A Pressure Swing Approach to Selective CO2 Sequestration Using Functionalized Hypercrosslinked Polymers
Abstract
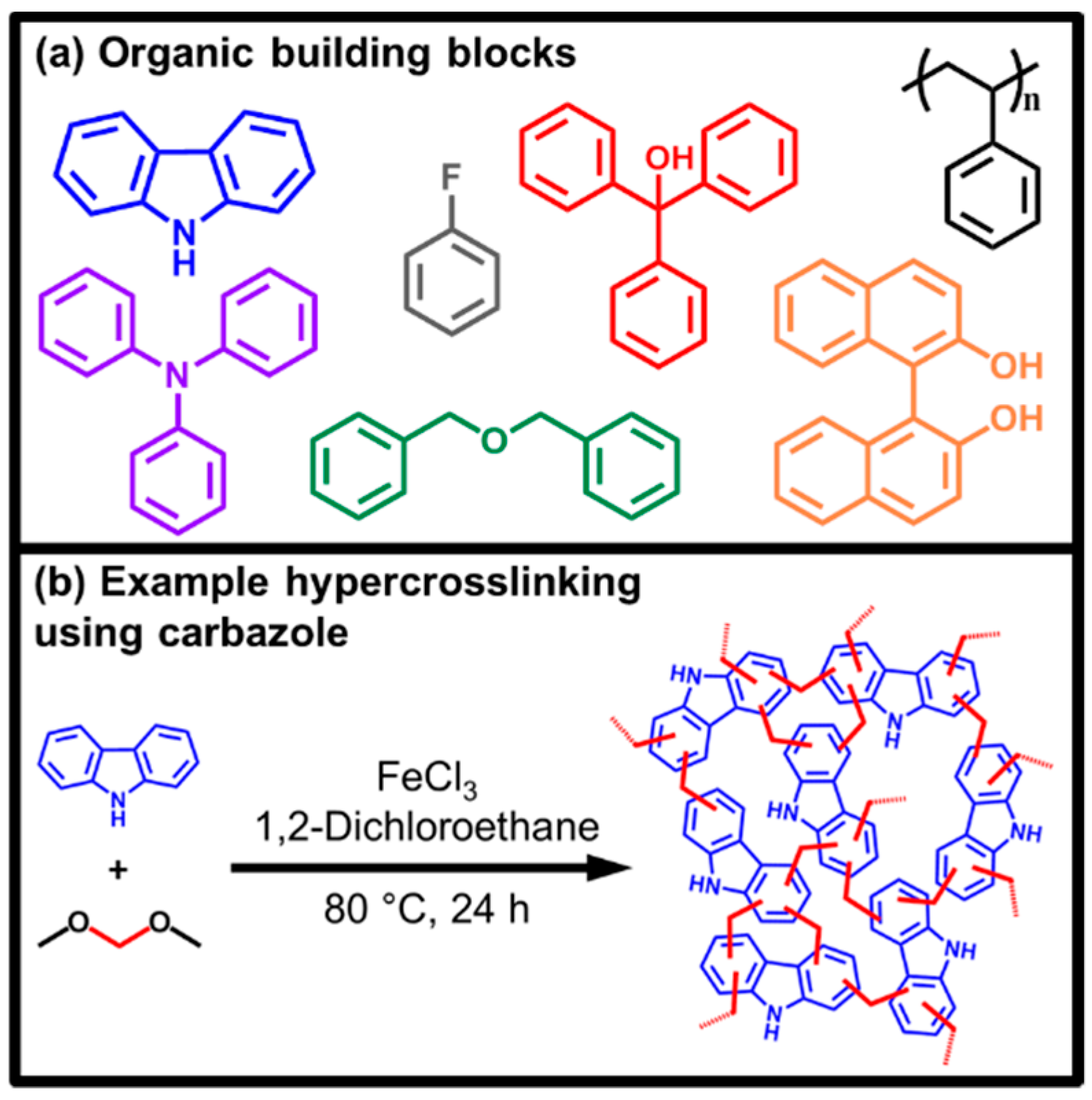
1. Introduction
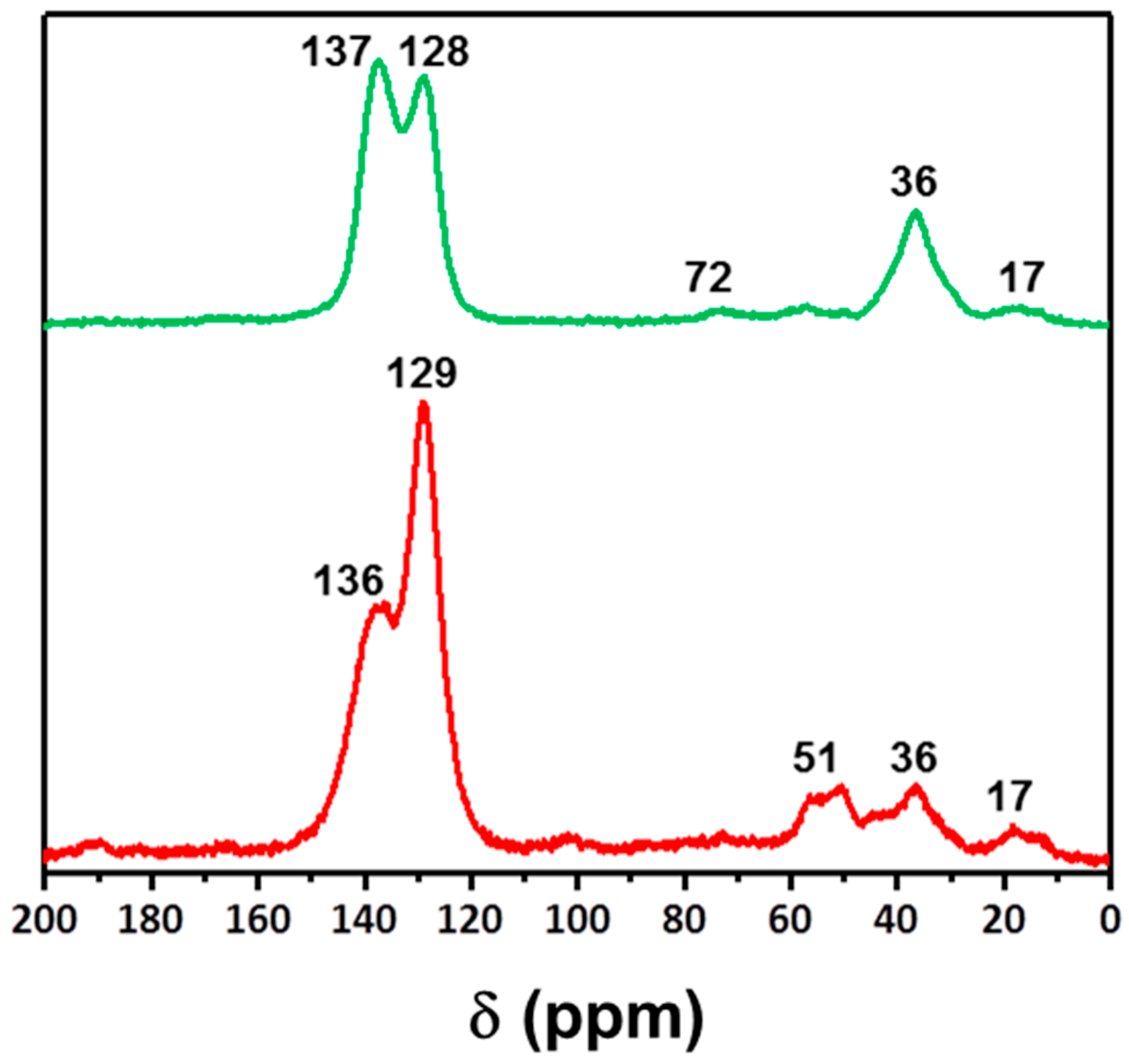
2. Results and Discussion
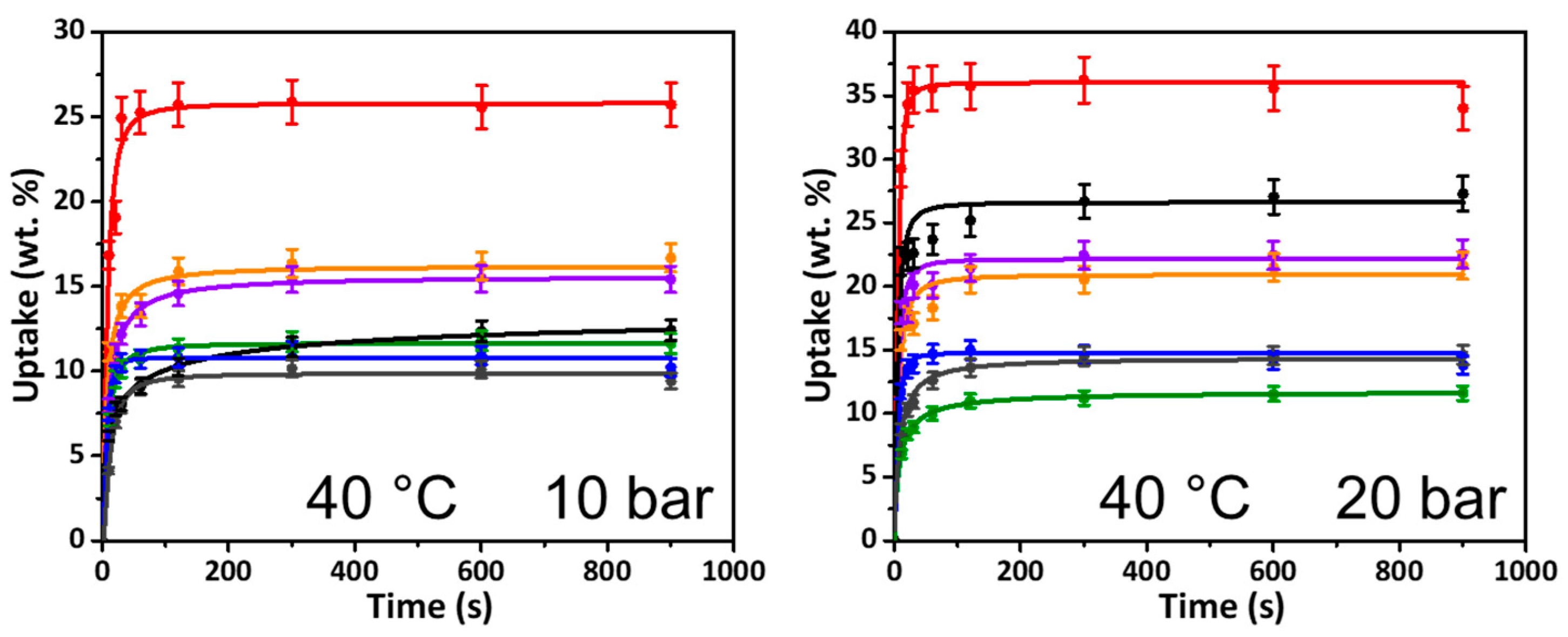
2.1. Kinetic Uptake of CO2
2.2. Selectivity Measurements
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. World Energy Outlook 2008; International Energy Agengy: Paris, France, 2008; Volume 23.
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Env. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Env. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Drage, T.C.; Snape, C.E.; Stevens, L.A.; Wood, J.; Wang, J.; Cooper, A.I.; Dawson, R.; Guo, X.; Satterley, C.; Irons, R. Materials challenges for the development of solid sorbents for post-combustion carbon capture. J. Mater. Chem. 2012, 22, 2815–2823. [Google Scholar] [CrossRef]
- Jung, D.; Chen, Z.; Alayoglu, S.; Mian, M.R.; Goetjen, T.A.; Idrees, K.B.; Kirlikovali, K.O.; Islamoglu, T.; Farha, O.K. Postsynthetically Modified Polymers of Intrinsic Microporosity (PIMs) for Capturing Toxic Gases. ACS Appl. Mater. Interfaces 2021, 13, 10409–10415. [Google Scholar] [CrossRef]
- Janeta, M.; Bury, W.; Szafert, S. Porous Silsesquioxane-Imine Frameworks as Highly Efficient Adsorbents for Cooperative Iodine Capture. ACS Appl. Mater. Interfaces 2018, 10, 19964–19973. [Google Scholar] [CrossRef]
- Janeta, M.; Lis, T.; Szafert, S. Zinc Imine Polyhedral Oligomeric Silsesquioxane as a Quattro-Site Catalyst for the Synthesis of Cyclic Carbonates from Epoxides and Low-Pressure CO2. Chem. A Eur. J. 2020, 26, 13686–13697. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Verheyen, T.V.; Adeloju, S.B.; Meuleman, E.; Feron, P. Towards commercial scale postcombustion capture of CO2 with monoethanolamine solvent: Key considerations for solvent management and environmental impacts. Environ. Sci. Technol. 2012, 46, 3643–3654. [Google Scholar] [CrossRef]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2017, 11, 57–70. [Google Scholar] [CrossRef]
- D’Alessandro, D.; Smit, B.; Long, J. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chemie Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Aschenbrenner, O.; Styring, P. Comparative study of solvent properties for carbon dioxide absorption. Energy Environ. Sci. 2010, 3, 1106–1113. [Google Scholar] [CrossRef]
- Schell, J.; Casas, N.; Marx, D.; Mazzotti, M. Precombustion CO2 Capture by Pressure Swing Adsorption (PSA): Comparison of Laboratory PSA Experiments and Simulations. Ind. Eng. Chem. Res. 2013, 52, 8311–8322. [Google Scholar] [CrossRef]
- Riboldi, L.; Bolland, O. Overview on Pressure Swing Adsorption (PSA) as CO2 Capture Technology: State-of-the-Art, Limits and Potentials. Energy Procedia 2017, 114, 2390–2400. [Google Scholar] [CrossRef]
- Borhan, A.; Yusuf, S. Activation of Rubber-Seed Shell Waste by Malic Acid as Potential CO2 Removal: Isotherm and Kinetics Studies. Materials 2020, 13, 4970. [Google Scholar] [CrossRef]
- Wang, G.; Graham, E.; Zheng, S.; Zhu, J.; Zhu, R.; He, H.; Sun, Z.; Mackinnon, I.D.R.; Xi, Y. Diatomite-Metal-Organic Framework Composite with Hierarchical Pore Structures for Adsorption/Desorption of Hydrogen, Carbon Dioxide and Water Vapor. Materials 2020, 13, 4700. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Shen, X.; Qiao, X.; Jia, L.; Xiang, J.; Jin, Y. Study on CO2 Capture Characteristics and Kinetics of Modified Potassium-Based Adsorbents. Materials 2020, 13, 877. [Google Scholar] [CrossRef]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Mueller, T.E. Worldwide Innovations in the Development of Carbon Capture Technologies and the Utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Ghalia, M.A.; Dahman, Y. Development and Evaluation of Zeolites and Metal-Organic Frameworks for Carbon Dioxide Separation and Capture. Energy Technol. 2017, 5, 356–372. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Dowson, G.R.M.; Reed, D.G.; Bellas, J.-M.; Charalambous, C.; Styring, P. Fast and selective separation of carbon dioxide from dilute streams by pressure swing adsorption using solid ionic liquids. Faraday Discuss. 2016, 192, 511–527. [Google Scholar] [CrossRef]
- Supasitmongkol, S.; Styring, P. High CO2 solubility in ionic liquids and a tetraalkylammonium- based poly(ionic liquid). Energy Environ. Sci. 2010, 3, 1961–1972. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.T.; Stevens, L.A.; Dawson, R.; Vijayaraghavan, M.; Hasell, T.; Silverwood, I.P.; Ewing, A.V.; Ratvijitvech, T.; Exley, J.D.; Chong, S.Y.; et al. Swellable, Water- and Acid-Tolerant Polymer Sponges for Chemoselective Carbon Dioxide Capture. J. Am. Chem. Soc. 2014, 136, 9028–9035. [Google Scholar] [CrossRef]
- Dawson, R.; Stevens, L.A.; Drage, T.C.; Snape, C.E.; Smith, M.W.; Adams, D.J.; Cooper, A.I. Impact of Water Coadsorption for Carbon Dioxide Capture in Microporous Polymer Sorbents. J. Am. Chem. Soc. 2012, 134, 10741–10744. [Google Scholar] [CrossRef]
- Martin, C.F.; Stöckel, E.; Clowes, R.; Adams, D.J.; Cooper, A.I.; Pis, J.J.; Rubiera, F.; Pevida, C. Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. J. Mater. Chem. 2011, 21, 5475–5483. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 2013, 62, 345–352. [Google Scholar] [CrossRef]
- Dawson, R.; Stöckel, E.; Holst, J.R.; Adams, D.J.; Cooper, A.I. Microporous organic polymers for carbon dioxide capture. Energy Environ. Sci. 2011, 4, 4239–4245. [Google Scholar] [CrossRef]
- Dawson, R.; Adams, D.J.; Cooper, A.I. Chemical tuning of CO2 sorption in robust nanoporous organic polymers. Chem. Sci. 2011, 2, 1173–1177. [Google Scholar] [CrossRef]
- Jiang, J.X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated microporous poly(aryleneethynylene) networks. Angew. Chemie Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef]
- Schmidt, J.; Werner, M.; Thomas, A. Conjugated Microporous Polymer Networks via Yamamoto Polymerization. Macromolecules 2009, 42, 4426–4429. [Google Scholar] [CrossRef]
- Woods, D.J.; Sprick, R.S.; Smith, C.L.; Cowan, A.J.; Cooper, A.I. A Solution-Processable Polymer Photocatalyst for Hydrogen Evolution from Water. Adv. Energy Mater. 2017, 1700479, 1700479. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheno, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An azine-linked covalent organic framework. J. Am. Chem. Soc. 2013, 46, 17310–17313. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chemie Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Esquivel, D.; Dey, S.; Fernandez-Teran, R.; Goto, Y.; Inagaki, S.; Van Der Voort, P.; Janiak, C. A photoluminescent covalent triazine framework: CO2 adsorption, light-driven hydrogen evolution and sensing of nitroaromatics. J. Mater. Chem. A 2016, 4, 13450–13457. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, W.; Zhang, W.; Huang, Q.; Yan, J.; Pan, C.; Yu, G. Sulfur-rich covalent triazine polymer nanospheres for environmental mercury removal and detection. Polym. Chem. 2018, 9, 4125–4131. [Google Scholar] [CrossRef]
- Trandafir, M.M.; Pop, L.; Hadade, N.D.; Florea, M.; Neațu, F.; Teodorescu, C.M.; Duraki, B.; van Bokhoven, J.A.; Grosu, I.; Parvulescu, V.I.; et al. An adamantane-based COF: Stability, adsorption capability, and behaviour as a catalyst and support for Pd and Au for the hydrogenation of nitrostyrene. Catal. Sci. Technol. 2016, 6, 8344–8354. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Parwaiz, S.; Lecoeuvre, F.; Schmidt, J.; Pradhan, D.; Van Der Voort, P. Illustrating the Role of Quaternary-N of BINOL Covalent Triazine-Based Frameworks in Oxygen Reduction and Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2020, 12, 44689–44699. [Google Scholar] [CrossRef]
- Yu, W.; Gu, S.; Fu, Y.; Xiong, S.; Pan, C.; Liu, Y.; Yu, G. Carbazole-decorated covalent triazine frameworks: Novel nonmetal catalysts for carbon dioxide fixation and oxygen reduction reaction. J. Catal. 2018, 362, 1–9. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 230–231. [Google Scholar] [CrossRef]
- McKeown, N.B. Polymers of Intrinsic Microporosity. ISRN Mater. Sci. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Memb. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Gray, E.M.; Webb, C.J. Review of polymers of intrinsic microporosity for hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 16944–16965. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, S.; Dai, Z.; Meng, X.; Xiao, F.-S. A hierarchical porous ionic organic polymer as a new platform for heterogeneous phase transfer catalysis. J. Mater. Chem. A 2015, 3, 23871–23875. [Google Scholar] [CrossRef]
- Deshmukh, A.; Bandyopadhyay, S.; James, A.; Patra, A. Trace level detection of nitroanilines using a solution processable fluorescent porous organic polymer. J. Mater. Chem. C 2016, 4, 4427–4433. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Xu, B.; Tong, H.; Wang, L. Solution-dispersed porous hyperbranched conjugated polymer nanoparticles for fluorescent sensing of TNT with enhanced sensitivity. Polym. Chem. 2014, 5, 4521–4525. [Google Scholar] [CrossRef]
- Luo, J.; Lu, J.; Zhang, J. Carbazole-triazine based donor-acceptor porous organic frameworks for efficient visible-light photocatalytic aerobic oxidation reactions. J. Mater. Chem. A 2018, 6, 15154–15161. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Kim, M.-R.; Kim, I. Sulfonic acid-functionalized, hyper-cross-linked porous polyphenols as recyclable solid acid catalysts for esterification and transesterification reactions. Ind. Eng. Chem. Res. 2018, 57, 11583–11591. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.J.; Wang, L.; Ma, B.C.; Ghasimi, S.; Lu, H.; Landfester, K.; Zhang, K.A.I. Photocatalytic Selective Bromination of Electron-Rich Aromatic Compounds Using Microporous Organic Polymers with Visible Light. ACS Catal. 2016, 6, 1113–1121. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Wang, C.; Laybourn, A.; Hasell, T.; Clowes, R.; Khimyak, Y.Z.; Xiao, J.; Higgins, S.J.; Adams, D.J.; Cooper, A.I. Metal–Organic Conjugated Microporous Polymers. Angew. Chemie Int. Ed. 2011, 50, 1072–1075. [Google Scholar] [CrossRef]
- Li, B.; Su, F.; Luo, H.-K.; Liang, L.; Tan, B. Hypercrosslinked microporous polymer networks for effective removal of toxic metal ions from water. Microporous Mesoporous Mater. 2011, 138, 207–214. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Xia, H.; Ding, X.; Luo, X.; Liu, X.; Mu, Y. Triarylboron-Linked Conjugated Microporous Polymers: Sensing and Removal of Fluoride Ions. Chem. A Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef]
- Yang, R.X.; Wang, T.T.; Deng, W.Q. Extraordinary Capability for Water Treatment Achieved by a Perfluorous Conjugated Microporous Polymer. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- James, A.M.; Harding, S.; Robshaw, T.; Bramall, N.; Ogden, M.D.; Dawson, R. Selective Environmental Remediation of Strontium and Cesium Using Sulfonated Hyper-Cross-Linked Polymers (SHCPs). ACS Appl. Mater. Interfaces 2019, 11, 22464–22473. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Wei, Z.; Zhou, H.-C. Polyamine-Tethered Porous Polymer Networks for Carbon Dioxide Capture from Flue Gas. Angew. Chemie Int. Ed. 2012, 51, 7480–7484. [Google Scholar] [CrossRef] [PubMed]
- Gulam, R.M.; Reich, T.E.; Kassab, R.; Jackson, K.T.; El-Kaderi, H.M. High CO2 Uptake and Selectivity by Triptycene-Derived Benzimidazole-Linked Polymers. Chem. Commun. 2012, 48, 1141–1143. [Google Scholar]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chemie 2009, 121, 9621–9624. [Google Scholar] [CrossRef]
- Yuan, D.; Lu, W.; Zhao, D.; Zhou, H.-C. Highly Stable Porous Polymer Networks with Exceptionally High Gas-Uptake Capacities. Adv. Mater. 2011, 23, 3723–3725. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Wang, G.; Yu, G.; Guan, J.; Pan, C.; Wang, Z. Control of porosity of novel carbazole-modified polytriazine frameworks for highly selective separation of CO2-N2. J. Mater. Chem. A 2014, 2, 7795–7801. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Xu, S.; Luo, Y.; Tan, B. Recent development of hypercrosslinked microporous organic polymers. Macromol. Rapid Commun. 2013, 34, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Turner, S.R. Hypercrosslinked Polymers: A Review. Polym. Rev. 2018, 58, 1–41. [Google Scholar] [CrossRef]
- Fontanals, N.; Cortés, J.; Galià, M.; Maria Marcé, R.; Cormack, P.A.G.; Borrull, F.; Sherrington, D.C. Synthesis of Davankov-type hypercrosslinked resins using different isomer compositions of vinylbenzyl chloride monomer, and application in the solid-phase extraction of polar compounds. J. Polym. Sci. Part. A Polym. Chem. 2005, 43, 1718–1728. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wood, C.D.; Bradshaw, D.; Rosseinsky, M.J.; Cooper, A.I. Hydrogen adsorption in microporous hypercrosslinked polymers. Chem. Commun. 2006, 25, 2670–2672. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu, C.; Tan, B. A New Strategy to Microporous Polymers: Knitting Rigid Aromatic Building Blocks by External Cross-Linker. Macromolecules 2011, 44, 2410–2414. [Google Scholar] [CrossRef]
- Wilson, C.; Main, M.J.; Cooper, N.J.; Briggs, M.E.; Cooper, A.I.; Adams, D.J. Swellable functional hypercrosslinked polymer networks for the uptake of chemical warfare agents. Polym. Chem. 2017, 8, 1914–1922. [Google Scholar] [CrossRef]
- Yang, X.; Yu, M.; Zhao, Y.; Zhang, C.; Wang, X.; Jiang, J.X. Hypercrosslinked microporous polymers based on carbazole for gas storage and separation. RSC Adv. 2014, 4, 61051–61055. [Google Scholar] [CrossRef]
- Tan, L.; Li, B.; Yang, X.; Wang, W.; Tan, B. Knitting hypercrosslinked conjugated microporous polymers with external crosslinker. Polymer 2015, 70, 336–342. [Google Scholar] [CrossRef]
- Dawson, R.; Ratvijitvech, T.; Corker, M.; Laybourn, A.; Khimyak, Y.Z.; Cooper, A.I.; Adams, D.J. Microporous copolymers for increased gas selectivity. Polym. Chem. 2012, 3, 2034–2038. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2017, 5, 1334–1347. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 1488. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, W.; You, Q.; Men, B.; Liao, G.; Wang, D. A green and low-cost strategy to synthesis of tunable pore sizes porous organic polymers derived from waste-expanded polystyrene for highly efficient removal of organic contaminants. Chem. Eng. J. 2019, 370, 251–261. [Google Scholar] [CrossRef]
- Ratvijitvech, T.; Barrow, M.; Cooper, A.I.; Adams, D.J. The effect of molecular weight on the porosity of hypercrosslinked polystyrene. Polym. Chem. 2015, 6, 7280–7285. [Google Scholar] [CrossRef]
- Zhu, X.; Mahurin, S.M.; An, S.-H.; Do-Thanh, C.-L.; Tian, C.; Li, Y.; Gill, L.W.; Hagaman, E.W.; Bian, Z.; Zhou, J.-H.; et al. Efficient CO2 capture by a task-specific porous organic polymer bifunctionalized with carbazole and triazine groups. Chem. Commun. 2014, 50, 7933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tao, L.; Wang, Q.; Wang, T. A facile synthesis of cost-effective triphenylamine-containing porous organic polymers using different crosslinkers. Polymers 2016, 82, 114–120. [Google Scholar] [CrossRef]
- Reed, D.G.; Dowson, G.R.M.; Styring, P. Cellulose-Supported Ionic Liquids for Low-Cost Pressure Swing CO2 Capture. Front. Energy Res. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Yao, S.; Yang, X.; Yu, M.; Zhang, Y.; Jiang, J.-X. High surface area hypercrosslinked microporous organic polymer networks based on tetraphenylethylene for CO2 capture. J. Mater. Chem. A 2014, 2, 8054–8059. [Google Scholar] [CrossRef]
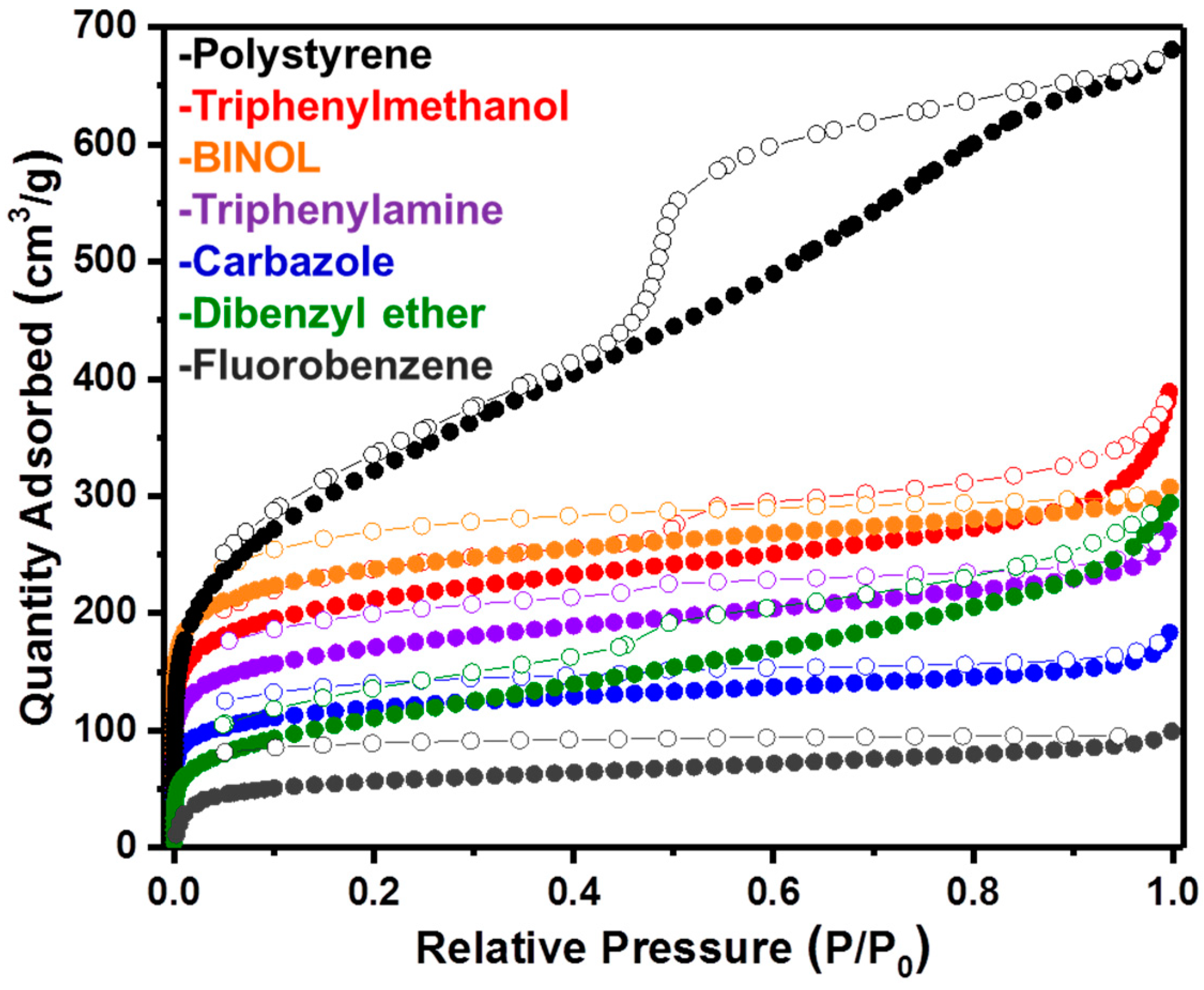


| Network | SABET (m2/g) a | Vtot (cm3/g) b | V0.1 (cm3/g) c | V0.1/Vtot |
|---|---|---|---|---|
| Poly(styrene) | 1124 | 1.01 | 0.42 | 0.42 |
| Triphenylmethanol | 781 | 0.48 | 0.30 | 0.63 |
| BINOL | 888 | 0.45 | 0.35 | 0.70 |
| Dibenzyl ether | 397 | 0.39 | 0.14 | 0.36 |
| Carbazole | 445 | 0.24 | 0.17 | 0.71 |
| Triphenylamine | 630 | 0.37 | 0.24 | 0.65 |
| Fluorobenzene | 213 | 0.14 | 0.10 | 0.71 |
| Network | CO2 Uptake (wt.%) | N2 Uptake (wt.%) | ||
|---|---|---|---|---|
| 10 bar | 20 bar | 10 bar | 20 bar | |
| Poly(styrene) | 15.59 | 24.94 | 1.20 | 2.79 |
| Triphenylmethanol | 15.25 | 28.91 | 2.20 | 6.13 |
| BINOL | 10.16 | 18.64 | 5.92 | 8.87 |
| Dibenzyl ether | 6.84 | 10.82 | 0.93 | 1.31 |
| Carbazole | 17.44 | 22.41 | 2.48 | 4.41 |
| Triphenylamine | 13.75 | 21.04 | 1.45 | 2.98 |
| Fluorobenzene | 6.86 | 11.89 | 0.82 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, A.M.; Reynolds, J.; Reed, D.G.; Styring, P.; Dawson, R. A Pressure Swing Approach to Selective CO2 Sequestration Using Functionalized Hypercrosslinked Polymers. Materials 2021, 14, 1605. https://doi.org/10.3390/ma14071605
James AM, Reynolds J, Reed DG, Styring P, Dawson R. A Pressure Swing Approach to Selective CO2 Sequestration Using Functionalized Hypercrosslinked Polymers. Materials. 2021; 14(7):1605. https://doi.org/10.3390/ma14071605
Chicago/Turabian StyleJames, Alex M., Jake Reynolds, Daniel G. Reed, Peter Styring, and Robert Dawson. 2021. "A Pressure Swing Approach to Selective CO2 Sequestration Using Functionalized Hypercrosslinked Polymers" Materials 14, no. 7: 1605. https://doi.org/10.3390/ma14071605
APA StyleJames, A. M., Reynolds, J., Reed, D. G., Styring, P., & Dawson, R. (2021). A Pressure Swing Approach to Selective CO2 Sequestration Using Functionalized Hypercrosslinked Polymers. Materials, 14(7), 1605. https://doi.org/10.3390/ma14071605








