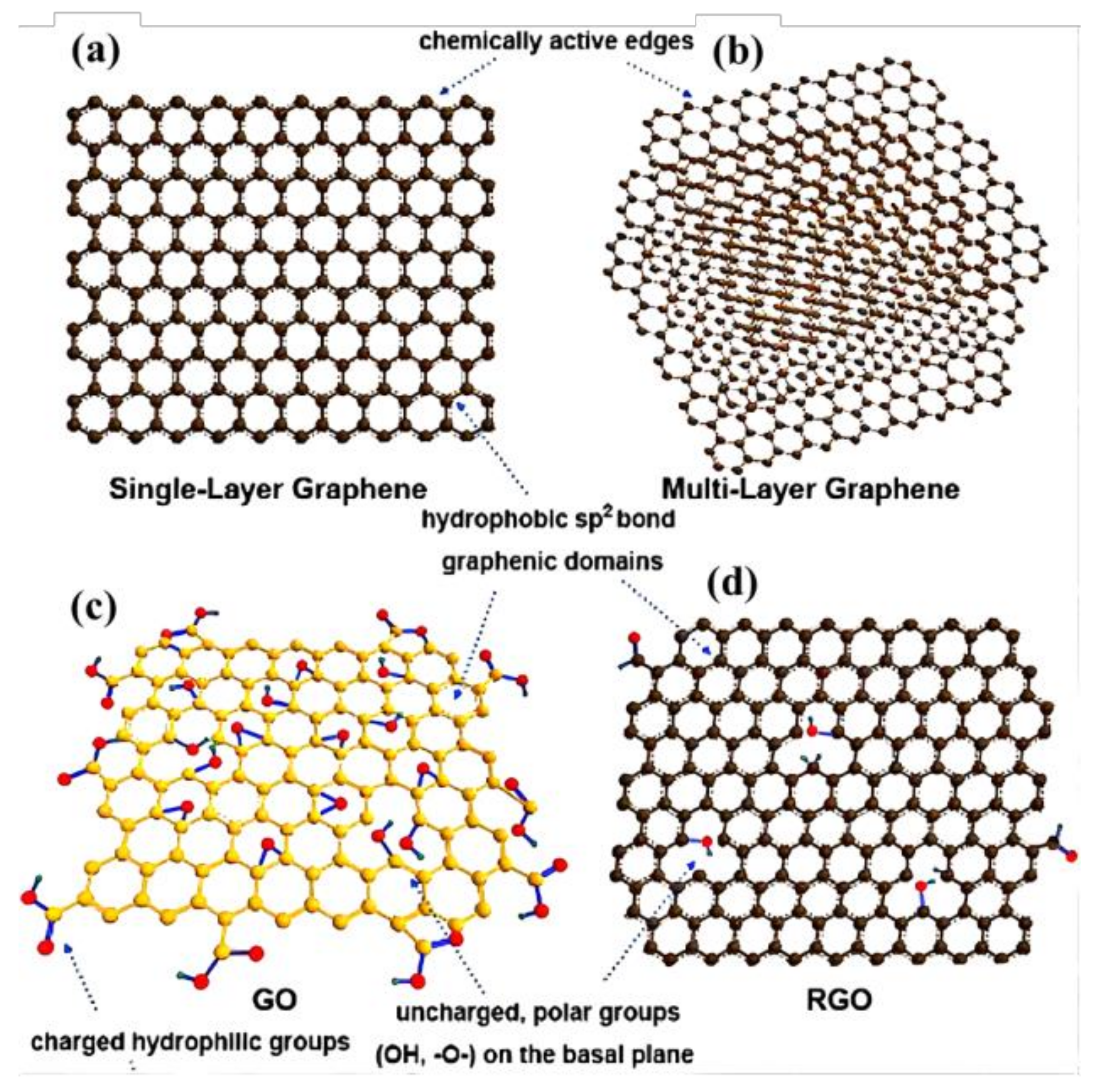
1.2. Preparation Principle of GCA
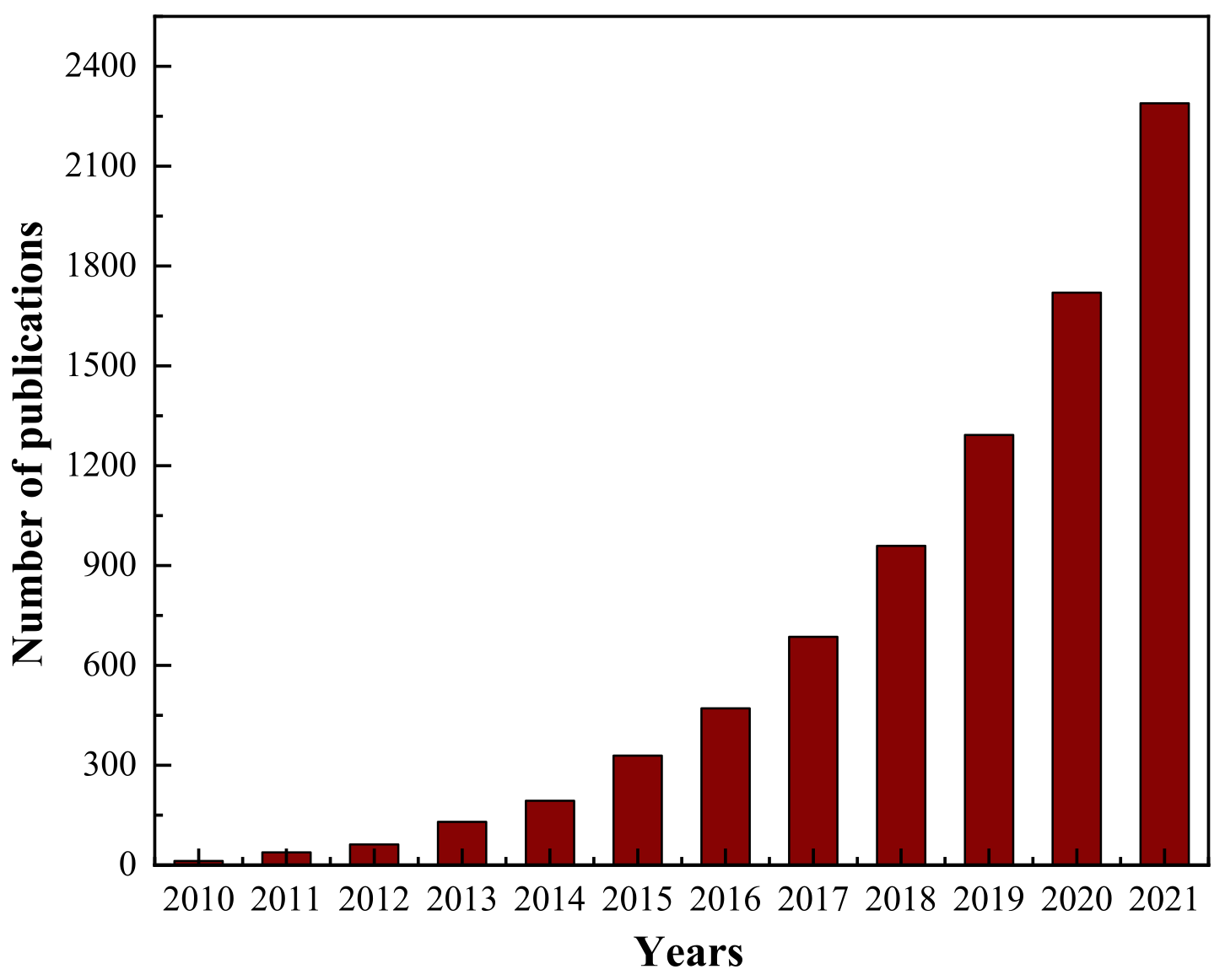
1.3. Current Research Situation
2. Composition of GCA
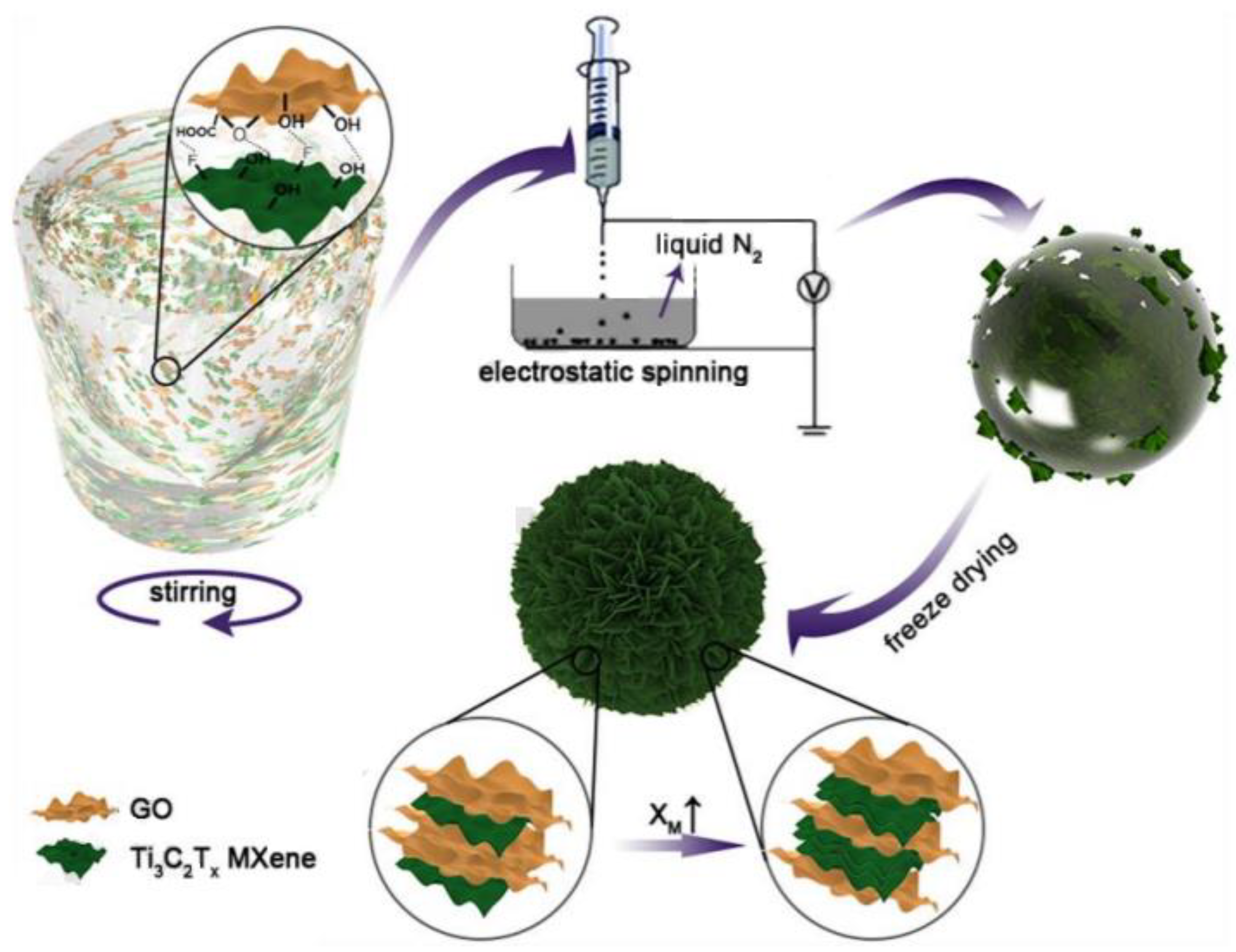
2.1. 2D Nanosheets/GCA
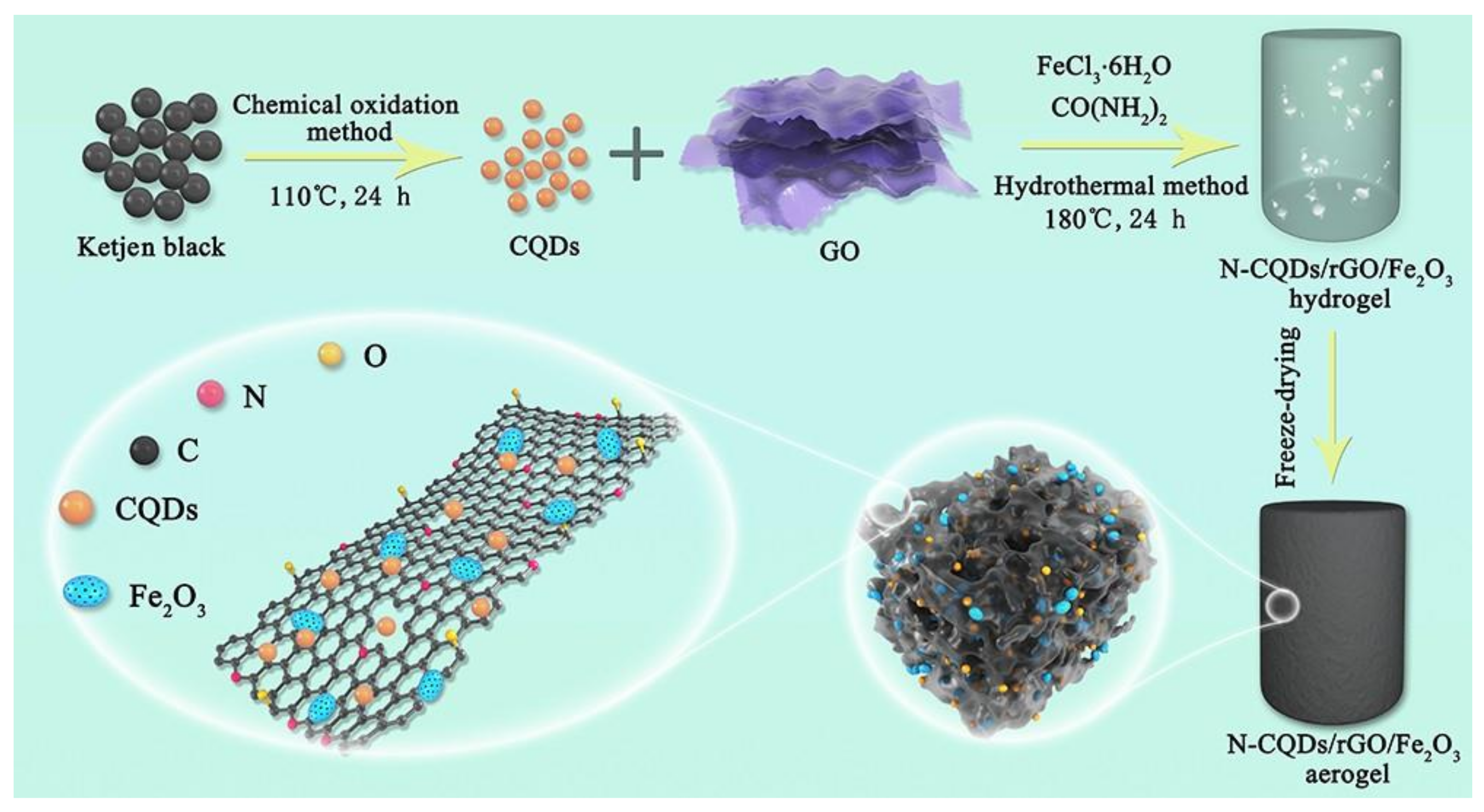
2.2. Inorganic Nanoparticle Materials/GCA
2.3. Synthetic Polymer/GCA
2.4. Natural Polymer/GCA
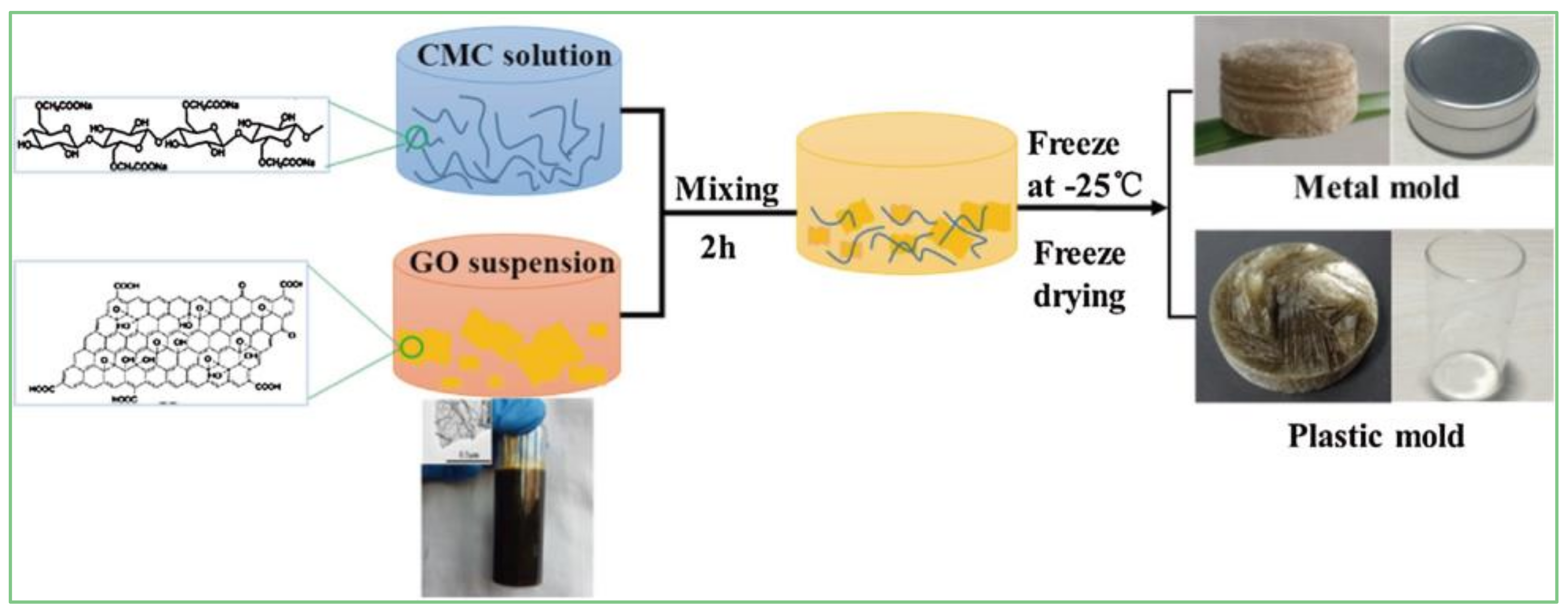
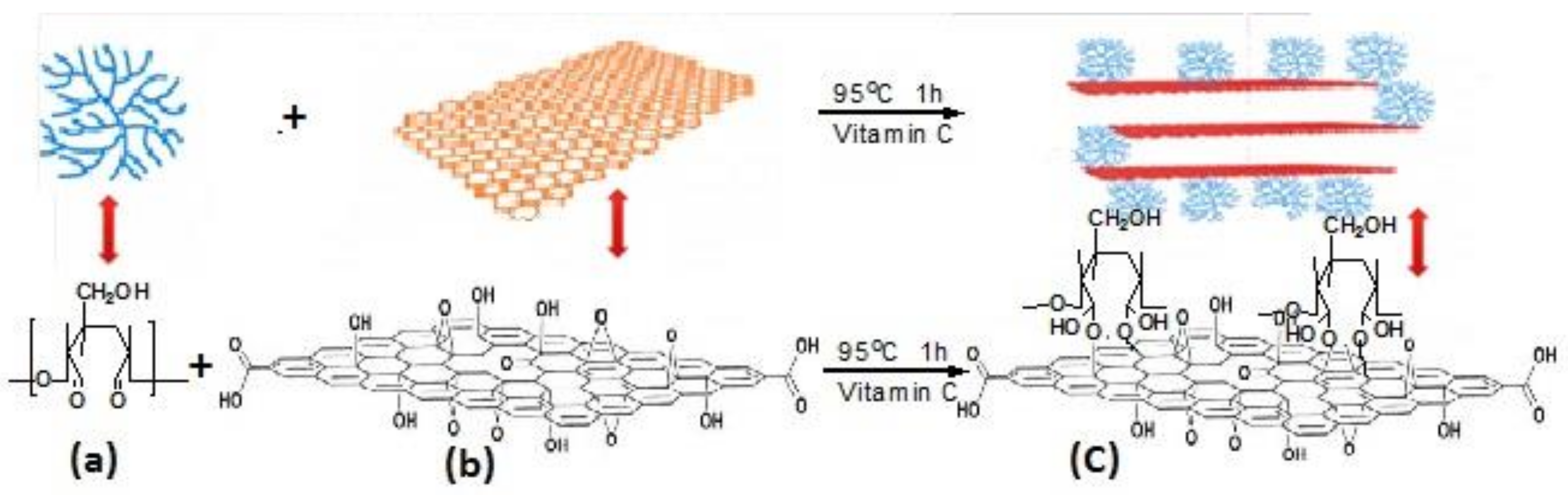
2.4.1. Cellulose/GCA
2.4.2. Starch/GCA
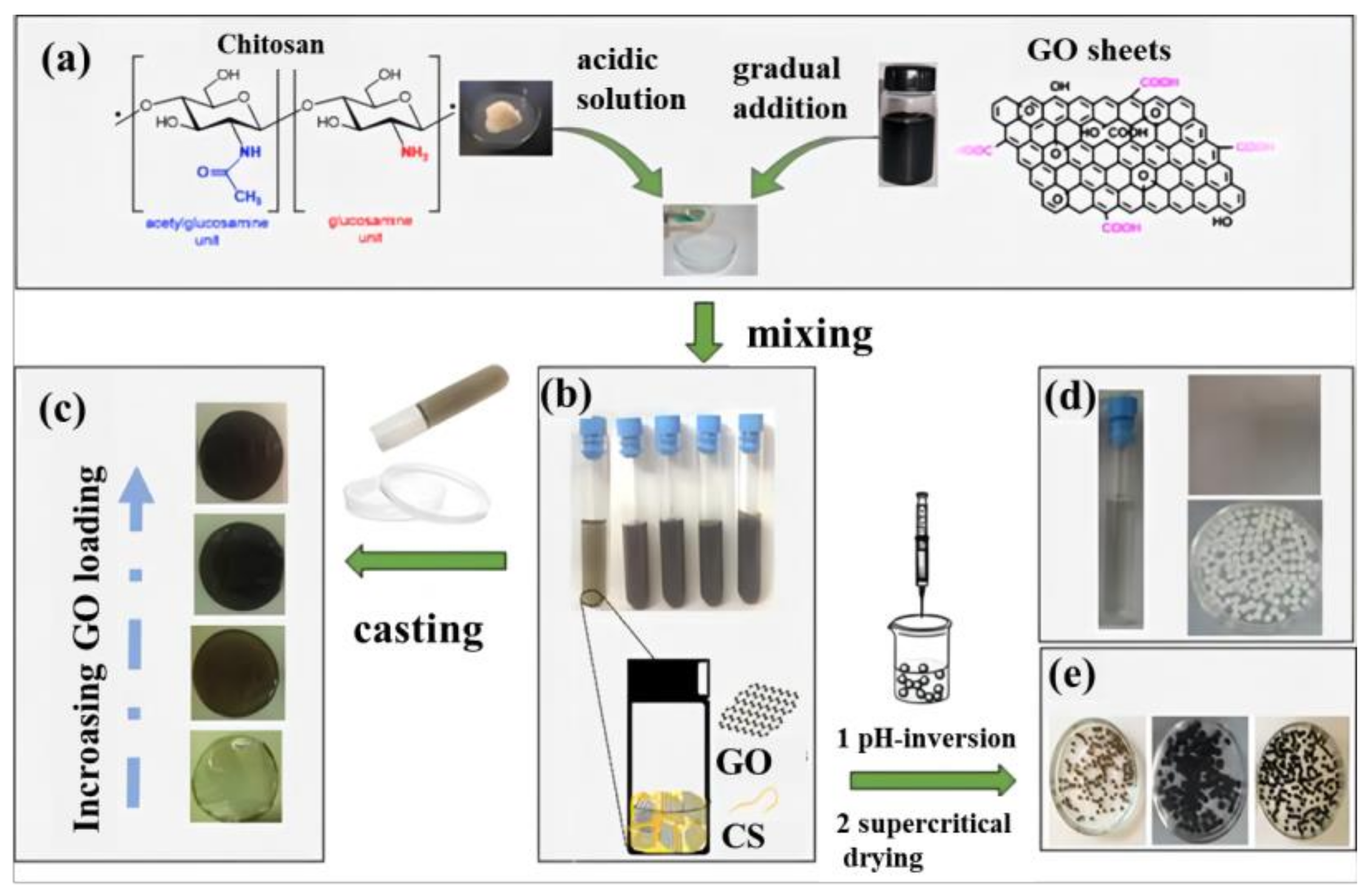
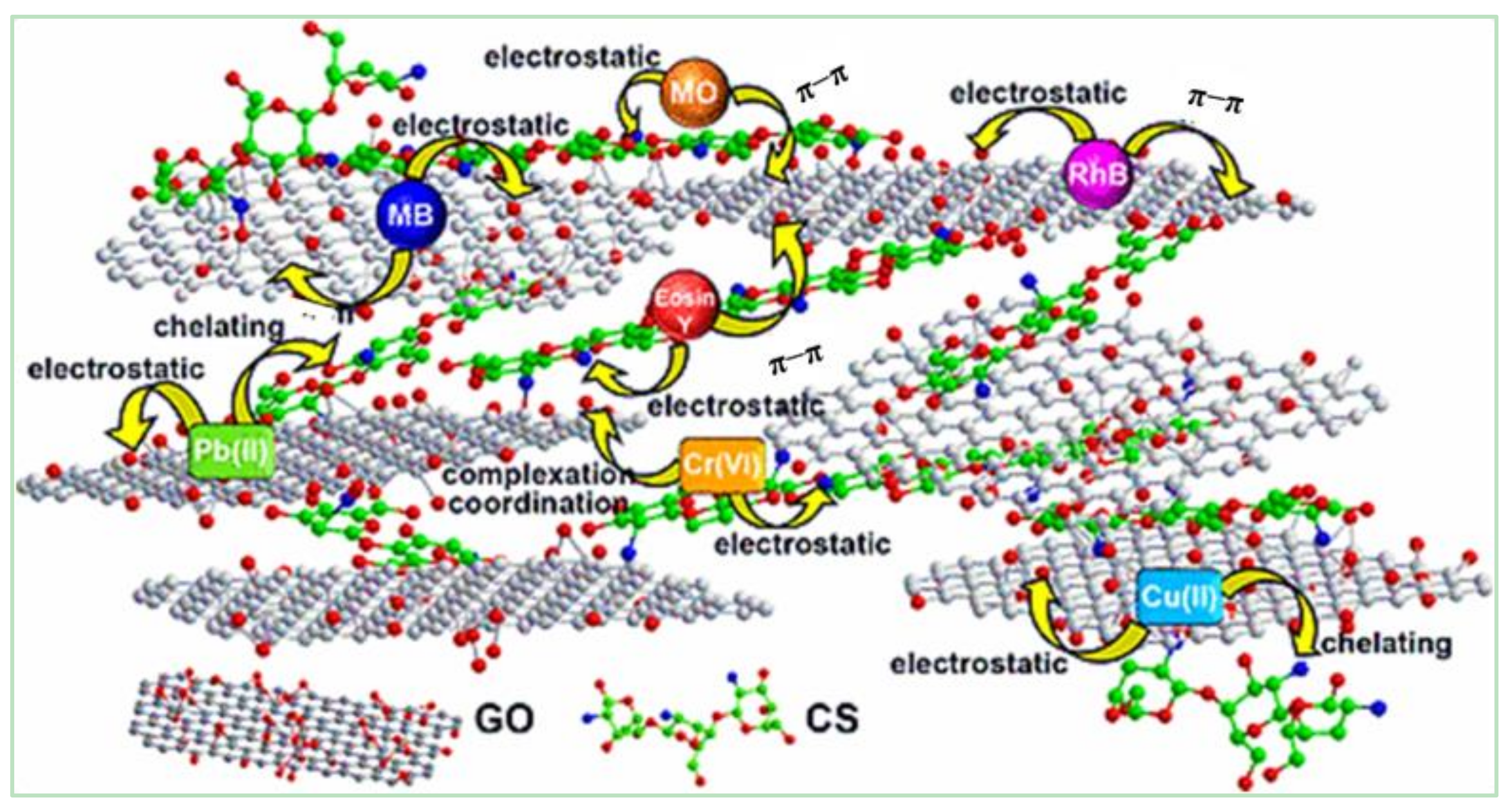
2.4.3. Chitosan/GCA
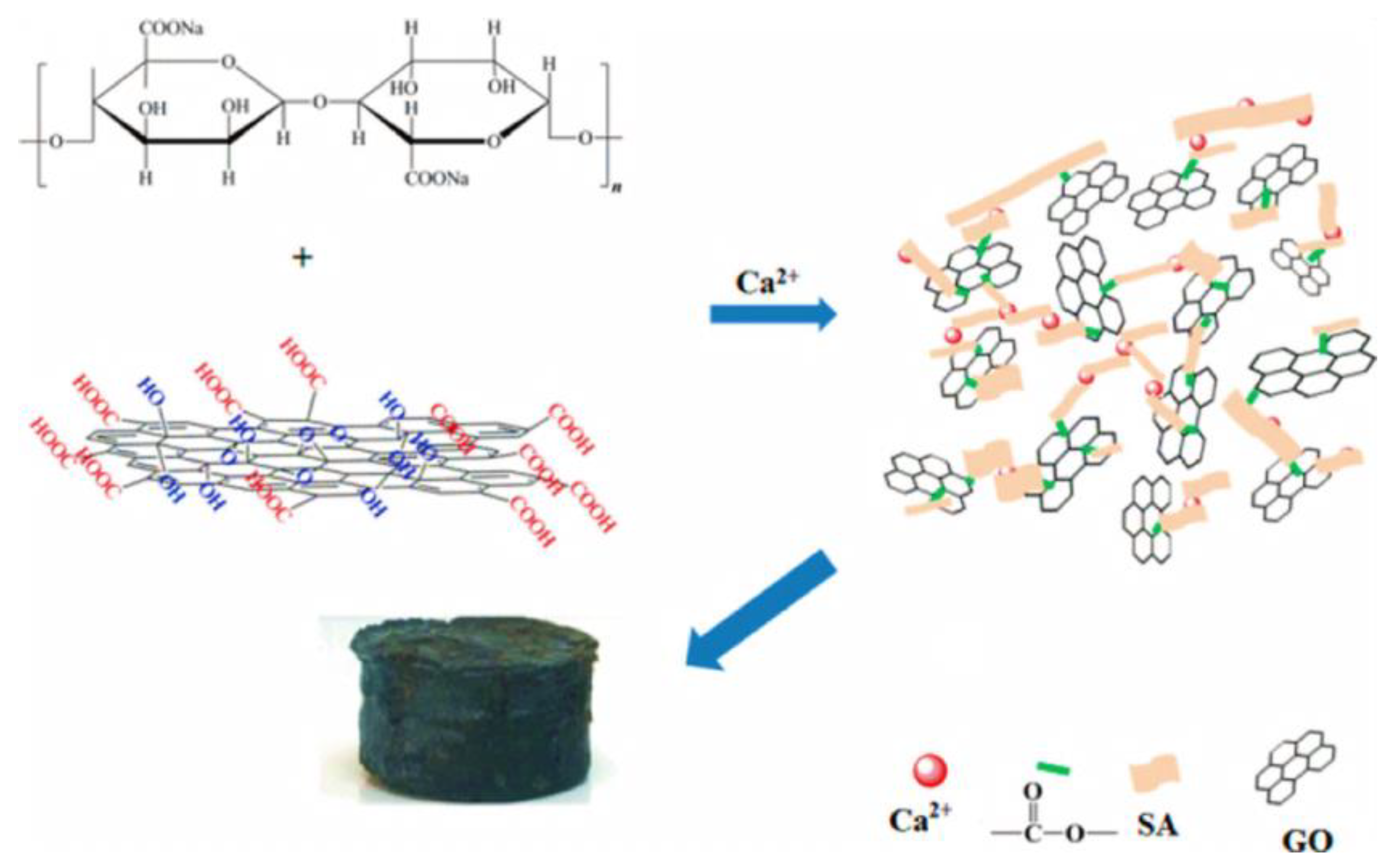
2.4.4. Sodium Alginate/GCA
3. Structure of GCA
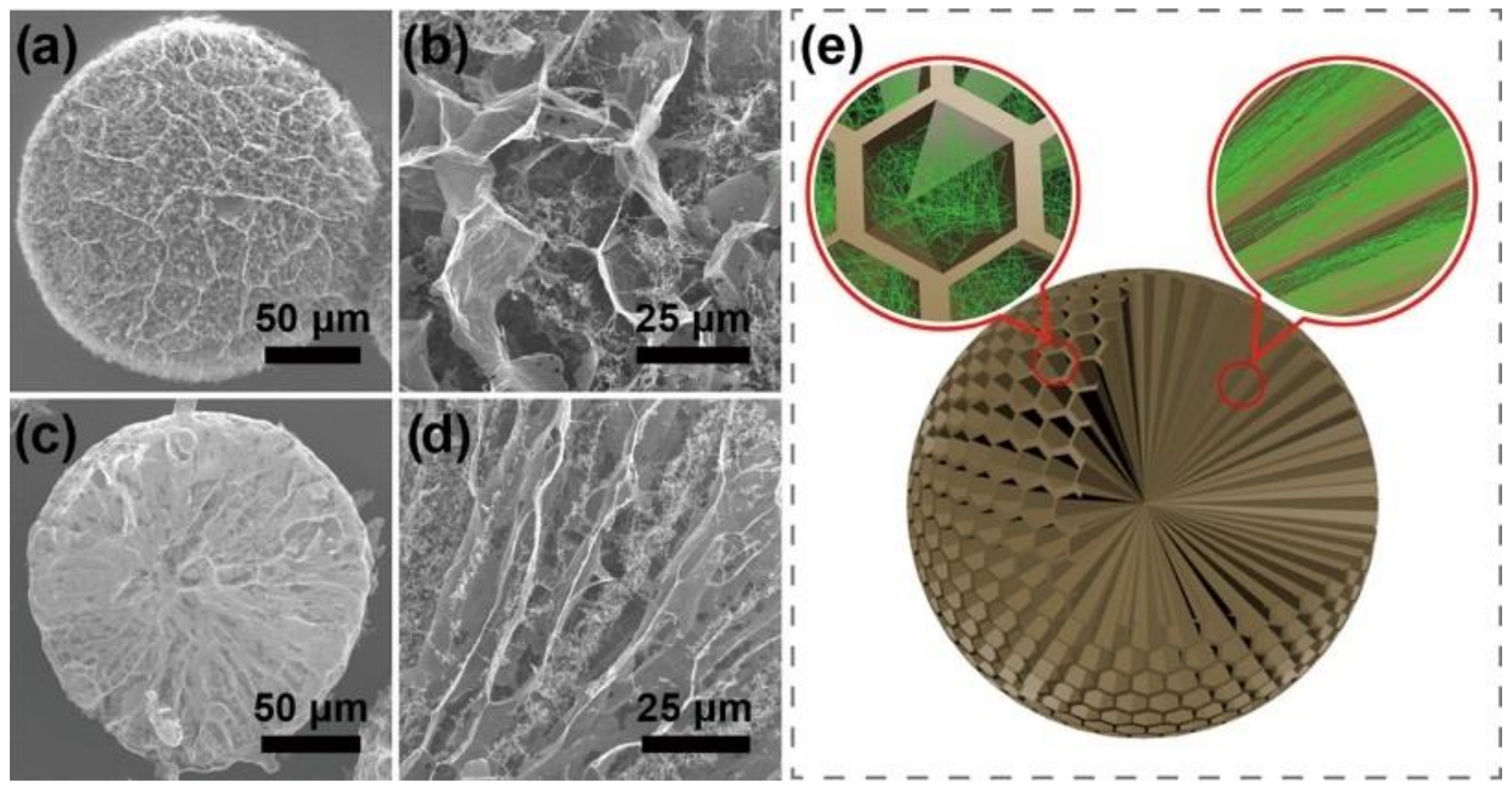
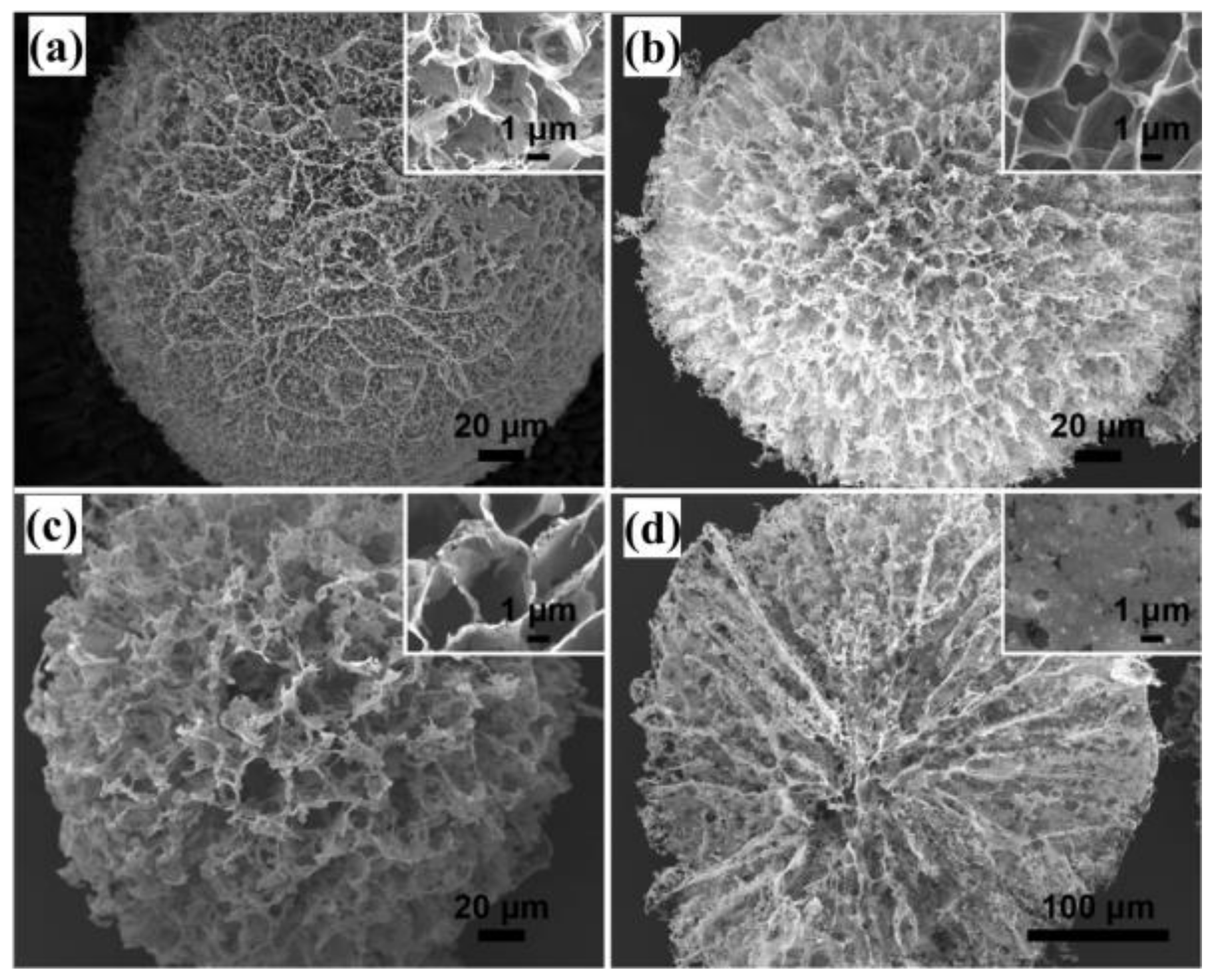
3.1. Porous Structure of GCA
3.2. Mesoporous Core–Shell Structure of GCA
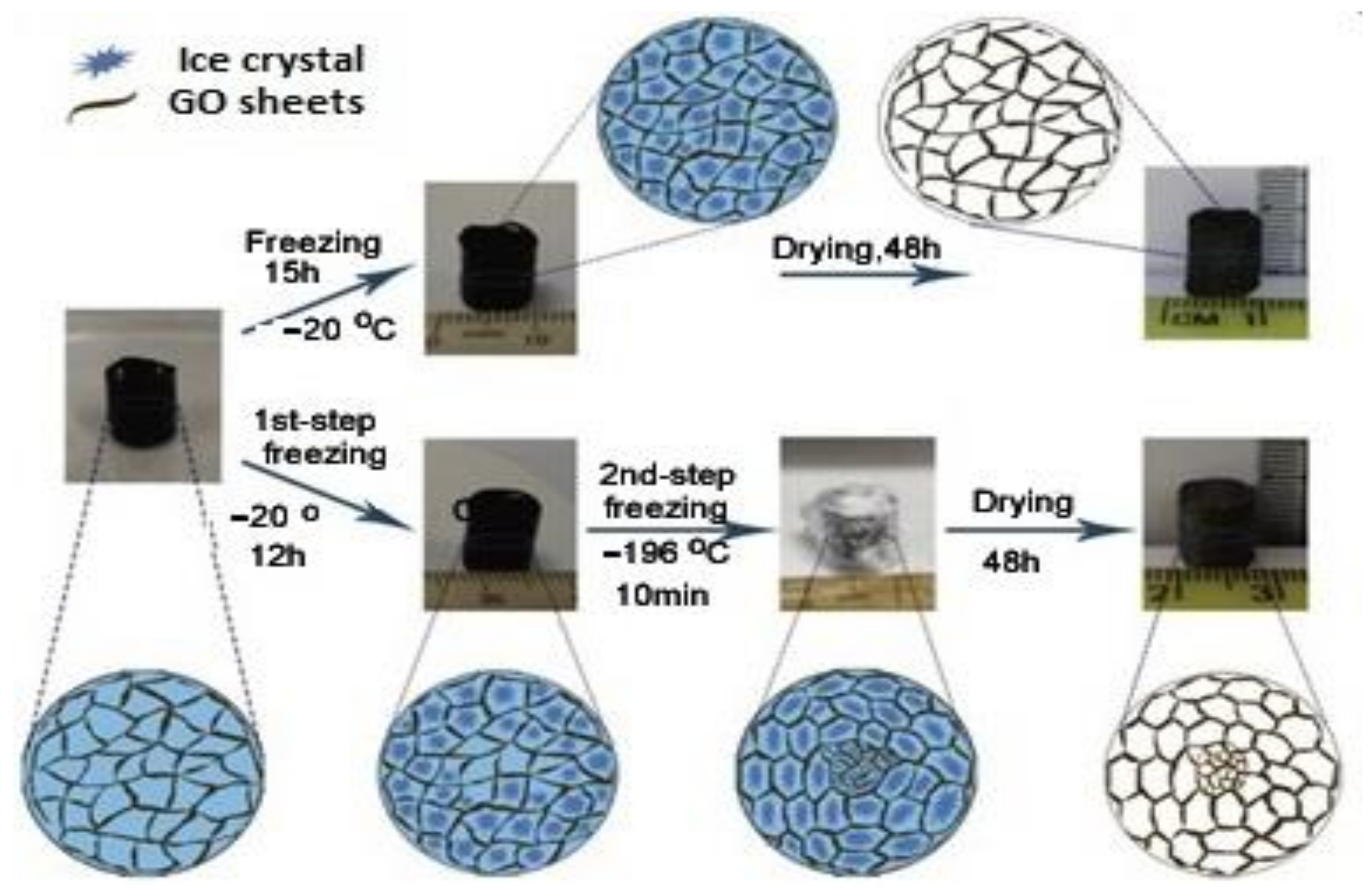
3.3. Methods for Adjusting the Porous Structure of GCA
4. Properties of GCA
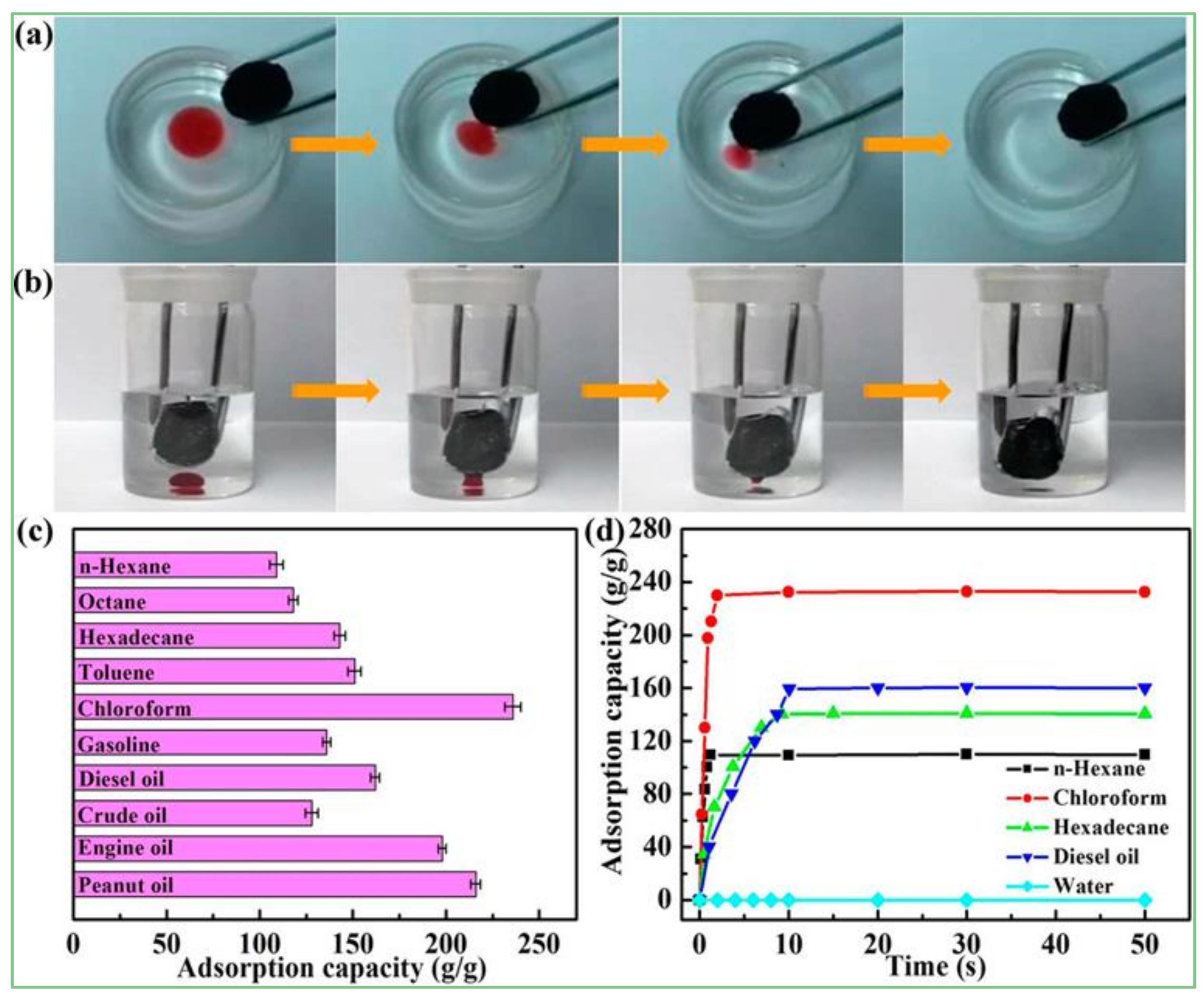
4.1. Adsorptive Properties
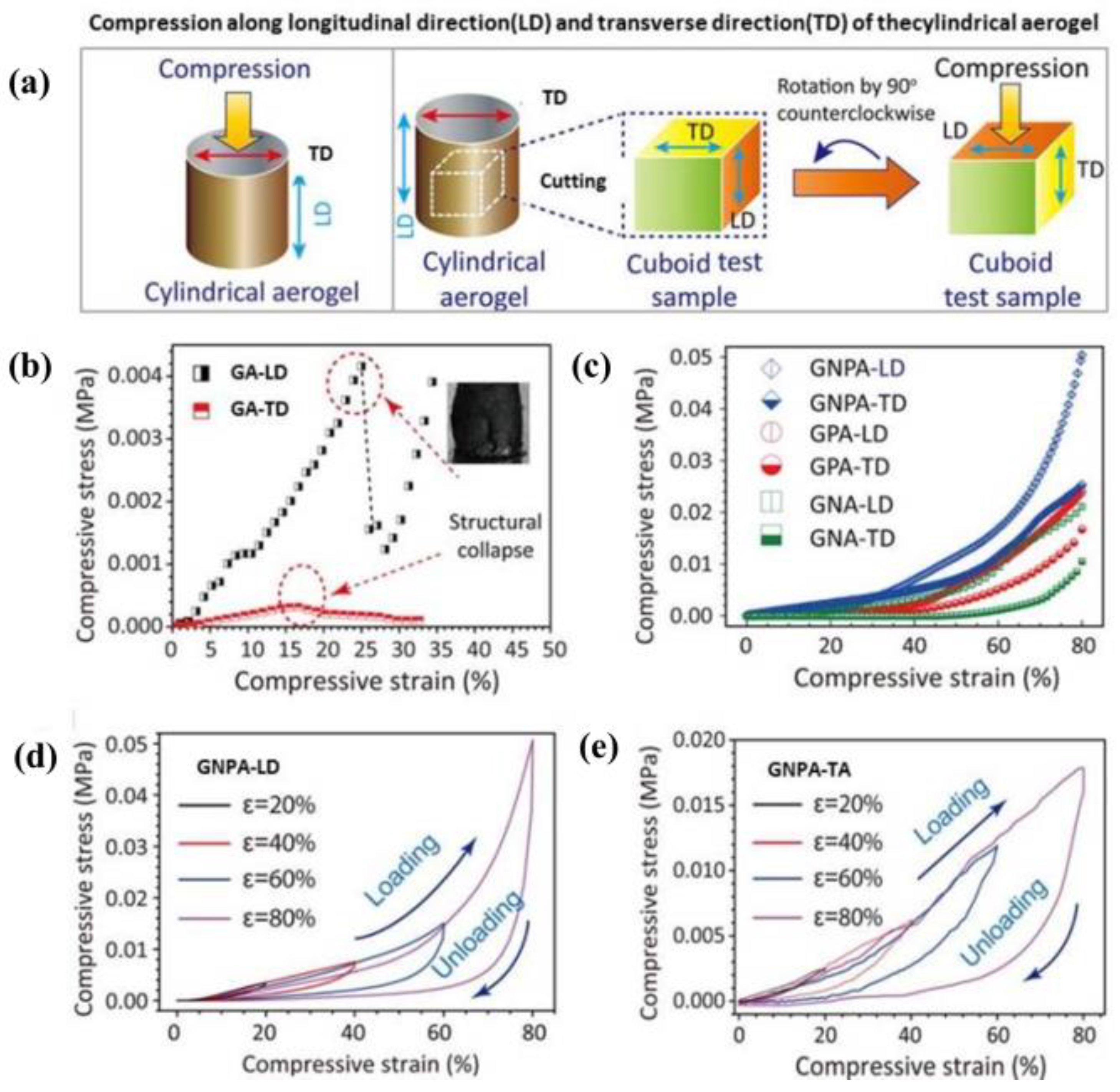
4.2. Mechanical Properties
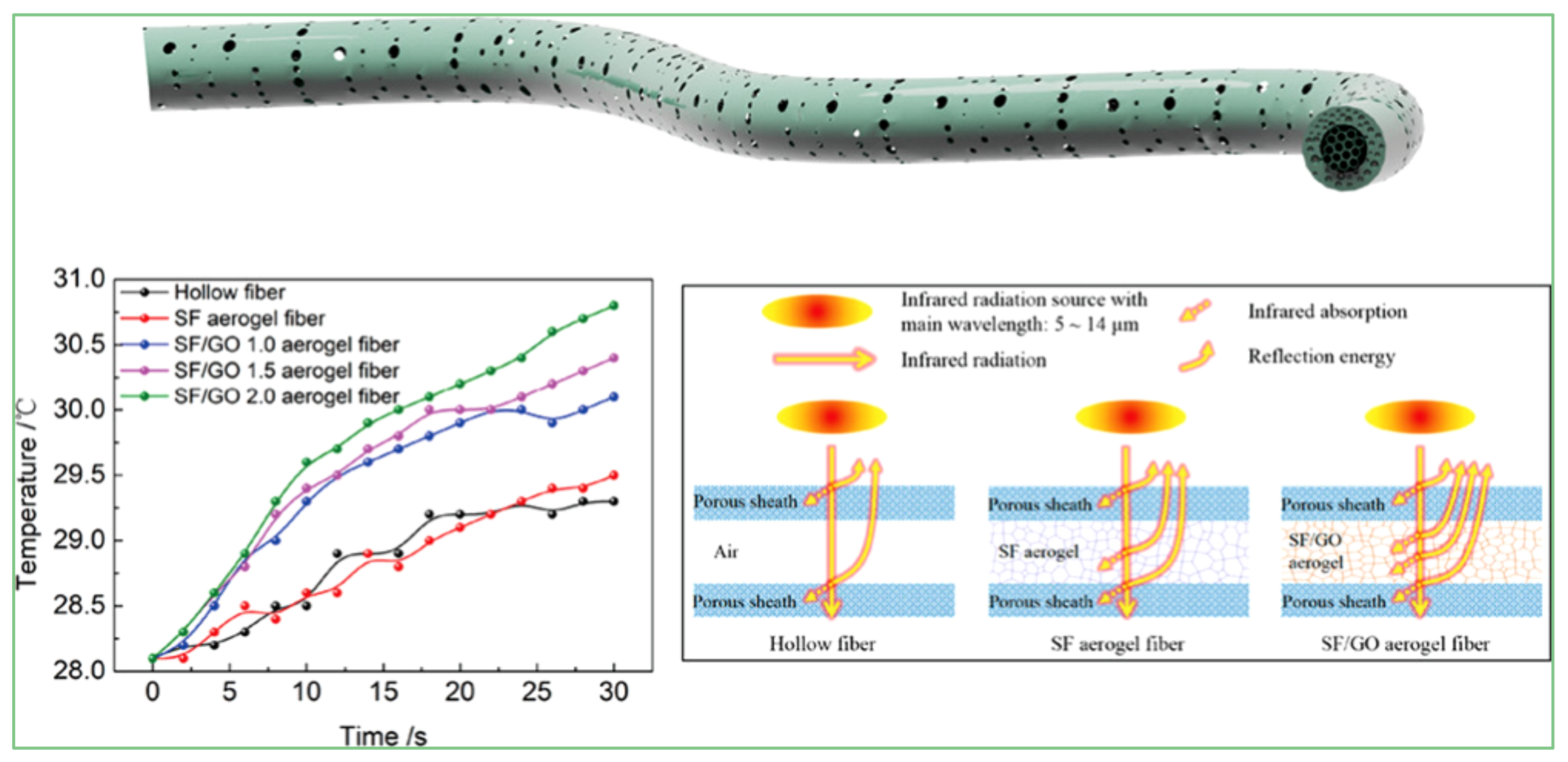
4.3. Thermal Properties
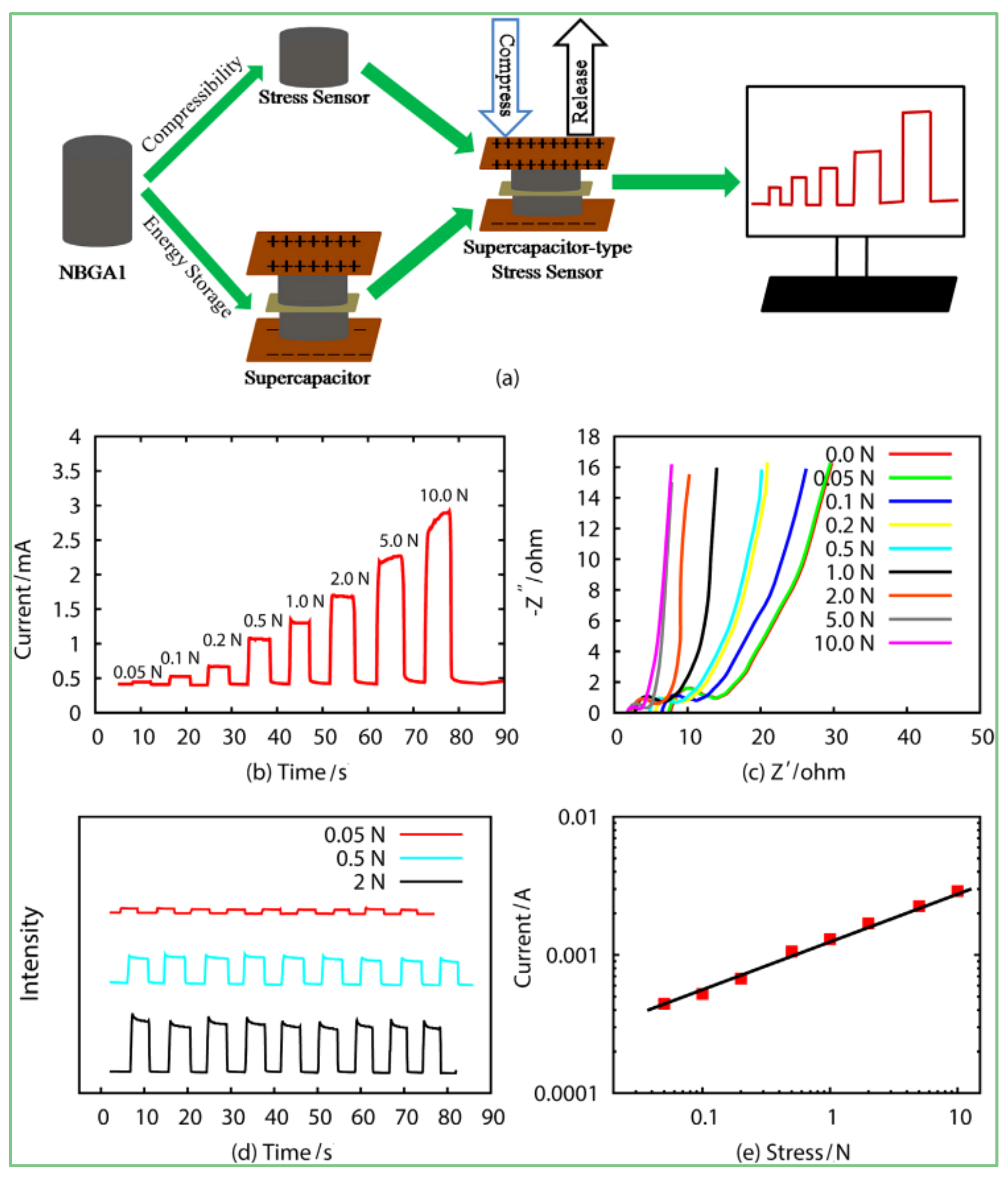
4.4. Electrochemical Properties
5. Application of GCA
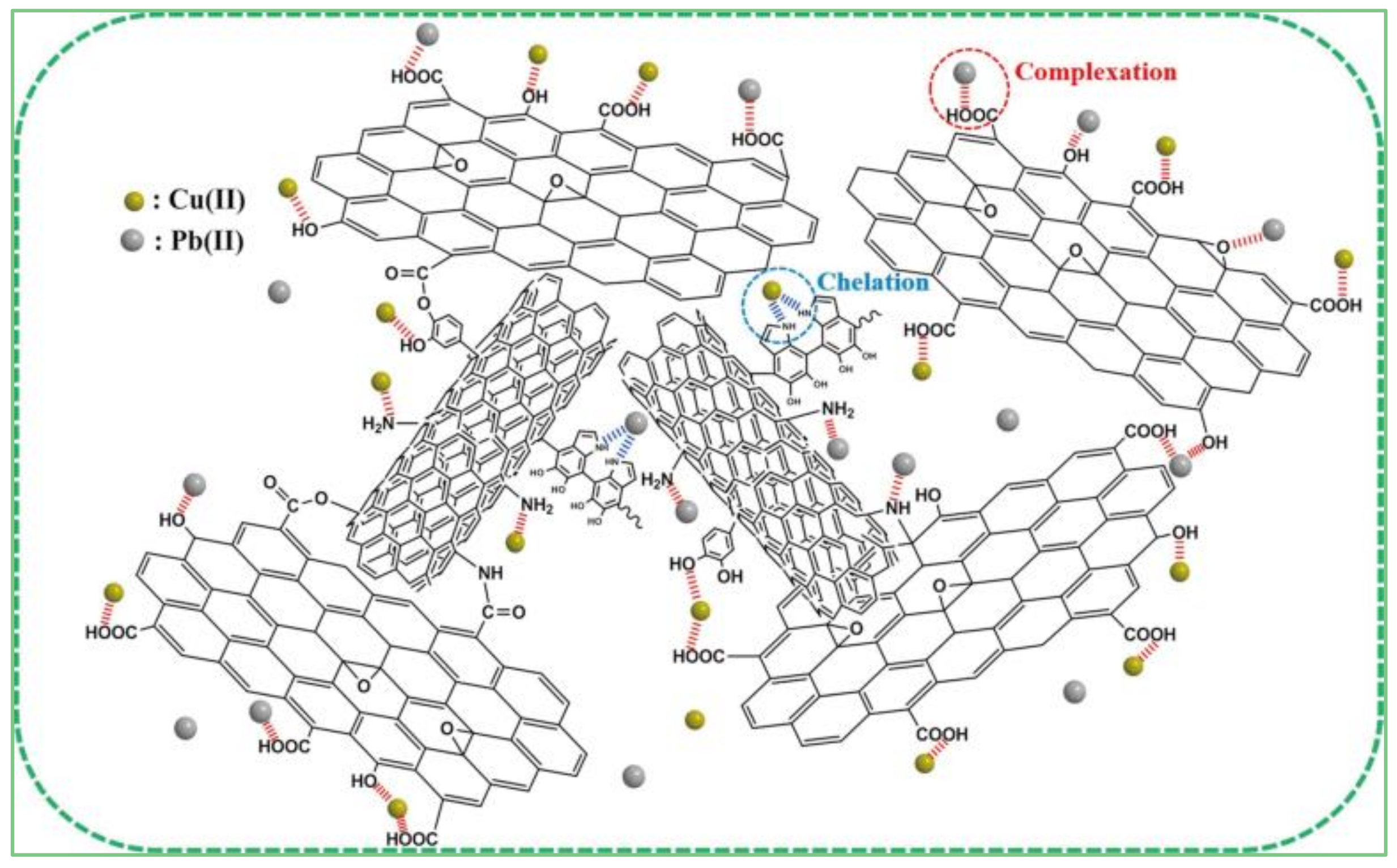
5.1. Adsorption Removal of Contaminants from Water
5.2. Application of Sensors and Supercapacitors
5.3. Application of Heat-Insulation and Flame-Retardant Materials
5.4. Biomedical Applications
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElKhatat, A.M.; Al-Muhtaseb, S.A. Advances in tailoring resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Jiang, Y.; Xu, Z.; Xiao, Y.; Fang, B.; Liu, Y.; Gao, W.; Zhao, P.; Wang, H.; Gao, C. Highly stretchable carbon aerogels. Nat. Commun. 2018, 9, 881. [Google Scholar] [CrossRef]
- Khetib, Y.; Alahmadi, A.A.; Alzaed, A.; Sajadi, S.M.; Cheraghian, G.; Sharifpur, M. Numerical study of the effect of graphene nanoparticles in calcium chloride hexahydrate-based phase change material on melting and freezing time in a circular cavity with a triangular obstacle. J. Energy Storage 2021, 43, 103243. [Google Scholar] [CrossRef]
- Abidi, A.; Rawa, M.; Khetib, Y.; Sindi, H.F.A.; Sharifpur, M.; Cheraghian, G. Simulation of melting and solidification of graphene nanoparticles-PCM inside a dual tube heat exchanger with extended surface. J. Energy Storage 2021, 44, 103265. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Zhou, Y.; Wan, N.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Low temperature casting of graphene with high compressive strength. Adv. Mater. 2012, 24, 5124–5129. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Thomas, A.; Kotov, N.A.; Haag, R. Functional graphene nanomaterials based architectures: Biointeractions, fabrications, and emerging biological applications. Chem. Rev. 2017, 117, 1826–1914. [Google Scholar] [CrossRef]
- Olszowska, K.; Pang, J.; Wrobel, P.S.; Zhao, L.; Ta, H.Q.; Liu, Z.; Trzebicka, B.; Bachmatiuk, A.; Rummeli, M.H. Three-dimensional nanostructured graphene: Synthesis and energy, environmental and biomedical applications. Synth. Met. 2017, 234, 53–85. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Brown, E.; Yan, P.; Tekik, H.; Elangovan, A.; Wang, J.; Lin, D.; Li, J. 3D printing of hybrid MoS2-graphene aerogels as highly porous electrode materials for sodium ion battery anodes. Mater. Des. 2019, 170, 107689. [Google Scholar] [CrossRef]
- Li, Y.; Meng, F.; Mei, Y.; Wang, H.; Guo, Y.; Wang, Y.; Peng, F.; Huang, F.; Zhou, Z. Electrospun generation of Ti3C2Tx MXene@ graphene oxide hybrid aerogel microspheres for tunable high-performance microwave absorption. Chem. Eng. J. 2020, 391, 123512. [Google Scholar] [CrossRef]
- Fu, X.; Choi, J.-Y.; Zamani, P.; Jiang, G.; Hoque, M.A.; Hassan, F.M.; Chen, Z. Co–N decorated hierarchically porous graphene aerogel for efficient oxygen reduction reaction in acid. ACS Appl. Mater. Interfaces 2016, 8, 6488–6495. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhu, H.W. Wearable and highly sensitive graphene strain sensors for human motion monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar]
- Cheng, L.; Qiao, D.; Zhao, P.; He, Y.; Sun, W.; Yu, H.; Jiao, Z. Template-free synthesis of mesoporous succulents-like TiO2/graphene aerogel composites for lithium-ion batteries. Electrochim. Acta 2019, 300, 417–425. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, X. Novel 3D graphene aerogel–ZnO composites as efficient detection for NO2 at room temperature. Sens. Actuators B Chem. 2015, 211, 220–226. [Google Scholar] [CrossRef]
- Li, W.; Gao, S.; Wu, L.; Qiu, S.; Guo, Y.; Geng, X.; Chen, M.; Liao, S.; Zhu, C.; Gong, Y. High-density three-dimension graphene macroscopic objects for high-capacity removal of heavy metal ions. Sci. Rep. 2013, 3, 2125. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wang, Z.; Wang, D.; Wang, J.-X.; Foster, N.R.; Pu, Y.; Chen, J.-F. Preparation of 3D graphene/iron oxides aerogels based on high-gravity intensified reactive precipitation and their applications for photo-Fenton reaction. Chem. Eng. Process. 2018, 129, 77–83. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, L.; Li, T.; Dang, Z. Compressible, amphiphilic graphene-based aerogel using a molecular glue to link graphene sheets and coated-polymer layers. Mater. Des. 2016, 110, 839–848. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, X.; Li, H.; Xiong, G.; Hu, H.; Fisher, T.S. Mechanically robust honeycomb graphene aerogel multifunctional polymer composites. Carbon 2015, 93, 659–670. [Google Scholar] [CrossRef]
- Ouyang, A.; Cao, A.; Hu, S.; Li, Y.; Xu, R.; Wei, J.; Zhu, H.; Wu, D. Polymer-coated graphene aerogel beads and supercapacitor application. ACS Appl. Mater. Interfaces 2016, 8, 11179–11187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Liu, W.; Liang, Q.; Liu, X.; Zhao, Z.; Yang, Y. Polypyrrole nanospheres@ graphene aerogel with high specific surface area, compressibility, and proper water wettability prepared in dimethylformamide-dependent environment. Polymer 2019, 185, 121974. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Politowicz, A.L.; Chen, E.; Huang, H.-X.; Turng, L.-S. Highly compressible ultra-light anisotropic cellulose/graphene aerogel fabricated by bidirectional freeze drying for selective oil absorption. Carbon 2018, 132, 199–209. [Google Scholar] [CrossRef]
- Ge, X.; Shan, Y.; Wu, L.; Mu, X.; Peng, H.; Jiang, Y. High-strength and morphology-controlled aerogel based on carboxymethyl cellulose and graphene oxide. Carbohydr. Polym. 2018, 197, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Liang, J.; Liao, Y.-H.; Lu, S.-Y. 3D porous graphene nanostructure from a simple, fast, scalable process for high performance flexible gel-type supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 4457–4467. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.; Kumar, S.; Bera, R.; Das, N. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Tang, H.; Zhao, Y.; Wang, W.; Cui, F. Highly porous zirconium-crosslinked graphene oxide/alginate aerogel beads for enhanced phosphate removal. Chem. Eng. J. 2019, 359, 779–789. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Behzadfar, E.; Naguib, H.E.; Park, C.B. Insights into in-situ sol-gel conversion in graphene modified polymer-based silica gels for multifunctional aerogels. Chem. Eng. J. 2020, 392, 123813. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Joseph, G.; Shinu, K.; Sreejesh, P.; Jose, S.; Salim, N.V.; Hameed, N. The role of SEBS in tailoring the interface between the polymer matrix and exfoliated graphene nanoplatelets in hybrid composites. Mater. Chem. Phys. 2015, 163, 182–189. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- Dai, J.; Xie, A.; Zhang, R.; Ge, W.; Chang, Z.; Tian, S.; Li, C.; Yan, Y. Scalable preparation of hierarchical porous carbon from lignin for highly efficient adsorptive removal of sulfamethazine antibiotic. J. Mol. Liq. 2018, 256, 203–212. [Google Scholar] [CrossRef]
- Li, Y.; Grishkewich, N.; Liu, L.; Wang, C.; Tam, K.C.; Liu, S.; Mao, Z.; Sui, X. Construction of functional cellulose aerogels via atmospheric drying chemically cross-linked and solvent exchanged cellulose nanofibrils. Chem. Eng. J. 2019, 366, 531–538. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced materials through assembly of nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, C.; Li, J. Graphene oxide/cellulose aerogels nanocomposite: Preparation, pyrolysis, and application for electromagnetic interference shielding. Carbohydr. Polym. 2016, 150, 172–179. [Google Scholar] [CrossRef]
- Dogenski, M.; Navarro-Díaz, H.J.; de Oliveira, J.V.; Ferreira, S.R.S. Properties of starch-based aerogels incorporated with agar or microcrystalline cellulose. Food Hydrocoll. 2020, 108, 106033. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Huang, T.; Shao, Y.-W.; Zhang, Q.; Deng, Y.-F.; Liang, Z.-X.; Guo, F.-Z.; Li, P.-C.; Wang, Y. Chitosan-cross-linked graphene oxide/carboxymethyl cellulose aerogel globules with high structure stability in liquid and extremely high adsorption ability. ACS Sustain. Chem. Eng. 2019, 7, 8775–8788. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Ennajih, H.; el Kacem Qaiss, A.; Bouhfid, R.; Lahcini, M.; Essassi, E.M.; Garcia, H.; El Kadib, A. Chitosan–graphene oxide films and CO2-dried porous aerogel microspheres: Interfacial interplay and stability. Carbohydr. Polym. 2017, 167, 297–305. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, H.; Yang, F. Hydrophilic and compressible aerogel: A novel draw agent in forward osmosis. ACS Appl. Mater. Interfaces 2017, 9, 33948–33955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, Q.; Chen, C.; Li, L.; Yuan, W. Developing a heat-insulating composite phase change material with light-to-thermal conversion performance from graphene oxide/silica hybrid aerogel. Appl. Therm. Eng. 2020, 174, 115303. [Google Scholar] [CrossRef]
- Chen, H.-C.; Lin, Y.-C.; Chen, Y.-L.; Chen, C.-J. Facile fabrication of three-dimensional hierarchical nanoarchitectures of VO2/graphene@ NiS2 hybrid aerogel for high-performance all-solid-state asymmetric supercapacitors with ultrahigh energy density. ACS Appl. Energy Mater. 2018, 2, 459–467. [Google Scholar] [CrossRef]
- Hou, S.; Wu, X.; Lv, Y.; Jia, W.; Guo, J.; Wang, L.; Tong, F.; Jia, D. Ultralight, highly elastic and bioinspired capillary-driven graphene aerogels for highly efficient organic pollutants absorption. Appl. Surf. Sci. 2020, 509, 144818. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.; He, X.; Gui, X.; Shang, Y.; Wang, C.; Wang, C.; Zhao, W.; Du, S.; Shi, E. Graphene nanoribbon aerogels unzipped from carbon nanotube sponges. Adv. Mater. 2014, 26, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Liu, W.; Liu, X.; Zhao, Z.; Yang, Y. A facile route to prepare nitrogen-doped carbon microspheres/graphene aerogel with high compressibility and superior capacitive property. Mater. Today Commun. 2020, 24, 101125. [Google Scholar] [CrossRef]
- Zhang, Z.; Kutana, A.; Yang, Y.; Krainyukova, N.V.; Penev, E.S.; Yakobson, B.I. Nanomechanics of carbon honeycomb cellular structures. Carbon 2017, 113, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength–toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Shi, Y.; Yang, D.; Liu, Y.; Qu, J.; Yu, Z.-Z. Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-spectrum and rapid adsorption of water contaminants. ACS Appl. Mater. Interfaces 2017, 9, 21809–21819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, D.; Shi, Y.; Song, L.; Yu, R.; Qu, J.; Yu, Z.-Z. Silver Phosphate/Graphene Oxide Aerogel Microspheres with Radially Oriented Microchannels for Highly Efficient and Continuous Removal of Pollutants from Wastewaters. ACS Sustain. Chem. Eng. 2019, 7, 11228–11240. [Google Scholar] [CrossRef]
- Afroze, J.D.; Abden, M.J.; Yuan, Z.; Wang, C.; Wei, L.; Chen, Y.; Tong, L. Core-shell structured graphene aerogels with multifunctional mechanical, thermal and electromechanical properties. Carbon 2020, 162, 365–374. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Han, T.Y.-J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors based on three-dimensional hierarchical graphene aerogels with periodic macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jin, Z.; Peng, L.; Zhao, F.; Xiao, D.; Jin, Y.; Yu, G. Stretchable all-gel-state fiber-shaped supercapacitors enabled by macromolecularly interconnected 3D graphene/nanostructured conductive polymer hydrogels. Adv. Mater. 2018, 30, 1800124. [Google Scholar] [CrossRef]
- Lv, S.H.; Zhu, L.L.; Li, Y.; Jia, C.M.; Sun, S.Y. The Adsorption capacity of GONs/CMC/Fe3O4 magnetic composite microspheres and applications for purifying dye wastewater. Materials 2017, 10, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, K.; Ren, X.; Alsaedi, A.; Hayat, T.; Chen, C. Exploring the aggregation mechanism of graphene oxide in the presence of radioactive elements: Experimental and theoretical studies. Environ. Sci. Technol. 2018, 52, 12208–12215. [Google Scholar] [CrossRef]
- Labiadh, L.; Kamali, A.R. 3D graphene nanoedges as efficient dye adsorbents with ultra-high thermal regeneration performance. Appl. Surf. Sci. 2019, 490, 383–394. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, H. Recent advances of 3D graphene-based adsorbents for sample preparation of water pollutants: A review. Chem. Eng. J. 2020, 393, 124691. [Google Scholar] [CrossRef]
- Hong, J.Y.; Bak, B.M.; Wie, J.J.; Kong, J.; Park, H.S. Reversibly compressible, highly elastic, and durable graphene aerogels for energy storage devices under limiting conditions. Adv. Funct. Mater. 2015, 25, 1053–1062. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, Y.; Zhang, H.; Cheng, F.; Zhao, W.; Rao, J.; Luo, S.; Wang, J.; Jiang, X.; Liu, Z. 3D synergistical MXene/reduced graphene oxide aerogel for a piezoresistive sensor. ACS Nano 2018, 12, 3209–3216. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Shanmuganathan, K.; Ellison, C.J. Mechanically stable thermally crosslinked poly (acrylic acid)/reduced graphene oxide aerogels. ACS Appl. Mater. Interfaces 2015, 7, 6220–6229. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Q.; Zhong, W.; Chen, J.; Sun, D.; Jiang, H.; Liu, K.; Wang, W.; Wang, Y.; Lu, Z.; et al. Strategy of Constructing Light-Weight and Highly Compressible Graphene-Based Aerogels with an ordered unique configuration for wearable piezoresistive sensors. ACS Appl. Mater. Interfaces 2019, 11, 19350–19362. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, M.; Huang, F.; Lin, T.; Wan, D. Effect of graphene aerogel on thermal behavior of phase change materials for thermal management. Sol. Energy Mater. Sol. Cells 2013, 113, 195–200. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Li, Y.; Zheng, X. Robust Silk Fibroin/Graphene Oxide Aerogel Fiber for Radiative Heating Textiles. ACS Appl. Mater. Interfaces 2020, 12, 15726–15736. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, M.; Panahi-Sarmad, M.; Abrisham, M.; Amirkiai, A.; Asghari, N.; Golbaten-Mofrad, H.; Karimpour-Motlagh, N.; Goodarzi, V.; Bahramian, A.R.; Zahiri, B. Nanostructure of Aerogels and Their Applications in Thermal Energy Insulation. ACS Appl. Energy Mater. 2019, 2, 5319–5349. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206. [Google Scholar] [CrossRef]
- Song, L.-T.; Wu, Z.-Y.; Liang, H.-W.; Zhou, F.; Yu, Z.-Y.; Xu, L.; Pan, Z.; Yu, S.-H. Macroscopic-scale synthesis of nitrogen-doped carbon nanofiber aerogels by template-directed hydrothermal carbonization of nitrogen-containing carbohydrates. Nano Energy 2016, 19, 117–127. [Google Scholar] [CrossRef]
- Song, Z.; Liu, W.; Xiao, P.; Zhao, Z.; Liu, G.; Qiu, J. Nano-iron oxide (Fe2O3)/three-dimensional graphene aerogel composite as supercapacitor electrode materials with extremely wide working potential window. Mater. Lett. 2015, 145, 44–47. [Google Scholar] [CrossRef]
- Yun, X.; Li, J.; Chen, X.; Chen, H.; Xiao, L.; Xiang, K.; Chen, W.; Liao, H.; Zhu, Y. Porous Fe2O3 modified by nitrogen-doped carbon quantum dots/reduced graphene oxide composite aerogel as a high-capacity and high-rate anode material for alkaline aqueous batteries. ACS Appl. Mater. Interfaces 2019, 11, 36970–36984. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Duan, S.; Zhang, L.; Wang, Z.; Li, C. Three-dimensional porous stretchable and conductive polymer composites based on graphene networks grown by chemical vapour deposition and PEDOT: PSS coating. Chem. Commun. 2015, 51, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Liu, N.; Ma, Y.; Wang, S.; Liu, W.; Luo, C.; Zhang, H.; Cheng, F.; Rao, J.; Hu, X. Highly self-healable 3D microsupercapacitor with MXene–graphene composite aerogel. ACS Nano 2018, 12, 4224–4232. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.-O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]
- Zhan, W.; Gao, L.; Fu, X.; Siyal, S.H.; Sui, G.; Yang, X. Green synthesis of amino-functionalized carbon nanotube-graphene hybrid aerogels for high performance heavy metal ions removal. Appl. Surf. Sci. 2019, 467, 1122–1133. [Google Scholar] [CrossRef]
- Mariana, M.; HPS, A.K.; Yahya, E.B.; Olaiya, N.; Alfatah, T.; Suriani, A.; Mohamed, A. Recent trends and future prospects of nanostructured aerogels in water treatment applications. J. Water Process Eng. 2022, 45, 102481. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, D.; Duan, L.; Chen, W. Adsorption of single-ringed N-and S-heterocyclic aromatics on carbon nanotubes. Carbon 2010, 48, 3906–3915. [Google Scholar] [CrossRef]
- Yang, S.; Shen, C.; Chen, L.; Wang, C.; Rana, M.; Lv, P. Vapor–liquid Deposition Strategy to Prepare Superhydrophobic and Superoleophilic Graphene Aerogel for Oil–Water Separation. ACS Appl. Nano Mater. 2018, 1, 531–540. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, R.; Ren, S.; Chen, C.; Chen, Z.; Yang, X. Three dimensional reduced graphene oxide/ZIF-67 aerogel: Effective removal cationic and anionic dyes from water. Chem. Eng. J. 2018, 348, 202–211. [Google Scholar] [CrossRef]
- Ye, Y.; Yin, D.; Wang, B.; Zhang, Q. Synthesis of three-dimensional Fe3O4/graphene aerogels for the removal of arsenic ions from water. J. Nanomater. 2015, 2015, 864864. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, K.; Wang, X.; Jia, Y.; Sun, B.; Luo, T.; Meng, F.; Jin, Z.; Lin, D.; Shen, W. Enhanced adsorption of cadmium ions by 3D sulfonated reduced graphene oxide. Chem. Eng. J. 2015, 262, 1292–1302. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.; Azari, S.; Losic, D. Graphene-diatom silica aerogels for efficient removal of mercury ions from water. ACS Appl. Mater. Interfaces 2015, 7, 11815–11823. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ge, X.; Ye, X.; Wang, G.; Zhang, H.; Zhou, H.; Zhang, Y.; Zhao, H. 3D graphene/δ-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 2016, 4, 1970–1979. [Google Scholar] [CrossRef]
- Platero, E.; Fernandez, M.E.; Bonelli, P.R.; Cukierman, A.L. Graphene oxide/alginate beads as adsorbents: Influence of the load and the drying method on their physicochemical-mechanical properties and adsorptive performance. J. Colloid Interface Sci. 2017, 491, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Du, Q.; Sun, J.; Chen, L.; Hu, S.; Wang, Z.; Xia, Y.; Xia, L. Highly effective removal of basic fuchsin from aqueous solutions by anionic polyacrylamide/graphene oxide aerogels. J. Colloid Interface Sci. 2015, 453, 107–114. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, W.; Shan, X.; Li, Z. Preparation of a porous graphene oxide/alkali lignin aerogel composite and its adsorption properties for methylene blue. Int. J. Biol. Macromol. 2020, 143, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, P.; Bi, W.; Yao, G.; Li, S.; Liu, H. Graphene oxide aerogels co-functionalized with polydopamine and polyethylenimine for the adsorption of anionic dyes and organic solvents. Chem. Eng. Res. Des. 2020, 154, 192–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Liao, J. Facile synthesis of reduced-graphene-oxide/rare-earth-metal-oxide aerogels as a highly efficient adsorbent for Rhodamine-B. Appl. Surf. Sci. 2020, 504, 144377. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, B.; Xiong, Y.; Jin, C.; Sun, Q.; Sheng, C. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.F.; Guo, B.B.; He, L.; Xia, P.F.; Wang, S.G. Electrically accelerated removal of organic pollutants by a three-dimensional graphene aerogel. AIChE J. 2016, 62, 2154–2162. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, L.; Hao, W.; Ren, W.; Cheng, H.-M. Synthesis and applications of three-dimensional graphene network structures. Mater. Today 2019, 5, 100027. [Google Scholar] [CrossRef]
- Wei, N.; Wang, Q.; Ma, Y.; Ruan, L.; Zeng, W.; Liang, D.; Xu, C.; Huang, L.; Zhao, J. Superelastic active graphene aerogels dried in natural environment for sensitive supercapacitor-type stress sensor. Electrochim. Acta 2018, 283, 1390–1400. [Google Scholar] [CrossRef]
- Wang, M.; Duan, X.; Xu, Y.; Duan, X. Functional three-dimensional graphene/polymer composites. ACS Nano 2016, 10, 7231–7247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pan, R.; Sun, R.; Wen, C.; Zhang, S.-L.; Wu, B.; Nyholm, L.; Zhang, Z.-B. High-conductivity reduced-graphene-oxide/copper aerogel for energy storage. Nano Energy 2019, 60, 760–767. [Google Scholar] [CrossRef]
- Wu, Z.S.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, R.; Li, M.; Li, Z. Flower-like Fe2O3@ multiple graphene aerogel for high-performance supercapacitors. J. Alloys Compd. 2018, 742, 759–768. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Zhang, X.; He, T.; Lu, F.; Li, H.; Ye, J. A facile method of preparing LiMnPO4/reduced graphene oxide aerogel as cathodic material for aqueous lithium-ion hybrid supercapacitors. Appl. Surf. Sci. 2018, 428, 977–985. [Google Scholar] [CrossRef]
- Sun, R.; Chen, H.; Li, Q.; Song, Q.; Zhang, X. Spontaneous assembly of strong and conductive graphene/polypyrrole hybrid aerogels for energy storage. Nanoscale 2014, 6, 12912–12920. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Guo, S.; Yu, C.; Zhong, S.; Liu, X. Multi-functional graphene/carbon nanotube aerogels for its applications in supercapacitor and direct methanol fuel cell. Electrochim. Acta 2018, 264, 12–19. [Google Scholar] [CrossRef]
- Wei, X.; Wan, S.; Gao, S. Self-assembly-template engineering nitrogen-doped carbon aerogels for high-rate supercapacitors. Nano Energy 2016, 28, 206–215. [Google Scholar] [CrossRef]
- Tingting, Y.; Ruiyi, L.; Xiaohuan, L.; Zaijun, L.; Zhiguo, G.; Guangli, W.; Junkang, L. Nitrogen and sulphur-functionalized multiple graphene aerogel for supercapacitors with excellent electrochemical performance. Electrochim. Acta 2016, 187, 143–152. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, S.M.; Jung, H.Y. Long term thermostable supercapacitor using in-situ SnO2 doped porous graphene aerogel. J. Power Sources 2020, 448, 227422. [Google Scholar] [CrossRef]
- Tian, W.; Cheng, D.; Wang, S.; Xiong, C.; Yang, Q. Phytic acid modified manganese dioxide/graphene composite aerogel as high-performance electrode materials for supercapacitors. Appl. Surf. Sci. 2019, 495, 143589. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, J.; Fang, D.; Zhang, X. Nanofibrous Kevlar aerogel threads for thermal insulation in harsh environments. ACS Nano 2019, 13, 5703–5711. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Liao, S.-F.; Shang, K.; Chen, M.-J.; Huang, J.-Q.; Wang, Y.-Z.; Schiraldi, D.A. Efficient approach to improving the flame retardancy of poly (vinyl alcohol)/clay aerogels: Incorporating piperazine-modified ammonium polyphosphate. ACS Appl. Mater. Interfaces 2015, 7, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef]
- Chen, H.-B.; Chiou, B.-S.; Wang, Y.-Z.; Schiraldi, D.A. Biodegradable pectin/clay aerogels. ACS Appl. Mater. Interfaces 2013, 5, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Feng, J.; Feng, J.; Jiang, Y. Ultralow-density and high-strength graphene aerogels composites for thermal insulation. Mater. Lett. 2017, 188, 169–171. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Gu, J.; Liu, H.; Liu, C.; Luo, C.; Kong, J.; Shao, Q.; Wang, N.; Guo, Z. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, D.; Deng, B.; Xu, X.; Nian, Q.; Jin, S.; Leedy, K.D.; Li, H.; Cheng, G.J. Flyweight, superelastic, electrically conductive, and flame-retardant 3D multi-nanolayer graphene/ceramic metamaterial. Adv. Mater. 2017, 29, 1605506. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Zhai, G. Biomedical applications of the graphene-based materials. Mater. Sci. Eng. C 2016, 61, 953–964. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, C.; Mou, S.; Li, J.; Zhou, M.; Zeng, Y.; Luo, C.; Sun, J.; Wang, Z.; Xu, W. Biocompatible graphene oxide–collagen composite aerogel for enhanced stiffness and in situ bone regeneration. Mater. Sci. Eng. C 2019, 105, 110137. [Google Scholar] [CrossRef] [PubMed]
- Ayazi, H.; Akhavan, O.; Raoufi, M.; Varshochian, R.; Motlagh, N.S.H.; Atyabi, F. Graphene aerogel nanoparticles for in-situ loading/pH sensitive releasing anticancer drugs. Colloids Surf. B 2020, 186, 110712. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic acid induced self-assembly of three-dimensional graphene with good adsorption and antibacterial properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef]
- López-Dolado, E.; González-Mayorga, A.; Portolés, M.T.; Feito, M.J.; Ferrer, M.L.; Del Monte, F.; Gutiérrez, M.C.; Serrano, M.C. Subacute tissue response to 3D graphene oxide scaffolds implanted in the injured rat spinal cord. Adv. Healthc. Mater. 2015, 4, 1861–1868. [Google Scholar] [CrossRef]
- Zheng, Q.; Lee, J.-h.; Shen, X.; Chen, X.; Kim, J.-K. Graphene-based wearable piezoresistive physical sensors. Mater. Today 2020, 36, 158–179. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, M.; Cai, B.; Wang, W.; Ye, Z.; Huang, J. 3D graphene foams decorated by CuO nanoflowers for ultrasensitive ascorbic acid detection. Biosen. Bioelectron. 2014, 59, 384–388. [Google Scholar] [CrossRef]
- Dong, X.; Wang, X.; Wang, L.; Song, H.; Zhang, H.; Huang, W.; Chen, P. 3D graphene foam as a monolithic and macroporous carbon electrode for electrochemical sensing. ACS Appl. Mater. Interfaces 2012, 4, 3129–3133. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Y.; Sun, W.; Han, R.; Luo, C.; Wang, X.; Wei, Q. A highly selective and sensitive detection of insulin with chemiluminescence biosensor based on aptamer and oligonucleotide-AuNPs functionalized nanosilica@ graphene oxide aerogel. Anal. Chim. Acta 2019, 1089, 152–164. [Google Scholar] [CrossRef] [PubMed]
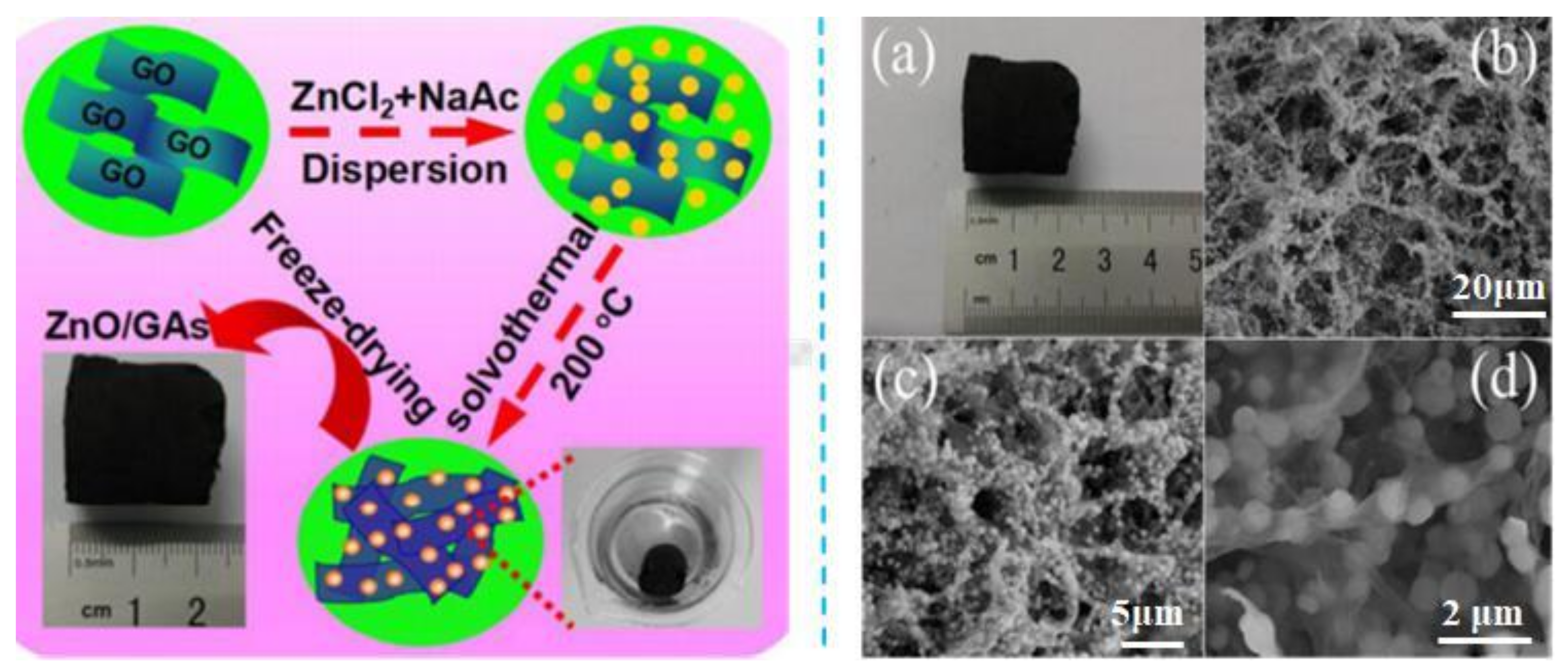
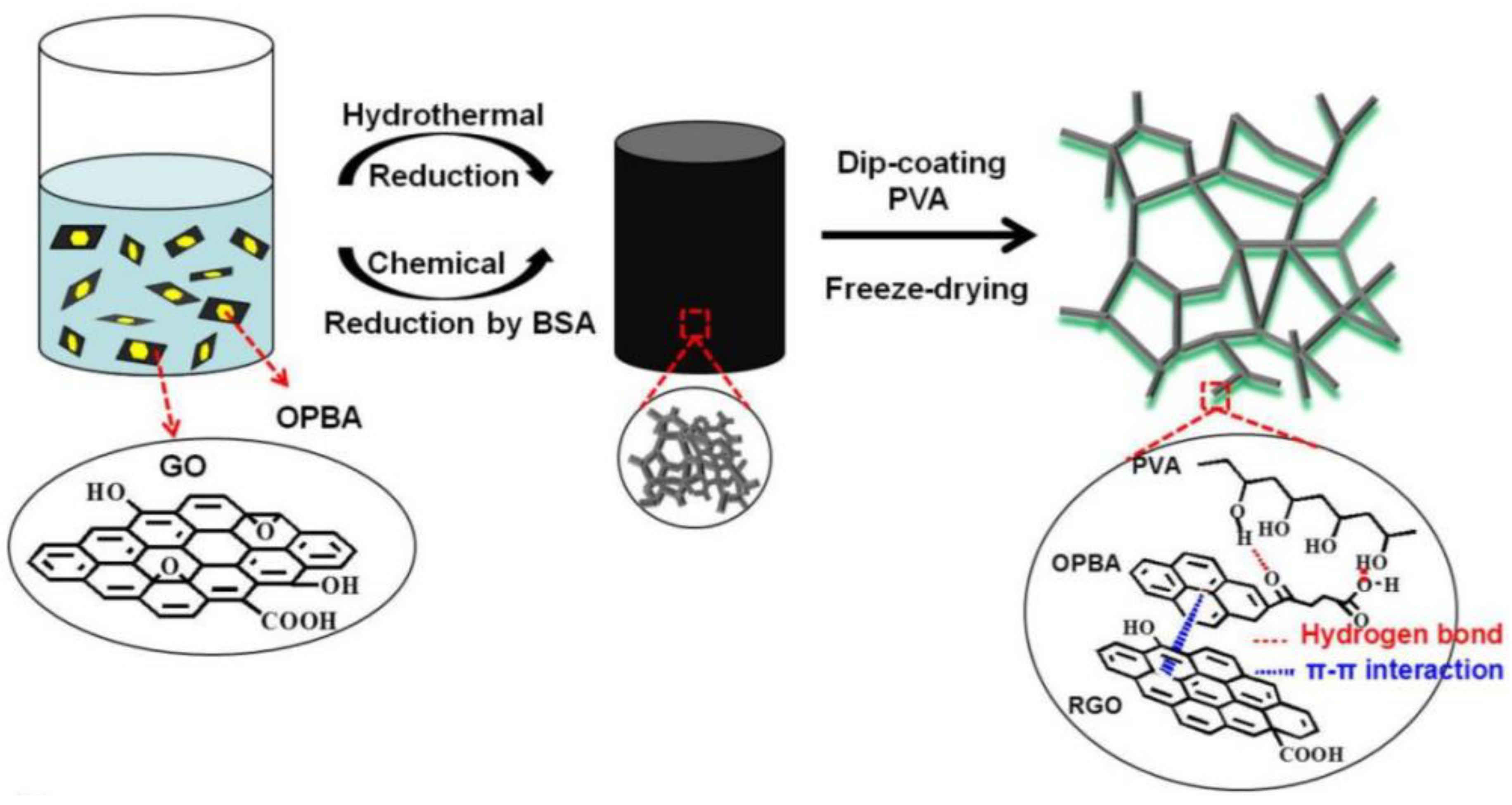
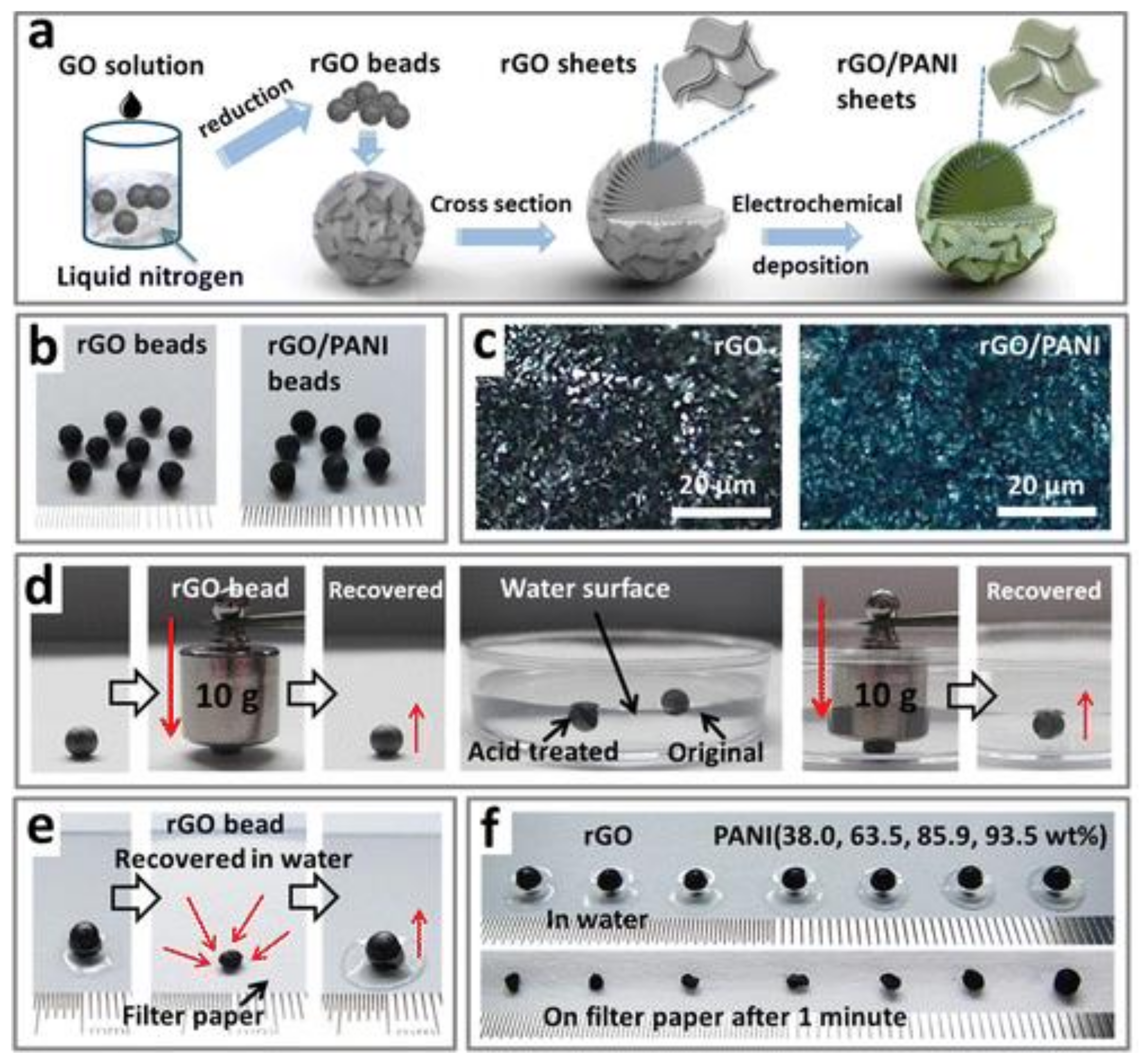
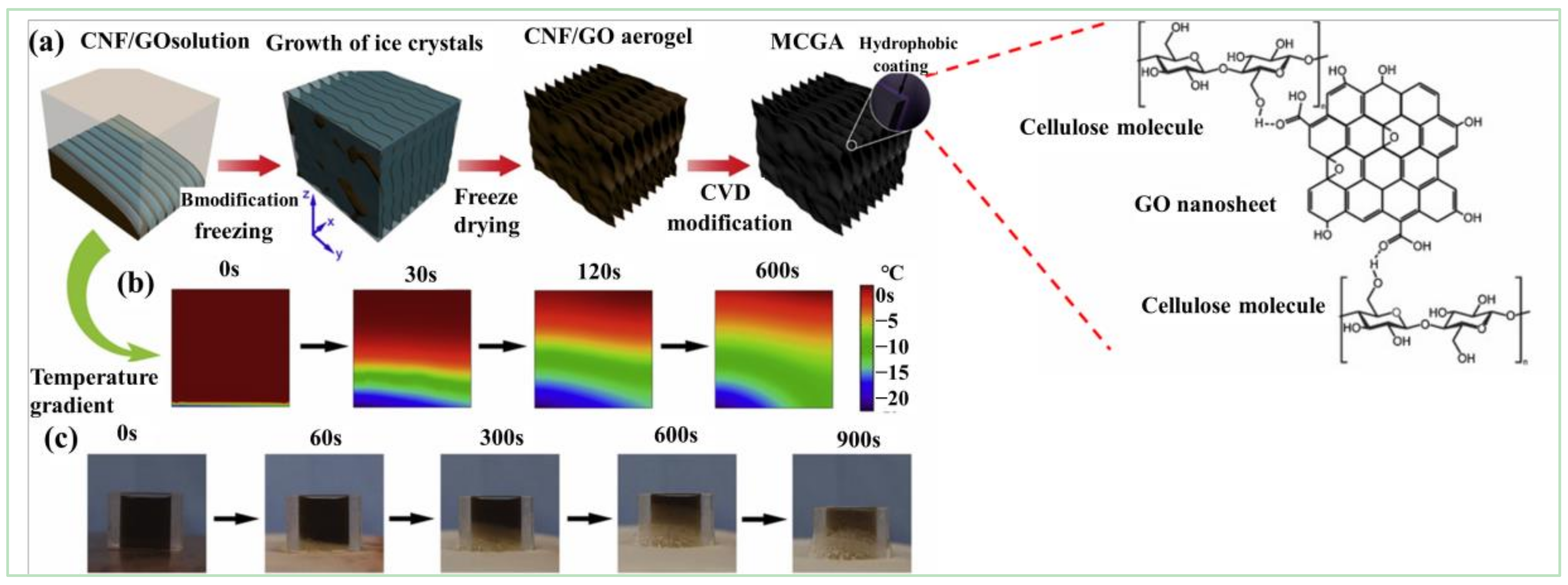
| GCA | Preparation Principle | Applications | Reference |
|---|---|---|---|
| 2D nanosheets/GCA | 3D printing | Energy storage materials | [9] |
| Electrospinning | Wave-absorbing materials | [10] | |
| Self-assembly | Electrocatalysts | [11] | |
| Inorganic nanoparticle materials/GCA | Self-assembly | Wearable piezoresistive sensors | [12] |
| Sol-gel method | Energy storage material | [13] | |
| Sol-gel method | Gas sensing materials | [14] | |
| CVD | Adsorption materials | [15] | |
| Self-assembly | Catalytic material | [16] | |
| Synthetic polymer/GCA | Sol-gel method | Pressure response sensor | [17] |
| Template method | Thermal insulation materials | [18] | |
| Immersion method | Porous electrode | [19] | |
| Self-assembly | Energy storage material | [20] | |
| Natural polymer/GCA | CVD | Adsorption materials | [21] |
| Template method | Thermal insulation materials | [22] | |
| Self-assembly | Supercapacitor materials | [23] | |
| Sol-gel method | Adsorption materials | [24] | |
| Chemical cross-linking | Adsorption materials | [25] |
| Composite Aerogel Material | Fabrication Method | Specific Surface Area (m2·g−1) | Density (mg·cm−3) | Pore Volume (cm3·g−1) | Diameter (nm) | Reference |
|---|---|---|---|---|---|---|
| GNPA | Self-assembly | / | 59.3 | / | 5 | [12] |
| GO/SiO2 | Sol-gel method | 889 | / | 3.72 | 16.75 | [40] |
| VO2/GA @NiS2 | Sol-gel method | 141.1 | / | / | 17.3 | [41] |
| Co-N-GA | Self-assembly | 485 | 0.29 | 0.71 | / | [11] |
| PPy@GA | Self-assembly | 686 | 7.8 | / | / | [20] |
| MGGNA | Freeze-drying | 45.1 | 2.32 | / | / | [42] |
| GNR | Self-assembly | 113.1 | 9.33 | / | / | [43] |
| MoS2-RGO | 3D printing | / | / | / | 100–200 | [9] |
| 3D-GMOs | CVD | 560 | 22 | / | / | [15] |
| N-CMS/GA | Mixed | 450 | / | / | 3.4–36.9 | [44] |
| Adsorbent | Contaminants | Adsorption Conditions | Isotherm Model | qm (mg·g−1) | Reference |
|---|---|---|---|---|---|
| RGO/ZIF-67 | CV MO | pH 3–9, T = 298 K, t = 0–16 h | Langmuir | 1714 426 | [79] |
| MWCNT-PDA | Cu2+ Pb2+ | pH 2–7, T = 298 K, t = 0–10 h | Langmuir | 318.47 350.87 | [74] |
| 3D-Fe3O4/GA | As5+ | pH 7, T = 298 K, t = 0–16 h | Langmuir | 40.05 | [80] |
| 3D-SRGO | Cd2+ | pH 2–9, T = 298 K, t = 0–24 h | Langmuir | 234.8 | [81] |
| GA/SiO2 | Hg2+ | pH 2–10, T = 296 K, t = 0–1.5 h | Langmuir | 500.0 | [82] |
| 3D δ-MnO2 | Pb2+ Cd2+ Cu2+ | pH 2–6, T = 298 K, t = 0–3 h | Langmuir | 643.62 250.31 228.46 | [83] |
| 3D GO/SA | MB | pH 4–9, T = 293 K, t = 0–24 h | Langmuir | 4.25 | [84] |
| PAM/GO | Magenta | pH 2.6–8.9, T = 303 K, t = 0–55 h | Langmuir | 1034.3 | [85] |
| GO-AL | MB | pH 3.0, T = 303 K, t = 0–4 h | Langmuir | 1185.98 | [86] |
| PPGA | N-hexane, MO | pH 3.0, T = 303 K, t = 0–4 h | Langmuir | 63.5 202.8 | [87] |
| RGO/REMO | RhB, | t = 0–24 h | Langmuir | 243.4 | [88] |
| GAS-MS | phenol, catechol, resorcinol, hydroquinone | pH 3.0, T = 298 K, t = 24 h | Langmuir&Freundlich | 90 66 22 67 | [28] |
| CNF/GO | Tetracycline | pH 2–12, T = 298 K, t = 24 h | Langmuir | 454.6 | [89] |
| 3DG | Methyl bromide | pH 7.5, T = 298 K, t = 0–5 h | Langmuir | 685 | [90] |
| Composite Aerogel Materials | Specific Capacitance (F·g−1) | Energy Density (Wh·kg−1) | Cyclic Behavior | Electrolyte | Reference |
|---|---|---|---|---|---|
| F-Fe2O3@MGA | 1119@1 Ag−1 | 800 | 98.9% after 2000 cycles | 3M KOH | [96] |
| LMP/rGO | 4645@0.5Ag−1 | 11.79 | 91.2% after 10,000 cycles | 6M KOH | [97] |
| PANI/CRGO/Co3O4 | 1247@1 Ag−1 | 190 | 92% after 3500 cycles | 6M KOH | [90] |
| PPy/CRGO | 304@0.5 Ag−1 | / | 58% after 50 cycles | 6M KOH | [98] |
| GR-CNT | 375@1 Ag−1 | / | 94.8% after 5000 cycles | 6M KOH | [99] |
| CA | 467@20 Ag−1 | 22.75 | 90.9% after 10,000 cycles | 1M KOH | [100] |
| N,S-MGA | 4929@2 Ag−1 | 686.7 | 98.7% after 5000 cycles | 1M KOH | [101] |
| SnO2-GA | 541@5 Ag−1 | 160 | 97.3% after 10,000 cycles | 3M KOH | [102] |
| MnO2/P-RGO | 645@1 Ag−1 | 59.2 | 94.6% after 10,000 cycles | 1M Na2SO4 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Liu, X.; Lv, S.; Liu, L.; Wu, L.; Li, Z.; Hou, Y. Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel. Materials 2022, 15, 299. https://doi.org/10.3390/ma15010299
Wei D, Liu X, Lv S, Liu L, Wu L, Li Z, Hou Y. Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel. Materials. 2022; 15(1):299. https://doi.org/10.3390/ma15010299
Chicago/Turabian StyleWei, Dequan, Xiang Liu, Shenghua Lv, Leipeng Liu, Lei Wu, Zexiong Li, and Yonggang Hou. 2022. "Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel" Materials 15, no. 1: 299. https://doi.org/10.3390/ma15010299
APA StyleWei, D., Liu, X., Lv, S., Liu, L., Wu, L., Li, Z., & Hou, Y. (2022). Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel. Materials, 15(1), 299. https://doi.org/10.3390/ma15010299





