XRD and TG-DTA Study of New Phosphate-Based Geopolymers with Coal Ash or Metakaolin as Aluminosilicate Source and Mine Tailings Addition
Abstract
:1. Introduction
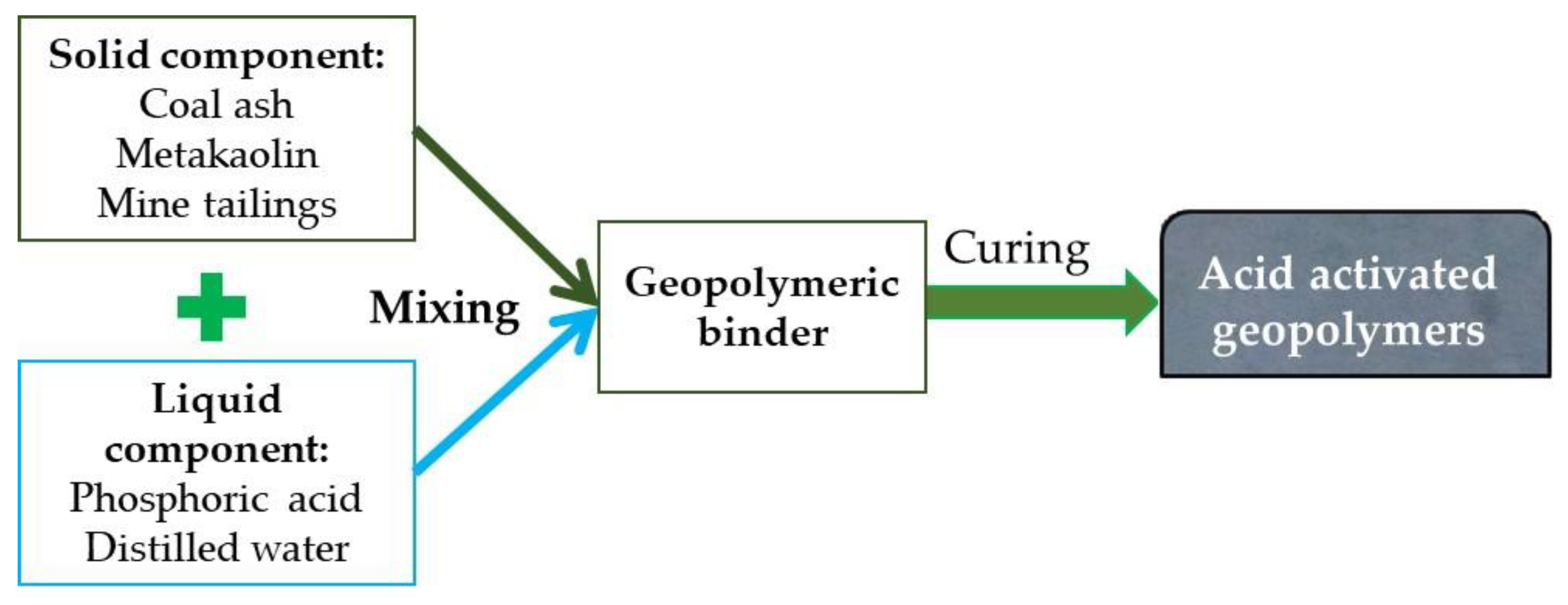
2. Materials and Methods
2.1. Materials
2.1.1. Coal Ash (CA)
2.1.2. Metakaolin (MK)
2.1.3. Mine Tailings (MT)
2.1.4. Activator Solution
2.2. Methods
2.2.1. X-ray Diffraction
2.2.2. Simultaneous Thermal Analysis
3. Results and Discussion
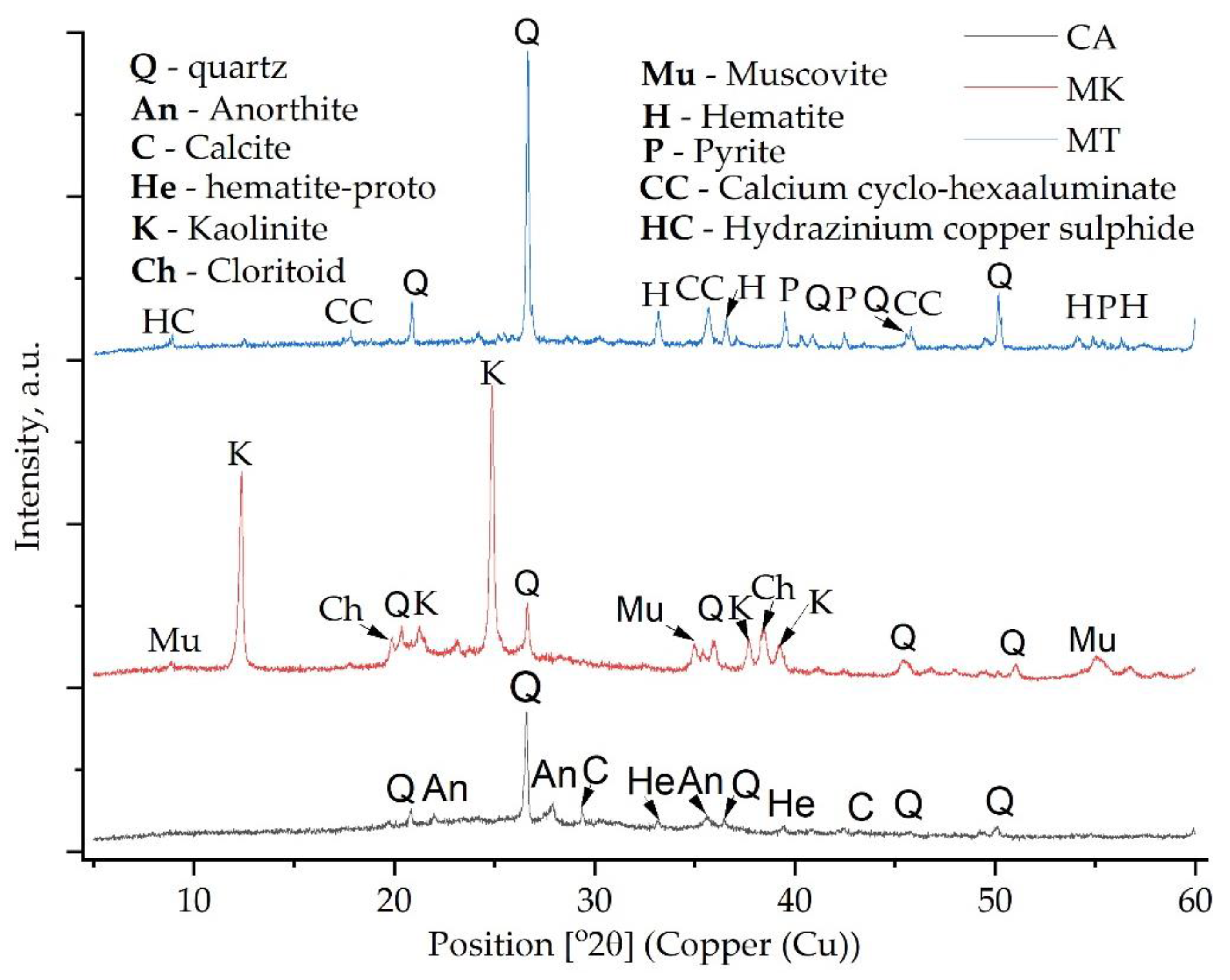
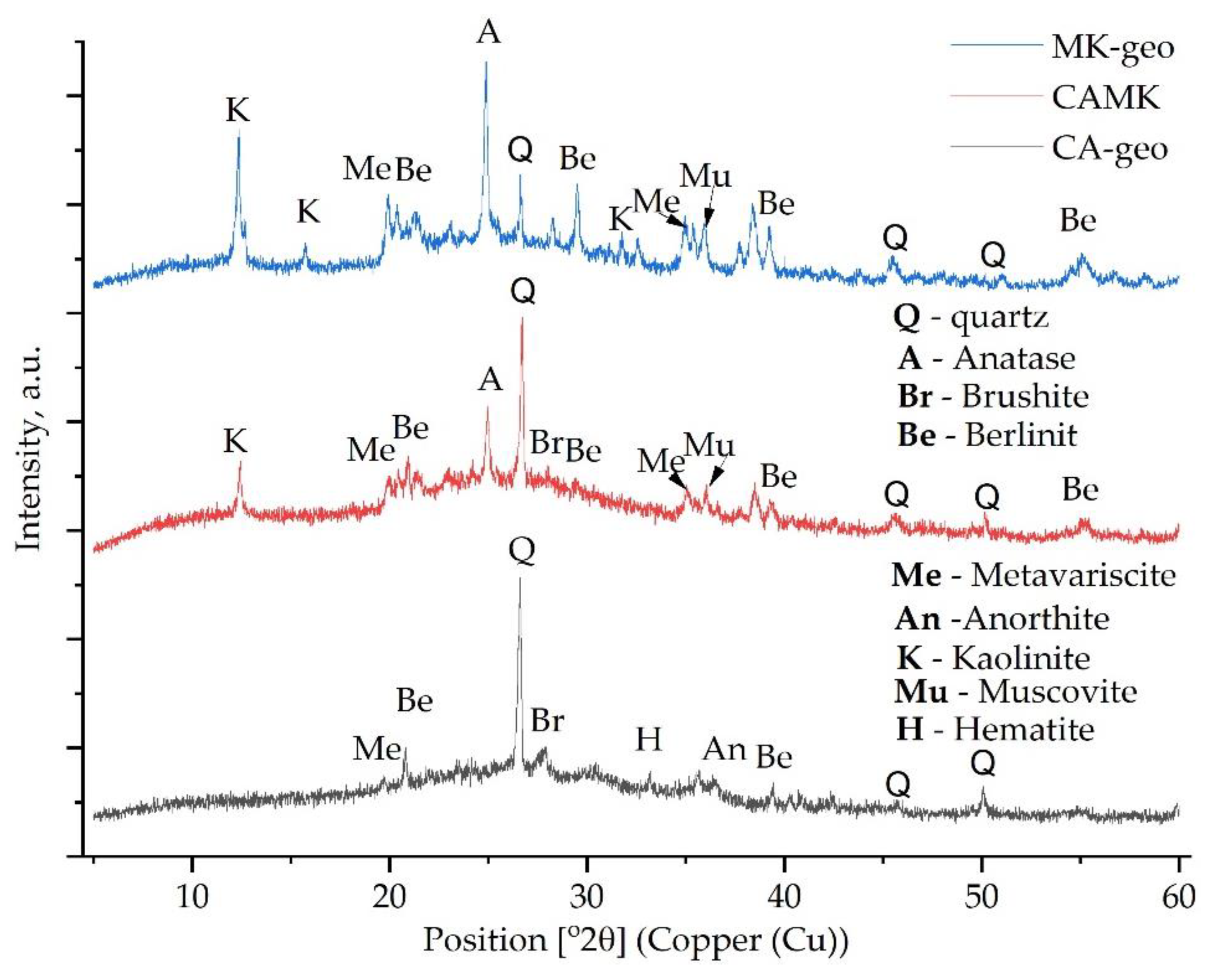
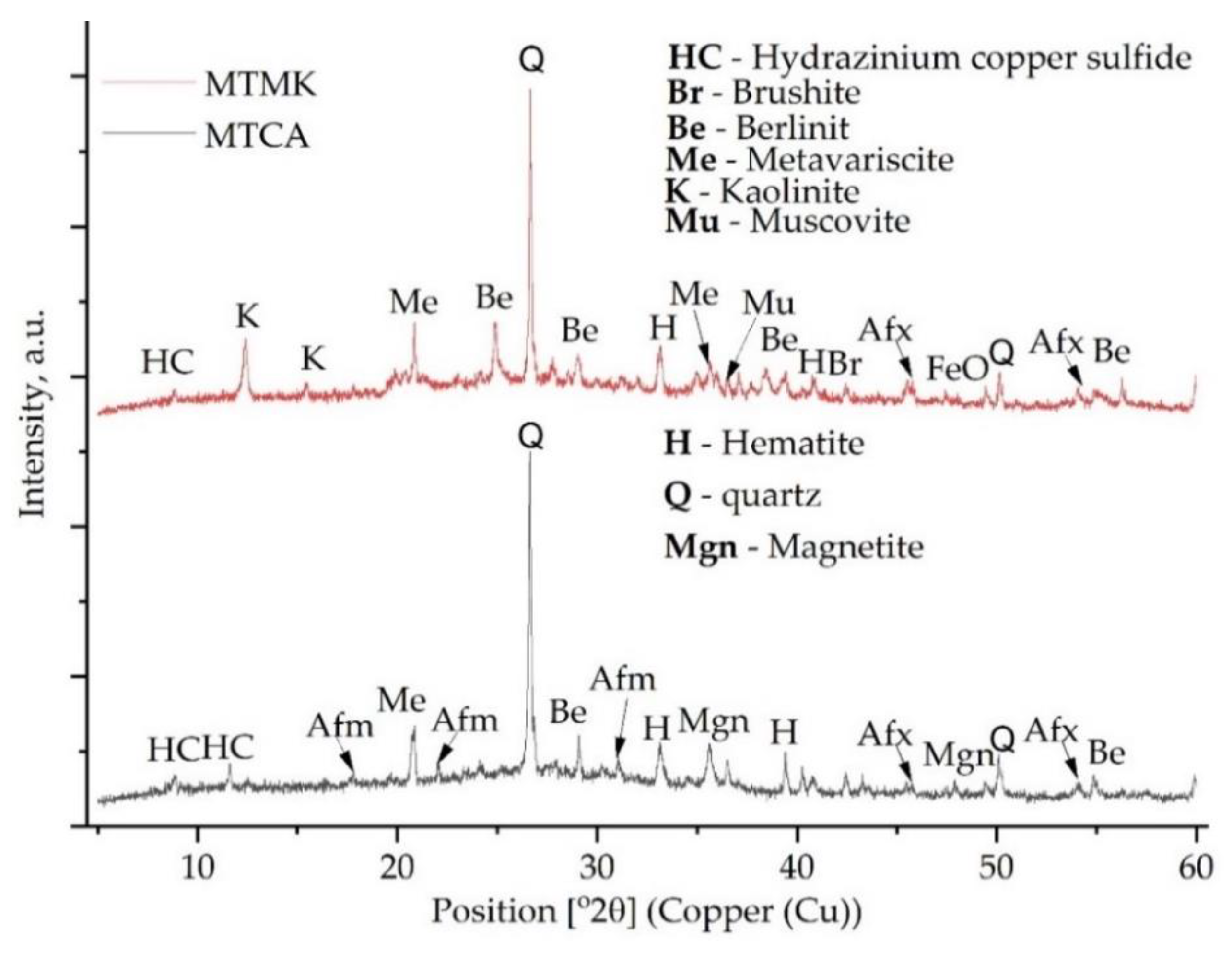
3.1. Phase Transition Analysis
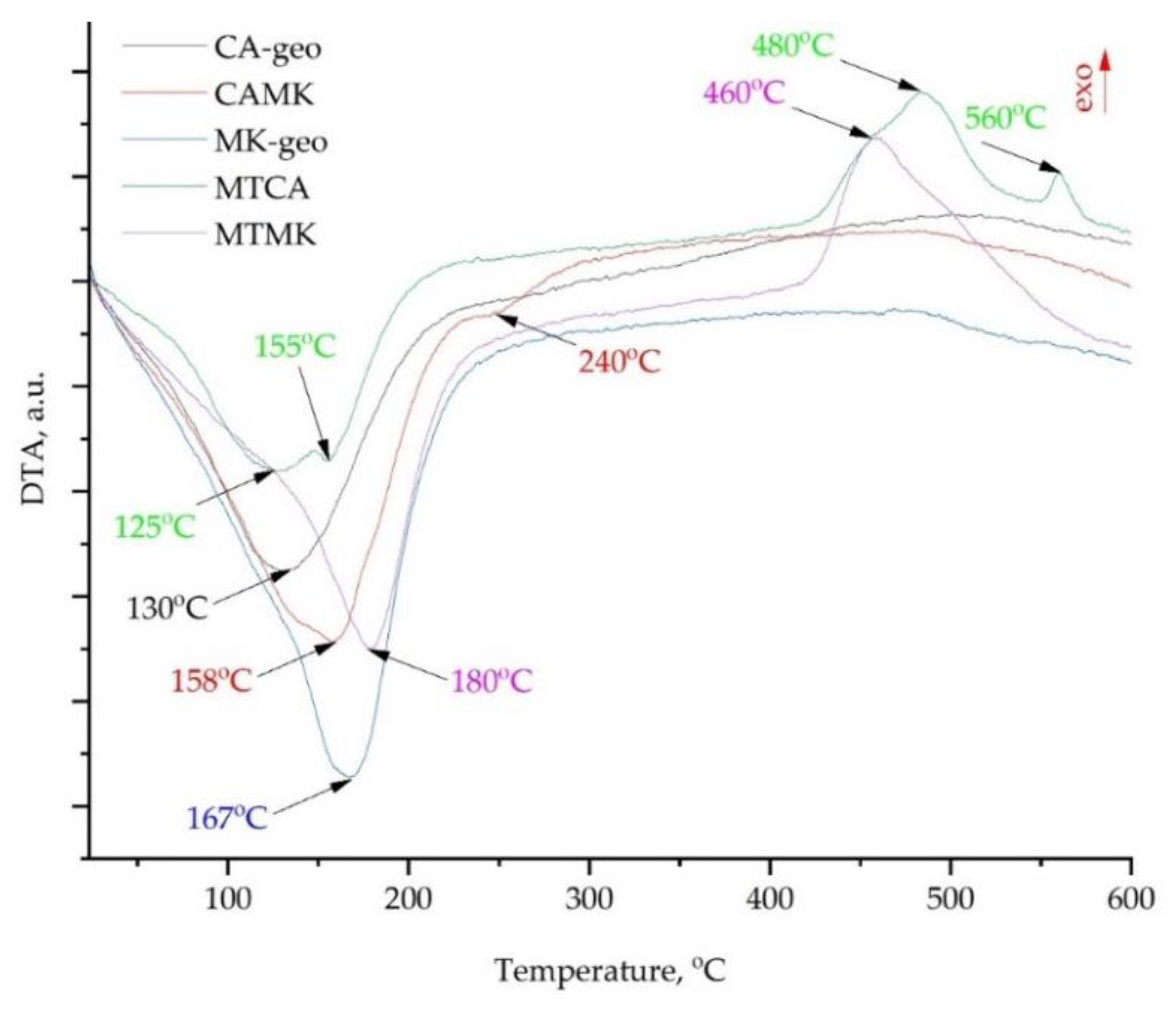
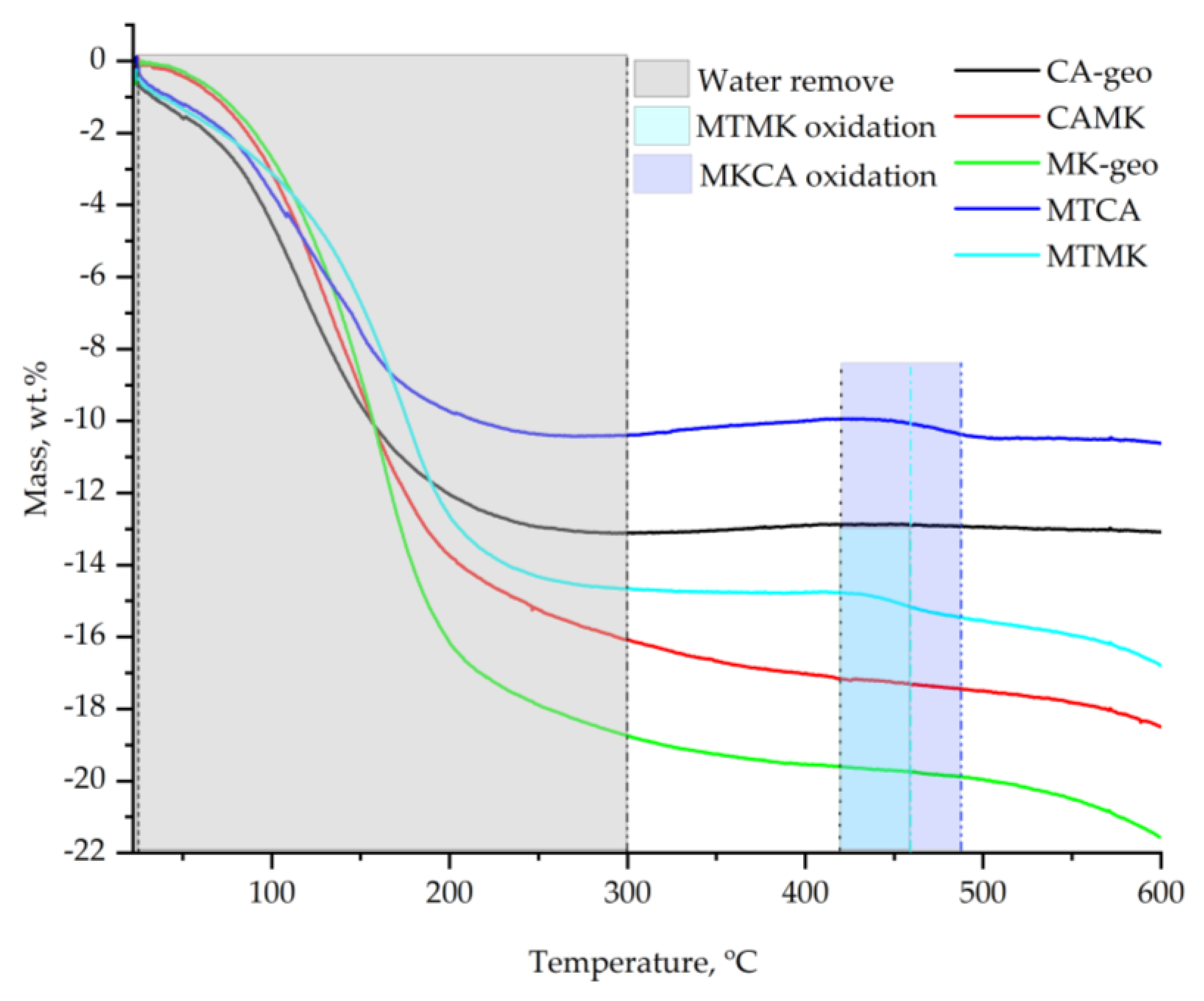
3.2. Thermal Behaviour Analysis
4. Conclusions
- In the 20–300 °C temperature range, the geopolymers obtained with H3PO4 acid exhibited similar thermal behaviour to those activated with a mix of NaOH and Na2SiO3,
- In the 400–600 °C temperature range, the geopolymers with mine tailings addition exhibited exothermic reactions, while those without mine tailings addition did not show significant phase transition,
- Up to 600 °C, the total mass loss of the Ca–geo was 12.9 wt.%, 21.5 wt.% for the MK and 18.5 wt.% for CAMK. The MT addition decreased the mass loss at 10.5 wt.% when mixed with CA and 14.4 wt.% when mixed with MK,
- The XRD analysis confirmed the formation of the ettringite phase in the geopolymers with MT addition and berlinite, brushite or metavariscite in those based on coal ash or metakaolin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habert, G.; Ouellet-Plamondon, C. Recent update on the environmental impact of geopolymers. RILEM Tech. Lett. 2016, 1, 17. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Zribi, M.; Adeniyi, F.; Saha, S.; Baklouti, S.; Shaikh, F.U.A.; Gökçe, H.S. A review on developments of environmentally friendly geopolymer technology. Materialia 2021, 20, 101212. [Google Scholar] [CrossRef]
- Yahya, Z.; Abdullah, M.M.A.B.; Mohd Ramli, N.; Burduhos-Nergis, D.D.; Abd Razak, R. Influence of Kaolin in Fly Ash Based Geopolymer Concrete: Destructive and Non-Destructive Testing. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012068. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Corbu, O. Synthesis and characteristics of local fly ash based geopolymers mixed with natural aggregates. Rev. Chim. 2019, 70, 1262–1267. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Magbool, H.M.; Tayeh, B.A.; Garcez de Azevedo, A.R.; Abutaleb, A.; Hussain, Q. Production of geopolymer concrete by utilizing volcanic pumice dust. Case Stud. Constr. Mater. 2022, 16, e00802. [Google Scholar] [CrossRef]
- Arpajirakul, S.; Pungrasmi, W.; Likitlersuang, S. Efficiency of microbially-induced calcite precipitation in natural clays for ground improvement. Constr. Build. Mater. 2021, 282, 122722. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, C.; Qiao, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous geopolymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106629. [Google Scholar] [CrossRef]
- Roy, S.; Adhikari, G.R.; Gupta, R.N. Use of gold mill tailings in making bricks: A feasibility study. Waste Manag. Res. 2007, 25, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, B.; Deng, L.; Yuan, P.; Li, M.; Wang, Q. Preparation of high-performance silico-aluminophosphate geopolymers using fly ash and metakaolin as raw materials. Appl. Clay Sci. 2021, 204, 106019. [Google Scholar] [CrossRef]
- Louati, S.; Baklouti, S.; Samet, B. Acid based geopolymerization kinetics: Effect of clay particle size. Appl. Clay Sci. 2016, 132–133, 571–578. [Google Scholar] [CrossRef]
- Wang, Y.S.; Dai, J.G.; Ding, Z.; Xu, W.T. Phosphate-based geopolymer: Formation mechanism and thermal stability. Mater. Lett. 2017, 190, 209–212. [Google Scholar] [CrossRef]
- Bewa, C.N.; Tchakouté, H.K.; Fotio, D.; Rüscher, C.H.; Kamseu, E.; Leonelli, C. Water resistance and thermal behavior of metakaolin-phosphate-based geopolymer cements. J. Asian Ceram. Soc. 2018, 6, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.; Conte, A.; Colombo, P. Open-cell phosphate-based geopolymer foams by frothing. Mater. Lett. 2017, 188, 379–382. [Google Scholar] [CrossRef]
- Zribi, M.; Samet, B.; Baklouti, S. Structural Characterization of Phosphate-Based Geopolymer. Lect. Notes Mech. Eng. 2020, 36–42. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Yanou Nkwaju, R. Preparation of acid aluminum phosphate solutions for metakaolin phosphate geopolymer binder. RSC Adv. 2021, 11, 32258–32268. [Google Scholar] [CrossRef]
- Xu, W.; Dai, J.G.; Ding, Z.; Wang, Y. Polyphosphate-modified calcium aluminate cement under normal and elevated temperatures: Phase evolution, microstructure, and mechanical properties. Ceram. Int. 2017, 43, 15525–15536. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Liu, M.; Mu, C.; Liang, Y.N.; Hu, X. Role of alkali cation in compressive strength of metakaolin based geopolymers. Ceram. Int. 2017, 43, 3811–3817. [Google Scholar] [CrossRef]
- Norkhairunnisa, M.; Fariz, M.N.M. Geopolymer: A review on physical properties of inorganic aluminosilicate coating materials. Mater. Sci. Forum 2015, 803, 367–373. [Google Scholar] [CrossRef]
- Siyal, A.A.; Azizli, K.A.; Man, Z.; Ullah, H. Effects of Parameters on the Setting Time of Fly Ash Based Geopolymers Using Taguchi Method. Procedia Eng. 2016, 148, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Louati, S.; Baklouti, S.; Samet, B. Geopolymers Based on Phosphoric Acid and Illito-Kaolinitic Clay. Adv. Mater. Sci. Eng. 2016, 2016, 2359759. [Google Scholar] [CrossRef] [Green Version]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar] [CrossRef]
- Lindsay, M.B.J.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Sargent, P. The development of alkali-activated mixtures for soil stabilisation. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Sawston, UK, 2015; pp. 555–604. [Google Scholar] [CrossRef]
- Daou, I.; Lecomte-Nana, G.L.; Tessier-Doyen, N.; Peyratout, C.; Gonon, M.F.; Guinebretiere, R. Probing the Dehydroxylation of Kaolinite and Halloysite by In Situ High Temperature X-ray Diffraction. Minerals 2020, 10, 480. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kamseu, E.; Andreola, F.; Leonelli, C. Influence of the molar concentration of phosphoric acid solution on the properties of metakaolin-phosphate-based geopolymer cements. Appl. Clay Sci. 2017, 147, 184–194. [Google Scholar] [CrossRef]
- Celerier, H.; Jouin, J.; Mathivet, V.; Tessier-Doyen, N.; Rossignol, S. Composition and properties of phosphoric acid-based geopolymers. J. Non. Cryst. Solids 2018, 493, 94–98. [Google Scholar] [CrossRef]
- Celerier, H.; Jouin, J.; Tessier-Doyen, N.; Rossignol, S. Influence of various metakaolin raw materials on the water and fire resistance of geopolymers prepared in phosphoric acid. J. Non. Cryst. Solids 2018, 500, 493–501. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Deng, L.; Fan, W.; Yu, T.; Wang, Q. Undehydrated kaolinite as materials for the preparation of geopolymer through phosphoric acid-activation. Appl. Clay Sci. 2020, 199, 105887. [Google Scholar] [CrossRef]
- Hamdi, N.; Ben Messaoud, I.; Srasra, E. Production of geopolymer binders using clay minerals and industrial wastes. Comptes Rendus Chim. 2019, 22, 220–226. [Google Scholar] [CrossRef]
- Ambroise, J.; Péra, J. Immobilization of calcium sulfate contained in demolition waste. J. Hazard. Mater. 2008, 151, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.N.; Jensen, T.R.; Hanson, J.C. Formation of ettringite, Ca6Al2(SO4)3(OH)12·26H2O, AFt, and monosulfate, Ca4Al2O6(SO4)·14H2O, AFm-14, in hydrothermal hydration of Portland cement and of calcium aluminum oxide—Calcium sulfate dihydrate mixtures studied by in situ synchrotron X-ray powder diffraction. J. Solid State Chem. 2004, 177, 1944–1951. [Google Scholar] [CrossRef]
- Li, J.; Mailhiot, S.; Sreenivasan, H.; Kantola, A.M.; Illikainen, M.; Adesanya, E.; Kriskova, L.; Telkki, V.V.; Kinnunen, P. Curing process and pore structure of metakaolin-based geopolymers: Liquid-state 1H NMR investigation. Cem. Concr. Res. 2021, 143, 106394. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Ardelean, I.; Sandu, A.V.; Corbu, O.C.; Matei, E. Revealing the influence of microparticles on geopolymers’ synthesis and porosity. Materials 2020, 13, 3211. [Google Scholar] [CrossRef]
- Kalombe, R.M.; Ojumu, V.T.; Eze, C.P.; Nyale, S.M.; Kevern, J.; Petrik, L.F. Fly Ash-Based Geopolymer Building Materials for Green and Sustainable Development. Materials 2020, 13, 5699. [Google Scholar] [CrossRef]
- Nergis, D.D.B.; Al Bakri Abdullah, M.M.; Sandu, A.V.; Vizureanu, P. XRD and TG-DTA study of new alkali activated materials based on fly ash with sand and glass powder. Materials 2020, 13, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandeep Kumar, T.K.; Viswanathan, N.N.; Ahmed, H.; Dahlin, A.; Andersson, C.; Bjorkman, B. Investigation of Magnetite Oxidation Kinetics at the Particle Scale. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2019, 50, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Rustschev, D.; Atanasov, O. Thermal and Group Analysis of Peat. J. Therm. Anal. 1983, 27, 439–442. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Pluduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]

| Sample | Oxide | SiO2 | Al2O3 | FexOy | CaO | K2O | MgO | TiO2 | CuO | Na2O | P2O5 | SO3 | Oth.* | L.O.I.** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | %, wt. | 46.1 | 27.6 | 9.8 | 6.2 | 2.3 | 1.9 | 1.3 | 0.0 | 0.6 | 0.4 | - | 0.3 | 3.5 |
| MK | %, wt. | 52.1 | 42.5 | 1.2 | 0.7 | 0.5 | 0.2 | 0.9 | 0.0 | - | 0.2 | - | 0.4 | 1.3 |
| MT | %, wt. | 16.2 | 2.6 | 38.9 | 0.4 | 0.6 | - | 0.2 | 0.5 | - | 0.3 | 11.4 | 0.9 | 28.1 |
| Sample Code | Coal Ash, wt.% | Metakaolin, wt.% | Mine Tailings, wt.% | Al/P Molar Ratio | Curing, °C |
|---|---|---|---|---|---|
| CA-geo | 100 | - | - | 1 | 22 ± 2 |
| MK-geo | - | 100 | - | 1 | 22 ± 2 |
| CAMK | 50 | 50 | - | 1 | 22 ± 2 |
| MTCA | 50 | - | 50 | 1 | 22 ± 2 |
| MTMK | - | 50 | 50 | 1 | 22 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burduhos Nergis, D.D.; Vizureanu, P.; Sandu, A.V.; Burduhos Nergis, D.P.; Bejinariu, C. XRD and TG-DTA Study of New Phosphate-Based Geopolymers with Coal Ash or Metakaolin as Aluminosilicate Source and Mine Tailings Addition. Materials 2022, 15, 202. https://doi.org/10.3390/ma15010202
Burduhos Nergis DD, Vizureanu P, Sandu AV, Burduhos Nergis DP, Bejinariu C. XRD and TG-DTA Study of New Phosphate-Based Geopolymers with Coal Ash or Metakaolin as Aluminosilicate Source and Mine Tailings Addition. Materials. 2022; 15(1):202. https://doi.org/10.3390/ma15010202
Chicago/Turabian StyleBurduhos Nergis, Dumitru Doru, Petrica Vizureanu, Andrei Victor Sandu, Diana Petronela Burduhos Nergis, and Costica Bejinariu. 2022. "XRD and TG-DTA Study of New Phosphate-Based Geopolymers with Coal Ash or Metakaolin as Aluminosilicate Source and Mine Tailings Addition" Materials 15, no. 1: 202. https://doi.org/10.3390/ma15010202
APA StyleBurduhos Nergis, D. D., Vizureanu, P., Sandu, A. V., Burduhos Nergis, D. P., & Bejinariu, C. (2022). XRD and TG-DTA Study of New Phosphate-Based Geopolymers with Coal Ash or Metakaolin as Aluminosilicate Source and Mine Tailings Addition. Materials, 15(1), 202. https://doi.org/10.3390/ma15010202










