Abstract
In one of our previously published articles, we reported the synthesis, spectroscopic, thermal, and catalytic properties of four new M(II) acetate (where M = Co, Ni, Cu, Zn) complexes with imidazole. Presented compounds exhibited activity in the reaction on catalytic oxidation of styrene. In this study we have synthesized and investigated properties of analogous compounds, however using formates or propionates of mentioned metal cations instead of acetates. Such an approach allowed us to draw valuable conclusions concerning the relationship between the carbon chain length and catalytic activity, which is an important factor for catalyst modeling. Synthesized compounds have been thoroughly investigated using appropriate analytic techniques: AAS (Atomic Absorption Spectrometry), FTIR (Fourier-Transform Infrared Spectroscopy), and TGA (Thermogravimetric Analysis). Catalytic properties have been studied under the same previous conditions, using GC-FID (GC-chromatograph equipped with FID detector).
Keywords:
metal(II) complexes; imidazole; formates; propionates; TG-DTG; FTIR; catalyst; styrene oxidation 1. Introduction
Incorporation of different ligands or counterions can significantly change physicochemical or biological properties of metal-based coordination compounds. One of the most popular ligands is imidazole. Imidazole and its derivatives are also known for their valuable properties, e.g., antifungal, antibacterial, anti-inflammatory, antiviral, and anticancer [1,2,3,4,5,6,7]. Imidazole itself is also a part of biologically important systems, e.g., one of the amino acids—histidine. Enormous variety of possible connections of different metal centres and compounds exhibiting useful properties or their derivatives create great opportunity to obtain outstanding results. It is thus important to determine the relationship between structure and activity, which is currently one of the most promising strategies in new drugs and catalyst design. All four mentioned metals, cobalt [8,9,10,11], nickel [12,13,14], copper [15,16,17], and zinc [17,18,19] are crucial and widely used in catalysis. One of the important reactions, problematic from an industrial point of view, is the reaction of the oxidation of styrene. This reaction is usually carried out in the presence of peracids however, since it leads to obtaining undesirable products, an ecological oxidizer (H2O2) can be used instead. In order to ensure proper conversion, a catalyst has to be used. Some catalysts have already been tested, like chromium-silica systems—resulting in obtaining benzaldehyde as the main product [20] or molecular sieves—in this case the main product was phenylacetaldehyde [21]. Benzaldehyde was also obtained in many other cases. Some examples comprise of modified cobalt or zinc oxide catalysts [22,23], as well as copper(II) complexes [24].
In one our previously published articles, we reported the synthesis, spectroscopic, thermal, and catalytic properties of four new M(II) acetate (where M = Co, Ni, Cu, Zn) complexes with imidazole [25]. Apart from experimental methods, such compounds can also be studied using computational techniques [26,27,28]. After physicochemical characterisation, synthesized compounds have additionally been tested for catalytic activity in a styrene oxidation reaction. Performed tests have given promising results with good percentage styrene conversion and almost 100% selectivity towards carbon dioxide formation. This is an important fact since one of the possible, undesired products for this reaction is benzaldehyde. In this paper, we report the synthesis and study of the physicochemical properties of analogous compounds, however using the formates or propionates of mentioned metal cations instead of acetates. Such an approach allows us to draw some valuable conclusions about the relationship between the carbon chain length and catalytic activity of investigated compounds, as well as thoroughly investigate their properties. (Abbreviations: L1—formate anion; Ac—acetate anion; L2—propionate anion; Im—imidazole; Imd—deprotonated imidazole)
2. Materials and Methods
All chemicals that were used for the synthesis were purchased from the following companies: Sigma-Aldrich, Pol-Aura, and Avantor Performance Materials Poland and were used without further purification. The contents of Co(II), Ni(II), Cu(II), and Zn(II) ions were determined by Atomic Absorption Spectrometry. Standard solutions from Merck (1000 mg/L, Darmstadt, Germany) were used for the preparation of diluted solutions used for calibration. For analysis, distilled water (electrical conductivity 0.05 µS) was used (obtained with Polwater system). Infrared spectra were recorded using IRTracer-100 Schimadzu Spectrometer (Japan) (3200–600 cm−1, accuracy of recording: 1 cm−1) in potassium bromide pellets. Thermal decompositions of compounds were studied using the TG-DTG thermogravimetric technique (range of temperature 25–800 °C; heating rate: 10 °C·min−1). TG and DTG curves were recorded using Netzsch TG 209 (Germany), in ceramic crucibles, under air atmosphere, v = 20 mL·min−1. As a reference material, ceramic crucibles were used. Catalytic properties have been studied in the liquid-phase styrene oxidation reaction. Reactions were carried out in 50-mL round-bottom flasks with the molar ratio of reagents being the same as in the previous part of the study [25]: C2H3N:H2O2:C8H8 = 1:1:1. The reaction mixture was stirred in a water bath at 60 °C and refluxed. After reaching this temperature, 10 mg of the catalyst was added. The optimum time of reaction for obtaining reliable results was found to be 2 h. After that time, the analysis of styrene content was performed using gas chromatography with a flame ionization detector (GC-FID, HP 5890, Hewlett Packard Corporation, (USA). The optimal parameters of method found for our study were as follows: Separation column: ZB-FFAP capillary column (30 m × 0.25 mm × 0.25 µm) with oven parameters: 60 °C for 8 min to 150 °C/min for 4 min; injection temperature of 225 °C; injection split: 11:8:1, 0.5 µL; and detector temperature of 250 °C. A carrier gas helium was used with a flow of 3.4 mL/min. As previously done, conversion degrees were determined according to the formula below:
where: A0 is the initial styrene concentration in reactant mixture and At is the concentration of styrene after 2 h of the reaction.
2.1. Synthesis of M(II) Formates and Propionates
M(II) formates and propionates for synthesis were prepared using concentrated formic and propionic acids. Mixtures of appropriate acid and basic M(II) carbonate have been heated, refluxed, and stirred for 5 h. After that time, the excess of the unreacted carbonates was filtered. Filtrates were cooled and left for several days for crystallization. Obtained crystals were filtered and ground in a mortar. The purity of synthesized compounds was proven with thermogravimetric analysis. It also allowed establishing the amount of water molecules in hydrated compounds. Synthesis of M(II) formates and propionates is shown in Figure 1.
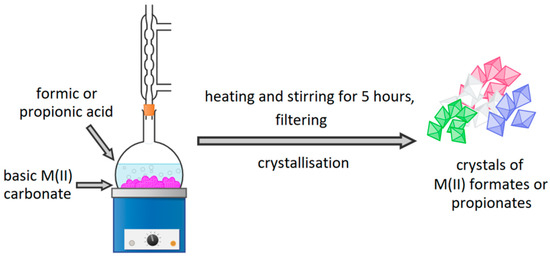
Figure 1.
Synthesis of appropriate M(II) formates and propionates.
2.2. Synthesis of M(II) Formate and Propionate Complexes with Imidazole
In the next step, complexes of M(II) formates and propionates with imidazole were synthesized. Appropriate M(II) formate or propionate was dissolved in distilled water and mixed with an ethanol solution of imidazole. Reaction mixtures were heated to 30 °C and stirred for 3 h. The volume of the reaction mixture did not exceed 60 mL. Figure 2 presents a synthesis of the described complexes.
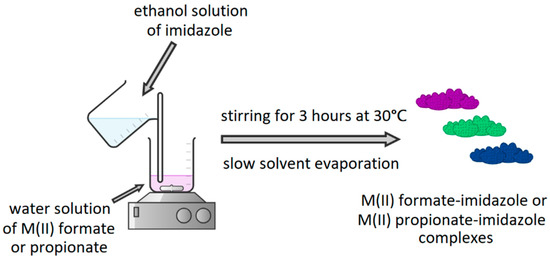
Figure 2.
Synthesis of M(II) formate-imidazole or M(II) propionate-imidazole complexes.
The molar ratio of metal cation and imidazole was determined based on the formulas of the complexes synthesized in our previous study [25]. This data is presented in Table 1.

Table 1.
Formulas of the synthesized M(II) formate and propionate complexes with imidazole and previously synthesized M(II) acetate complexes with imidazole [25].
After 3 h of stirring, clear reaction mixtures were left for slow solvent evaporation. In the case of Cu(II) propionate—imidazole synthesis, the complex precipitated immediately after adding the imidazole solution. As a result, the molar ratio of Cu(II) and imidazole is in this case different than previously assumed. After 3 h, the precipitate was filtered and washed several times with distilled water. In the next step, the formulas of all synthesized complexes were established and their properties were investigated.
3. Results and Discussion
3.1. Atomic Absorption Spectrometry
This technique allowed us to determine the contents of metal cations in investigated compounds, which confirms obtaining the compounds that are described by the presented formulas. Table 2 presents measured values in comparison with theoretical ones.

Table 2.
Measured and theoretical percentage M(II) contents for investigated compounds.
3.2. FTIR Spectra
The FTIR spectra of synthesized complexes prove their purity and allow establishing the manner in which carboxylate anions and imidazole bind the metal cation. All analyses have been performed in a manner analogous to the previously studied compounds [25].
Figure 3 and Figure 4 present the FTIR spectra of M(II) formate-imidazole and M(II) propionate-imidazole complexes, respectively. The FTIR spectra of sodium formate and sodium propionate have also been added in order to determine how formate and propionate anions coordinate metal centres, according to the spectroscopic criteria described by Nakamoto [29] and Alcock and co-authors [30].
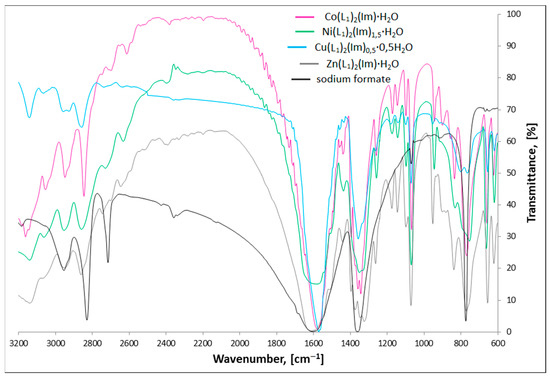
Figure 3.
FTIR spectra of studied M(II) formate-imidazole complexes and sodium formate.
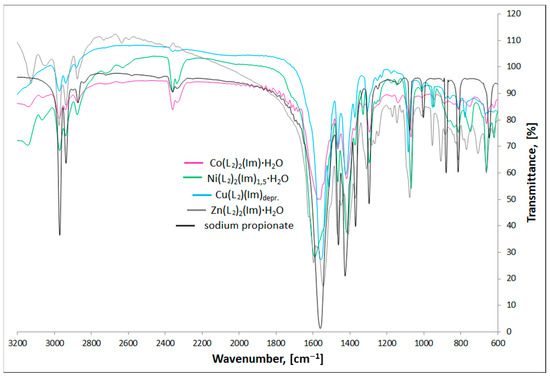
Figure 4.
FTIR spectra of studied M(II) propionate-imidazole complexes and sodium propionate.
The most important modes observed for studied complexes, sodium formate, and sodium propionate are presented in Table 3.

Table 3.
FTIR modes (cm−1) observed for studied M(II) formate- or propionate-imidazole complexes, and for sodium formate and sodium propionate.
Stretching ν(NH) bands can be found in the range 3139–3037 cm−1. It is important to notice that these bands are not observed in the Cu(L2)(Im)d spectrum, which is a proof of deprotonation of the NH group in the imidazole molecule for this compound. Stretching (CH)alifat. bands can be found in their characteristic area, 2973–2826 cm−1. In the region, 955–746 cm−1, we can observe a characteristic band that can be ascribed to π(CH) and δ(imidazole ring) vibrations. Recognizing the ν(COO)as. and ν(COO)sym. bands allowed us to calculate Δν(COO) values, according to the formula: Δν(COO) = ν(COO)as.−ν(COO)sym. Based on spectroscopic criteria. Nakamoto [29], Alcock and co-authors [30] compared values of separation of ν(COO)as. And ν(COO)sym. frequencies of studied compounds with analogous bands found in the spectra of appropriate carboxylate sodium salts. The Δν(COO) values characterize the nature of metal-carboxylate bond. When ΔνNa > Δνcomplex, the carboxylate group is considered to be a bidentate chelating ligand, in case of ΔνNa < Δνcomplex, it coordinates as monodentate ligand and for ΔνNa ≈ Δνcomplex, it acts as a bidentate-bridging donor [29,30]. On this basis, we can say that in the case of Co(L1)2(Im)⸱H2O, Ni(L1)2(Im)1.5⸱H2O, Cu(L1)2(Im)0.5⸱0.5H2O, Co(L2)2(Im)⸱H2O, Cu(L2)(Im)d, and Zn(L2)2(Im)⸱H2O compounds, the carboxylate groups bind the metal centres in a bidentate-bridging manner. In the case of Zn(L1)2(Im)⸱H2O, we can find both higher and lower band separation values in comparison with sodium salt, which indicates two ways of binding the zinc cation: monodentate and bidentate-chelating. Analyzed values suggest the monodentate way of binding in the case of the Ni(L2)2(Im)1.5⸱H2O compound.
3.3. Thermogravimetric Studies in Air
3.3.1. Thermolysis of M(II) Formate Complexes with Imidazole
Thermal properties have been studied using the TG-DTG method under air atmosphere. All eight compounds are stable at room temperature and decompose gradually when heated.
Thermolysis of Co(L1)2(Im)⸱H2O begins at 50 °C (Figure 5a, Table 4). First step of decomposition is dehydration (mass loss: found. 8.0%; calc. 7.67%). In the temperature range 170–280 °C we observe partial thermolysis of imidazole molecule (mass loss: found. 21.5%; calc. 21.72%). Horizontal mass appears at 450 °C with CoO as a solid decomposition product (mass loss: found. 38.0%; calc. 38.74%).
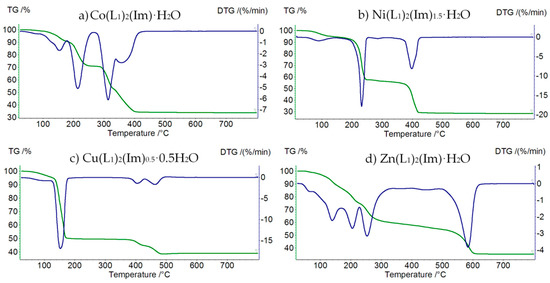
Figure 5.
TG (green) and DTG (blue) curves of decomposition of: (a) Co(L1)2(Im)⸱H2O, (b) Ni(L1)2(Im)1.5⸱H2O, (c) Cu(L1)2(Im)0.5⸱0.5 H2O, and (d) Zn(L1)2(Im)⸱H2O.

Table 4.
TG-DTG analysis data of decomposition of Co(L1)2(Im)⸱H2O.
Ni(L1)2(Im)1.5⸱H2O is thermally stable up to 50 °C (Figure 5b, Table 5). In the first step a water molecule is released (mass loss: found. 6.0%; calc. 6.70%). Above 170 °C, decomposition of imidazole begins (mass loss: found. 38.0%; calc. 37.99%). In the temperature range 310–600 °C, the thermodestruction of formate ions takes place (mass loss: found. 27.0%; calc. 27.54%). The final product of decomposition is NiO.

Table 5.
TG-DTG analysis data of decomposition of Ni(L1)2(Im)1.5⸱H2O.
Cu(L1)2(Im)0.5⸱0.5H2O starts to decompose at 40 °C (Figure 5c, Table 6). Additionally, in this case, the first step of decomposition is dehydration (mass loss: found. 5.0%; calc. 4.58%). In the temperature range 140–280 °C, we observe the thermolysis of organic molecule, as well as partial decomposition of formate ions (mass loss: found. 46.0%; calc. 45.93%). In the temperature range 280–560 °C, the total decomposition of formates takes place (mass loss: found. 9.5%; calc. 9.03%). Above 560 °C, the horizontal mass for CuO appears.

Table 6.
TG-DTG analysis data of decomposition of Cu(L1)2(Im)0.5⸱0.5H2O.
First step of decomposition of the Zn(L1)2(Im)⸱H2O compound (Figure 5d, Table 7) is dehydration connected with the decomposition of the imidazole molecule in the temperature range 50–270 °C (mass loss: found. 6.0%; calc. 6.70%). In the temperature range 270–620 °C, we observe the thermodesctruction of formate ions (mass loss: found. 30.0%; calc. 30.65%). The final product of decomposition is ZnO.

Table 7.
TG-DTG analysis data of decomposition of Zn(L1)2(Im)⸱H2O.
3.3.2. Thermolysis of M(II) Propionate Complexes with Imidazole
Decomposition of Co(L2)2(Im)⸱H2O starts at 40 °C (Figure 6a, Table 8). At this temperature, a water molecule is released (mass loss: found. 5.0%; calc. 6.19%). In the temperature range 140–330 °C, the thermodestruction of the imidazole molecule takes place (mass loss: found. 23.0%; calc. 23.38%). In the next step, up to 440 °C, we observe the destruction of propionate anions (mass loss: found. 42.0%; calc. 41.95%). Above 440 °C, horizontal mass for Co2O3 appears.
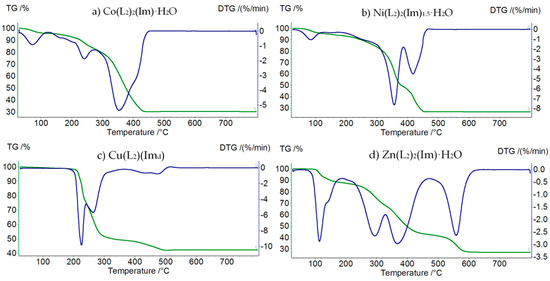
Figure 6.
TG (green) and DTG (blue) curves of decomposition of: (a) Co(L2)2(Im)⸱H2O, (b) Ni(L2)2(Im)1.5⸱H2O, (c) Cu(L2)(Imd), and (d) Zn(L2)2(Im)⸱H2O.

Table 8.
TG-DTG analysis data of decomposition of Co(L2)2(Im)⸱H2O.
Ni(L2)2(Im)1.5⸱H2O is thermally stable up to 60 °C (Figure 6b, Table 9). Additionally, in this case, the first step of decomposition is dehydration (mass loss: found. 5.0%; calc. 5.54%). In the next step, in the temperature range 140–370 °C, we can observe the thermolysis of imidazole and partial thermolysis of propionate ions (mass loss: found. 42.5%; calc. 42.67%). In this case, the final product of decomposition is Ni2O3, formed in the final step (mass loss: found. 26.5%; calc. 26.34%).

Table 9.
TG-DTG analysis data of decomposition of Ni(L2)2(Im)1.5⸱H2O.
Decomposition of Cu(L2)(Imd) begins at 160 °C (Figure 6c, Table 10). In the temperature range 160–340 °C, the total thermodestruction of imidazole and partial thermodestruction of propionate anions occurs (mass loss: found. 49.5%; calc. 50.86%). Above 340 °C, we observe the total decomposition of propionate anions (mass loss: found. 9.0%; calc. 10.27%). This process stops at 540 °C, when the final solid product of decomposition is formed—CuO.

Table 10.
TG-DTG analysis data of decomposition of Cu(L2)(Imd).
In the case of Zn(L2)2(Im)⸱H2O (Figure 6d, Table 11), the first step of decomposition is dehydration, occurring at a temperature range of 80–130 °C (mass loss: found. 7.0%; calc. 6.05%). In the second step, up to 320 °C, the imidazole molecule is released (mass loss: found. 23.0%; calc. 22.88%). The last step takes place in the temperature range 320–610 °C and is associated with the themodestruction of propionate anions (mass loss: found. 43.5%; calc. 43.73%). The final solid product of the thermolysis of this compound is ZnO.

Table 11.
TG-DTG analysis data of decomposition of Zn(L2)2(Im)⸱H2O.
3.4. Catalytic Activity
Figure 7 presents the scheme of the apparatus used for carrying out the reaction of styrene oxidation described in the Materials and Methods section.
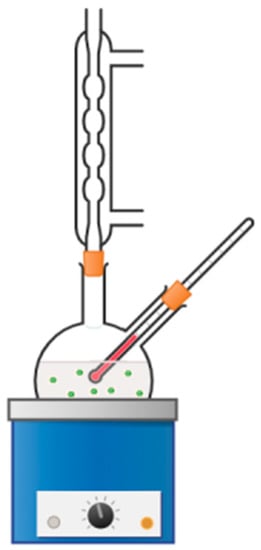
Figure 7.
Scheme of the apparatus used for carrying out the reaction of styrene oxidation. The molar ratio of reagents was: C2H3N:H2O2:C8H8 = 1:1:1. After reaching 60 °C, 10 mg of the catalyst was added. Reaction was then carried out for 2 h. After that time, samples for gas chromatography were probed.
Table 12 and Figure 8 present conversion degrees for eight described compounds (M(II) formate or propionate complexes with imidazole) as well as for four previously studied compounds [25] (M(II) acetate complexes with imidazole). In case of studied compounds, the main product of oxidation is carbon dioxide.

Table 12.
Conversion degrees for eight described compounds (M(II) formate or propionate complexes with imidazole) and for four previously studied compounds [25] (M(II) acetate complexes with imidazole).
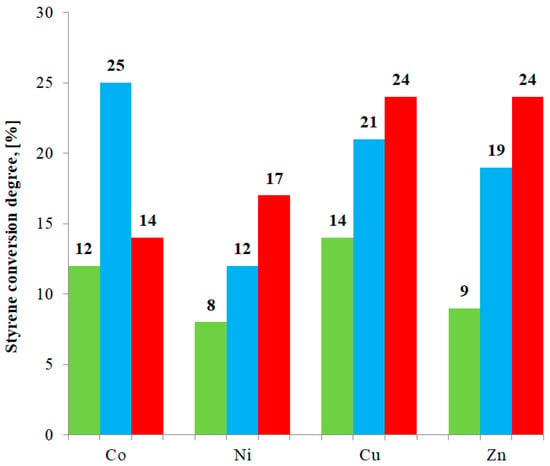
Figure 8.
Styrene conversion degree for investigated compounds. Green bars: M(II) formate-imidazole complexes; blue bars: Previously studied M(II) acetate-imidazole complexes [25]; and red bars: M(II) propionate-imidazole complexes.
4. Conclusions
In this work, we presented a synthesis procedure, as well as an investigation of properties of eight new compounds: Co(L1)2(Im)⸱H2O, Ni(L1)2(Im)1.5⸱H2O, Cu(L1)2(Im)0.5⸱0.5H2O, Zn(L1)2(Im)⸱H2O, Co(L2)2(Im)⸱H2O, Ni(L2)2(Im)1.5⸱H2O, and Cu(L2)(Im)d determine the way organic ligands bind metal centers. It also allowed us to confirm the deprotonation of an imidazole molecule in the case of a Cu(L2)(Im)d compound. All eight compounds form solids that are stable at room temperature and decompose gradually when heated. Taking into consideration the thermal stability of investigated compounds, we can say that the carbon chain length had very little effect on this aspect. In all cases, the solid products of decomposition are metal oxides. Of the eight synthesized compounds, Cu(L2)(Imd) and Zn(L2)2(Im)⸱H2O exhibited the highest catalytic activity. In all cases, M(II) formate-imidazole complexes showed the lowest activity. This study also allowed us to draw some valuable conclusions concerning the relationship between the carbon chain length and catalytic activity. For all Ni(II), Cu(II), and Zn(II) compounds, an increase of the carbon chain length went hand in hand with the increase of conversion degree. In case of Co(II) compounds, Co(Ac)2(Im)⸱H2O showed the highest activity. The remaining Co(II) compounds exhibited lower activity.
Author Contributions
Conceptualization, B.R. and A.C.; methodology, B.R., T.M. and A.C.; validation, B.R., R.C. and A.C.; formal analysis, B.R. and A.C.; investigation, B.R.; data curation, B.R.; writing—original draft preparation, B.R. and A.C.; writing—review and editing, B.R. and A.C.; visualization, B.R. and A.C.; supervision, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shalini, K.; Sharma, P.K.; Kumar, N. Imidazole and its biological activities: A review. Der. Chem. Sin. 2010, 1, 36–47. [Google Scholar]
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kant, V. A Review on Biological Activity of Imidazole and Thiazole Moieties and their Derivatives. Sci. Int. 2013, 1, 253–260. [Google Scholar] [CrossRef] [Green Version]
- De Luca, L. Naturally occurring and synthetic imidazoles: Their chemistry and their biological activities. Curr. Med. Chem. 2006, 13, 1–23. [Google Scholar]
- Sharma, D.; Narasimhan, B.; Kumar, P.; Judge, V.; Narang, R.; De Clercq, E.; Balzarini, J. Synthesis, antimicrobial and antiviral evaluation of substituted imidazole derivatives. Eur. J. Med. Chem. 2009, 44, 2347–2353. [Google Scholar] [CrossRef]
- Sharma, P.; LaRosa, C.; Antwi, J.; Govindarajan, R.; Werbovetz, K.A. Imidazoles as Potential Anticancer Agents: An Update on Recent Studies. Molecules. 2021, 26, 4213. [Google Scholar] [CrossRef]
- Mlostoń, G.; Celeda, M.; Poper, W.; Kowalczyk, M.; Gach-Janczak, K.; Janecka, A.; Jasiński, M. Synthesis, Selected Transformations, and Biological Activity of Alkoxy Analogues of Lepidilines A and C. Materials 2020, 13, 4190. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors. Catal. Rev. 2021, 63, 512–595. [Google Scholar] [CrossRef]
- Mirzaei, A.A.; Arsalanfar, M.; Bozorgzadeh, H.R.; Samimi, A. A review of Fischer-Tropsch synthesis on the cobalt based catalysts. Phys. Chem. Res. 2014, 2, 179–201. [Google Scholar] [CrossRef]
- Zhou, H.; Song, J.; Fan, H.; Zhang, B.; Yang, Y.; Hu, J.; Zhu, Q.; Han, B. Cobalt catalysts: Very efficient for hydrogenation of biomass-derived ethyl levulinate to gammavalerolactone under mild conditions. Green Chem. 2014, 16, 3870. [Google Scholar] [CrossRef]
- Zybert, M.; Tarka, A.; Mierzwa, B.; Raróg-Pilecka, W. Cobalt-lanthanum catalyst precursors for ammonia synthesis: Determination of calcination temperature and storage conditions. Pol. J. Chem. Tech. 2017, 19, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Izumi, Y.; Imaida, M.; Fukawa, H.; Akabori, S. Asymmetric Hydrogenation with Modified Raney Nickel. Studies on Modified Hydrogenation Catalyst. Bull. Chem. Soc. Jpn. 1963, 36, 155–160. [Google Scholar] [CrossRef]
- Harada, T.; Izumi, Y. Improved modified Raney Nickel catalyst for enantioface-differentiating (asymmetric) hydrogenation of methyl acetoacetate. Chem. Lett. 1978, 7, 1195–1196. [Google Scholar] [CrossRef]
- Studentschnig, A.F.H.; Schober, S.; Mittelbach, M. Conversion of Crude Palm Oil into Hydrocarbons over Commercial Raney Nickel. Energy Fuels 2013, 27, 7480–7484. [Google Scholar] [CrossRef]
- Abudayyeh, A.M.; Schott, O.; Feltham, H.L.C.; Hanan, G.S.; Brooker, S. Copper catalysts for photo- and electro-catalytic hydrogen production. Inorg. Chem. Front. 2021, 8, 1015–1029. [Google Scholar] [CrossRef]
- Bluhm, H.; Hävecker, M.; Knop-Gericke, A.; Kleimenov, E.; Schlogl, R. Methanol Oxidation on a Copper Catalyst Investigated Using in Situ X-ray Photoelectron Spectroscopy. J. Phys. Chem. B 2004, 108, 14340–14347. [Google Scholar] [CrossRef]
- Górecka, S.; Pacultová, K.; Fridrichová, D.; Górecki, K.; Bílková, T.; Žebrák, R.; Obalová, L. Catalytic Oxidation of Ammonia over Cerium-Modified Copper Aluminium Zinc Mixed Oxides. Materials 2021, 14, 6581. [Google Scholar] [CrossRef] [PubMed]
- Nisa, R.U.; Mahmood, T.; Ludwig, R.; Ayub, K. Theoretical mechanistic investigation of zinc(II) catalyzed oxidation of alcohols to aldehydes and esters. RSC Adv. 2016, 6, 31876–31883. [Google Scholar] [CrossRef] [Green Version]
- Cheung, E.; Alberti, C.; Enthaler, S. Chemical Recycling of End-of-Life Poly(lactide) via Zinc-Catalyzed Depolymerization and Polymerization. ChemistryOpen 2020, 9, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Iqbal, A. The oxidation of styrene by chromium–silica heterogeneous catalyst prepared from rice husk. Chem. Eng. J. 2010, 160, 742–750. [Google Scholar] [CrossRef]
- Kumar, S.B.; Mirajkar, S.P.; Pais, G.C.G.; Kumar, P.; Kumar, R. Epoxidation of Styrene over a Titanium Silicate Molecular Sieve TS1 Using Dilute H2O2 as Oxidizing Agent. J. Catal. 1995, 156, 163–166. [Google Scholar] [CrossRef]
- Gao, D.; Gao, Q. Selective oxidation of styrene to benzaldehyde over VSB-5 and isomorphously substituted cobalt VSB-5. Catal. Commun. 2007, 8, 681–685. [Google Scholar] [CrossRef]
- Aberkouks, A.; Mekkaoui, A.A.; Boualy, B.; El Houssame, S.; Ali, M.A.; El Firdoussi, L. Selective oxidation of styrene to benzaldehyde by Co-Ag codoped ZnO catalyst and H2O2 as oxidant. Adv. Mater. Sci. Eng. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Titinchi, S.J.; VonWillingh, G.; Abbo, H.S.; Prasad, R. Tri- and tetradentate copper complexes: A comparative study on homogeneous and heterogeneous catalysis over oxidation reactions. Catal. Sci. Technol. 2015, 5, 325–338. [Google Scholar] [CrossRef]
- Czylkowska, A.; Rogalewicz, B.; Raducka, A.; Błaszczyk, N.; Maniecki, T.; Wieczorek, K.; Mierczyński, P. Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands. Materials 2020, 13, 3217. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Pacheco, J.M. Shapes of cagelike metal carbide clusters: First-principles calculations. Phys. Rev. B 2003, 68, 241401(R). [Google Scholar] [CrossRef]
- Dos Santos, R.B.; Rivelino, R.; de Brito Mota, F.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Dopant species with Al–Si and N–Si bonding in the MOCVD of AlN implementing trimethylaluminum, ammonia and silane. J. Phys. D Appl. Phys. 2015, 48, 295104. [Google Scholar] [CrossRef]
- Tavares, S.R.; Vaiss, V.S.; Novais Antunes, F.P.; Fonseca, C.G.; Nangoi, I.M.; Moraes, P.I.R.; Soares, C.V.; Haddad, J.F.S.; Lima, L.L.; Silva, B.N.N.; et al. DFT calculations for structural prediction and applications of intercalated lamellar compounds. Dalton Trans. 2018, 47, 2852–2866. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John and Wiley and Sons: New York, NY, USA, 2009. [Google Scholar]
- Alcock, N.W.; Tracy, V.M.; Waddington, T.C. Acetates and acetato-complexes. Part 2. Spectroscopic studies. J. Chem. Soc. Dalton Trans. 1976, 21, 2243–2246. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).