Carbon Nanotubes and Polydopamine Modified Poly(dimethylsiloxane) Sponges for Efficient Oil–Water Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
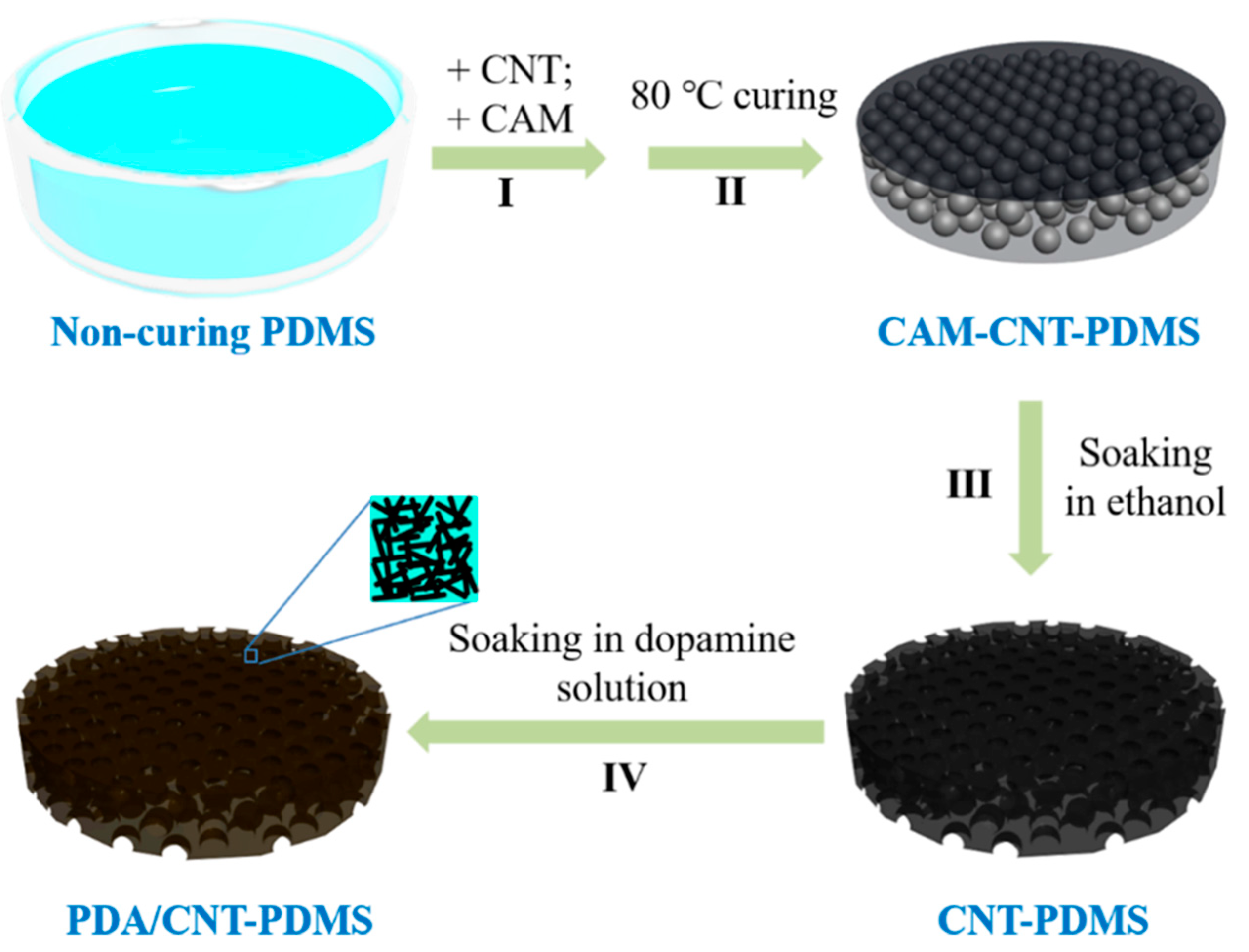
2.2. Preparation of Porous PDMS Sponges
2.3. Preparation of CNT-PDMS Sponges
2.4. Preparation of PDA/PDMS and PDA/CNT-PDMS Sponges
2.5. Oil Absorption Experiments
2.6. Characterization
3. Results and Discussion
3.1. Preparation of PDMS-Based Sponges
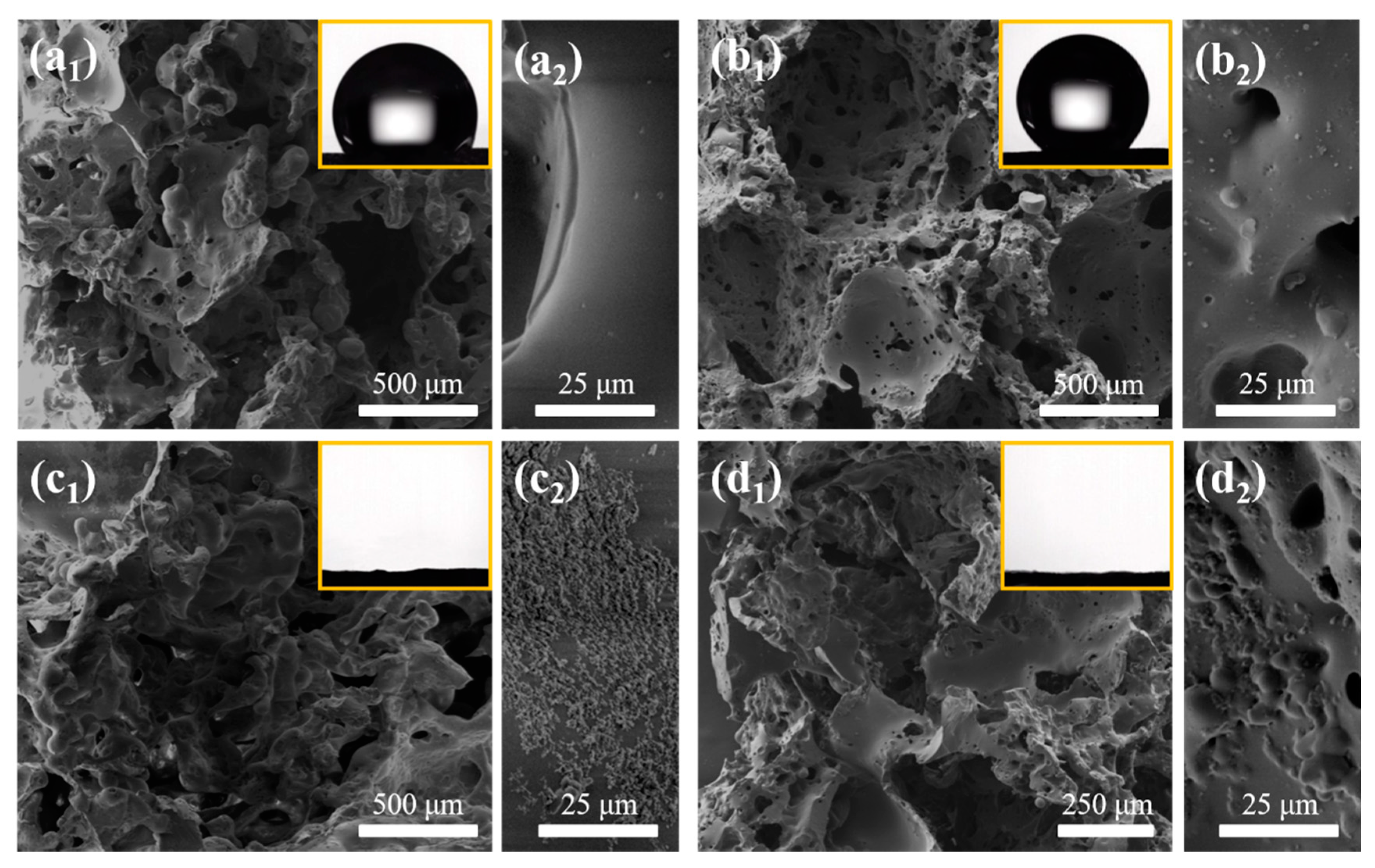
3.2. Morphology and Wettability Characterization of PDMS-Based Sponges
3.3. Performance of Oil Absorption for PDMS-Based Sponges
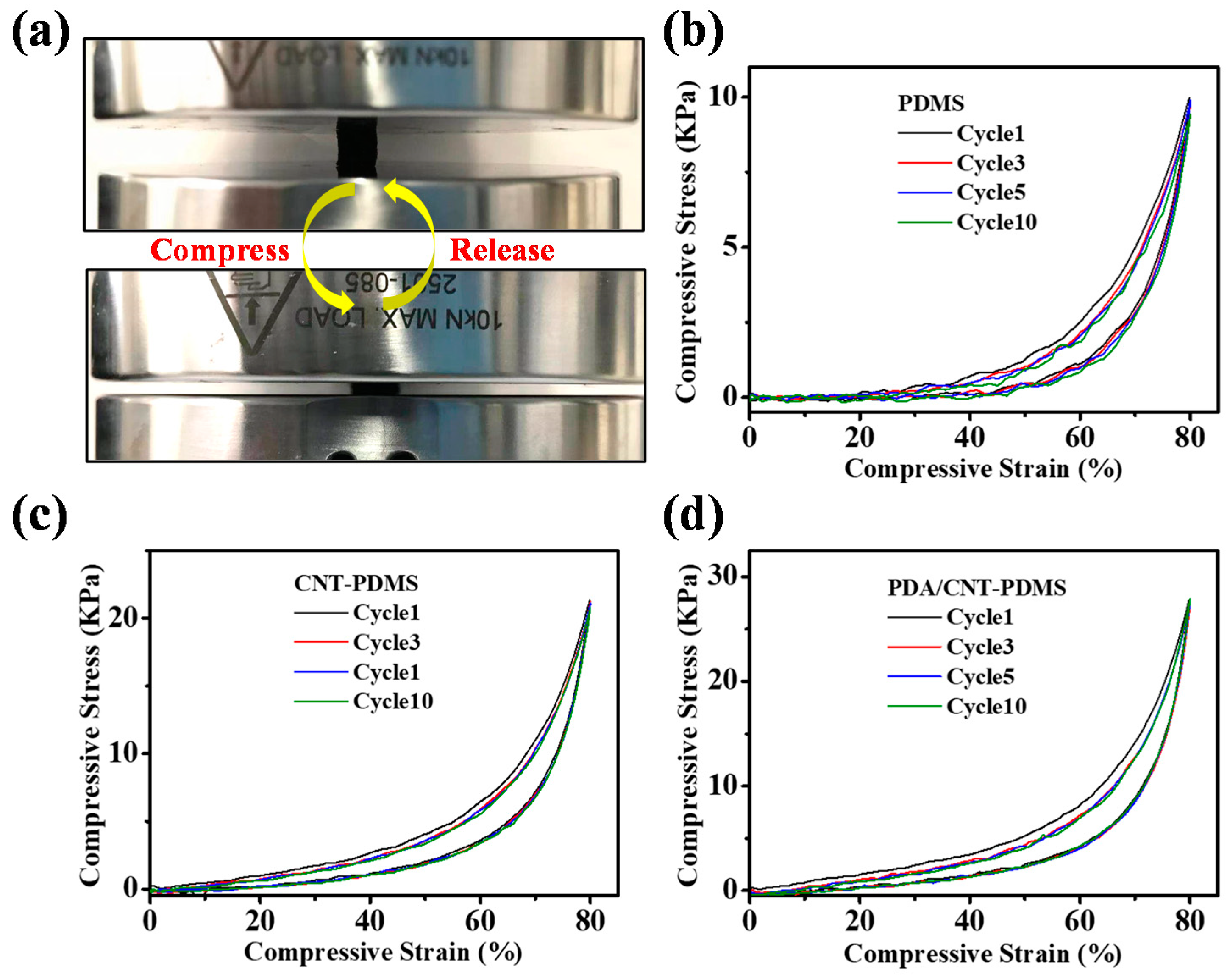
3.4. Mechanical Property of PDMS-Based Sponges
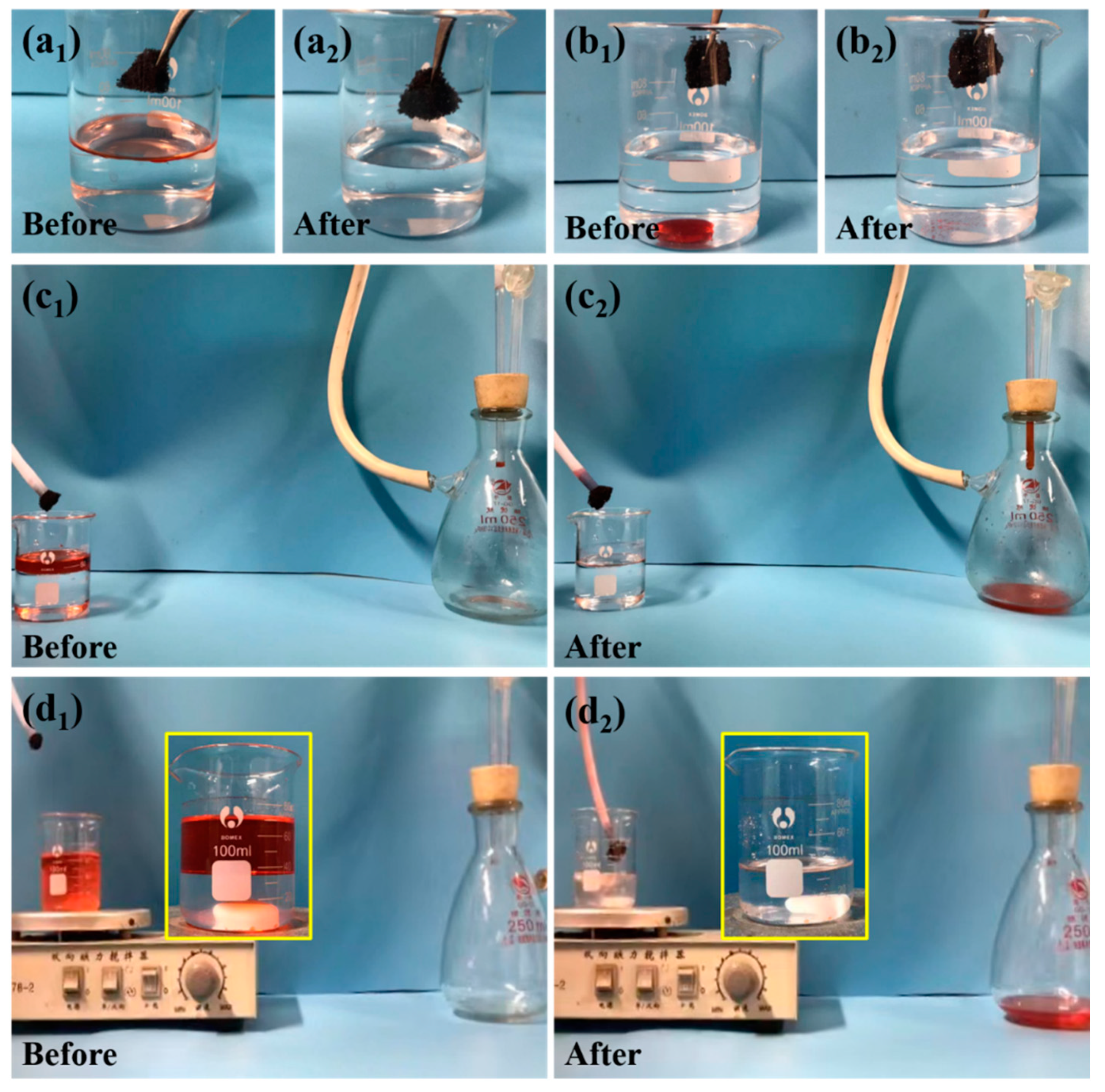
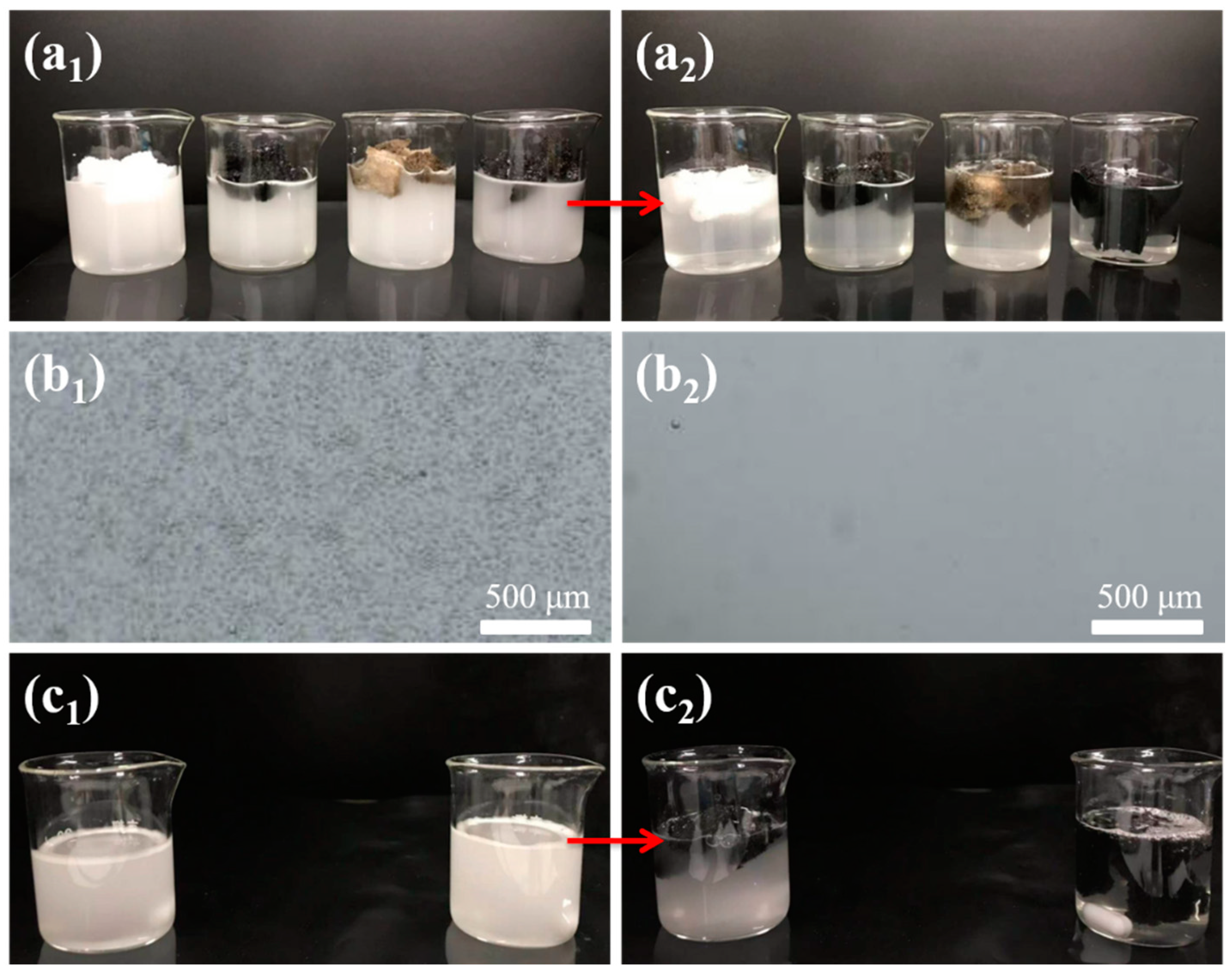
3.5. Application in Selective Oil Absorption from Immiscible Oil–Water Mixtures
3.6. Application in the Separation of O/W Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Liu, N.; Zhang, W.; Feng, L.; Wei, Y. One-Step Coating toward Multifunctional Applications: Oil/Water Mixtures and Emulsions Separation and Contaminants Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 3333–3339. [Google Scholar] [CrossRef]
- Liu, D.; He, L.; Lei, W.W.; Klika, K.D.; Kong, L.X.; Chen, Y. Multifunctional Polymer/Porous Boron Nitride Nanosheet Membranes for Superior Trapping Emulsified Oils and Organic Molecules. Adv. Mater. Interfaces 2015, 2, 1500228. [Google Scholar] [CrossRef]
- Thilagavathi, G.; Karan, C.P.; Das, D. Oil Sorption and Retention Capacities of Thermally-Bonded Hybrid Nonwovens Prepared from Cotton, Kapok, Milkweed and Polypropylene Fibers. J. Environ. Manag. 2018, 219, 340–349. [Google Scholar] [CrossRef]
- Farooq, U.; Taban, I.C.; Daling, P.S. Study of the Oil Interaction Towards Oil Spill Recovery Skimmer Material: Effect of the Oil Weathering and Emulsification Properties. Mar. Pollut. Bull. 2018, 135, 119–128. [Google Scholar] [CrossRef]
- Duan, M.; He, Z.; Wang, X.; Jing, B.; Xu, Z.; Xiong, Y.; Fang, S. A Novel Interface-Active Cationic Flocculant for the Oil-Water Separation of Oily Wastewater Produced from Polymer Flooding. J. Mol. Liq. 2019, 286, 110868. [Google Scholar] [CrossRef]
- Chandrakant, T.; Vimal, C.S.; Indra, D.M.; Ajay, D. Mechanistic Study and Multi-Response Optimization of the Electrochemical Treatment of Petroleum Refinery Wastewater. Clean-Soil Air Water 2018, 46, 1700624. [Google Scholar]
- Sayed, K.; Baloo, L.; Sharma, N.K. Bioremediation of Total Petroleum Hydrocarbons (TPH) by Bioaugmentation and Biostimulation in Water with Floating Oil Spill Containment Booms as Bioreactor Basin. Int. J. Environ. Res. Public Health 2021, 18, 2226. [Google Scholar] [CrossRef]
- Bu, X.; Lu, Y.; Chen, S.; Li, D.; Zhang, Z.; Qian, P. Fabrication of Porous Carbon Nitride Foams/Acrylic Resin Composites for Efficient Oil and Organic Solvents Capture. Chem. Eng. J. 2019, 355, 299–308. [Google Scholar] [CrossRef]
- Kimura, K.; Niisaka, S.; Kakuta, Y.; Kurihara, K. Effect of Hydrogen Donation of Palladium on Active Carbon on Woody Biomass Pyrolysis Using Diesel Oil as a Solvent. J. Jpn. Inst. Energy 2020, 99, 8–15. [Google Scholar] [CrossRef]
- Sampaio, C.J.S.; de Souza, J.R.B.; Damiao, A.O.; Bahiense, T.C.; Roque, M.R.A. Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHS) in a Diesel Oil-Contaminated Mangrove by Plant Growth-Promoting Rhizobacteria. 3 Biotech 2019, 9, 155. [Google Scholar] [CrossRef]
- Tejero, M.D.; Jove, E.; Carmona, P.; Gomez, V.; Garcia-Molina, V.; Villa, J.; Das, S. Treatment of Oil-Water Emulsions by Adsorption onto Resin and Activated Carbon. Desalin. Water Treat. 2017, 100, 21–28. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, M. Sorption of Styrene from Aqueous Solutions with Oil Absorptive Resin. J. Environ. Manag. 2009, 90, 217–221. [Google Scholar] [CrossRef]
- Radetic, M.M.; Jocic, D.M.; Jovancic, P.M.; Petrovic, Z.L.; Thomas, H.F. Recycled Wool-Based Nonwoven Material as an Oil Sorbent. Environ. Sci. Technol. 2003, 37, 1008–1012. [Google Scholar] [CrossRef]
- Turco, A.; Primiceri, E.; Frigione, M.; Maruccio, G.; Malitesta, C. An Innovative, Fast and Facile Soft-Template Approach for the Fabrication of Porous PDMS for oil–Water Separation. J. Mater. Chem. A 2017, 5, 23785–23793. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable Superhydrophobic/Superoleophilic PDMS Sponges and Their Applications in Selective Oil Absorption and in Plugging Oil Leakages. J. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Tran, D.N.H.; Kabiri, S.; Sim, T.R.; Losic, D. Selective Adsorption of Oil-Water Mixtures Using Polydimethylsiloxane (PDMS)-Graphene Sponges. Environ. Sci. Water Res. Technol. 2015, 1, 298–305. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, H.; Ye, H.; Zhang, B.; Xu, L. Poly(dimethylsiloxane)/Graphene Oxide Composite Sponge: A Robust and Reusable Adsorbent for Efficient Oil/Water Separation. Soft Matter 2019, 15, 9224–9232. [Google Scholar] [CrossRef]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D.I.; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Chen, M.; Du, C.; Guo, H.; Bai, H.; Li, L. Poly(dimethylsiloxane) Oil Absorbent with a Three-Dimensionally Interconnected Porous Structure and Swellable Skeleton. ACS Appl. Mater. Interfaces 2013, 5, 10201–10206. [Google Scholar] [CrossRef]
- Yu, C.; Yu, C.; Cui, L.; Song, Z.; Zhao, X.; Ma, Y.; Jiang, L. Facile Preparation of the Porous PDMS Oil-Absorbent for Oil/Water Separation. Adv. Mater. Interfaces 2017, 4, 1600862. [Google Scholar] [CrossRef]
- Turco, A.; Malitesta, C.; Barillaro, G.; Greco, A.; Maffezzoli, A.; Mazzotta, E. A Magnetic and Highly Reusable Macroporous Superhydrophobic/Superoleophilic PDMS/MWNT Nanocomposite for Oil Sorption from Water. J. Mater. Chem. A 2015, 3, 17685–17696. [Google Scholar] [CrossRef]
- Mo, S.Q.; Mei, J.; Liang, Q.; Li, Z. Repeatable Oil-Water Separation with a Highly-Elastic and Tough Amino-Terminated Polydimethylsiloxane-based Sponge Synthesized Using a Self-Foaming Method. Chemosphere 2021, 271, 129827. [Google Scholar] [CrossRef]
- Yao, Q.; Zhao, P.; Li, R.; Li, C.; Luo, Y.; Zhou, G.; Yang, M. Fabrication of Recyclable Carbonized Asphalt-Melamine Sponges with High Oil-Absorption Capability. J. Chem. Technol. Biotechnol. 2017, 92, 1415–1420. [Google Scholar] [CrossRef]
- Hashim, D.P.; Narayanan, N.T.; Romo-Herrera, J.M.; Cullen, D.A.; Hahm, M.G.; Lezzi, P.; Suttle, J.R.; Kelkhoff, D.; Munoz-Sandoval, E.; Ganguli, S.; et al. Covalently Bonded Three-Dimensional Carbon Nanotube Solids Via Boron Induced Nanojunctions. Sci. Rep. 2012, 2, 363. [Google Scholar] [CrossRef]
- Beshkar, F.; Khojasteh, H.; Salavati-Niasari, M. Recyclable Magnetic Superhydrophobic Straw Soot Sponge for Highly Efficient Oil/Water Separation. J. Colloid Interface Sci. 2017, 497, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.C.; Murthe, S.S.; Mohamed, N.M.; Perumal, V.; Saheed, M.S.M. Nanoscaled Surface Modification of Poly(dimethylsiloxane) Using Carbon Nanotubes for Enhanced Oil and Organic Solvent Absorption. ACS Omega 2018, 3, 15907–15915. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Scolaro, C.; Visco, A. Surface Modifications Induced in UHMWPE Based Nanocomposites during the Ageing in Simulated Synovial Fluid. Macromol. Symp. 2020, 389, 1900055. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Feng, S. Nonflammable and Magnetic Sponge Decorated with Polydimethylsiloxane Brush for Multitasking and Highly Efficient Oil-Water Separation. Adv. Funct. Mater. 2019, 29, 1902488. [Google Scholar] [CrossRef]
- Li, X.; Cao, M.; Shan, H.; Handan Tezel, F.; Li, B. Facile and Scalable Fabrication of Superhydrophobic and Superoleophilic PDMS-Co-PMHS Coating on Porous Substrates for Highly Effective Oil/Water Separation. Chem. Eng. J. 2019, 358, 1101–1113. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, X.; Li, L.; Zhao, B.; Ma, F.; Zhang, J. Polydopamine and Poly(dimethylsiloxane) Modified Superhydrophobic Fiberglass Membranes for Efficient Water-in-Oil Emulsions Separation. J. Colloid Interface Sci. 2020, 559, 178–185. [Google Scholar] [CrossRef]
- Abdelhafeez, I.A.; Zhou, X.; Yao, Q.; Yu, Z.; Gong, Y.; Chen, J. Multifunctional Edge-Activated Carbon Nitride Nanosheet-Wrapped Polydimethylsiloxane Sponge Skeleton for Selective Oil Absorption and Photocatalysis. ACS Omega 2020, 5, 4181–4190. [Google Scholar] [CrossRef]
- Meng, J.; Xie, J.; Han, X.; Lu, C. Surface Wrinkling on Polydopamine Film. Appl. Surf. Sci. 2016, 371, 96–101. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, J.; Ma, X.; Peng, X.; Qiu, Z.; Ying, J.; Wang, J. Smart PDMS Sponge with Switchable PH-Responsive Wetting Surface for Oil/Water Separation. N. J. Chem. 2017, 41, 8940–8946. [Google Scholar] [CrossRef]
- Kollarigowda, R.H.; Bhyrappa, H.M.; Cheng, G. Stimulus-Responsive Biopolymeric Surface: Molecular Switches for Oil/Water Separation. ACS Appl. Bio Mater. 2019, 2, 4249–4257. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, J.; Han, X.; Li, L.; Liu, E.; Lu, C. Carbon Nanotubes and Polydopamine Modified Poly(dimethylsiloxane) Sponges for Efficient Oil–Water Separation. Materials 2021, 14, 2431. https://doi.org/10.3390/ma14092431
Zhang W, Wang J, Han X, Li L, Liu E, Lu C. Carbon Nanotubes and Polydopamine Modified Poly(dimethylsiloxane) Sponges for Efficient Oil–Water Separation. Materials. 2021; 14(9):2431. https://doi.org/10.3390/ma14092431
Chicago/Turabian StyleZhang, Wen, Juanjuan Wang, Xue Han, Lele Li, Enping Liu, and Conghua Lu. 2021. "Carbon Nanotubes and Polydopamine Modified Poly(dimethylsiloxane) Sponges for Efficient Oil–Water Separation" Materials 14, no. 9: 2431. https://doi.org/10.3390/ma14092431
APA StyleZhang, W., Wang, J., Han, X., Li, L., Liu, E., & Lu, C. (2021). Carbon Nanotubes and Polydopamine Modified Poly(dimethylsiloxane) Sponges for Efficient Oil–Water Separation. Materials, 14(9), 2431. https://doi.org/10.3390/ma14092431





