Insight towards Nucleation Mechanism and Change in Morphology for Nanostructured Platinum Thin Film Directly Grown on Carbon Substrate via Electrochemical Deposition
Abstract
1. Introduction
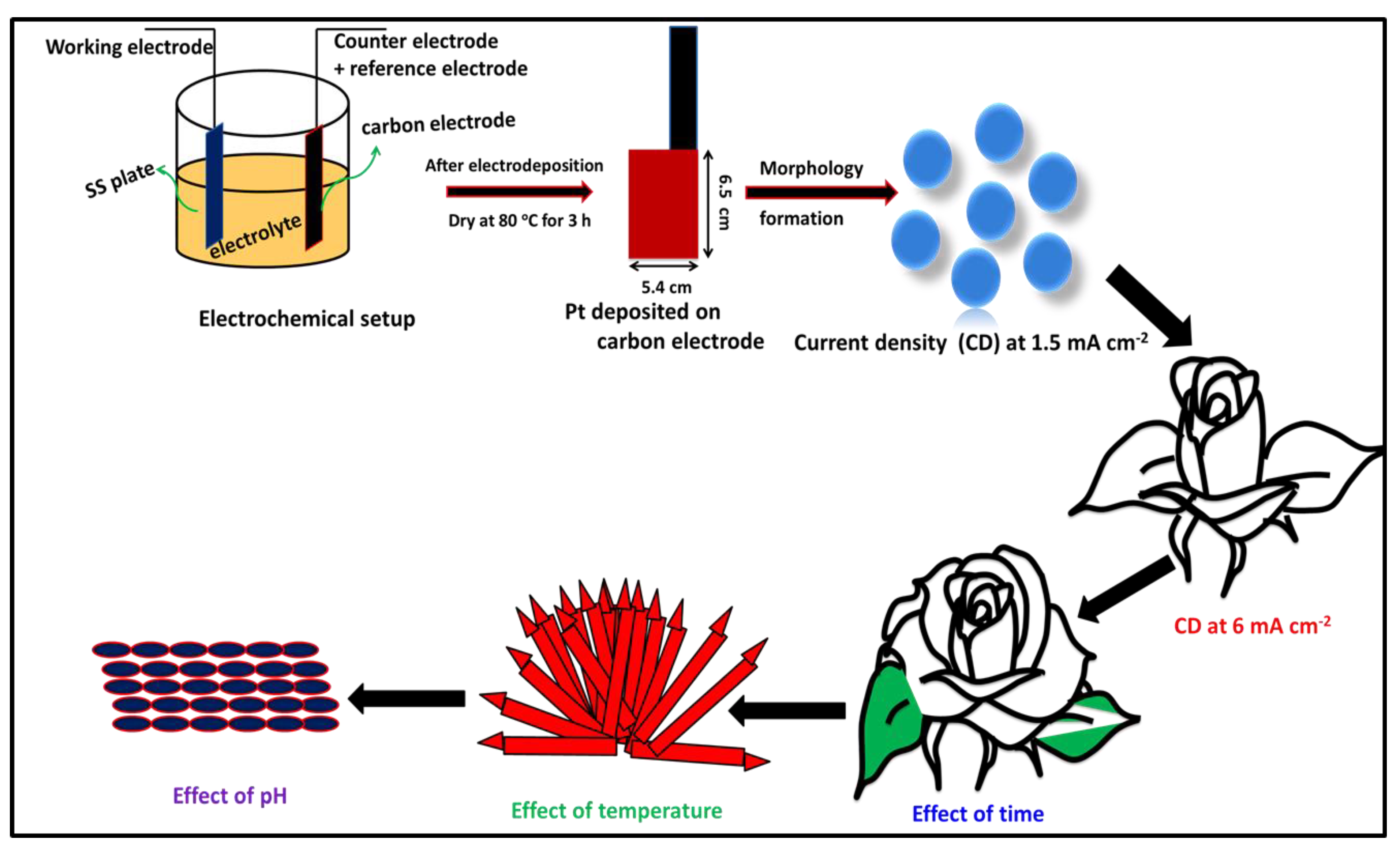
2. Experimental Section
2.1. Electrodeposition of Pt Metal Nanoparticles
2.2. Materials Characterization
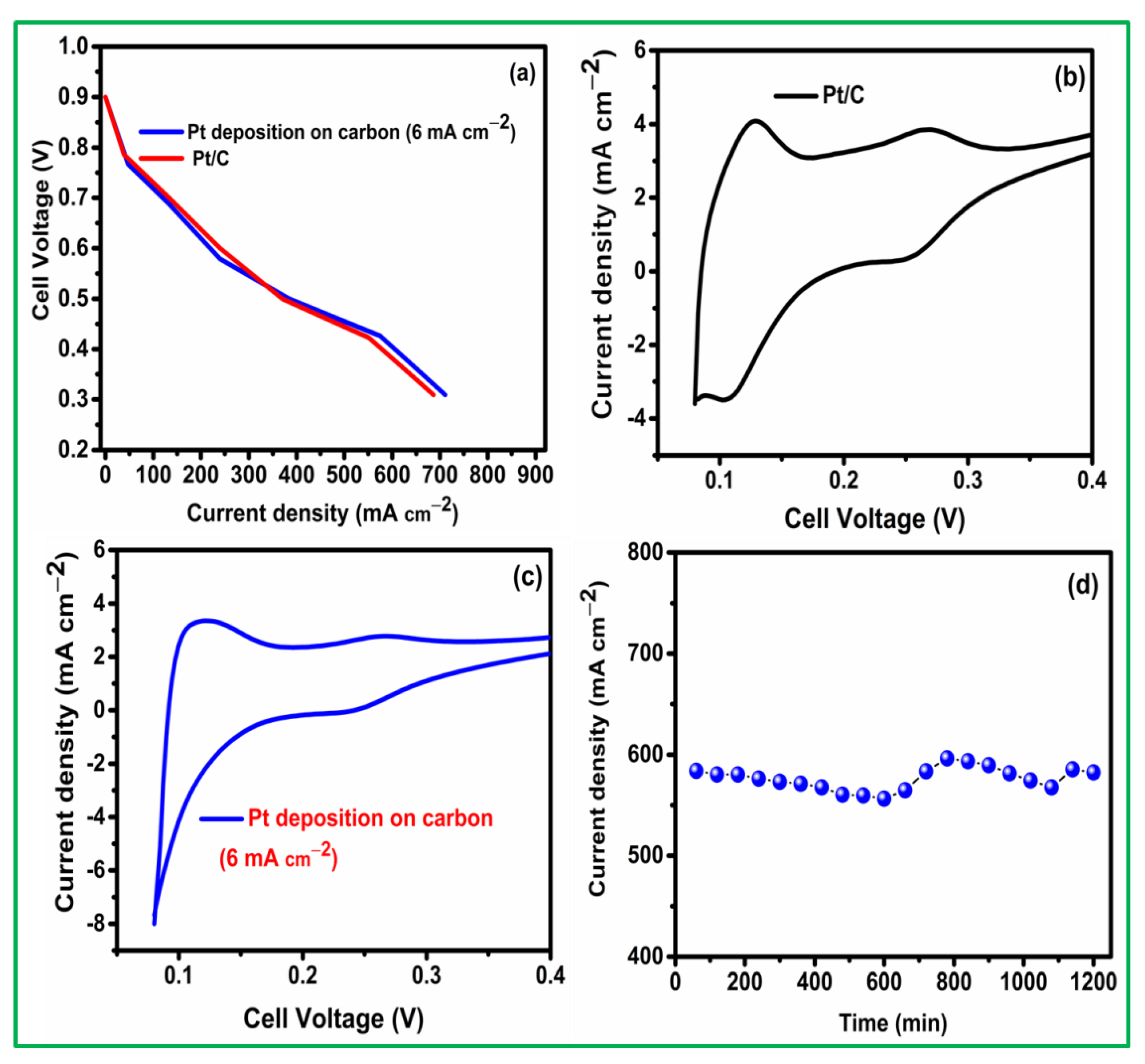
3. Results and Discussion
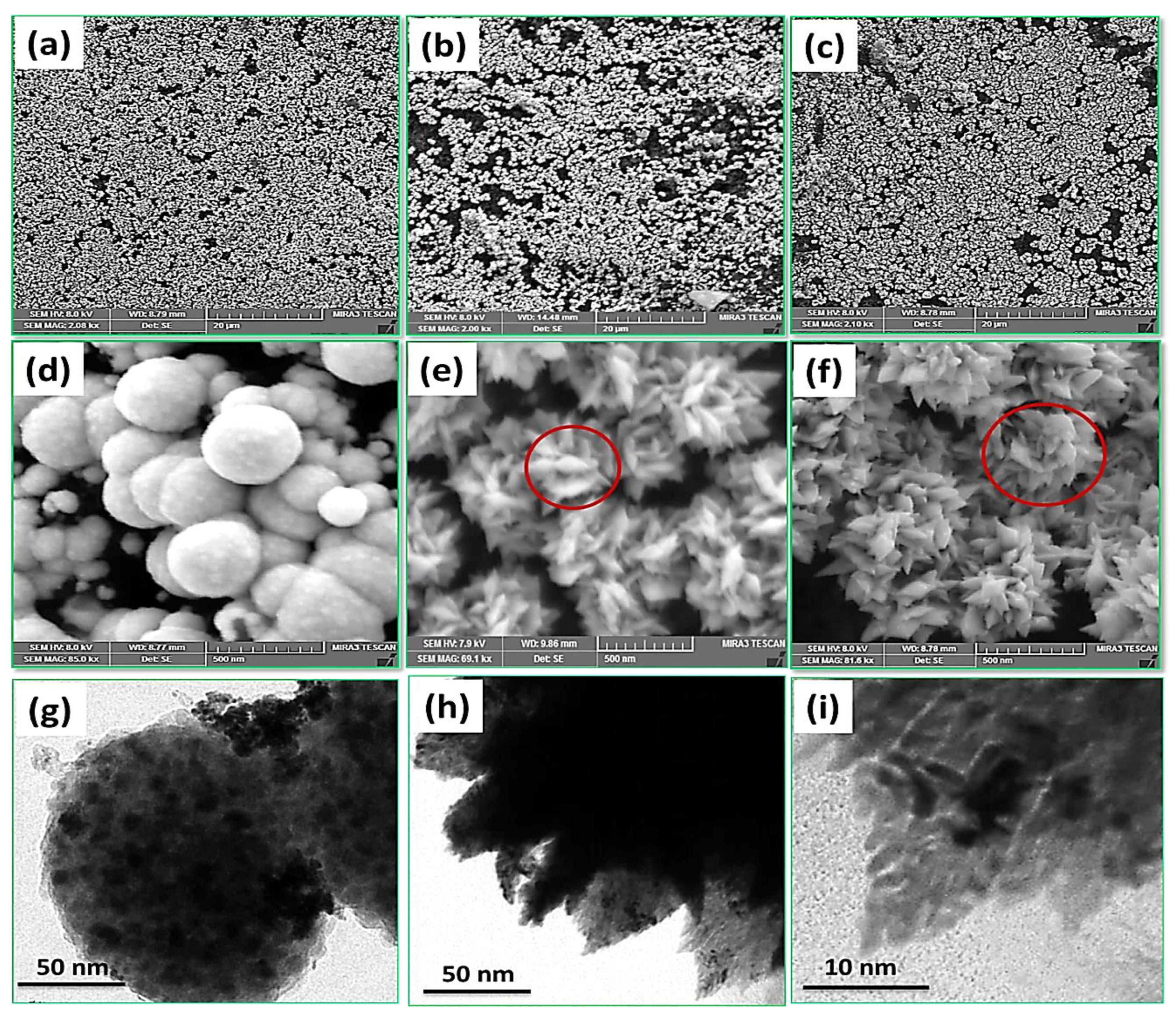
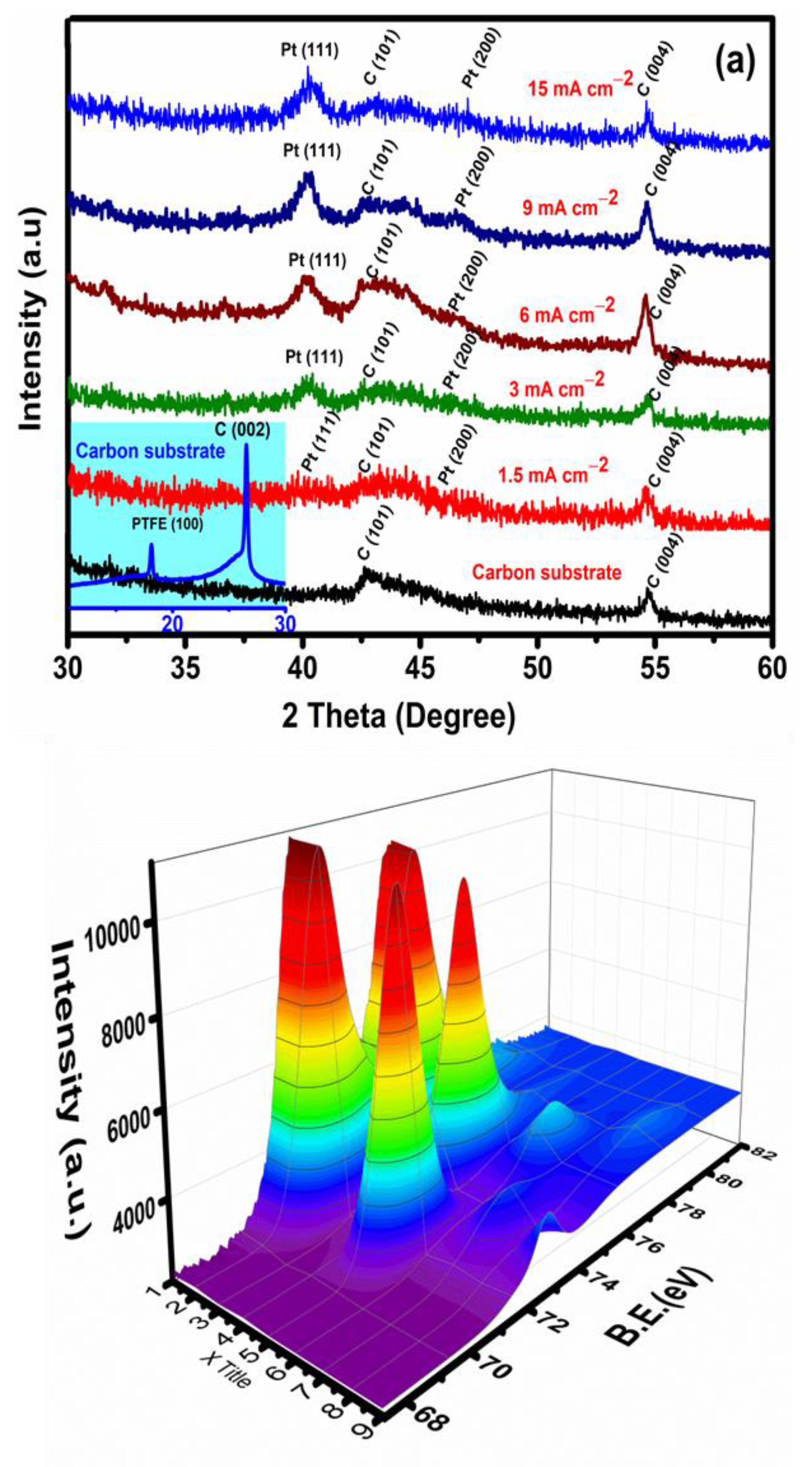
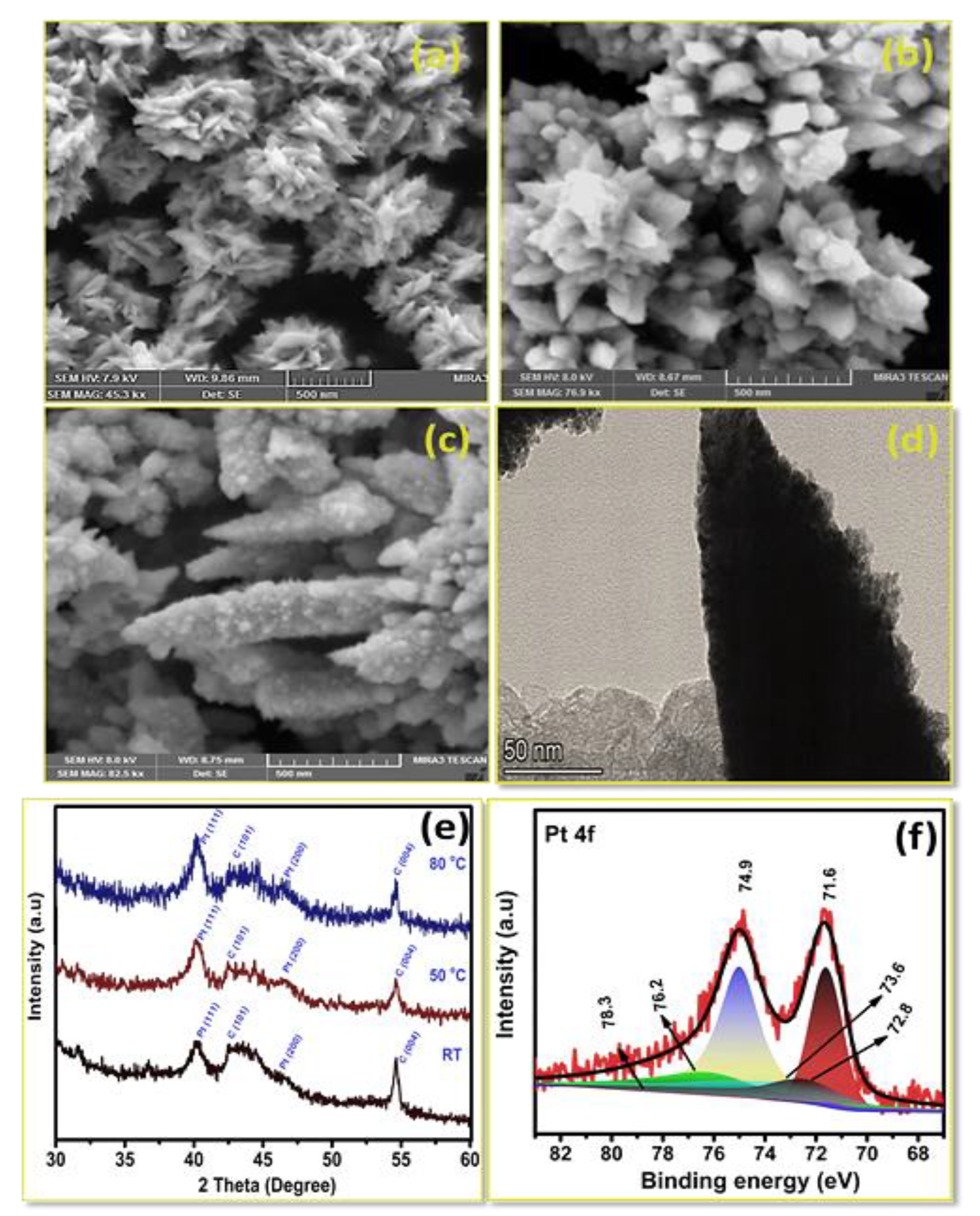
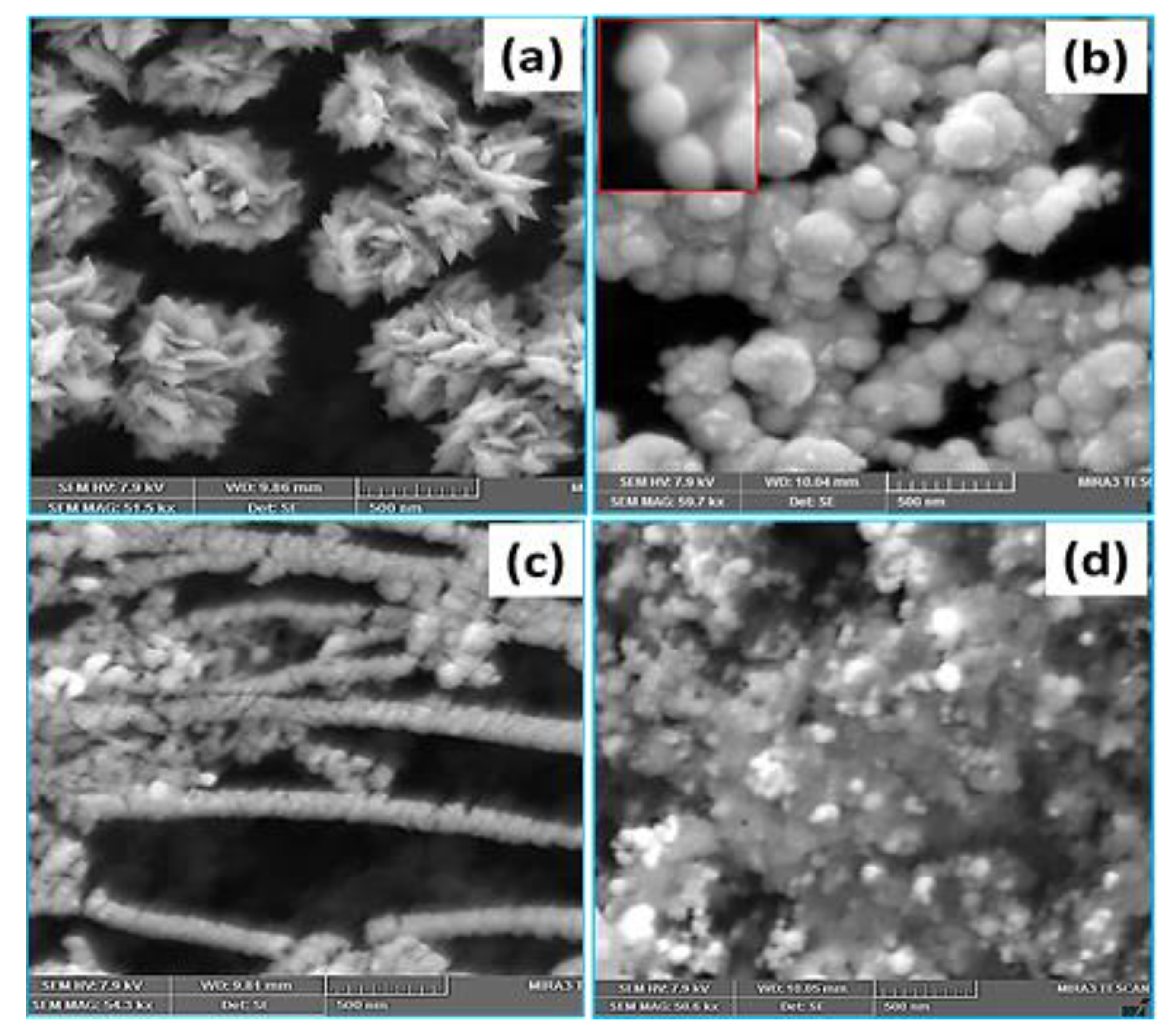
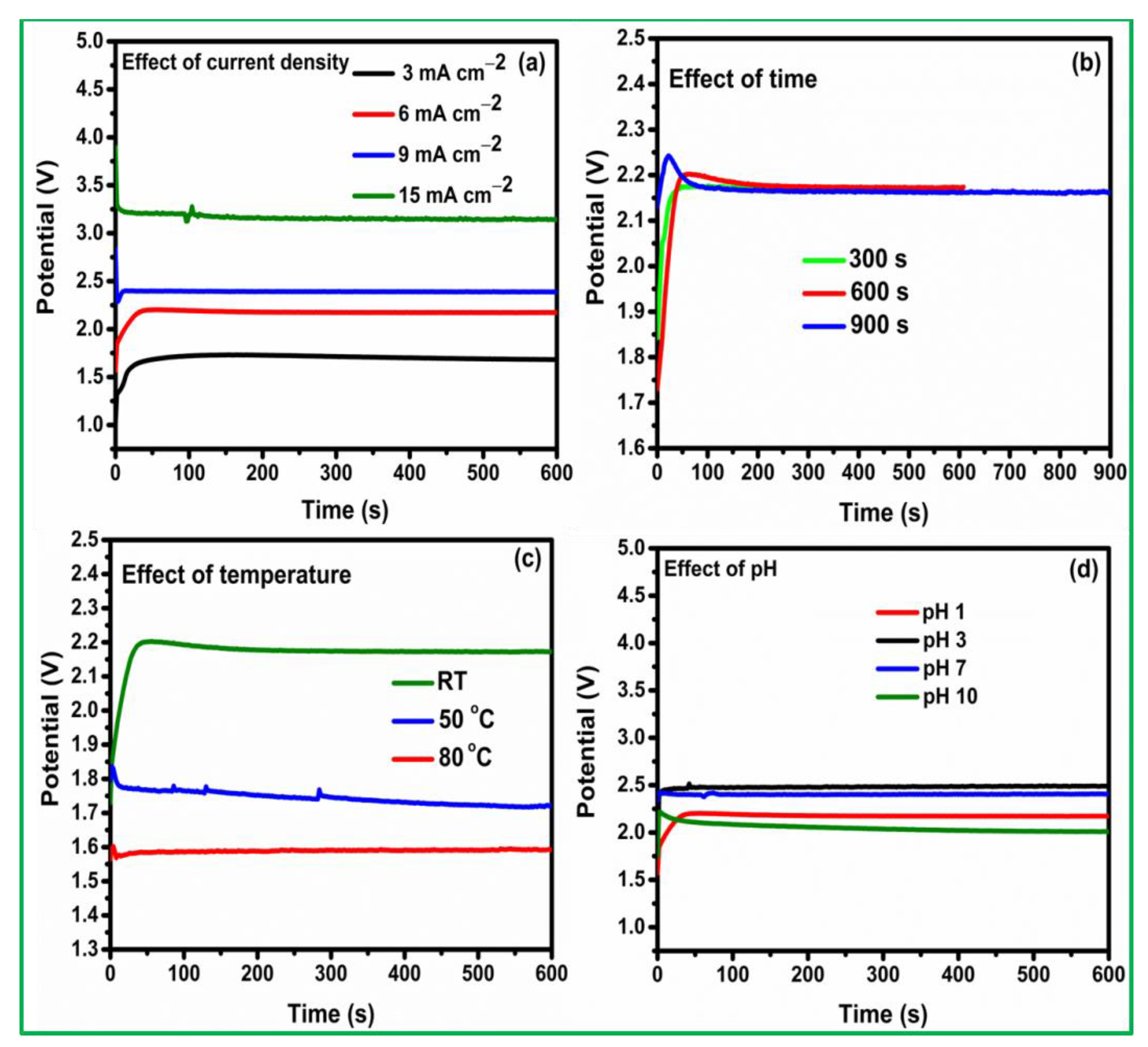
3.1. Pt Particle Electrochemically Deposited on A Carbon Substrate and Their Response to Varying Current Density
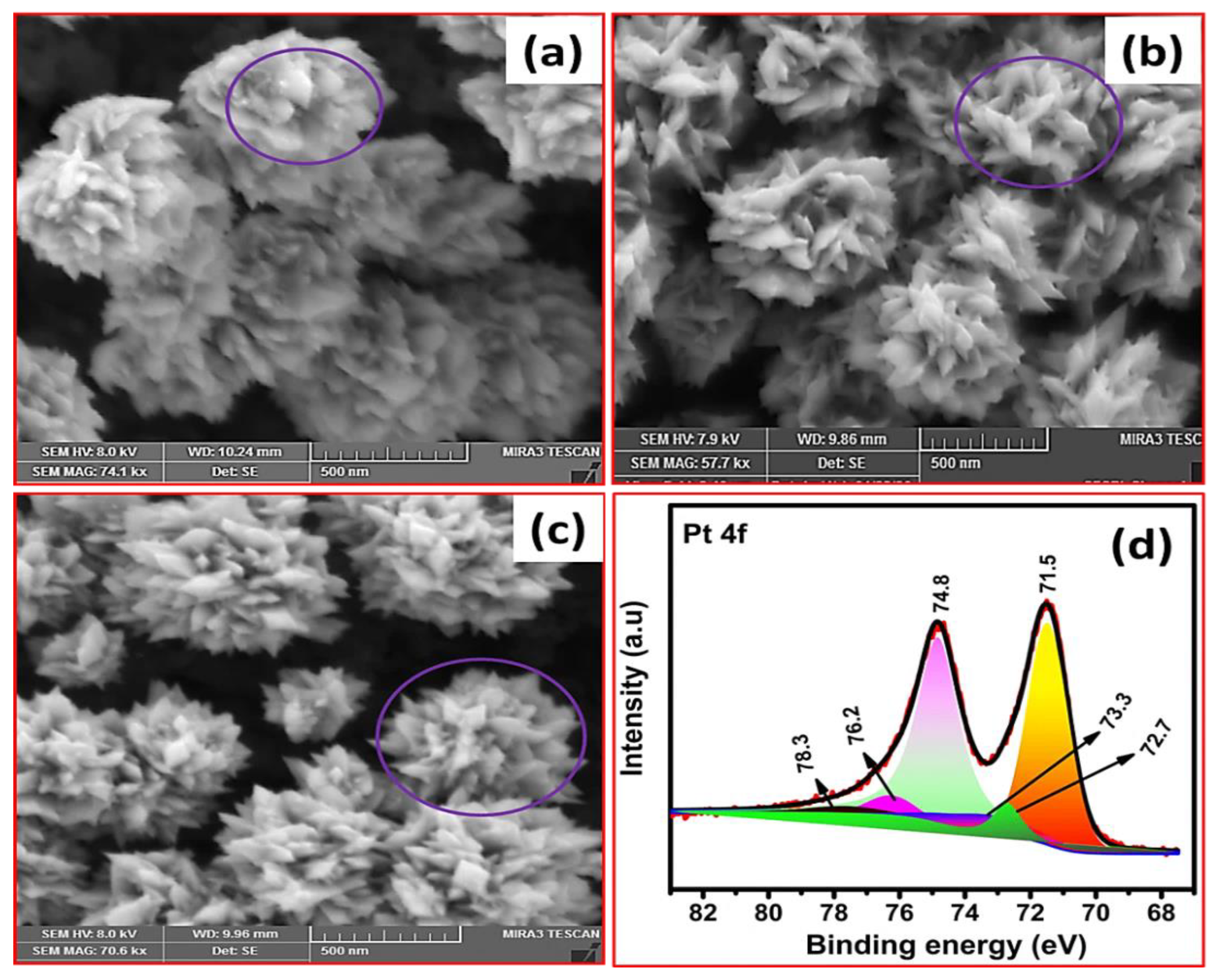
3.2. Influence of Time on Pt Particle Deposited on the Carbon Substrate
3.3. Effect of Temperature on Pt Structure Fabricated on a Carbon Substrate
3.4. Pt Structure on A Carbon Substrate with the Influence of pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bing, Y.; Liu, H.; Zhang, L.; Ghosh, D.; Zhang, J.J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184–2202. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Shukla, A.; Vinod Selvaganesh, S.; Mohan, S.; Bhat, S.D. Silica-decorated carbon-Pt electrocatalyst synthesis via single-step polyol method for superior polymer electrolyte fuel cell performance, durability and stack operation under low relative humidity. J. Power Sources 2019, 438, 226999. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, W.B.; Cheng, Y.F. Recent advances in electrocatalysts for electro-oxidation of ammonia. J. Mater. Chem. A 2013, 1, 3216–3238. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Tian, J.Z.; Cai, Y.; Ye, Y.; Yuan, Q.; Liang, C. Highly dispersed ultrafine Pt nanoparticles on reduced graphene oxide nanosheets: In situ sacrificial template synthesis and superior electrocatalytic performance for methanol oxidation. ACS Appl. Mater. Interfaces 2015, 7, 22935–22940. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Sun, Y.; Pan, S.; Dai, Y.; Hao, L.; Zou, J. Self-stable WP/C support with excellent co-catalytic functionality for Pt: Enhanced catalytic activity and durability for methanol electro-oxidation. ACS Appl. Mater. Interfaces 2016, 8, 33572–33582. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, C.; Du, X.; Wu, Y.; Xu, P.; Liu, J.; Hu, W. Pulsed electrodeposition of Pt particles on indium tin oxide substrates and their electrocatalytic properties for methanol oxidation. Electrochim. Acta 2013, 100, 164–170. [Google Scholar] [CrossRef]
- Baingane, A.; Narayanan, J.S.; Slaughter, G. Sensitive electrochemical detection of glucose via a hybrid self-powered biosensing system. Sens. Bio-Sens. Res. 2018, 20, 41–46. [Google Scholar] [CrossRef]
- Kocemba, I.; Rynkowski, J. The Influence of catalytic activity on the response of Pt/SnO2 gas sensors to carbon monoxide and hydrogen. Sens. Actuators B Chem. 2011, 155, 659–666. [Google Scholar] [CrossRef]
- Leite, L.; Benazzi, E.; George, N.M. Hydrocracking of phenanthrene over bifunctional Pt catalysts. Catal. Today 2001, 65, 241–247. [Google Scholar] [CrossRef]
- Kariya, N.; Fukuoka, A.; Utagawa, T.; Sakuramoto, M.; Goto, Y.; Ichikawa, M. Efficient hydrogen production using cyclohexane and decalin by pulse-spray mode reactor with Pt catalysts. Appl. Catal. A Gen. 2003, 247, 247–259. [Google Scholar] [CrossRef]
- Skander, M.; Retailleau, P.; Bourrie, B.; Schio, L.; Mailliet, P.; Marinetti, A. N-heterocyclic carbene-amine Pt(II) complexes, a new chemical space for the development of platinum-based anticancer drugs. J. Med. Chem. 2010, 53, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.C. Supported metal clusters: Synthesis, structure, and catalysis. Chem. Rev. 1995, 95, 511–522. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Vinod Selvaganesh, S.; Shukla, A.; Nagaraju, N.; Bhat, S.D. Boosting Pt oxygen reduction reaction activity and durability by carbon semi-coated titania nanorods for proton exchange membrane fuel cells. Electrochim. Acta 2018, 263, 596–609. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Vinod Selvaganesh, S.; Bhat, S.D. Nitrogen and carbon doped titanium oxide as an alternative and durable electrocatalyst support in polymer electrolyte fuel cells. J. Power Sources 2016, 304, 360–372. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Xie, Z.; Zhao, F.; Zhou, Z.; Yuan, Q. Hollow PtCu octahedral nanoalloys: Efficient bifunctional electrocatalysts towards oxygen reduction reaction and methanol oxidation reaction by regulating near-surface composition. J. Colloid. Interface Sci. 2020, 562, 244–251. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Wang, Z.L.; Green, T.C.; Henglein, A.; El-Said, M.A. Shape-controlled synthesis of colloidal platinum nanoparticles. Science 1996, 272, 1924–1926. [Google Scholar] [CrossRef]
- Ramirez, E.; Erades, L.; Philippot, K.; Lecante, P.; Chaudret, B. Shape control of platinum nanoparticles. Adv. Funct. Mater. 2007, 17, 2219–2228. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Vinod Selvaganesh, S.; Bhat, S.D. Enhanced catalytic activity and stability of copper and nitrogen doped titania nanorod supported Pt electrocatalyst for oxygen reduction reaction in polymer electrolyte fuel cells. New J. Chem. 2017, 41, 13012–13026. [Google Scholar] [CrossRef]
- Paunovic, M. Electrochemical Deposition; Bard, A.J., Stratmann, M., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2007. [Google Scholar]
- Paunovic, M.; Schlesinger, M. Fundamentals of Electrochemical Deposition, 2nd ed.; Wiley-Interscience: New York, NY, USA, 2006. [Google Scholar]
- Dhanasekaran, P.; Lokesh, K.; Ojha, P.K.; Sahu, A.K.; Bhat, S.D.; Kalpana, D. Electrochemical deposition of three-dimensional platinum nanoflowers for high-performance polymer electrolyte fuel cells. J. Colloid. Interface Sci. 2020, 572, 198–206. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Liu, X.; Song, Z.; Deng, Y.; Han, X.; Hu, W. Synthesis of cubic-shaped Pt particles with (100) preferential orientation by a quick, one-step and clean electrochemical method. ACS Appl. Mater. Interfaces 2017, 9, 18856–18864. [Google Scholar] [CrossRef]
- Song, Y.J.; Kim, J.Y.; Park, K.W. Synthesis of Pd dendritic nanowires by electrochemical deposition. Cryst. Growt. Des. 2009, 9, 505–507. [Google Scholar] [CrossRef]
- Jia, F.; Wong, K.; Zhang, L. Electrochemical synthesis of nanostructured palladium of different morphology directly on gold substrate through a cyclic deposition/dissolution route. J. Phys. Chem. C 2009, 113, 7200–7206. [Google Scholar] [CrossRef]
- Takai, A.; Yamauchi, Y.; Kuroda, K. Tailored electrochemical synthesis of 2D-hexagonal, lamellar, and cage-type mesostructured Pt thin films with extralarge periodicity. J. Am. Chem. Soc. 2010, 132, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Cheruvally, G. Influence of various carbon nano-forms as supports for Pt catalyst on proton exchange membrane fuel cell performance. J. Power Sources 2017, 360, 196–205. [Google Scholar] [CrossRef]
- Sadiku-Agboola, O.; Sadiku, E.R.; Ojo, O.I.; Akanji, O.L.; Biotidara, O.F. Influence of operation parameters on metal deposition in bright nickel-plating process. Electrochim. Acta 2011, 29, 91. [Google Scholar] [CrossRef]
- Kumar, S.; Pande, S.; Verma, P. Factor effecting electrodeposition process. Int. J. Curr. Eng. Technol. 2015, 5, 700–704. [Google Scholar]
- Ye, F.; Xu, C.; Liu, G.; Li, J.; Wang, X.; Du, X.; Lee, J. A novel PtRuIr nanoclusters synthesized by selectively electrodepositing Ir on PtRu as highly active bifunctional electrocatalysts for oxygen evolution and reduction. Energy Convers. Manag. 2018, 155, 182–187. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Liu, G.; Chen, M.; Ye, F.; Zhang, W.; Yang, W.; Wang, X. Octahedral Pt-Ni nanoparticles prepared by pulse-like hydrothermal method for oxygen reduction reaction. Ionics 2020, 26, 293–300. [Google Scholar] [CrossRef]
- Ye, F.; Wang, Z.; Xu, C.; Yuan, M.; Liu, P.; Yang, W.; Liu, G. Mechanism and kinetic study of pulse electrodeposition process of Pt/C catalysts for fuel cells. Renew. Energy 2020, 145, 514–520. [Google Scholar] [CrossRef]
- Ye, F.; Xu, C.; Liu, G.; Yuan, M.; Wang, Z.; Du, X. Effect of pulse electrodeposition parameters on electrocatalytic the activity of methanol oxidation and morphology of Pt/C catalyst for direct methanol fuel cells. Energy Convers. Manag. 2018, 160, 85–92. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanasekaran, P.; Rajavarman, S.; Selvaganesh, S.V.; Bhat, S.D. Insight towards Nucleation Mechanism and Change in Morphology for Nanostructured Platinum Thin Film Directly Grown on Carbon Substrate via Electrochemical Deposition. Materials 2021, 14, 2330. https://doi.org/10.3390/ma14092330
Dhanasekaran P, Rajavarman S, Selvaganesh SV, Bhat SD. Insight towards Nucleation Mechanism and Change in Morphology for Nanostructured Platinum Thin Film Directly Grown on Carbon Substrate via Electrochemical Deposition. Materials. 2021; 14(9):2330. https://doi.org/10.3390/ma14092330
Chicago/Turabian StyleDhanasekaran, Prabhakaran, Swaminathan Rajavarman, Sivasuriyanarayanan Vinod Selvaganesh, and Santoshkumar Dattatray Bhat. 2021. "Insight towards Nucleation Mechanism and Change in Morphology for Nanostructured Platinum Thin Film Directly Grown on Carbon Substrate via Electrochemical Deposition" Materials 14, no. 9: 2330. https://doi.org/10.3390/ma14092330
APA StyleDhanasekaran, P., Rajavarman, S., Selvaganesh, S. V., & Bhat, S. D. (2021). Insight towards Nucleation Mechanism and Change in Morphology for Nanostructured Platinum Thin Film Directly Grown on Carbon Substrate via Electrochemical Deposition. Materials, 14(9), 2330. https://doi.org/10.3390/ma14092330




