Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
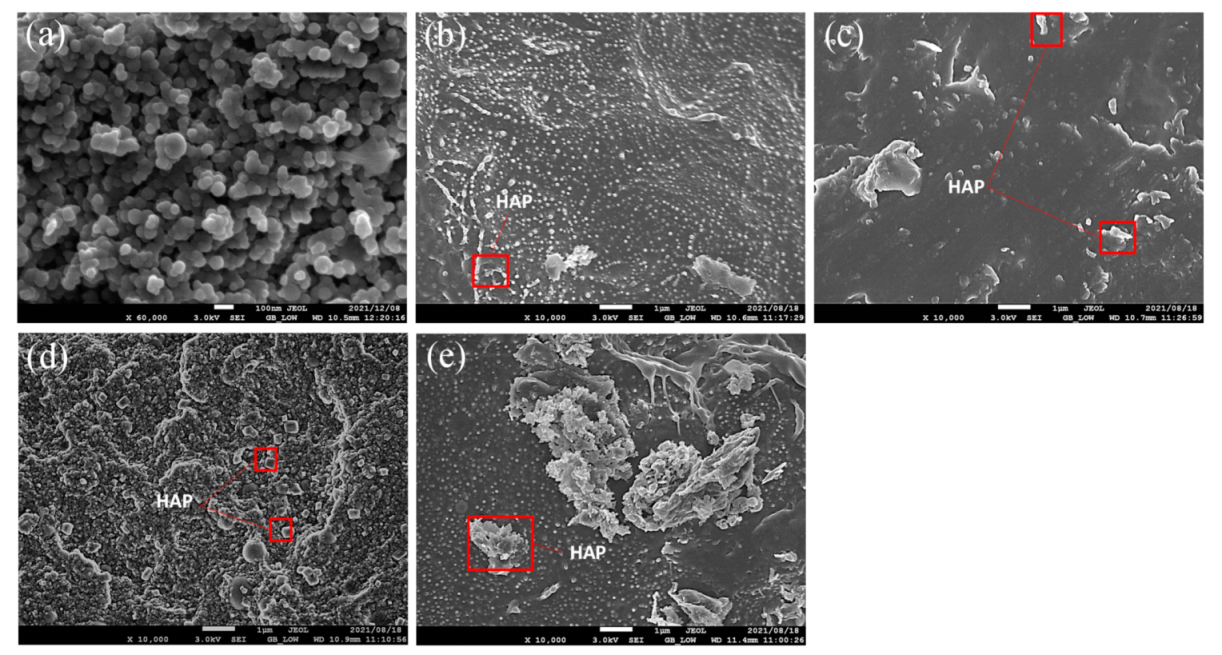
2.3.1. Scanning Electron Microscopy
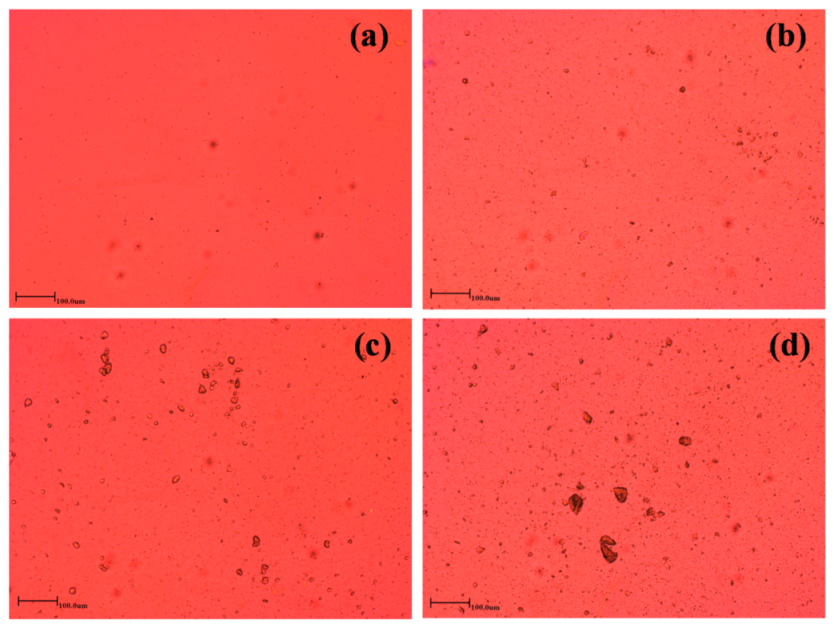
2.3.2. Polarized Optical Microscopy
2.3.3. Chemical Structures
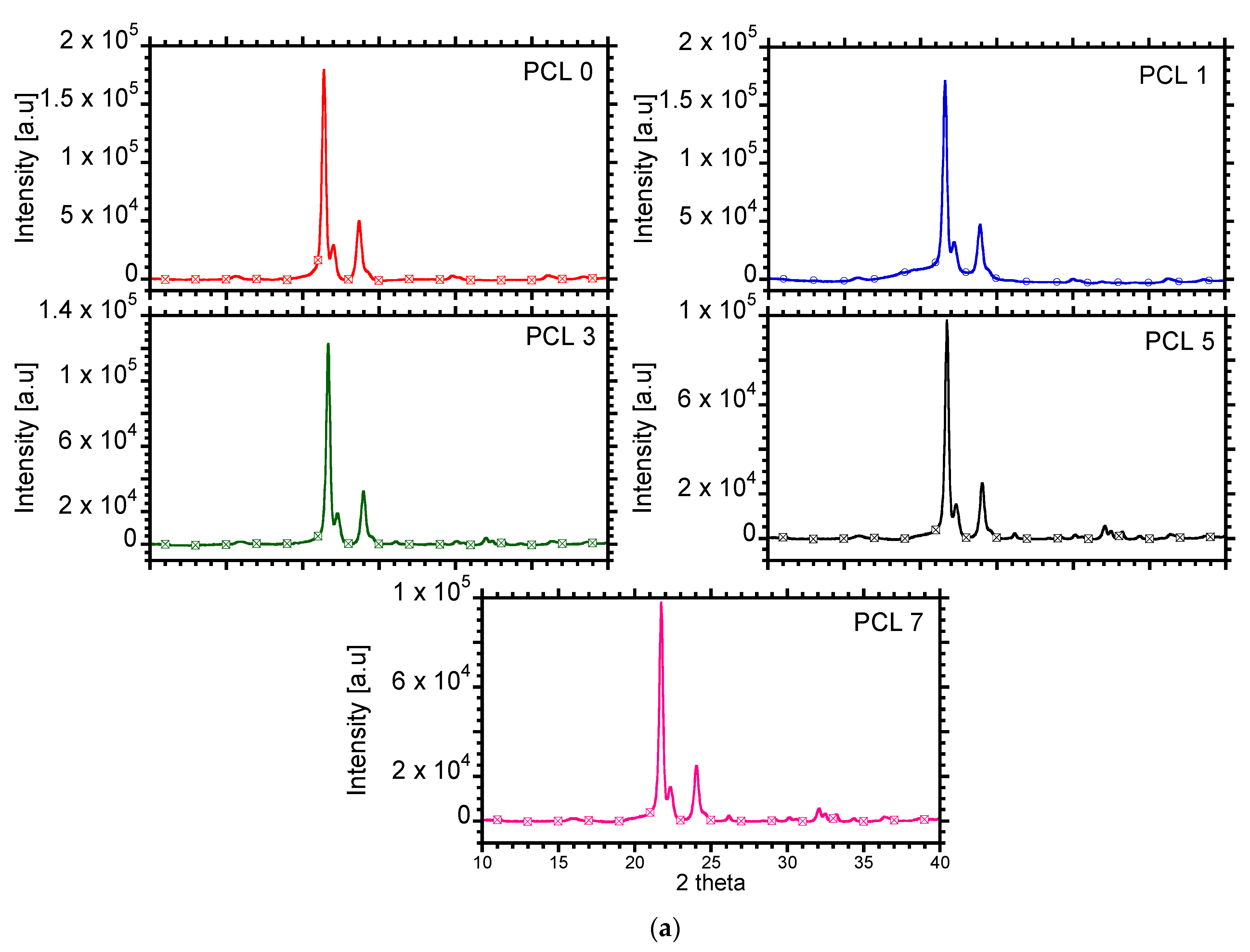
2.3.4. Crystalline Structures
2.3.5. Rheological Properties
2.3.6. Thermal Properties
2.3.7. Mechanical Properties
3. Results and Discussion
3.1. Surface Morphology and Chemical Structures
3.2. Viscoelastic Properties
3.2.1. Complex Viscosity
3.2.2. Complex Viscosity Modelling
3.2.3. Storage and Loss Modulus
3.2.4. Damping Factor
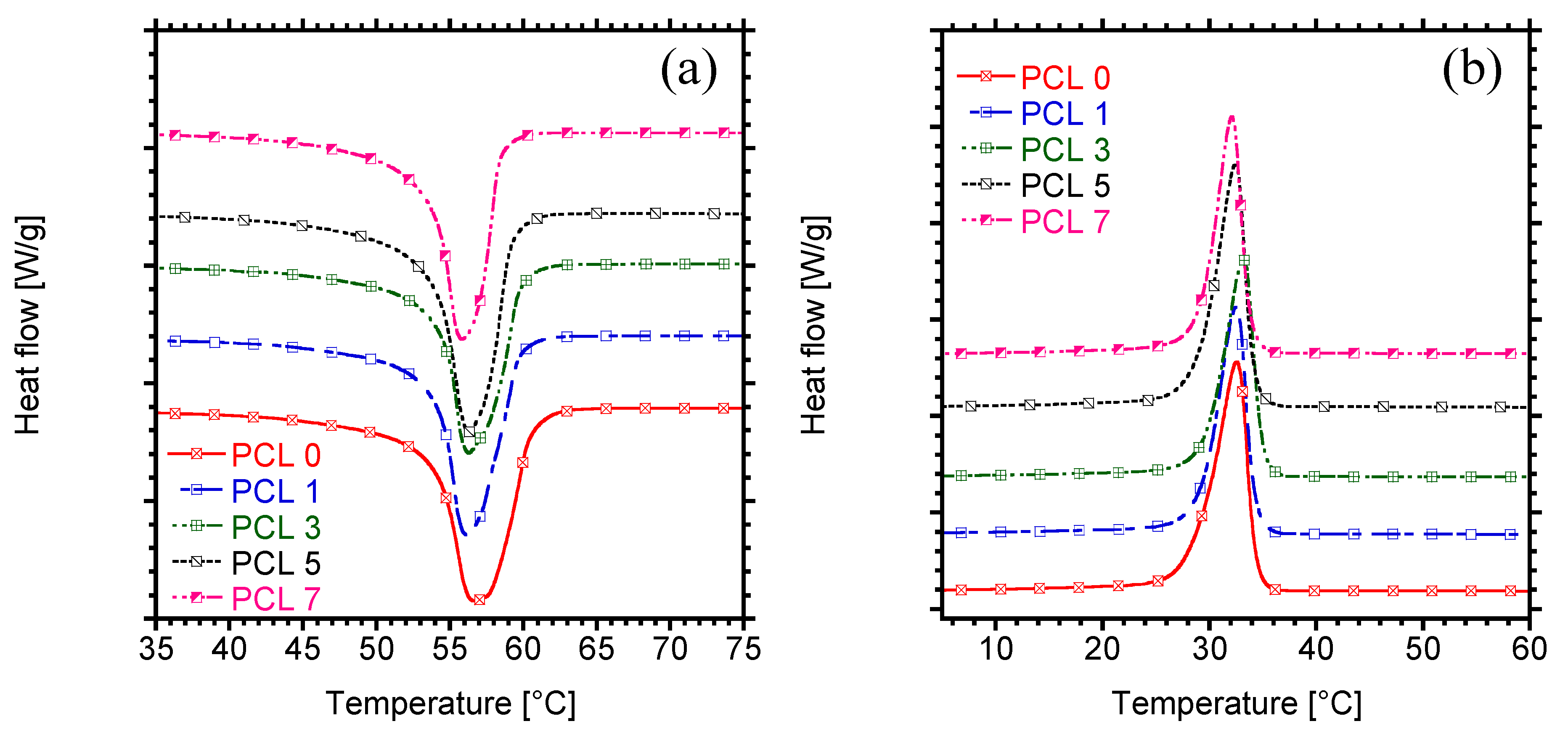
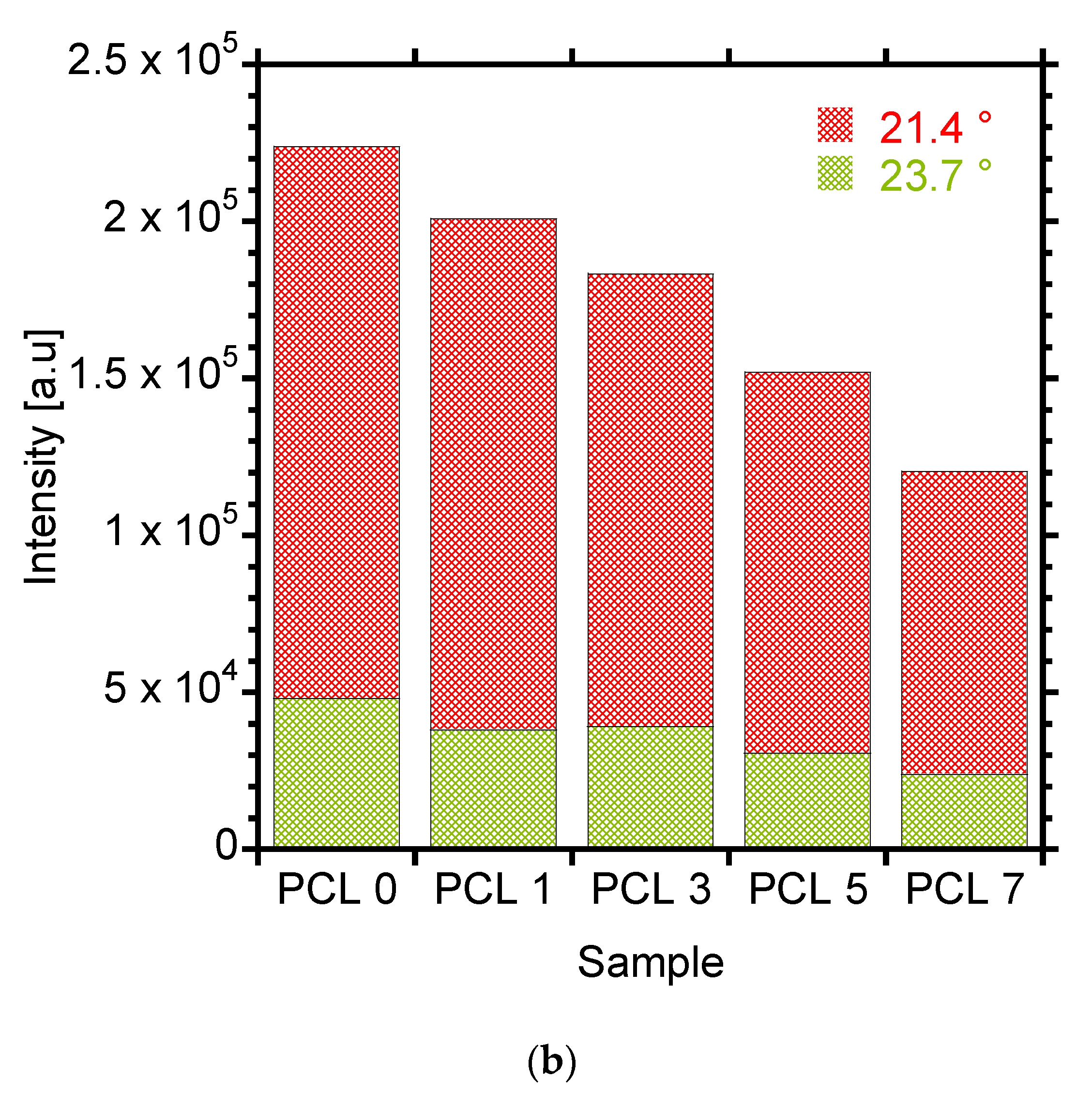
3.3. Melting and Crystallization Behaviour
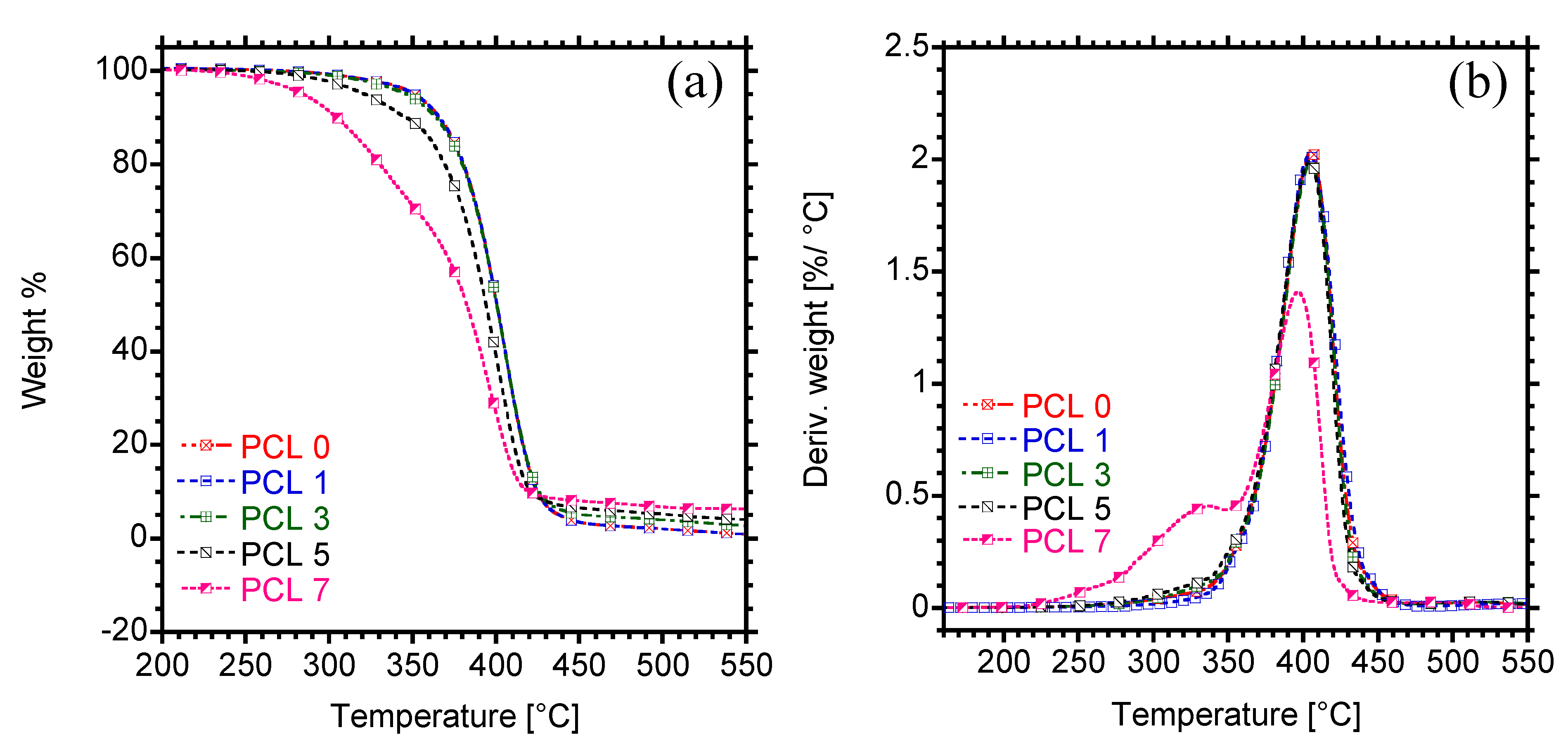
3.4. Thermal Stability
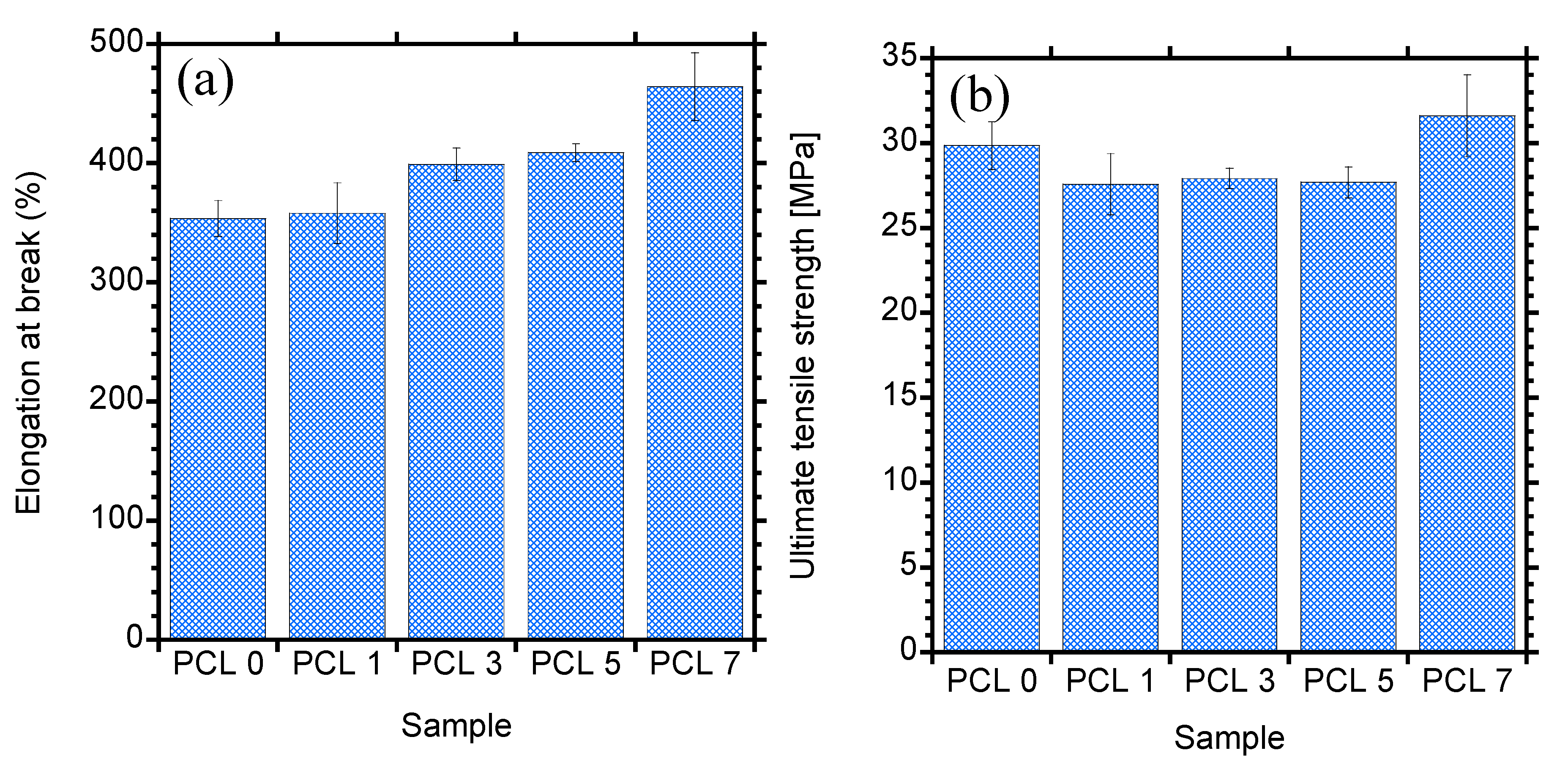
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Correa, A.S.; Carmona, V.B.; Simão, J.A.; Mattoso, L.H.C.; Marconcini, J.M. Biodegradable blends of urea plasticized thermoplastic starch (UTPS) and poly (ε-caprolactone) (PCL): Morphological, rheological, thermal, and mechanical properties. Carbohydr. Polym. 2017, 167, 177–184. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.E.; Henry, L.; McGennisken, E.; Onofrillo, C.; di Bella, C.; Duchi, S.; O’Connell, C.; Pirogova, E. Characterization of polycaprolactone nanohydroxyapatite composites with tunable degradability suitable for indirect printing. Polymers 2021, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.K.; Rao, R.U.; Suman, K.N.S.; Rambabu, V. Preparation and characterization of biodegradable PLA/PCL polymeric blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef] [Green Version]
- Vozniak, I.; Hosseinnezhad, R.; Morawiec, J.; Galeski, A. Microstructual evolution of poly (ε-caprolactone), its immiscible blend, and in situ generated nanocomposites. Polymers 2020, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Endogan, T.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, F.; Benhacine, F.; Ferfera-Harrar, H.; Habi, A.; Hadj-Hamou, A.S.; Grohens, Y. Development of antimicrobial PCL/nanoclay nanocomposite films with enhanced mechanical and water vapor barrier properties for packaging applications. Polym. Bull. 2015, 72, 235–254. [Google Scholar] [CrossRef]
- Piyasin, P.; Yensano, R. Pinitsoontorn, size-controllable melt-electrospun polycaprolactone (PCL) fibers with a sodium chloride additive. Polymers 2019, 11, 1768. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Chen, S.; Tang, X.; Fang, H.; Tian, H.; Li, D.; Peng, X. Thermal conductivity of polycaprolactone/three-dimensional hexagonal boron nitride composites and multi-orientation model investigation, Compos. Sci. Technol. 2020, 197, 108245. [Google Scholar]
- Wurm, A.; Lellinger, D.; Minakov, A.A.; Skipa, T.; Pötschke, P.; Nicula, R.; Alig, I.; Schick, C. Crystallization of poly (ε-caprolactone)/MWCNT composites: A combined SAXS/WAXS, electrical and thermal conductivity study. Polymer 2014, 55, 2220–2232. [Google Scholar] [CrossRef]
- Gain, O.; Espuche, E.; Pollet, E.; Alexandre, M.; Dubois, P.H. Gas barrier properties of poly (ε-caprolactone)/clay nanocomposites: Influence of the morphology and polymer/clay interactions. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 205–214. [Google Scholar] [CrossRef]
- Castro, M.; Lu, J.; Bruzaud, S.; Kumar, B.; Feller, J. Carbon nanotubes/poly (ε-caprolactone) composite vapour sensors. Carbon 2009, 47, 1930–1942. [Google Scholar] [CrossRef]
- Malysheva, K.; Kwaśniak, K.; Gnilitskyi, I.; Barylyak, A.; Zinchenko, V.; Fahmi, A.; Korchynskyi, O.; Bobitski, Y. Functionalization of polycaprolactone electrospun osteoplastic scaffolds with fluorapatite and hydroxyapatite nanoparticles: Biocompatibility comparison of human versus mouse mesenchymal stem cells. Materials 2021, 14, 1333. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, P.; Shokrolahi, F.; Hassanzadeh, P. Structural and biodegradation characterization of supramolecular PCL/Hap nanocomposites for application in tissue engineering, In Handbook of Composites from Renewable Materials; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Huang, G.; Du, Z.; Yuan, Z.; Gu, L.; Cai, Q.; Yang, X. Poly (l-lactide) nanocomposites containing poly (D-lactide) grafted nanohydroxyapatite with improved interfacial adhesion via stereocomplexation. J. Mech. Behav. Biomed. Mater. 2018, 78, 10–19. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442. [Google Scholar] [CrossRef] [Green Version]
- Behera, K.; Sivanjineyulu, V.; Chang, Y.; Chiu, F. Thermal properties, phase morphology and stability of biodegradable PLA/PBSL/Hap composites. Polym. Degrad. Stab. 2018, 154, 248–260. [Google Scholar] [CrossRef]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D printing of HA/PCL composite tissue engineering scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Elhassani, C.E.; Essamlali, Y.; Aqlil, M.; Nzenguet, A.M.; Ganetri, I.; Zahouily, M. Urea-impregnated HAP encapsulated by lignocellulosic biomass-extruded composites: A novel slow-release fertilizer. Environ. Technol. Innov. 2019, 15, 100403. [Google Scholar] [CrossRef]
- Maghsoodi, M.R.; Najafi, N.; Reyhanitabar, A.; Oustan, S. Hydroxyapatite nanorods, hydrochar, biochar, and zeolite for controlled release urea fertilizers. Geoderma 2020, 379, 114644. [Google Scholar] [CrossRef]
- Hao, J.; Yuan, M.; Deng, X. Biodegradable and biocompatible nanocomposites of poly (ε-caprolactone) with hydroxyapatite nanocrystals: Thermal and mechanical properties. J. Appl. Polym. Sci. 2002, 86, 676–683. [Google Scholar] [CrossRef]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. A 2010, 94, 241–251. [Google Scholar] [CrossRef]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Improvement of dual-leached polycaprolactone porous scaffolds by incorporating with hydroxyapatite for bone tissue regeneration. J. Biomater. Sci. Polym. Ed. 2014, 25, 1986–2008. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, S.; Mostafavi, A.; Ramesh, S.; Memic, A.; Rivero, I.V.; Rao, P.; Tamayol, A. Process-structure-quality relationships of three-dimensional printed poly (caprolactone)-hydroxyapatite scaffolds. Tissue Eng. Part A 2020, 26, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zavřel, F.; Novák, M.; Kroupová, J.; Beveridge, C.; Štěpánek, F.; Ruphuy, G. Development of hot-melt extrusion method to produce hydroxyapatite/polycaprolactone composite filaments. Adv. Eng. Mater. 2021, 2100820. [Google Scholar] [CrossRef]
- Akhbar, S.; Subuki, I.; Sharudin, R.W. Performance of polycaprolactone/hydroxyapatite (PCL/HAP) composite blended by ultrasound assisted melt blending. J. Mech. Eng. 2018, 5, 235–250. [Google Scholar]
- Backes, E.H.; Beatrice, C.A.G.; Shimomura, K.M.B.; Pachane, B.C.; Selistre-de-Araujo, H.S.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Development of poly (ε-caprolactone)/hydroxyapatite composites for bone tissue regeneration. J. Mater. Res. 2021, 36, 3050–3062. [Google Scholar] [CrossRef]
- R-Rochina, J.; Ribelles, J.L.G.; Lebourg, M. Comparative study of PCL-Hap and PCL-bioglass composite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1293–1308. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Jin, X.; Ke, Q. An electrospun poly (ε-caprolactone) nanocomposite fibrous mat with a high content of hydroxyapatite to promote cell infiltration. RSC Adv. 2018, 8, 25228. [Google Scholar] [CrossRef] [Green Version]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Medeiros, G.S.; Muñoz, P.A.R.; de Oliviera, C.F.P.; da Silva, L.C.E.; Malhotra, R.; Gonçalves, M.C.; Rosa, V.; Fechine, G.J.M. Polymer nanocomposites based on poly (ε-caprolactone), hydroxyapatite and graphene oxide. J. Polym. Environ. 2020, 28, 331–342. [Google Scholar] [CrossRef]
- Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and characterization of 3D printed multifunctional bioscaffolds based on PLA/PCL/Hap/BaTiO3 composites. Appl. Sci. 2020, 11, 4253. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Kulandaivelu, R.; Panneer, D.S.; Vivekananthan, S.; Sagadevan, S.; Lett, J.A. Microwave synthesis of hydroxyapatite encumbered with ascorbic acid intended for drug leaching studies. Mater. Res. Innov. 2020, 24, 171–178. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, A.; Mandal, N.; Mondal, B.; Mukhopadhyay, S.S.; Dey, A.; Singh, S. Physico-chemical characterization and biological response of Labeo rohita-derived hydroxyapatite scaffold. Bioprocess Biosyst. Eng. 2014, 37, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Bouakaz, B.S.; Habi, A.; Grohens, Y.; Pillin, I. Effect of combinations of nanofillers on rheology-structure relations in biodegradable poly (ε-caprolactone) nanocomposites. Appl. Clay Sci. 2018, 161, 35–47. [Google Scholar] [CrossRef]
- Jyoti, J.; Dhakate, S.R.; Singh, B.P. Phase transition and anomalous rheological properties of graphene oxide-carbon nanotube acrylonitrile butadiene styrene hybrid composites. Compos. B Eng. 2018, 154, 337350. [Google Scholar] [CrossRef]
- Tesfaye, M.; Patwa, R.; Dhar, P.; Katiyar, V. Nanosilk-grafted poly (lactic acid) films: Influence of cross-linking on rheology and thermal stability. ACS Omega 2017, 2, 7071–7084. [Google Scholar] [CrossRef]
- Patrício, T.; Bártolo, P. Thermal stability of PCL/PLA blends produced by physical blending process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Li, D.; Zhou, L.; Wang, X.; He, L.; Yang, X. Effect of crystallinity of polyethylene with different densities on breakdown strength and conductance property. Materials 2019, 12, 1746. [Google Scholar] [CrossRef] [Green Version]



| Sample | Carreau–Yasuda Model | Cross Model | |||||
|---|---|---|---|---|---|---|---|
| η0 | λ | n | a | η0 | λ | n | |
| PCL 0 | 667.54 | 0.026 | 0.818 | 1.197 | 556.7 | 0.003 | 0.00 |
| PCL 1 | 1030.3 | 0.046 | 0.904 | 1.681 | 613.8 | 0.003 | 0.02 |
| PCL 3 | 951.3 | 0.041 | 0.873 | 1.435 | 604.3 | 0.003 | 0.08 |
| PCL 5 | 1071.2 | 0.039 | 0.849 | 1.299 | - | - | - |
| PCL 7 | - | - | - | - | - | - | - |
| Sample | Tm (°C) | ΔHm (J/g) | Tc (°C) | % Crystallinity |
|---|---|---|---|---|
| PCL 0 | 56.7 | 76.6 | 31.6 | 55.1 |
| PCL 1 | 56.2 | 76.7 | 32.5 | 55.7 |
| PCL 3 | 56.3 | 77.5 | 33.1 | 57.5 |
| PCL 5 | 56.4 | 73.7 | 32.5 | 55.8 |
| PCL 7 | 55.8 | 69.7 | 32.1 | 53.9 |
| Sample | T5% | Tmax |
|---|---|---|
| PCL 0 | 351.3 | 406.2 |
| PCL 1 | 360.5 | 407.1 |
| PCL 3 | 346.6 | 405.2 |
| PCL 5 | 321.5 | 400.6 |
| PCL 7 | 284.6 | 396.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites. Materials 2022, 15, 104. https://doi.org/10.3390/ma15010104
Motloung MP, Mofokeng TG, Ray SS. Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites. Materials. 2022; 15(1):104. https://doi.org/10.3390/ma15010104
Chicago/Turabian StyleMotloung, Mpho Phillip, Tladi Gideon Mofokeng, and Suprakas Sinha Ray. 2022. "Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites" Materials 15, no. 1: 104. https://doi.org/10.3390/ma15010104
APA StyleMotloung, M. P., Mofokeng, T. G., & Ray, S. S. (2022). Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites. Materials, 15(1), 104. https://doi.org/10.3390/ma15010104







