Qualitative Evaluation of the Effects of Professional Oral Hygiene Instruments on Prosthetic Ceramic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poggio, C.E.; Ercoli, C.; Rispoli, L.; Maiorana, C.; Esposito, M. Metal-free materials for fixed prosthodontic restorations. Cochrane Database Syst. Rev. 2017, 12, CD009606. [Google Scholar] [CrossRef] [PubMed]
- Ortensi, L.; Vitali, T.; Bonfiglioli, R.; Grande, F. New Tricks in the Preparation Design for Prosthetic Ceramic Laminate Veeners. Prosthesis 2019, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Mobilio, N.; Catapano, S. The use of monolithic lithium disilicate for posterior screw-retained implant crowns. J. Prosthet. Dent. 2017, 118, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Mobilio, N.; Fasiol, A.; Catapano, S. Survival Rates of Lithium Disilicate Single Restorations: A Retrospective Study. Int. J. Prosthodont. 2018, 31, 283–286. [Google Scholar] [CrossRef]
- Wolfart, S.; Harder, S.; Eschbach, S.; Lehmann, F.; Kern, M. Four-year clinical results of fixed dental prostheses with zirconia substructures (Cercon): End abutments vs. cantilever design. Eur. J. Oral Sci. 2009, 117, 741–749. [Google Scholar] [CrossRef]
- Schmitter, M.; Mussotter, K.; Rammelsberg, P.; Gabbert, O.; Ohlmann, B. Clinical performance of long-span zirconia frameworks for fixed dental prostheses: 5-year results. J. Oral Rehabil. 2012, 39, 552–557. [Google Scholar] [CrossRef]
- Celeghin, G.; Franceschetti, G.; Mobilio, N.; Fasiol, A.; Catapano, S.; Corsalini, M.; Grande, F. Complete-Arch Accuracy of Four Intraoral Scanners: An In Vitro Study. Healthcare 2021, 9, 246. [Google Scholar] [CrossRef]
- Koch, G.K.; Gallucci, G.O.; Lee, S.J. Accuracy in the digital workflow: From data acquisition to the digitally milled cast. J. Prosthet. Dent. 2016, 115, 749–754. [Google Scholar] [CrossRef]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef] [Green Version]
- Willard, A.; Gabriel Chu, T.-M. The science and application of IPS e.Max dental ceramic. Kaohsiung J. Med. Sci. 2018, 34, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S152–S157. [Google Scholar] [CrossRef] [Green Version]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, G.; Basile, F.; Relics, D.; Ferri, A.; Grande, F.; Tarsitano, A.; Marchetti, C. Computer-Aided Rehabilitation Supported by Zygomatic Implants: A Cohort Study Comparing Atrophic with Oncologic Patients after Five Years of Follow-Up. J. Clin. Med. 2020, 9, 3254. [Google Scholar] [CrossRef]
- Mengel, R.; Buns, C.; Stelzel, M.; Flores-de-Jacoby, L. An in vitro study of oscillating instruments for root planing. J. Clin. Periodontol. 1994, 21, 513–518. [Google Scholar] [CrossRef]
- Mengel, R.; Stelzel, M.; Mengel, C.; Flores-de-Jacoby, L.; Diekwisch, T. An in vitro study of various instruments for root planing. Int. J. Periodontics Restor. Dent. 1997, 17, 592–599. [Google Scholar]
- Torfason, T.; Kiger, R.; Selvig, K.A.; Egelberg, J. Clinical improvement of gingival conditions following ultrasonic versus hand instrumentation of periodontal pockets. J. Clin. Periodontol. 1979, 6, 165–176. [Google Scholar] [CrossRef]
- Badersten, A.; Nilveus, R.; Egelberg, J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. J. Clin. Periodontol. 1984, 11, 63–76. [Google Scholar] [CrossRef]
- Henry, P.J.; Johnston, J.F.; Mitchell, D.F. Tissue changes beneath fixed partial dentures. J. Prosthet. Dent. 1966, 16, 937–947. [Google Scholar] [CrossRef]
- Checketts, M.R.; Turkyilmaz, I.; Asar, N.V. An investigation of the effect of scaling-induced surface roughness on bacterial adhesion in common fixed dental restorative materials. J. Prosthet. Dent. 2014, 112, 1265–1270. [Google Scholar] [CrossRef]
- Vigolo, P.; Motterle, M. An in vitro evaluation of zirconia surface roughness caused by different scaling methods. J. Prosthet. Dent. 2010, 103, 283–287. [Google Scholar] [CrossRef]
- Coldiron, N.B.; Yukna, R.A.; Weir, J.; Caudill, R.F. A quantitative study of cementum removal with hand curettes. J. Periodontol. 1990, 61, 293–299. [Google Scholar] [CrossRef]
- Walmsley, A.D.; Laird, W.R.; Williams, A.R. A model system to demonstrate the role of cavitational activity in ultrasonic scaling. J. Dent. Res. 1984, 63, 1162–1165. [Google Scholar] [CrossRef]
- Peng, Z.; Rahman, M.I.A.; Zhang, Y.; Yin, L. Wear behavior of pressable lithium disilicate glass ceramic. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 968–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cura, C.; Özcan, M.; Isik, G.; Saracoglu, A. Comparison of alternative adhesive cementation concepts for zirconia ceramic: Glaze layer vs. zirconia primer. J. Adhes. Dent. 2012, 14, 75–82. [Google Scholar] [CrossRef]
- Ntala, P.; Chen, X.; Niggli, J.; Cattell, M. Development and testing of multi-phase glazes for adhesive bonding to zirconia substrates. J. Dent. 2010, 38, 773–781. [Google Scholar] [CrossRef]
- Allahkarami, M.; Hanan, J.C. Mapping the tetragonal to monoclinic phase transformation in zirconia core dental crowns. Dent. Mater. 2011, 27, 1279–1284. [Google Scholar] [CrossRef]

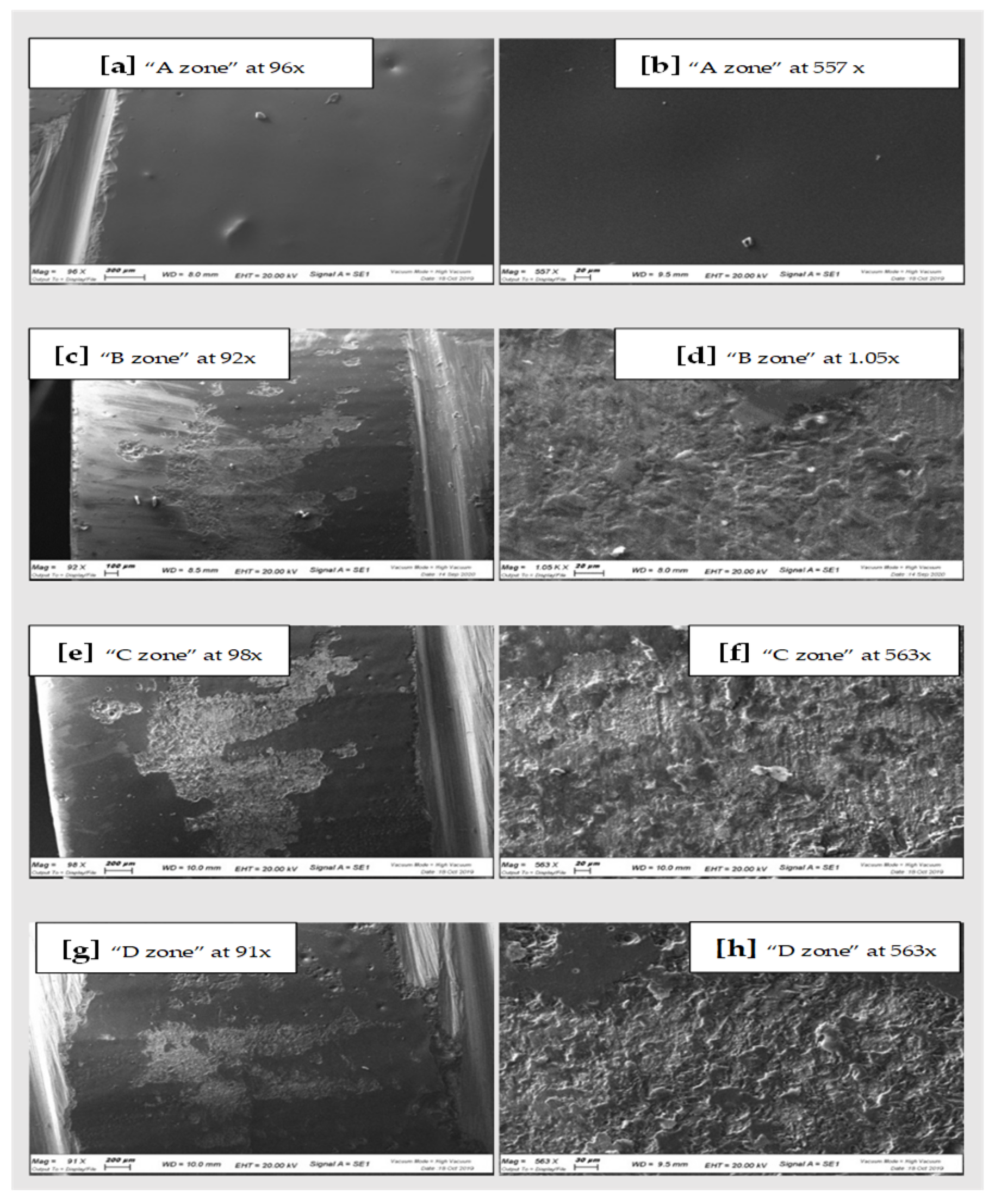
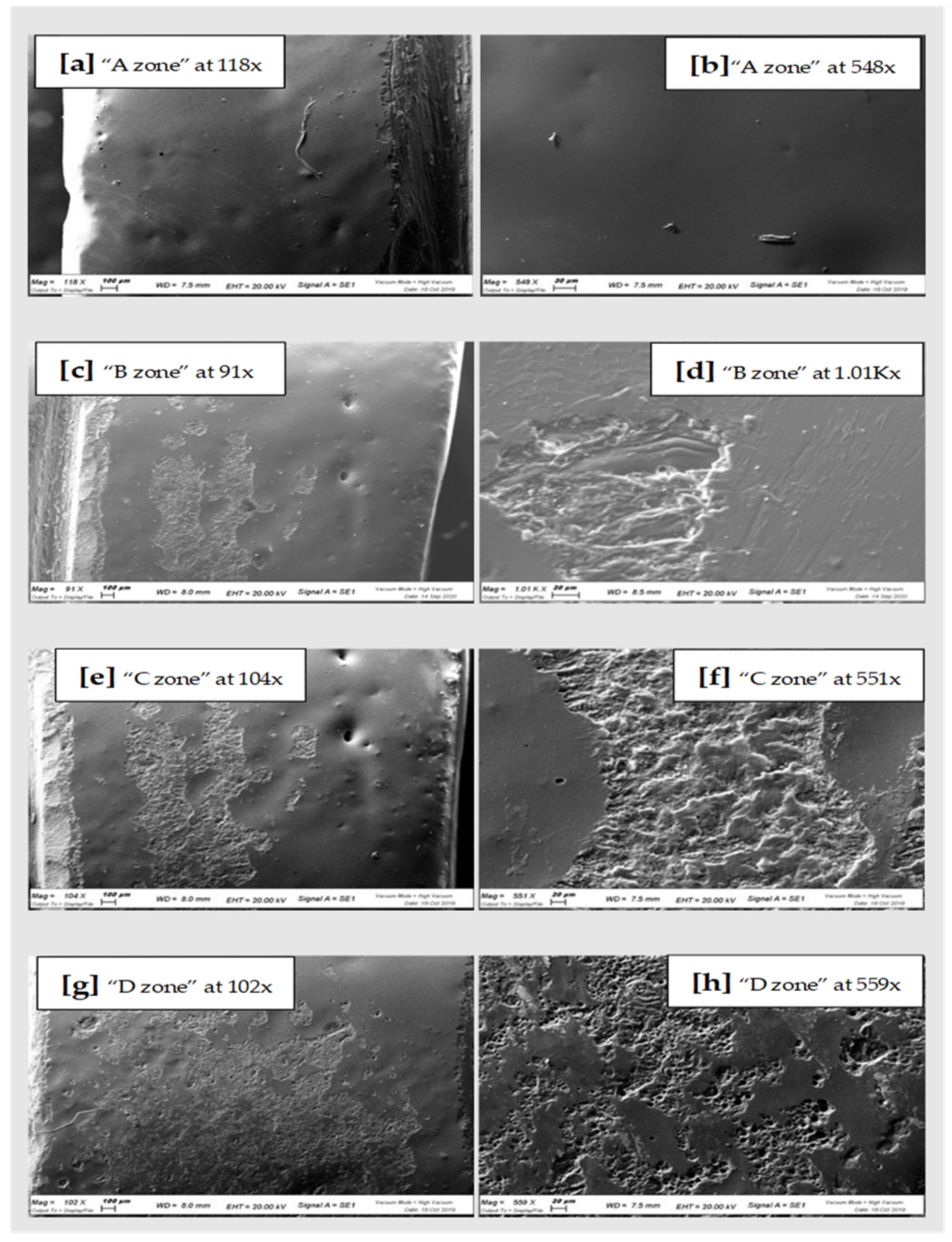
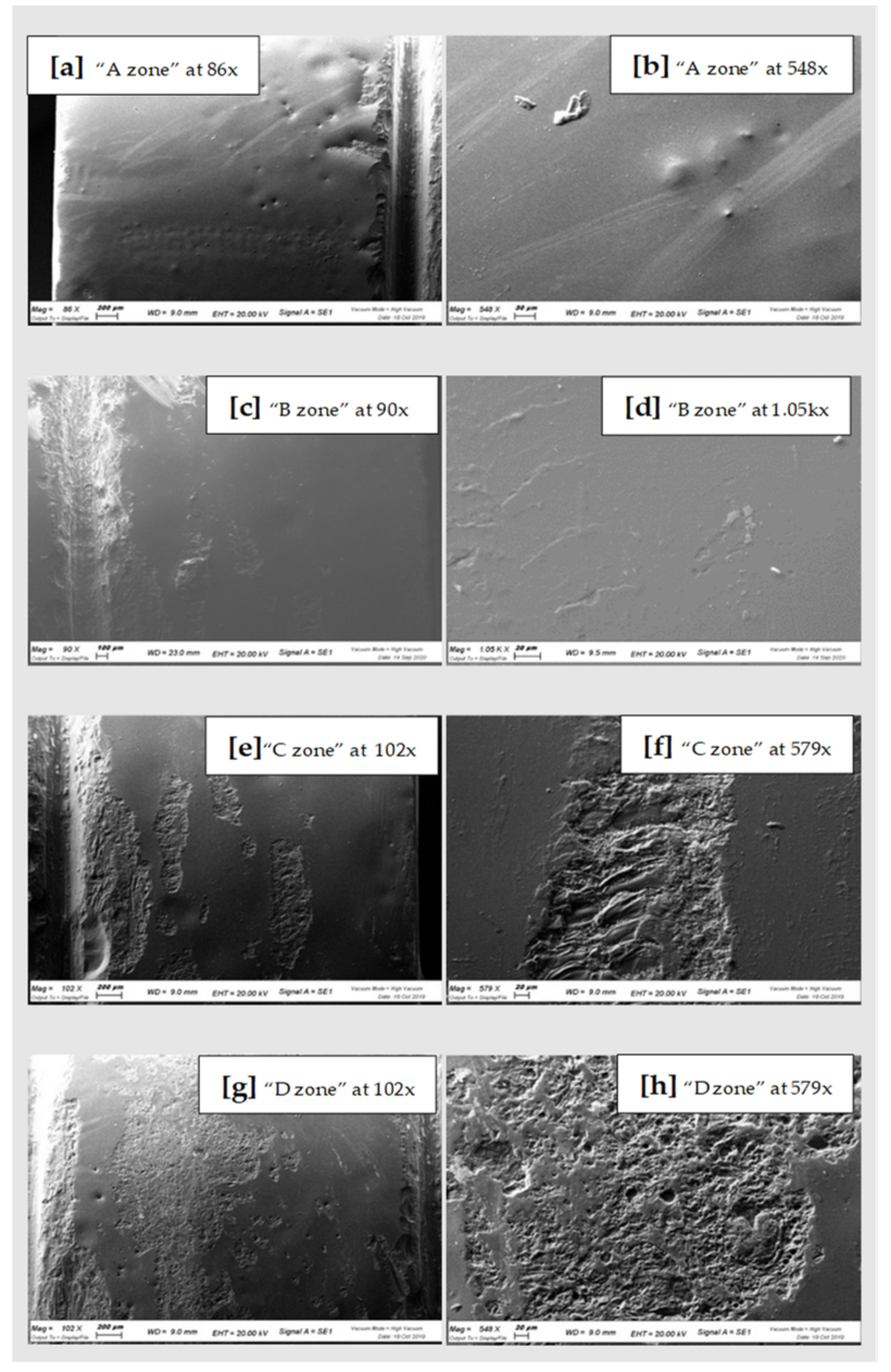
| Qualitative Analysis Zirconia | O | Si | Zr | Na | Al | K | Zn | Ca | Mg | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| “A zone”- non treated | wt%: 56.8% σ: 0.2 | wt%: 27.4% σ: 0.1 | wt%: - σ: - | wt%: 7.2% σ: 0.1 | wt%: 2.6% σ: 0.0 | wt%: 2.9% σ: 0.0 | wt%: 1.9% σ: 0.1 | wt%: 1.1% σ: 0.0 | wt%: 0.3% σ: 0.0 | wt%: - σ: - |
| “B zone”-treated with plastic curettes | wt%: 46.6% σ: 0.3 | wt%: 13.4% σ: 0.1 | wt%: 28.6% σ: 0.2 | wt%: 3.3 σ: 0.0 | wt%: 1.9% σ: 0.0 | wt%: 1.8% σ: 0.0 | wt%: 1.2% σ: 0.1 | wt%: 0.8% σ: 0.0 | wt%: - σ: - | wt%: 1.5% σ: 0.1 |
| “C zone”-treated with SS curettes | wt%: 48.7% σ: 0.2 | wt%: 8.2% σ: 0.1 | wt%: 33.2% σ: 0.2 | wt%: 2.4% σ: 0.1 | wt%: 1.4% σ: 0.0 | wt%: 0.9% σ: 0.0 | wt%: 0.6% σ: 0.1 | wt%: 0.4% σ: 0.0 | wt%: - σ: - | wt%: - σ:- |
| “D zone”- treated with piezoelectric | wt%: 54.7% σ:0.2 | wt%: 18.8% σ: 0.1 | wt%: 13.9% σ: 0.2 | wt%: 4.9% σ: 0.1 | wt%: 2.3% σ: 0.0 | wt%: 2.1% σ: 0.0 | wt%: 1.2% σ: 0.1 | wt%: 0.8% σ:0.0 | wt%: 0.2% σ: 0.0 | wt%: 1.1% σ: 0.1 |
| Qualitative Analysis Cad-Cam Disilicate | O | Si | Na | Al | K | Zn | Ca | Mg | Ce | Fe | P | Cr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| “A zone” - non treated | wt%: 64.2% σ: 0.2 | wt%: 24.7% σ: 0.1 | wt%: 3.3% σ: 0.1 | wt%: 2.5% σ: 0.0 | wt%: 3.5% σ: 0.0 | wt%: 0.7% σ: 0.1 | wt%: 0.9% σ: 0.0 | wt%: 0.3% σ: 0.0 | wt%: - σ: - | wt%: - σ: - | wt%: - σ: - | wt%: - σ: - |
| “B zone”-treated with plastic curettes | wt%: 55.7% σ: 0.2 | wt%: 29.0% σ: 0.2 | wt%: 2.4% σ: 0.1 | wt%: 2.7% σ: 0.1 | wt%: 5.7% σ: 0.1 | wt%: 2.3% σ: 0.1 | wt%: 1.2 % σ: 0.0 | wt%: 0.5% σ: 0.0 | wt%: 0.5% σ: 0.0 | wt%: - σ: - | wt%: - σ: - | wt%: - σ: - |
| “C zone”-treated with SS curettes | wt%: 53.8% σ: 0.2 | wt%: 28.9% σ: 0.2 | wt%: 1.6% σ: 0.1 | wt%: 2.4% σ: 0.1 | wt%: 4.6% σ: 0.1 | wt%: 1.0% σ: 0.1 | wt%: 0.9% σ: 0.0 | wt%: 1.4% σ: 0.0 | wt%: 0.8% σ: 0.1 | wt%: 2.8% σ: 0.1 | wt%: 0.5% σ: 0.0 | wt%: 0.5% σ: 0.1 |
| “D zone”- treated with piezoelectric | wt%: 63.3% σ: 0.2 | wt%: 24.7% σ: 0.1 | wt%: 1.5% σ: 0.1 | wt%: 2.2% σ: 0.0 | wt%: 2.8% σ: 0.0 | wt%: - σ: - | wt%: 0.4% σ: 0.0 | wt%: - σ: - | wt%: - σ: - | wt%: 3.6% σ: 0.1 | wt%: 0.8% σ: 0.0 | wt%: 0.7% σ: 0.0 |
| Qualitative Analysis Pressed Lithium Disilicate | O | Si | Na | Al | K | Zn | Ca | Mg | Ce | Fe | Cr | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| “A zone” - non treated | wt%: 55.0% σ: 0.2 | wt%: 29.7% σ: 0.1 | wt%: 2.4% σ: 0.1 | wt%: 2.8% σ: 0.0 | wt%: 6.1% σ: 0.1 | wt%: 2.6% σ: 0.1 | wt%: 1.1% σ: 0.0 | wt%: 0.4% σ: 0.0 | wt%: - σ: - | wt%: - σ: - | wt%: - σ: - | wt%: - σ: - |
| “B zone”-treated with plastic curettes | wt%: 55.7% σ: 0.2 | wt%: 30.3% σ: 0.2 | wt%: 2.2% σ: 0.1 | wt%: 2.6% σ: 0.0 | wt%: 6.1% σ: 0.1 | wt%: 3.6% σ: 0.1 | wt%: 1.1 % σ: 0.0 | wt%: 0.5% σ: 0.1 | wt%: 0.5% σ: 0.0 | wt%: 0.3% σ: 0.01 | wt%: - σ: - | wt%: - σ: - |
| “C zone”-treated with SS curettes | wt%: 57.4% σ: 0.2 | wt%: 28.7% σ: 0.1 | wt%: 2.0% σ: 0.1 | wt%: 2.6% σ: 0.0 | wt%: 5.5% σ: 0.1 | wt%: 2.7% σ: 0.1 | wt%: 0.9% σ: 0.0 | wt%: - σ: - | wt%: - σ: - | wt%: 0.3% σ: 0.0 | wt%: - σ: - | wt%: - σ: - |
| “D zone”- treated with piezoelectric | wt%: 57.5% σ: 0.2 | wt%: 18.8% σ: 0.1 | wt%: 1.8% σ: 0.1 | wt%: 2.3% σ: 0.0 | wt%: 5.4% σ: 0.1 | wt%: 3.0% σ: 0.1 | wt%: 0.8% σ: 0.0 | wt%: 0.5% σ: 0.0 | wt%: - σ: - | wt%: 0.9% σ: 0.1 | wt%: 0.1% σ: 0.0 | wt%: 0.4% σ: 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, F.; Mochi Zamperoli, E.; Pozzan, M.C.; Tesini, F.; Catapano, S. Qualitative Evaluation of the Effects of Professional Oral Hygiene Instruments on Prosthetic Ceramic Surfaces. Materials 2022, 15, 21. https://doi.org/10.3390/ma15010021
Grande F, Mochi Zamperoli E, Pozzan MC, Tesini F, Catapano S. Qualitative Evaluation of the Effects of Professional Oral Hygiene Instruments on Prosthetic Ceramic Surfaces. Materials. 2022; 15(1):21. https://doi.org/10.3390/ma15010021
Chicago/Turabian StyleGrande, Francesco, Edoardo Mochi Zamperoli, Mario Cesare Pozzan, Fabio Tesini, and Santo Catapano. 2022. "Qualitative Evaluation of the Effects of Professional Oral Hygiene Instruments on Prosthetic Ceramic Surfaces" Materials 15, no. 1: 21. https://doi.org/10.3390/ma15010021
APA StyleGrande, F., Mochi Zamperoli, E., Pozzan, M. C., Tesini, F., & Catapano, S. (2022). Qualitative Evaluation of the Effects of Professional Oral Hygiene Instruments on Prosthetic Ceramic Surfaces. Materials, 15(1), 21. https://doi.org/10.3390/ma15010021









