Effect of Cr on Aqueous and Atmospheric Corrosion of Automotive Carbon Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
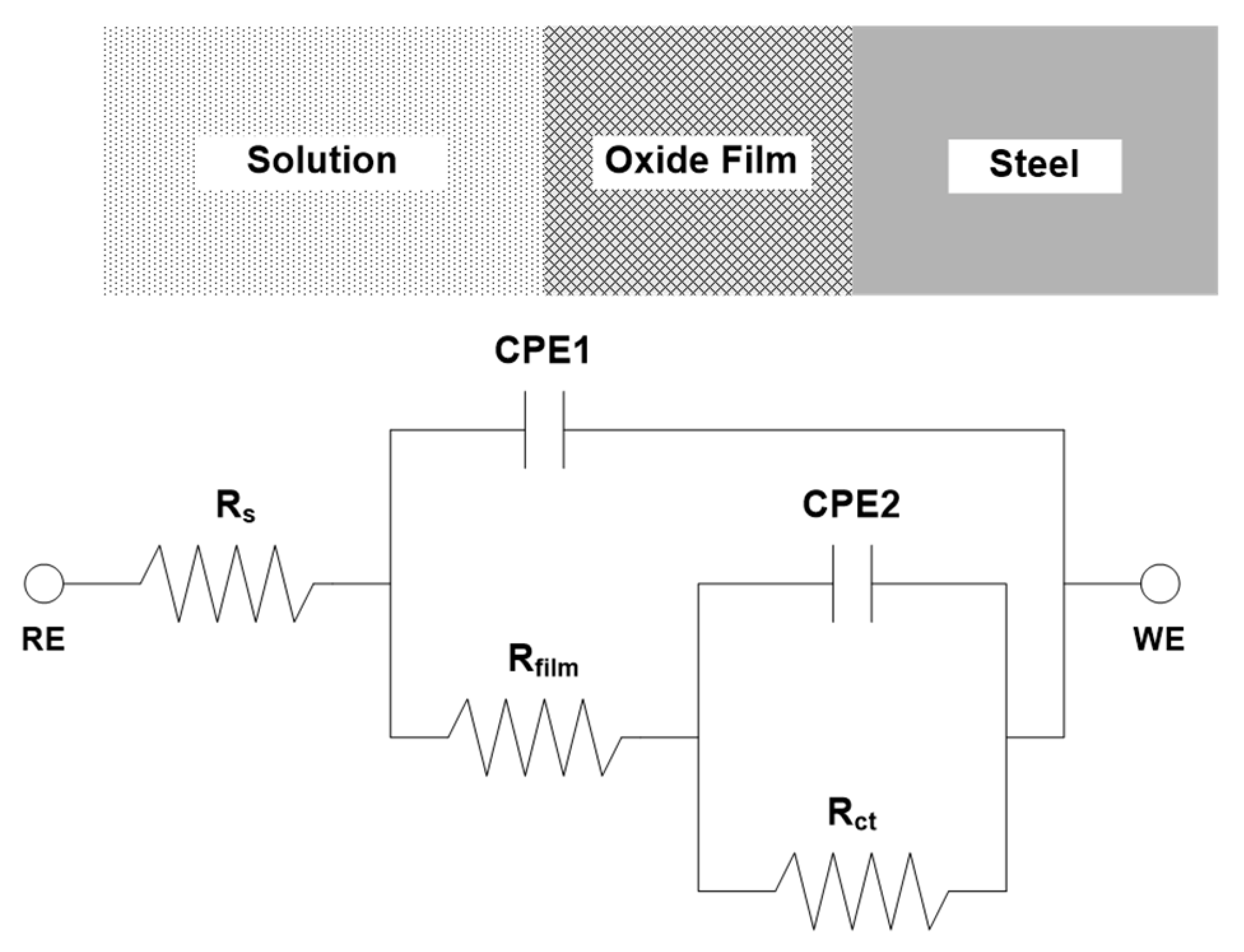
3.1. Electrochemical Measurement
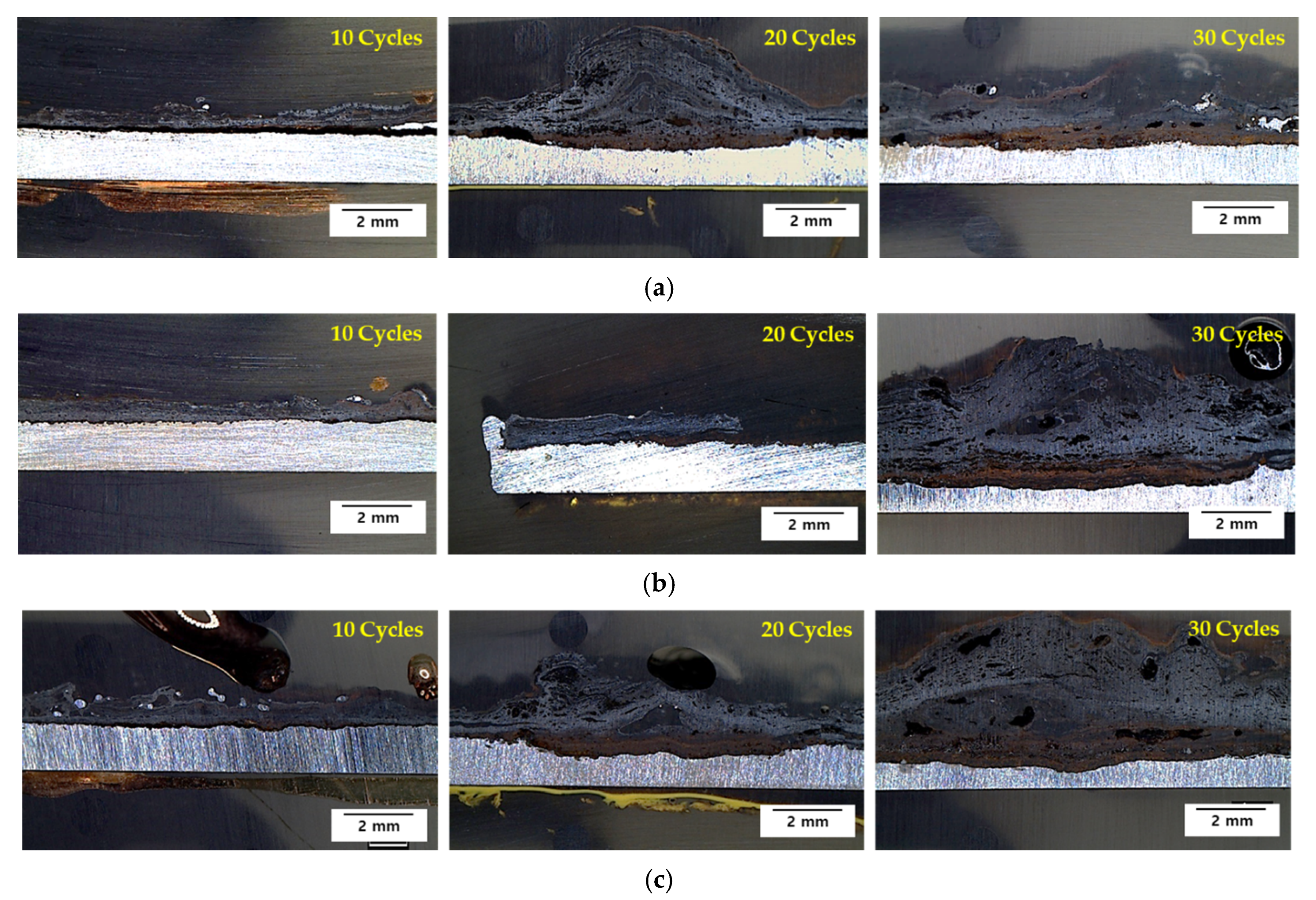
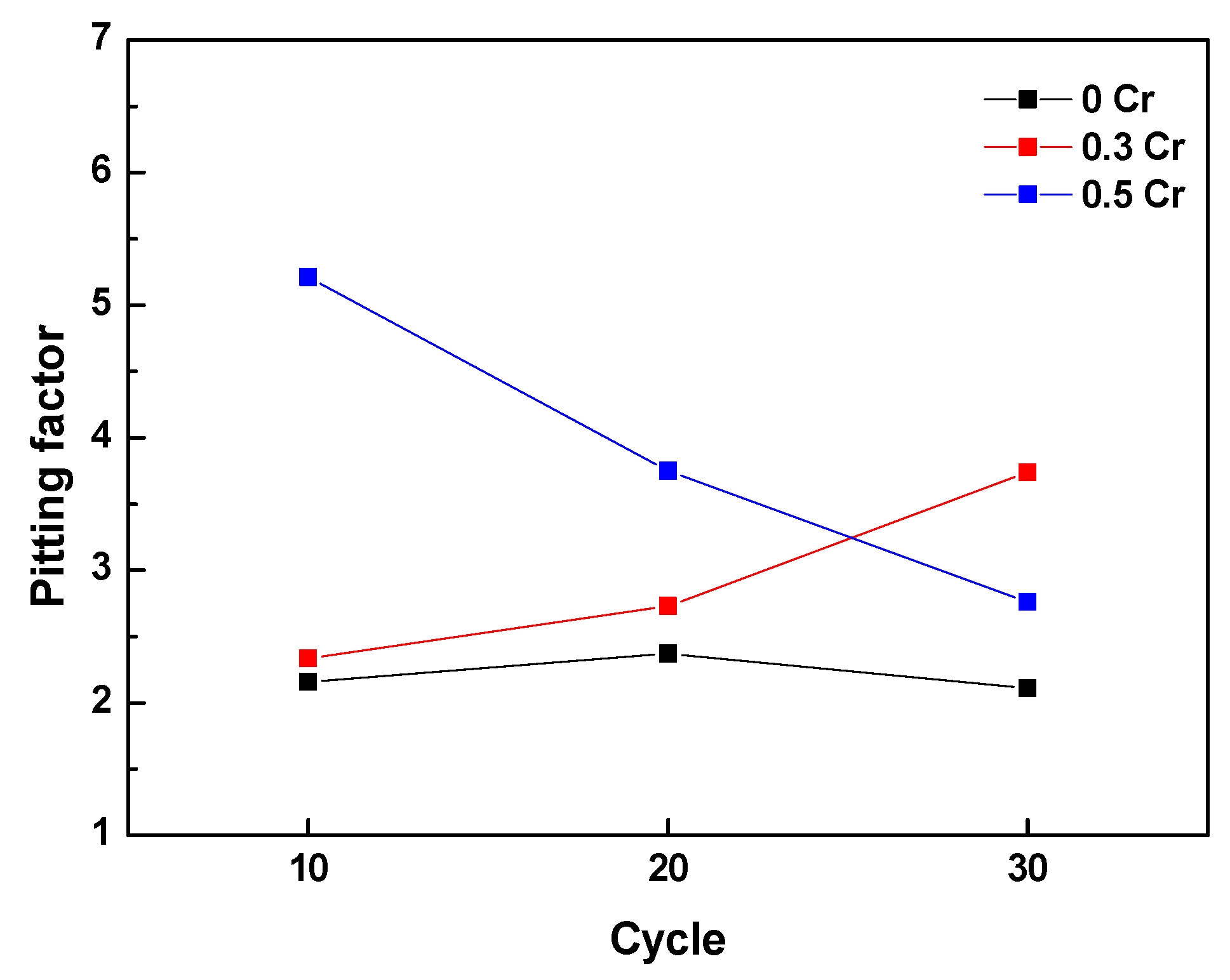
3.2. Cyclic Corrosion Test Results
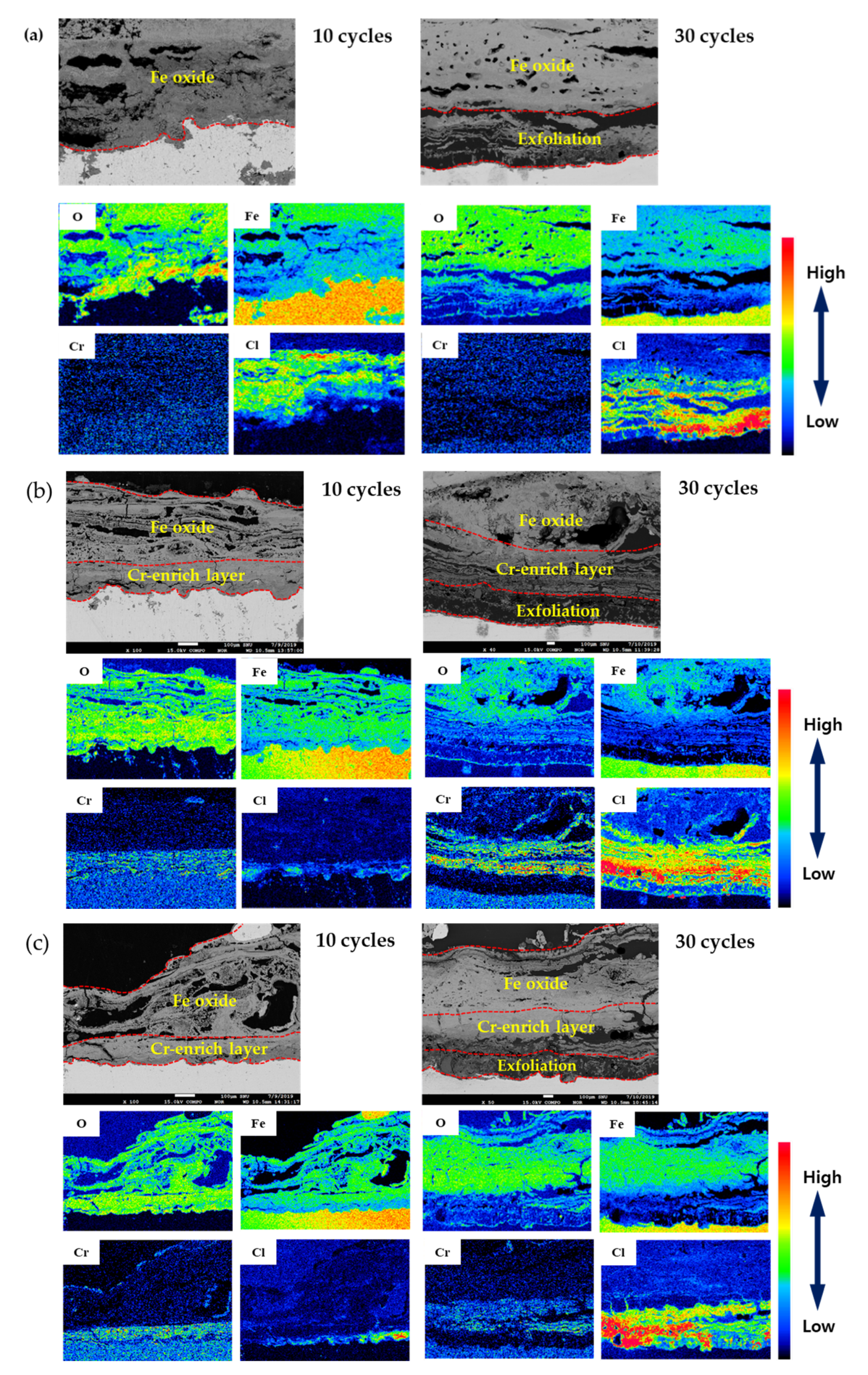
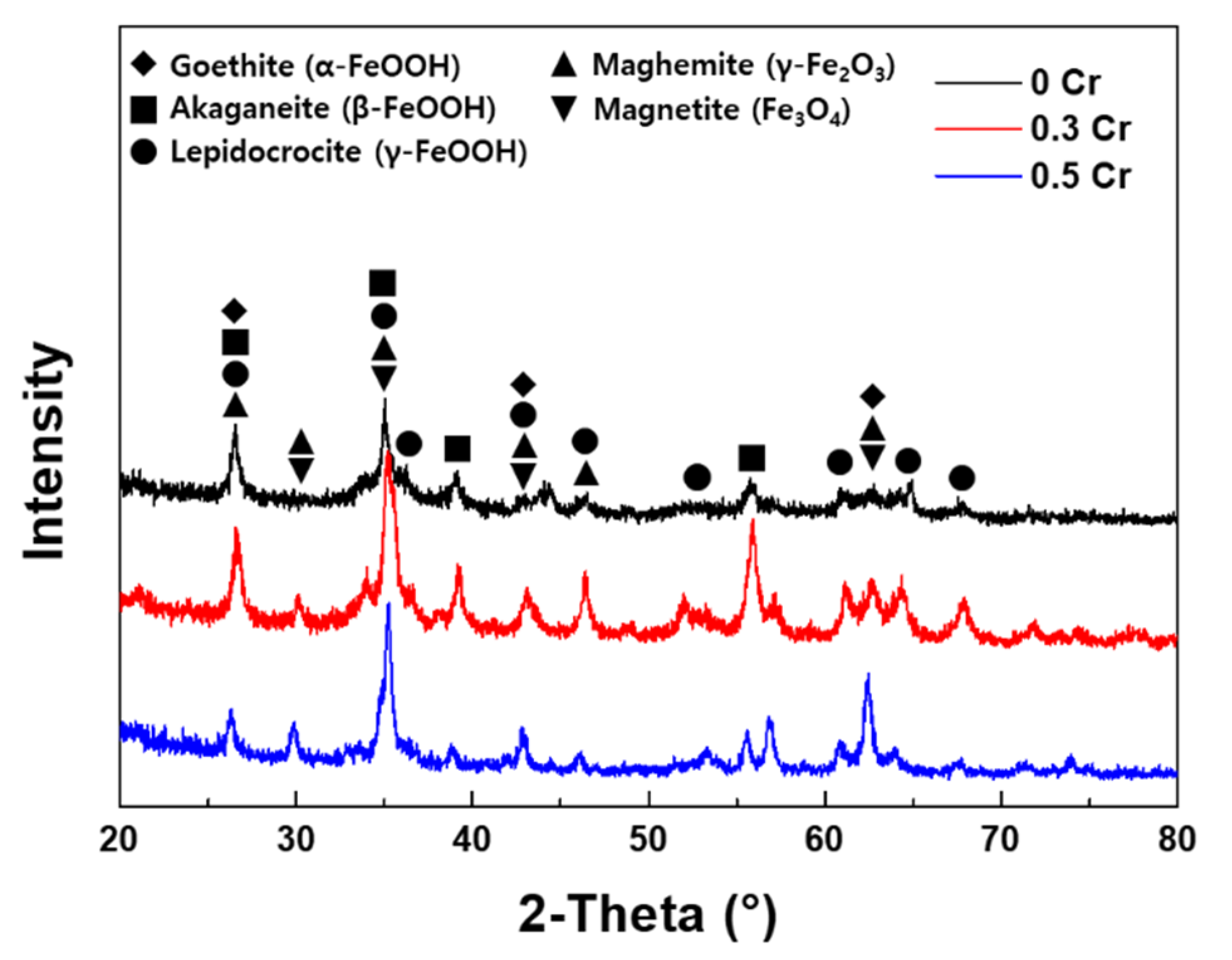
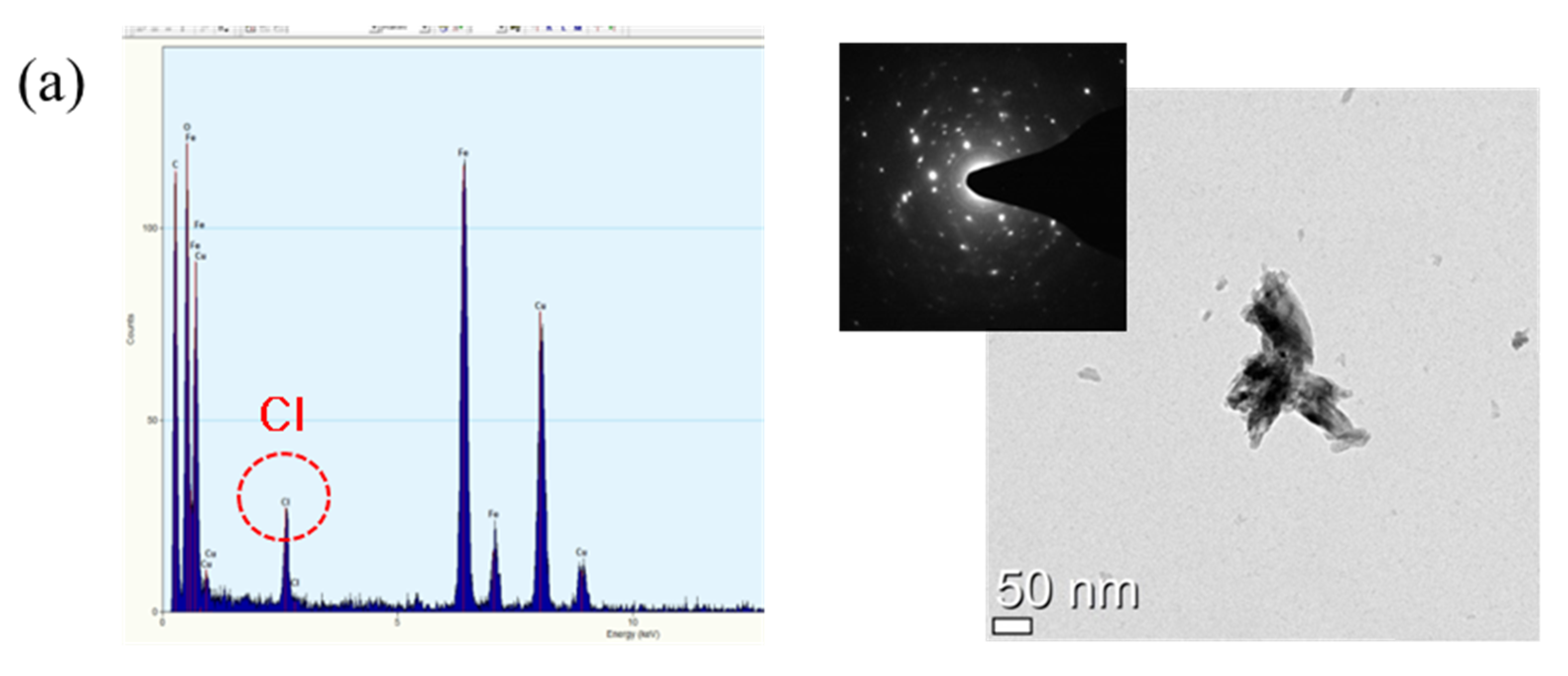
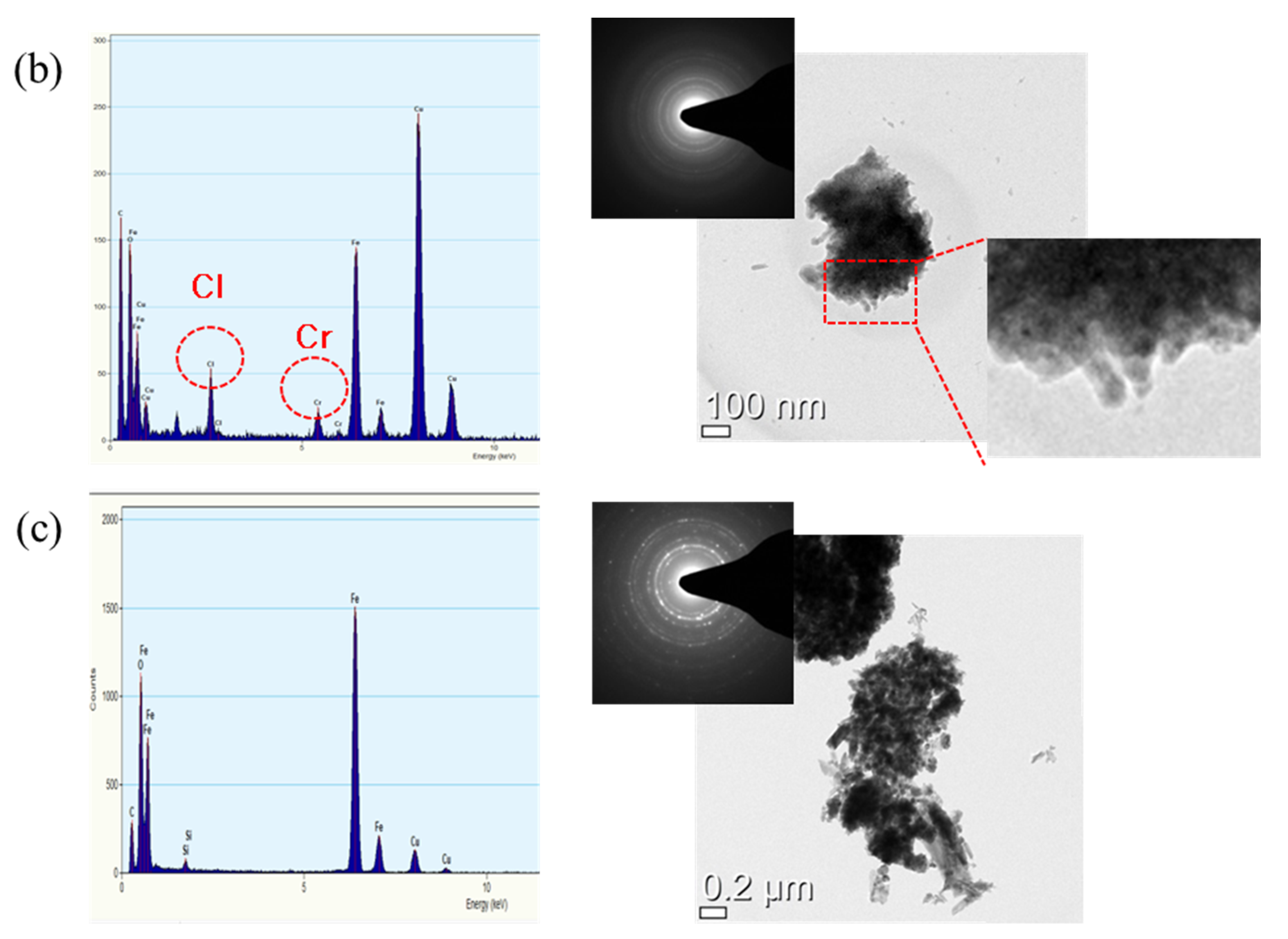
3.3. Rust Constituent Analysis
3.4. Localized Corrosion Mechanism of Cr-Added Steel under Wet/Dry Conditions
4. Conclusions
- In the electrochemical measurement results, the Cr alloying element improves the corrosion resistance of the ACS that was immersed in the Cl-containing aqueous solution.
- Cl is concentrated at the metal/rust interface in all of the specimens regardless of Cr content after the CCT. The Cl is uniformly concentrated and distributed on the 0 Cr steel, whereas Cl is localized and non-uniformly concentrated on the Cr-added steels. The PF of the Cr-added steels is higher than that of the 0 Cr steel during the CCT.
- The inner rust layer consists of Cl-containing akaganeite and Cr-goethite, while the outer rust layer is composed of amorphous iron oxyhydroxide mixed with various types of rust.
- FeCl2 and CrCl3 are formed from the Cl nest developed in the early stage, and the pitting at CrCl3-formed regions is locally accelerated because Cr is strongly hydrolyzed to a very low pH.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, C.; Kang, B.; Choi, B.-H.; Lee, J.; Lee, K. Observation and characterization of squeak noises of polymeric materials for automotive interior parts under field-degradation. Trans. KSAE 2017, 25, 257–265. [Google Scholar] [CrossRef]
- Adikari, A.; Munasinghe, R.D.S.; Jayatileke, S. Prediction of atmospheric corrosion—A Review. Engineer 2014, 47, 75–83. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine atmospheric corrosion of carbon steel: A review. Materials 2017, 10, 406. [Google Scholar] [CrossRef]
- Kamimura, T.; Stratmann, M. The influence of chromium on the atmospheric corrosion of steel. Corros. Sci. 2001, 43, 429–447. [Google Scholar] [CrossRef]
- Asami, K.; Kikuchi, M. Characterization of rust layers on weathering steels air-exposed for a long period. Mater. Trans. 2002, 43, 2818–2825. [Google Scholar] [CrossRef]
- Yamashita, M.; Shimizu, T.; Konishi, H.; Mizuki, J.; Uchida, H. Structure and protective performance of atmospheric corrosion product of Fe–Cr alloy film analyzed by Mössbauer spectroscopy and with synchrotron radiation X-rays. Corros. Sci. 2003, 45, 381–394. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. Corros. Sci. 1994, 36, 283–299. [Google Scholar] [CrossRef]
- Zhao, Q.-H.; Liu, W.; Zhu, Y.-C.; Zhang, B.-L.; Li, S.-Z.; Lu, M.-X. Effect of small content of chromium on wet–dry acid corrosion behavior of low alloy steel. Acta Metall. Sin-Engl. 2017, 30, 164–175. [Google Scholar] [CrossRef]
- Park, S.-A.; Kim, J.-G.; Lee, B.-H.; Yoon, J.-B. Development of sulfuric and hydrochloric acid dew-point corrosion-resistant steels: 1. Effect of alloying elements on the corrosion resistance of low-alloy steels. Korean J. Met. Mater. 2014, 52, 837–855. [Google Scholar]
- Kim, S.-H.; Lee, J.-H.; Kim, J.-G.; Kim, W.-C. Effect of the crevice former on the corrosion behavior of 316L stainless steel in chloride-containing synthetic tap water. Met. Mater. Int. 2018, 24, 516–524. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, Z. The cause analysis of the incomplete semi-circle observed in high frequency region of EIS obtained from TEL-covered pure copper. Int. J. Electrochem. Sci 2013, 8, 8282–8290. [Google Scholar]
- Keddam, M.; Mottos, O.R.; Takenouti, H. Reaction model for iron dissolution studied by electrode impedance: I. Experimental results and reaction model. J. Electrochem. Soc. 1981, 128, 257–266. [Google Scholar] [CrossRef]
- Liu, W.; Dou, J.; Lu, S.; Zhang, P.; Zhao, Q. Effect of silty sand in formation water on CO2 corrosion behavior of carbon steel. Appl. Surf. Sci. 2016, 367, 438–448. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.; Guo, X. Erosion–corrosion at different locations of X65 carbon steel elbow. Corros. Sci. 2014, 85, 318–330. [Google Scholar] [CrossRef]
- Srinivasan, A.; Blawert, C.; Huang, Y.; Mendis, C.; Kainer, K.; Hort, N. Corrosion behavior of Mg–Gd–Zn based alloys in aqueous NaCl solution. J. Magnes. Alloy 2014, 2, 245–256. [Google Scholar] [CrossRef]
- Kim, M.; Jang, S.; Woo, S.; Kim, J.; Kim, Y. Corrosion resistance of ferritic stainless steel in exhaust condensed water containing aluminum cations. Corrosion 2015, 71, 285–291. [Google Scholar] [CrossRef]
- Cho, S.; An, J.-H.; Lee, S.-H.; Kim, J.-G. Effect of pH on the passive film characteristics of lean duplex stainless steel in chloride-containing synthetic tap water. Int. J. Electrochem. Sci 2020, 15, 4406–4420. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, T.; Gopal, M.; Jepson, W. EIS studies of a corrosion inhibitor behavior under multiphase flow conditions. Corros. Sci. 2000, 42, 979–990. [Google Scholar] [CrossRef]
- Hamdy, A.S.; El-Shenawy, E.; El-Bitar, T. Electrochemical impedance spectroscopy study of the corrosion behavior of some niobium bearing stainless steels in 3.5% NaCl. Int. J. Electrochem. Sci. 2006, 1, 171–180. [Google Scholar]
- Bentiss, F.; Lebrini, M.; Vezin, H.; Chai, F.; Traisnel, M.; Lagrené, M. Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corros. Sci. 2009, 51, 2165–2173. [Google Scholar] [CrossRef]
- Lopez, D.A.; Simison, S.; De Sanchez, S. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Mansfeld, F. Recording and analysis of AC impedance data for corrosion studies. Corrosion 1981, 37, 301–307. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scripta Mater. 2010, 63.12, 1201–1204. [Google Scholar] [CrossRef]
- Xiao, H.; Ye, W.; Song, X.; Ma, Y.; Li, Y. Evolution of akaganeite in rust layers formed on steel submitted to wet/dry cyclic tests. Materials 2017, 10, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Asami, K.; Kikuchi, M. In-depth distribution of rusts on a plain carbon steel and weathering steels exposed to coastal–industrial atmosphere for 17 years. Corros. Sci. 2003, 45, 2671–2688. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Díaz, I.; De la Fuente, D.; Morcillo, M. Airborne chloride deposit and its effect on marine atmospheric corrosion of mild steel. Corros. Sci. 2015, 97, 74–88. [Google Scholar] [CrossRef]
- Senthilnathan, A.; Dissanayake, D.; Chandrakumara, G.; Mantilaka, M.; Rajapakse, R.; Pitawala, H.; Nalin de Silva, K. Akaganeite nanorices deposited muscovite mica surfaces as sunlight active green photocatalyst. R. Soc. Open Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Baig, R.N.; Nadagouda, M.N.; Varma, R.S. Oxidative CH activation of amines using protuberant lychee-like goethite. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kihira, H. Nanoscopic mechanism of protective-rusts formation on weathering steel surfaces. SHINNITTETSU GIHO 2004, 91, 77–81. [Google Scholar]
- Xu, Q.; Gao, K.; Lv, W.; Pang, X. Effects of alloyed Cr and Cu on the corrosion behavior of low-alloy steel in a simulated groundwater solution. Corros. Sci. 2016, 102, 114–124. [Google Scholar] [CrossRef]
- Melchers, R.E. Effect of small compositional changes on marine immersion corrosion of low alloy steels. Corros. Sci. 2004, 46, 1669–1691. [Google Scholar] [CrossRef]
- Henriksen, J. The distribution of NaCl on Fe during atmospheric corrosion. Corros. Sci. 1969, 9, 573–576. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. The effect of β-FeOOH on the corrosion behavior of low carbon steel exposed in tropic marine environment. Mater. Chem. Phys. 2008, 112, 844–852. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Yang, W.; Ni, R.-C.; Hua, H.-Z.; Pourbaix, A. The behavior of chromium and molybdenum in the propagation process of localized corrosion of steels. Corros. Sci. 1984, 24, 691–707. [Google Scholar] [CrossRef]
- SA, P.; DP, L. Alloying effect of chromium on the corrosion behavior of low-alloy steels. Meter. Trans. 2013, 54, 1770–1778. [Google Scholar]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 1996; p. 217. [Google Scholar]
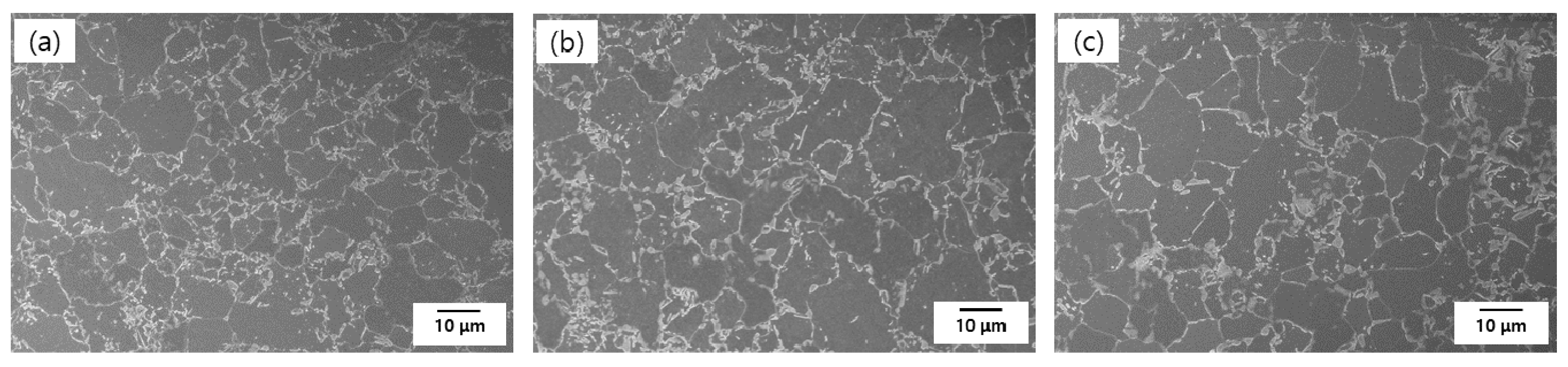
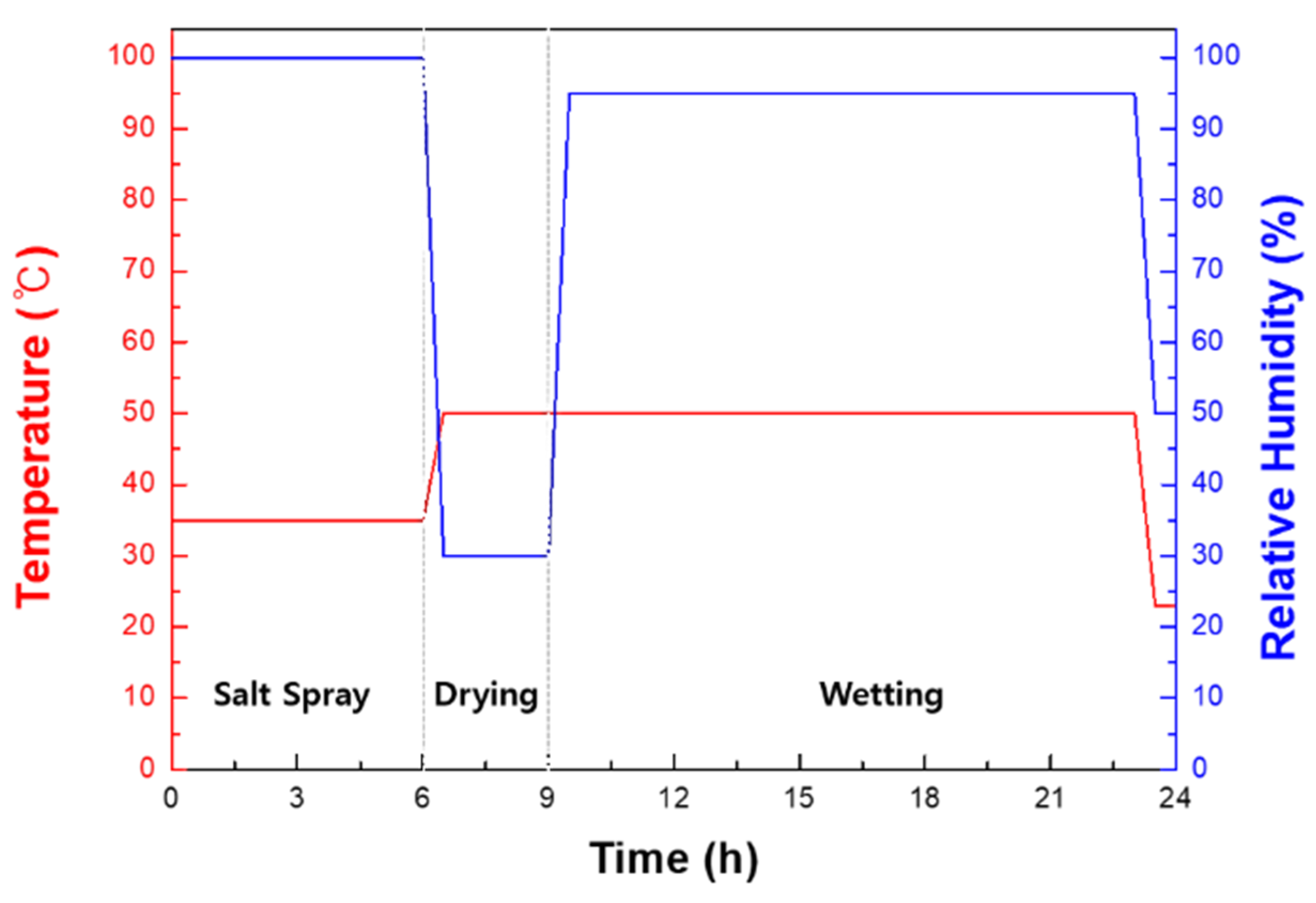



| Steels | Cr | C | Si | Mn | Fe |
|---|---|---|---|---|---|
| 0 Cr | 0.01 | 0.10 | 0.52 | 2.49 | Bal. |
| 0.3 Cr | 0.32 | 0.10 | 0.52 | 2.49 | Bal. |
| 0.5 Cr | 0.50 | 0.10 | 0.52 | 2.49 | Bal. |
| Parameter | 0 Cr | 0.3 Cr | 0.5 Cr |
|---|---|---|---|
| Ecorr (mVSCE) | −686.5 ± 1.4 | −683.6 ± 5.4 | −693.2 ± 12.6 |
| Icorr (μA/cm2) | 21.3 | 20.2 | 17.4 |
| Steel | Immersion Time | Rs (Ω·cm−2) | CPE1 | Rfilm (Ω·cm−2) | CPE2 | Rct (Ω·cm−2) | Rp (Ω·cm−2) | ||
|---|---|---|---|---|---|---|---|---|---|
| Qfilm (Ω−1 cm−2·sn) | n1 | Qct (Ω−1· cm−2·sn) | n1 | ||||||
| 0 Cr | 0 h | 1.424 | 7.52 × 10−4 | 0.8355 | 29.9 | 3.97 × 10−4 | 0.9671 | 636.5 | 665.7 |
| 1 h | 2.531 | 6.11 × 10−4 | 0.8146 | 192.6 | 1.29 × 10−4 | 0.9516 | 951.6 | 1144.2 | |
| 2 h | 2.522 | 6.36 × 10−4 | 0.8037 | 294 | 1.48 × 10−4 | 0.9883 | 801.2 | 1095.2 | |
| 3 h | 2.542 | 6.65 × 10−4 | 0.801 | 239.9 | 1.55 × 10−4 | 0.9724 | 831.3 | 1071.2 | |
| 4 h | 2.549 | 6.84 × 10−4 | 0.796 | 267.4 | 1.61 × 10−4 | 0.997 | 752.1 | 1019.5 | |
| 5 h | 2.574 | 6.72 × 10−4 | 0.7959 | 217.5 | 1.73 × 10−4 | 0.9593 | 766.3 | 983.8 | |
| 6 h | 2.589 | 6.81 × 10−4 | 0.7953 | 203.7 | 1.85 × 10−4 | 0.9509 | 733.1 | 936.8 | |
| 0.3 Cr | 0 h | 1.773 | 1.71 × 10−4 | 1 | 3 | 9.06 × 10−4 | 0.7621 | 1304 | 1307 |
| 1 h | 1.227 | 2.05 × 10−4 | 0.9653 | 32.3 | 3.76 × 10−4 | 0.7516 | 1210 | 1242.3 | |
| 2 h | 1.22 | 1.92 × 10−4 | 0.9714 | 29.2 | 3.79 × 10−4 | 0.7541 | 1151 | 1180.2 | |
| 3 h | 1.212 | 1.83 × 10−4 | 0.9759 | 26.5 | 3.90 × 10−4 | 0.7497 | 1215 | 1241.5 | |
| 4 h | 1.212 | 1.69 × 10−4 | 0.9843 | 24.3 | 4.00 × 10−4 | 0.7475 | 1223 | 1247.3 | |
| 5 h | 1.217 | 1.59 × 10−4 | 0.9914 | 23.7 | 4.10 × 10−4 | 0.7442 | 1266 | 1289.7 | |
| 6 h | 1.215 | 1.57 × 10−4 | 0.993 | 23.1 | 4.08 × 10−4 | 0.7424 | 1285 | 1308.1 | |
| 0.5 Cr | 0 h | 2.809 | 4.71 × 10−4 | 0.8449 | 21.6 | 2.14 × 10−4 | 0.8069 | 1414 | 1435.6 |
| 1 h | 1.986 | 5.09 × 10−4 | 0.8474 | 59 | 1.63 × 10−4 | 0.7983 | 1543 | 1602 | |
| 2 h | 1.978 | 5.18 × 10−4 | 0.8405 | 63.5 | 1.67 × 10−4 | 0.7818 | 1419 | 1482.5 | |
| 3 h | 1.964 | 3.86 × 10−4 | 0.8637 | 20.6 | 2.95 × 10−4 | 0.7261 | 1270 | 1290.6 | |
| 4 h | 1.963 | 3.24 × 10−4 | 0.8782 | 14.9 | 3.39 × 10−4 | 0.7095 | 1489 | 1503.9 | |
| 5 h | 2.001 | 1.18 × 10−4 | 0.9798 | 4.5 | 5.31 × 10−4 | 0.7418 | 1502 | 1506.5 | |
| 6 h | 2.013 | 9.42 × 10−4 | 0.9999 | 3.6 | 5.44 × 10−4 | 0.748 | 1542 | 1545.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-w.; Ko, S.-J.; Yoo, J.-S.; Yoo, Y.-H.; Song, Y.-K.; Kim, J.-G. Effect of Cr on Aqueous and Atmospheric Corrosion of Automotive Carbon Steel. Materials 2021, 14, 2444. https://doi.org/10.3390/ma14092444
Cho S-w, Ko S-J, Yoo J-S, Yoo Y-H, Song Y-K, Kim J-G. Effect of Cr on Aqueous and Atmospheric Corrosion of Automotive Carbon Steel. Materials. 2021; 14(9):2444. https://doi.org/10.3390/ma14092444
Chicago/Turabian StyleCho, Sang-won, Sang-Jin Ko, Jin-Seok Yoo, Yun-Ha Yoo, Yon-Kyun Song, and Jung-Gu Kim. 2021. "Effect of Cr on Aqueous and Atmospheric Corrosion of Automotive Carbon Steel" Materials 14, no. 9: 2444. https://doi.org/10.3390/ma14092444
APA StyleCho, S.-w., Ko, S.-J., Yoo, J.-S., Yoo, Y.-H., Song, Y.-K., & Kim, J.-G. (2021). Effect of Cr on Aqueous and Atmospheric Corrosion of Automotive Carbon Steel. Materials, 14(9), 2444. https://doi.org/10.3390/ma14092444







