Microtensile Bond Strength of Fiber-Reinforced and Particulate Filler Composite to Coronal and Pulp Chamber Floor Dentin
Abstract
1. Introduction
2. Materials and Methods
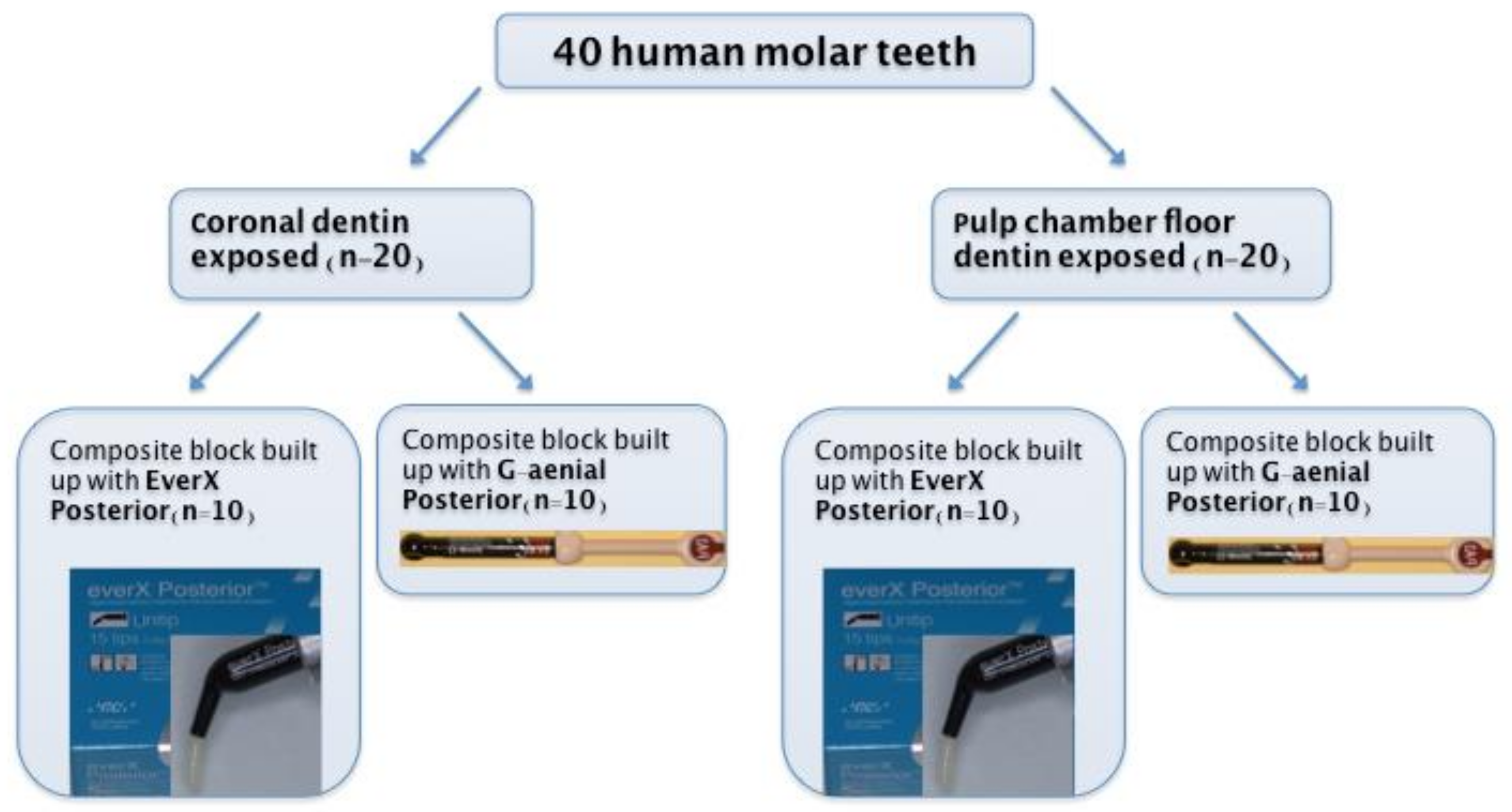
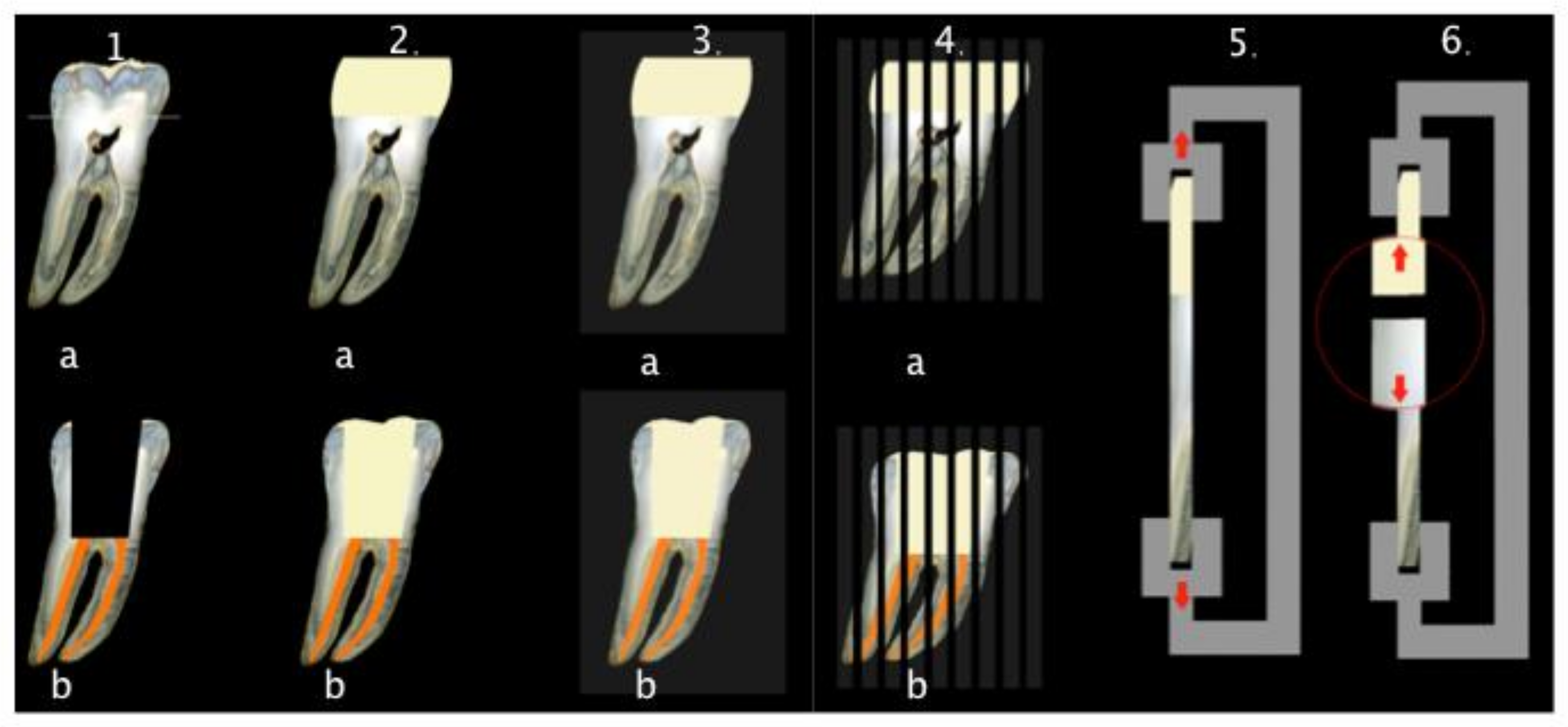
2.1. Specimen Preparation
2.2. Microtensile Bond Strength Test
2.3. Statistical Analysis
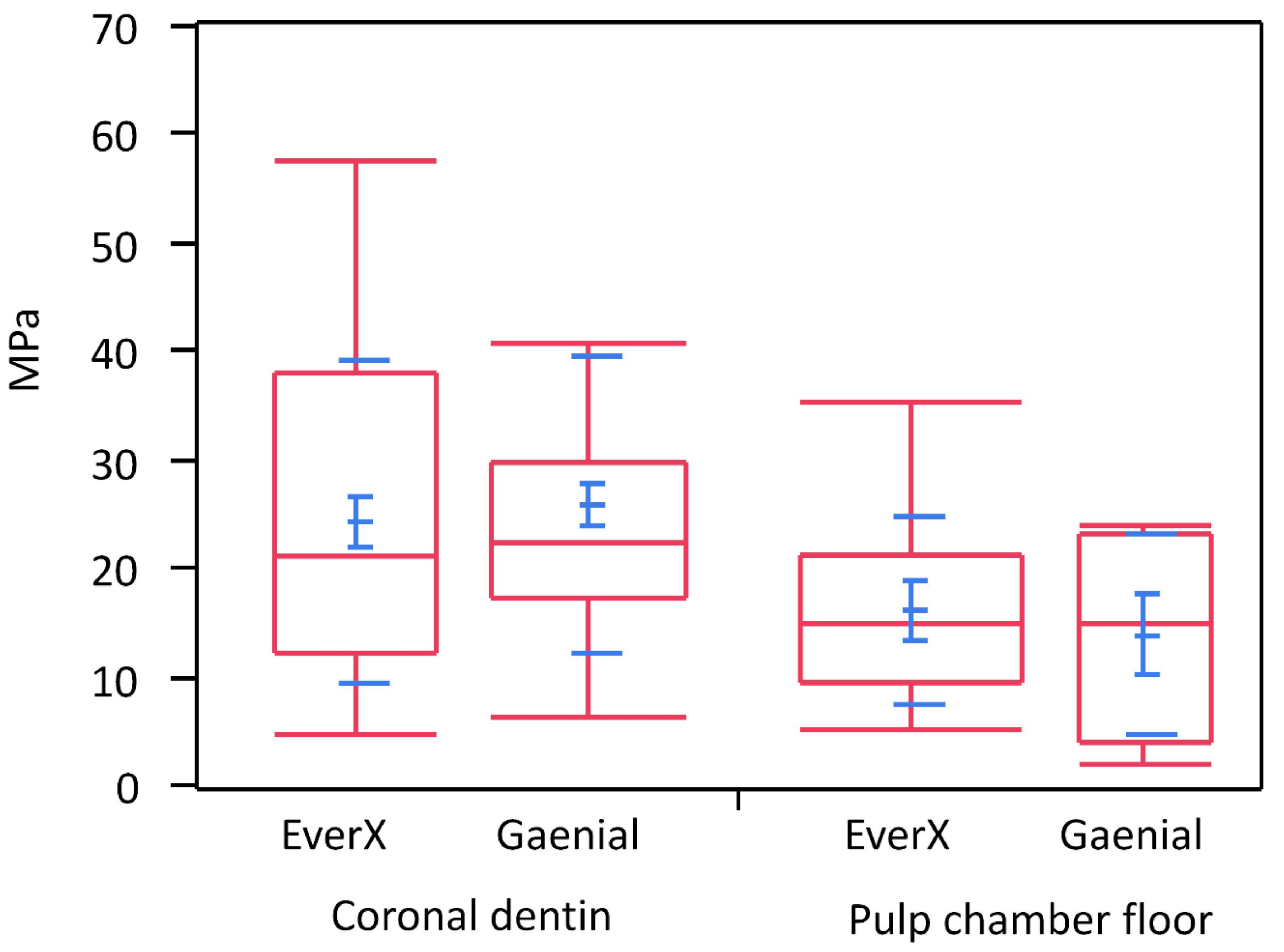
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.L.; Chang, C.H.; Ko, C.C. Multifactorial analysis of an MOD restored human premolar using auto-mesh finite element approach. J. Oral Rehabil. 2001, 28, 576–585. [Google Scholar] [CrossRef]
- Rao, M.S.; Shameem, A.; Nair, R.; Ghanta, S.; Thankachan, R.P.; Issac, J.K. Comparison of the remaining dentin thickness in the root after hand and four rotary instrumentation techniques: An in vitro study. J. Contemp. Dent. Pract. 2013, 14, 712–717. [Google Scholar]
- Panitvisai, P.; Messer, H.H. Cuspal deflection in molars in relation to endodontic and restorative procedures. J. Endod. 1995, 21, 57–61. [Google Scholar] [CrossRef]
- Kijsamanmith, K.; Timpawat, S.; Harnirattisai, C.; Messer, H.H. Micro-tensile bond strengths of bonding agents to pulpal floor dentine. Int. Endod. J. 2002, 35, 833–839. [Google Scholar] [CrossRef]
- Lenzi, T.L.; Guglielmi, C.A.B.; Arana-Chavez, V.E.; Raggio, D.P. Tubule density and diameter in coronal dentin from primary and permanent human teeth. Microsc. Microanal. 2013, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aslantas, E.E.; Buzoglu, H.D.; Altundasar, E.; Serper, A. Effect of EDTA, sodium hypochlorite, and chlorhexidine gluconate with or without surface modifiers on dentin microhardness. J. Endod. 2014, 40, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Lovasz, B.V.; Bihari, E.; Krajczár, K.; Jeges, S.; Tóth, Á.; Szalma, J. Long-term clinical evaluation of direct resin composite restorations in vital vs. endodontically treated posterior teeth—Retrospective study up to 13 years. Dent. Mater. 2019, 35, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Alshabib, A.; Silikas, N.; Watts, D.C. Hardness and fracture toughness of resin-composite materials with and without fibers. Dent. Mater. 2019, 35, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Garoushi, S.; Gargoum, A.; Vallittu, P.K.; Lasilla, L. Short fiber-reinforced composite restorations: A review of the current literature. J. Investig. Clin. Dent. 2018, 9, e12330. [Google Scholar] [CrossRef]
- Bijelic-Donova, J.; Garoushi, S.; Vallittu, P.K.; Lassila, L.V. Mechanical properties, fracture resistence, and fatigue limits of short fiber reinforced composite resin. J. Prosthet. Dent. 2016, 115, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Garoushi, S.; Vallittu, P.K.; Lassila, L.V. Direct restoration of severely damaged incisors using short fiber-reinforced composite resin. J. Dent. 2007, 35, 731–736. [Google Scholar] [CrossRef]
- Garlapati, T.G.; Krithikadatta, J.; Natanasabapathy, V. Fracture resistance of endodontically treated teeth restored with short fiber composite used as a core material—An in vitro study. J. Prosthodont. Res. 2017, 61, 464–470. [Google Scholar] [CrossRef]
- Ozsevik, A.S.; Yildrim, C.; Aydin, U.; Culha, E.; Surmelioglu, D. Effect of fiber-reinforced composite on the fracture resistence of endodontically treated teeth. Aust. Endod. J. 2016, 42, 82–87. [Google Scholar] [CrossRef]
- Arbildo-Vega, H.I.; Lapinska, B.; Panda, S.; Lamas-Lara, C.; Khan, A.S.; Lukomska-Szymanska, M. Clinical Effectiveness of Bulk-Fill and Conventional Resin Composite Restorations: Systematic Review and Meta-Analysis. Polymer 2020, 12, 1786. [Google Scholar] [CrossRef]
- Chesterman, J.; Jowett, A.; Gallacher, A.; Nixon, P. Bulk-fill resin-based composite restorative materials: A review. Br. Dent. J. 2017, 222, 337–344. [Google Scholar] [CrossRef]
- Schreiner, R.F.; Chappell, R.P.; Glaros, A.G.; Eick, J.D. Micro-tensile testing of dentin adhesives. Dent. Mater. 1998, 14, 194–201. [Google Scholar] [CrossRef]
- Tanumiharja, M.; Burrow, M.F.; Tyas, M.J. Micro-tensile bond strengths of seven dentin adhesive systems. Dent. Mater. 2007, 16, 180–187. [Google Scholar] [CrossRef]
- Marshall, G.W.; Marshall, S.J.; Kinney, J.H.; Balooch, M. The dentin substrate: Structure and properties related to bonding. J. Dent. 1997, 25, 441–458. [Google Scholar] [CrossRef]
- Pashley, D.H.; Carvalho, R.M. Dentine permeability and dentine adhesion. J. Dent. 1997, 25, 355–372. [Google Scholar] [CrossRef]
- Schellenberg, U.; Krey, G.; Bosshardt, D.; Nair, P. Numerical density of dentinal tubules at the pulpal wall of human permanent premolars and third molars. J. Endod. 1992, 18, 104–109. [Google Scholar] [CrossRef]
- Gwinnett, A.J. Quantitative contribution of resin infiltration/hybridization to dentin bonding. Am. J. Dent. 1993, 6, 7–9. [Google Scholar]
- Pashley, D.H.; Sano, H.; Ciucchi, B.; Carvalho, R.M.; Russell, C.M. Bond strength versus dentin structures: A modeling approach. Arch. Oral Biol. 1995, 40, 110991118. [Google Scholar] [CrossRef]
- Lohbauer, U.; Nikolaenko, S.A.; Petschelt, A.; Frankenberg, R. Resin tags do not contribute to dentin adhesion in self-etching adhesives. J. Adhes. Dent. 2008, 10, 97–103. [Google Scholar]
- Haapasalo, M.; Endal, U.; Zandi, H.; Coil, J.M. Eradication of endodontic infection by instrumentation and irrigation solutions. Endodontics 2005, 10, 77–102. [Google Scholar] [CrossRef]
- Peters, O.A.; Schönenberger, K.; Laib, A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int. Endod. J. 2001, 34, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in endodontics. Dent. Clin. N. Am. 2010, 54, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.C.; Mak, Y.F.; Cheung, G.S.; Osorio, R.; Toledano, M.; Carvalho, R.M.; Tay, F.R.; Pashley, D.H. Reversal of compromised bonding to oxidized etched dentin. J. Dent. Res. 2001, 80, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.S.; Huang, X.Q.; Griffin, B.; Bergeron, B.R.; Pashley, D.H.; Niu, L.; Tay, F.R. Primum non nocere—The effects of sodium hypochlorite on dentin as used in endodontics. Acta Biomater. 2017, 61, 144–156. [Google Scholar] [CrossRef]
- Sim, T.P.; Knowles, J.C.; Ng, Y.L.; Shelton, J.; Gulaivala, K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int. Endod. J. 2001, 34, 120–132. [Google Scholar] [CrossRef]
- Jacques, P.; Hebling, J. Effect of dentin conditioners on the microtensile bond strength of a conventional and a self-etching primer adhesive system. Dent. Mater. 2005, 21, 103–109. [Google Scholar] [CrossRef]
- Shafier, F.; Memarpour, M. Effect of EDTA conditioning on microleakage of four adhesive systems in composite restorations. J. Dent. 2008, 5, 150–155. [Google Scholar]
- Kasraei, S.; Azarsina, M.; Khamverdi, Z. Effect of Ethylene diamine tetra acetic acid and sodium hypochlorite solution conditioning on microtensile bond strength of one-step self-etch adhesive. J. Conserv. Dent. 2013, 16, 243–246. [Google Scholar]
- Torri, Y.; Hikasa, R.; Iwate, S.; Oyama, F.; Itou, K.; Yoshiyama, M. Effect of EDTA conditioning on bond strength to bovine dentin promoted by four current adhesives. Am. J. Dent. 2003, 16, 395–400. [Google Scholar]
- Soares, C.J.; Castro, C.G.; Santos Filho, P.C.; da Mota, A.S. Effect of previous treatments on bond strength of two self-etching adhesive systems to dental substrate. J. Adhes. Dent. 2007, 9, 291–296. [Google Scholar]
- Abbas, G.; Fleming, G.J.; Harrington, E.; Shortell, A.C.; Burke, F.J. Cuspal movement and microleakage in premolar teeth restored with a packable composite cured in bulk or in increments. J. Dent. 2003, 31, 437–444. [Google Scholar] [CrossRef]
- Goncu Basaran, E.; Ayna, E.; Uctasli, S.; Vallittu, P.K.; Lassila, L.V. Load-bearing capacity of fiber reinforced fixed composite bridges. Acta Odontol. Scand. 2013, 71, 65–71. [Google Scholar] [CrossRef]
- Vallittu, P.K. High-aspect ratio fillers: Fiber-reinforced composites and their anisotropic properties. Dent. Mater. 2015, 31, 1–7. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Watts, D.C.; Lassila, L.V. Polymerization shrinkage of experimental short glass fiber-reinforced composite with semi-inter penetrating polymer network matrix. Dent. Mater. 2008, 24, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Sfondrini, M.F.; Cacciafesta, V.; Scribante, A. Shear bond strength of fiber-reinforced composite nets using two different ad- hesive systems. Eur. J. Orthod. 2011, 33, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Kessler, A.; Durner, J. Influence of various irradiation processes on the mechanical properties and polymerisation kinetics of bulk-fill resin based composites. J. Dent. 2013, 41, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A.; Asmussen, E. Determination of in vitro gap formation of resin composites. J. Dent. 2004, 32, 109–115. [Google Scholar] [CrossRef]
- Dehoff, P.H.; Anusavice, K.J.; Wang, Z. Three dimensional finite analysis of the shear bond test. Dent. Mater. 1995, 11, 126–131. [Google Scholar] [CrossRef]
- Amussen, E.; Peutzdeldt, A. Resin composites: Strength of the bond to dentin versus surface free energy parameters. Dent. Mater. 2005, 21, 1039–1043. [Google Scholar] [CrossRef]
- Steiner, R.; Edelhoff, D.; Stawarczyk, B.; Dumfahrt, H.; Lente, I. Effect of dentin bonding agents, various resin composites and curing modes on bond strength to human dentin. Materials 2019, 12, 3395. [Google Scholar] [CrossRef] [PubMed]
- Cekic, I.; Ergun, G.; Uctsdli, S.; Lassila, L.V. In vitro evaluation of push-out bond strength of direct ceramic inlays to tooth surface with fiber-reinforced composite at the interface. J. Prosthet. Dent. 2007, 97, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Bonding performance and interfacial characteristics of short fiber-reinforced resin composite in comparison with other composite restoratives. Eur. J. Oral Sci. 2016, 124, 301–308. [Google Scholar] [CrossRef]
- Freedman, G. Contemporary Aesthetic Dentistry; Mosby: St. Louis, MI, USA; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Garoushi, S.; Sailynoja, A.; Vallittu, P.K.; Lsassila, L. Physical properties and depth of cure of a new short fiber reinforced composite. Dent. Mater. 2013, 29, 835–841. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, S.H. Comparison of polymerization shrinkage, physical properties, and marginal adaptation of flowable and restorative bulk fill resin-based composites. Oper. Dent. 2017, 42, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Fernandes, C.; Tay, F. The microtensile bond test: A review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar]
- Neves Ade, A.; Coutinho, E.; Cardoso, M.V.; Jaecques, S.; Lambrechts, P.; Sloten, J.V.; van Oosterwyck, H.; van Meerbeek, B. Influence of notch geometry and interface on stress concentration and distribution in micro-tensile bond strength specimens. J. Dent. 2008, 36, 808–815. [Google Scholar] [CrossRef]

| Material | Manufacturer | Composition |
|---|---|---|
| EverX Posterior (test) | GC, Tokyo, Japan | Bis-GMA, PMMA, TEGDMA, 74.2 wt%, 53.6 vol% short E-glass fibers, barium glass |
| G-aenial Posterior (control) | GC, Tokyo, Japan | UDMA, dimethacrylate-comonomers, 77 wt%, 65 vol% pre-polymerized silica/lanthanoid fluride fluoraluminosilicate/silica |
| Source | Nparm | Sum of Squares | F Ratio | Prob > F |
|---|---|---|---|---|
| Dentin location | 1 | 1258.7711 | 6.9517 | 0.0098 * |
| Composite | 1 | 0.3628 | 0.0020 | 0.9644 |
| Dentin location*Composite | 1 | 32.6946 | 0.1806 | 0.6719 |
| Level | Mean | Std Dev | Std Err Mean |
|---|---|---|---|
| Coronal, EverX | 22.9089 | 14.6572 | 2.3777 |
| Coronal, Gaenial | 24.4409 | 13.7245 | 2.0019 |
| Pulp, EverX | 13.9967 | 5.8355 | 1.9452 |
| Pulp, Gaenial | 12.1040 | 8.8892 | 3.9754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraba, A.; Cimic, S.; Basso, M.; Ionescu, A.C.; Brambilla, E.; Miletić, I. Microtensile Bond Strength of Fiber-Reinforced and Particulate Filler Composite to Coronal and Pulp Chamber Floor Dentin. Materials 2021, 14, 2400. https://doi.org/10.3390/ma14092400
Baraba A, Cimic S, Basso M, Ionescu AC, Brambilla E, Miletić I. Microtensile Bond Strength of Fiber-Reinforced and Particulate Filler Composite to Coronal and Pulp Chamber Floor Dentin. Materials. 2021; 14(9):2400. https://doi.org/10.3390/ma14092400
Chicago/Turabian StyleBaraba, Anja, Samir Cimic, Matteo Basso, Andrei C. Ionescu, Eugenio Brambilla, and Ivana Miletić. 2021. "Microtensile Bond Strength of Fiber-Reinforced and Particulate Filler Composite to Coronal and Pulp Chamber Floor Dentin" Materials 14, no. 9: 2400. https://doi.org/10.3390/ma14092400
APA StyleBaraba, A., Cimic, S., Basso, M., Ionescu, A. C., Brambilla, E., & Miletić, I. (2021). Microtensile Bond Strength of Fiber-Reinforced and Particulate Filler Composite to Coronal and Pulp Chamber Floor Dentin. Materials, 14(9), 2400. https://doi.org/10.3390/ma14092400








