Stabilization of Ferroelectric Phase in Highly Oriented Quinuclidinium Perrhenate (HQReO4) Thin Films
Abstract
1. Introduction
2. Materials and Methods
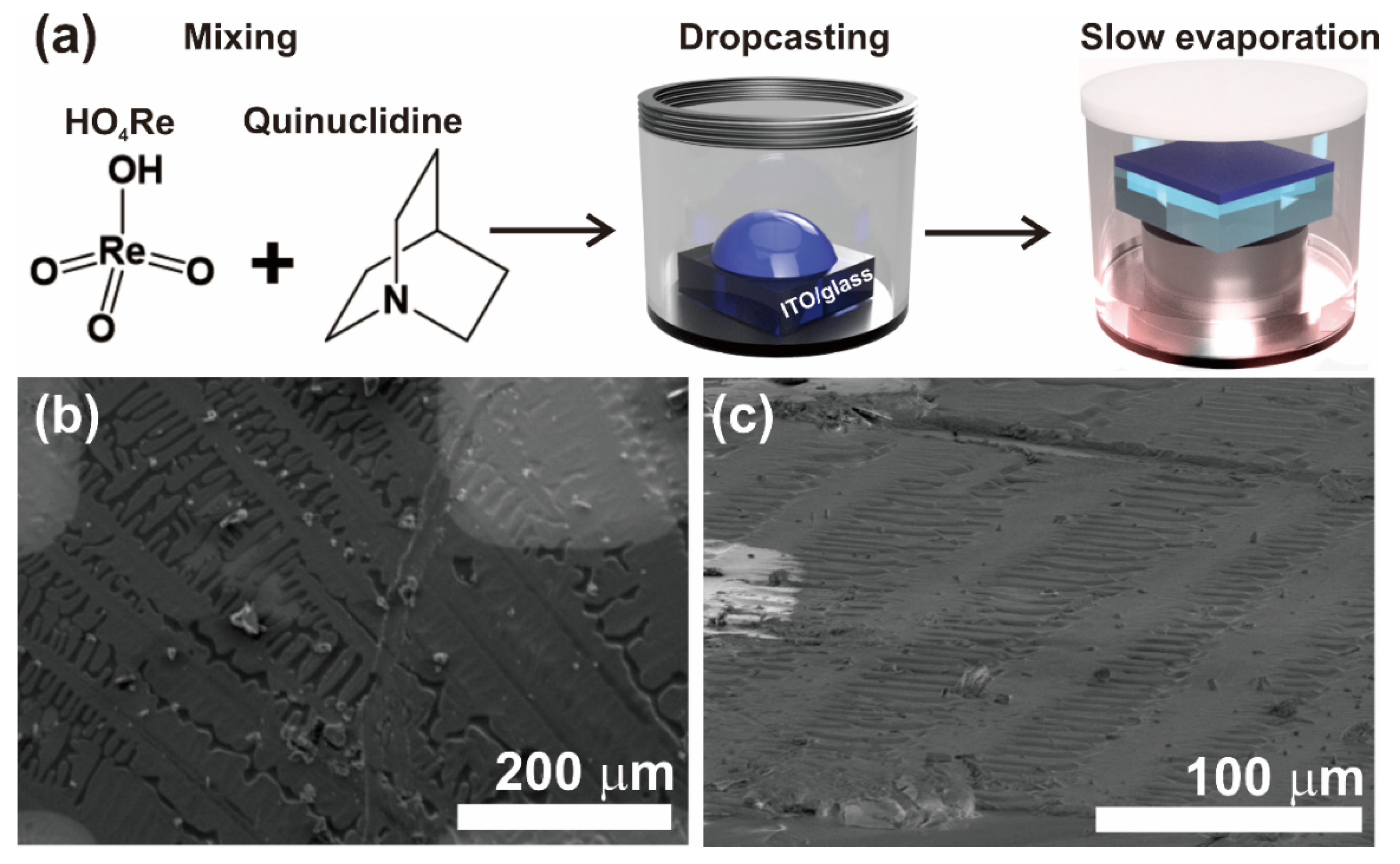
2.1. Preparation of Quinuclidine Perrhenate Thin Films
2.2. Characterizations
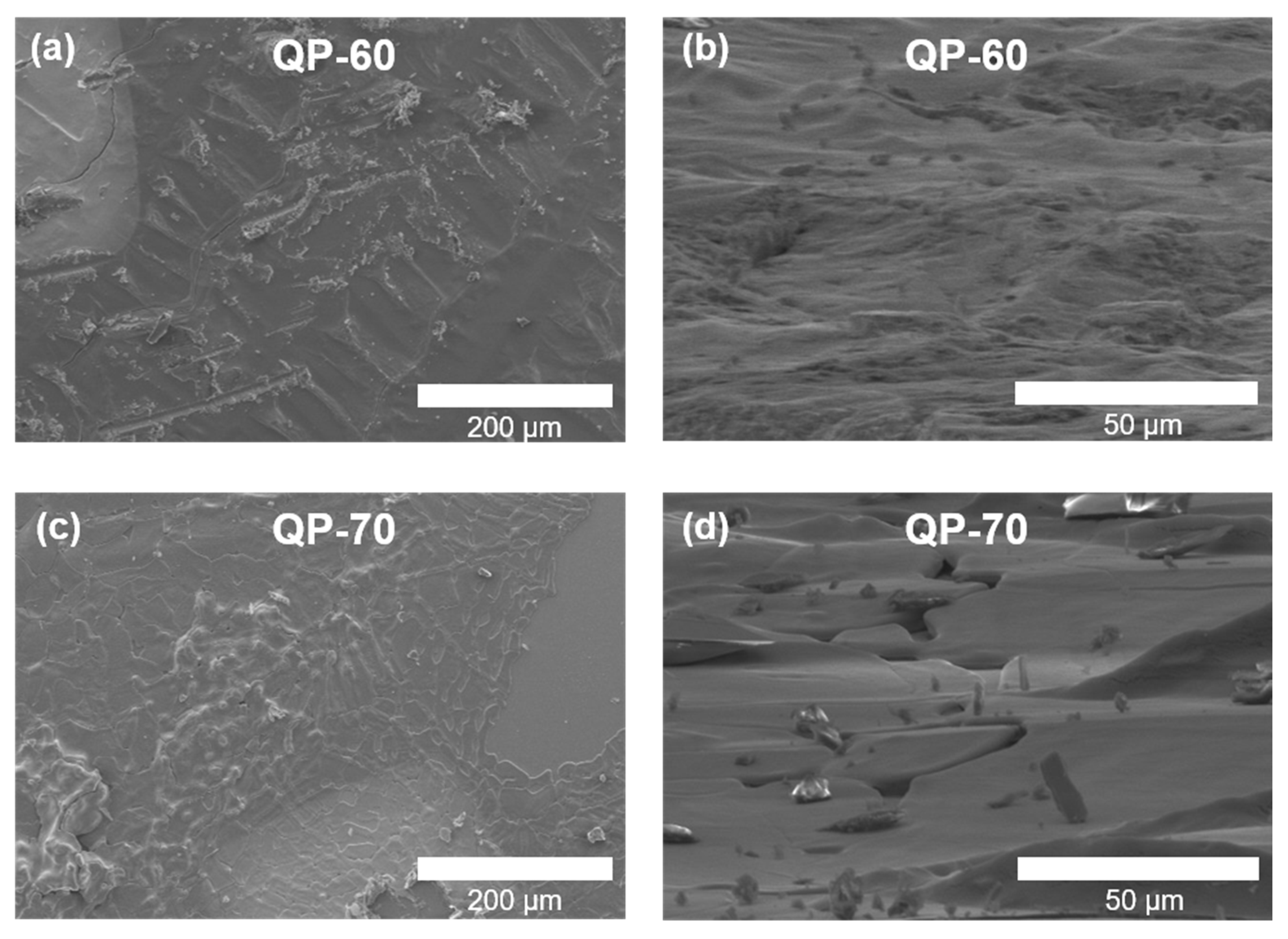
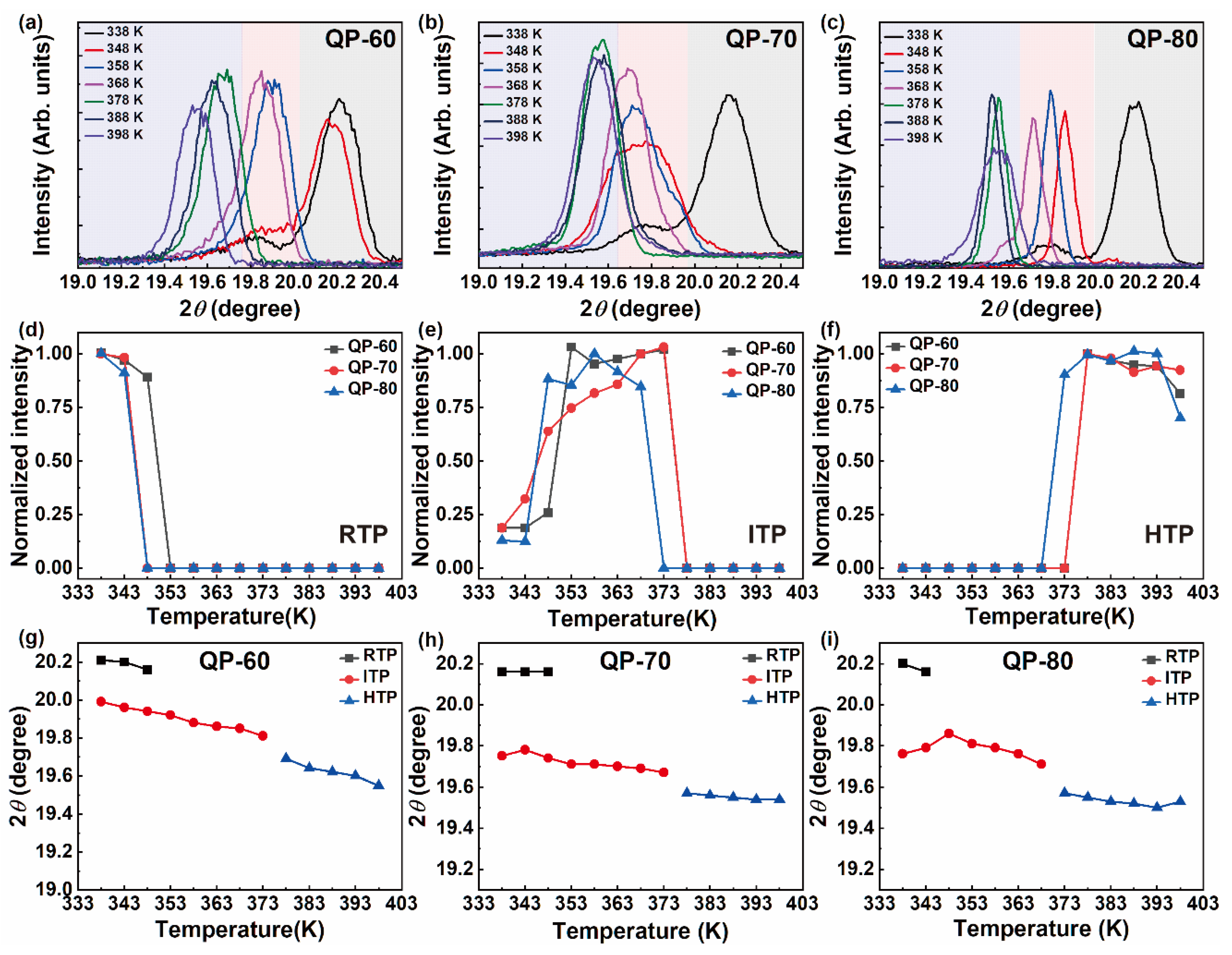
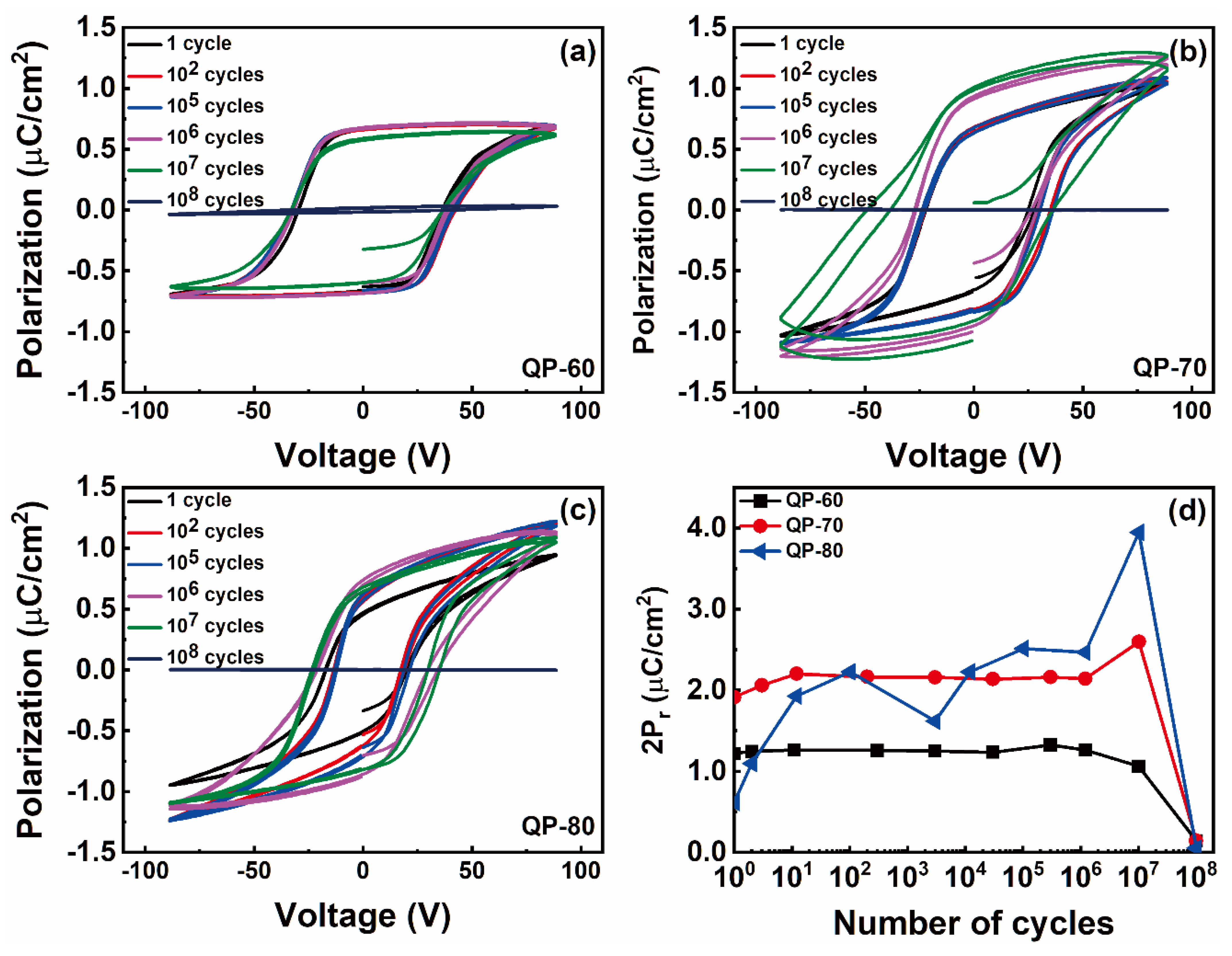
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.Y.; Zhang, H.Y.; Chen, X.G.; Xiong, R.G. Molecular Design Principles for Ferroelectrics: Ferroelectrochemistry. J. Am. Chem. Soc. 2020, 142, 15205–15218. [Google Scholar] [CrossRef]
- Harada, J.; Shimojo, T.; Oyamaguchi, H.; Hasegawa, H.; Takahashi, Y.; Satomi, K.; Suzuki, Y.; Kawamata, J.; Inabe, T. Directionally tunable and mechanically deformable ferroelectric crystals from rotating polar globular ionic molecules. Nat. Chem. 2016, 8, 946–952. [Google Scholar] [CrossRef]
- Wang, C.F.; Gao, J.X.; Li, C.; Yang, C.S.; Xiong, J.B.; Tang, Y.Z. A novel co-crystallization molecular ferroelectric induced by the ordering of sulphate anions and hydrogen atoms. Inorg. Chem. Front. 2018, 5, 2413–2419. [Google Scholar] [CrossRef]
- Harada, J. Plastic/ferroelectric molecular crystals: Ferroelectric performance in bulk polycrystalline forms. APL Mater. 2021, 9, 020901. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Li, P.F.; Shi, P.P.; Zhang, W.Y.; Wang, Z.X.; You, Y.M.; Ye, H.Y.; Nakamura, T.; Xiong, R.G. Visualization of Room-Temperature Ferroelectricity and Polarization Rotation in the Thin Film of Quinuclidinium Perrhenate. Phys. Rev. Lett. 2017, 119, 207602. [Google Scholar] [CrossRef]
- Yang, C.K.; Chen, W.N.; Ding, Y.T.; Wang, J.; Rao, Y.; Liao, W.Q.; Xie, Y.; Zou, W.; Xiong, R.G. Directional Intermolecular Interactions for Precise Molecular Design of a High- T c Multiaxial Molecular Ferroelectric. J. Am. Chem. Soc. 2019, 141, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zeng, X.C. Design of ferroelectric organic molecular crystals with ultrahigh polarization. J. Am. Chem. Soc. 2014, 136, 6428–6436. [Google Scholar] [CrossRef] [PubMed]
- Harada, J.; Kawamura, Y.; Takahashi, Y.; Uemura, Y.; Hasegawa, T.; Taniguchi, H.; Maruyama, K. Plastic/Ferroelectric Crystals with Easily Switchable Polarization: Low-Voltage Operation, Unprecedentedly High Pyroelectric Performance, and Large Piezoelectric Effect in Polycrystalline Forms. J. Am. Chem. Soc. 2019, 141, 9349–9357. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mondal, A.; Reddy, C.M. Harnessing molecular rotations in plastic crystals: A holistic view for crystal engineering of adaptive soft materials. Chem. Soc. Rev. 2020, 49, 8878–8896. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Li, P.F.; Liao, W.Q.; Shi, P.P.; You, Y.M.; Xiong, R.G. Multiaxial Molecular Ferroelectric Thin Films Bring Light to Practical Applications. J. Am. Chem. Soc. 2018, 140, 8051–8059. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Li, K.; Li, Z.; Zhu, S.; Li, Q.; Zhang, Z.; Ji, L.; Li, W.; Bu, X. Engineering Elastic Properties of Isostructural Molecular Perovskite Ferroelectrics via B-Site Substitution. Small 2021, 2006021. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, P.P.; Liu, X.M.; Feng, J.C.; Ye, Q.; Yao, Y.F.; Fu, D.W.; Li, P.F.; You, Y.M.; Zhang, Y.; et al. An above-room-temperature phosphonium-based molecular ferroelectric perovskite, [(CH3)4P]CdCl3, with Sb3+-doped luminescence. NPG Asia Mater. 2019, 11, 1–9. [Google Scholar] [CrossRef]
- You, Y.M.; Tang, Y.Y.; Li, P.F.; Zhang, H.Y.; Zhang, W.Y.; Zhang, Y.; Ye, H.Y.; Nakamura, T.; Xiong, R.G. Quinuclidinium salt ferroelectric thin-film with duodecuple-rotational polarization-directions. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Y.; Ge, J.Z.; Tang, Y.Y.; Li, P.F.; Zhang, Y.; You, Y.M.; Xiong, R.G. Molecular Ferroelectric with Most Equivalent Polarization Directions Induced by the Plastic Phase Transition. J. Am. Chem. Soc. 2016, 138, 13175–13178. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-J.; Zhang, T.; Gu, Z.-X.; Zhang, Z.-X.; Fu, D.-W.; Chen, X.-G.; Zhang, H.-Y.; Xiong, R.-G. Record Enhancement of Curie Temperature in Host–Guest Inclusion Ferroelectrics. J. Am. Chem. Soc. 2021, 143, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Tang, Y.Y.; Shi, P.P.; Xiong, R.G. Toward the targeted design of molecular ferroelectrics: Modifying molecular symmetries and homochirality. Acc. Chem. Res. 2019, 52, 1928–1938. [Google Scholar] [CrossRef]
- Gao, K.; Su, Z.; Li, C.; Wu, D.; Zhang, B. Spontaneous self-formation of molecular ferroelectric heterostructures. Phys. Chem. Chem. Phys. 2021, 23, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, G.; Zhang, G.; Jafri, H.M.; Zhou, J.; Liu, D.; Liu, Y.; Wang, J.; Jin, K.; Hu, Y.; et al. Improper molecular ferroelectrics with simultaneous ultrahigh pyroelectricity and figures of merit. Sci. Adv. 2021, 7, 3068–3097. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Z.-B.; Zhang, H.-Y.; Zhang, W.-Y.; Tang, Y.-Y.; You, Y.-M.; Li, P.-F.; Liao, W.-Q.; Shi, P.-P.; Ma, R.-W.; et al. A Molecular Polycrystalline Ferroelectric with Record-High Phase Transition Temperature. Adv. Mater. 2017, 29, 1700831. [Google Scholar] [CrossRef]
- Ye, H.Y.; Tang, Y.Y.; Li, P.F.; Liao, W.Q.; Gao, J.X.; Hua, X.N.; Cai, H.; Shi, P.P.; You, Y.M.; Xiong, R.G. Metal-free three-dimensional perovskite ferroelectrics. Science 2018, 361, 151–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ye, H.-Y.; Fu, D.-W.; Gao, W.; Ma, H.; Liu, Z.; Liu, Y.; Zhang, W.; Li, J.; et al. A Molecular Ferroelectric Thin Film of Imidazolium Perchlorate That Shows Superior Electromechanical Coupling. Angew. Chem. Int. Ed. 2014, 53, 5064–5068. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, Y.; Cai, H.L.; Xiong, R.G. Molecular ferroelectrics: Where electronics meet biology. Phys. Chem. Chem. Phys. 2013, 15, 20786–20796. [Google Scholar] [CrossRef]
- Fu, D.W.; Cai, H.L.; Liu, Y.; Ye, Q.; Zhang, W.; Zhang, Y.; Chen, X.Y.; Giovannetti, G.; Capone, M.; Li, J.; et al. Diisopropylammonium bromide is a high-temperature molecular ferroelectric crystal. Science 2013, 339, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Setter, N.; Damjanovic, D.; Eng, L.; Fox, G.; Gevorgian, S.; Hong, S.; Kingon, A.; Kohlstedt, H.; Park, N.Y.; Stephenson, G.B.; et al. Ferroelectric thin films: Review of materials, properties, and applications. J. Appl. Phys. 2006, 100, 51606. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Chen, X.; Hou, Y.; Chen, L. A novel ferroelectric based on quinuclidine derivatives. Chin. Chem. Lett. 2020, 31, 1686–1689. [Google Scholar] [CrossRef]
- Yoneya, M.; Harada, J. Molecular Dynamics Simulation Study of the Plastic/Ferroelectric Crystal Quinuclidinium Perrhenate. J. Phys. Chem. C 2020, 124, 2171–2177. [Google Scholar] [CrossRef]
- Hiroshima, T.; Tanaka, K.; Kimura, T. Effects of Microstructure and Composition on the Curie Temperature of Lead Barium Niobate Solid Solutions. J. Am. Ceram. Soc. 1996, 79, 3235–3242. [Google Scholar] [CrossRef]
- Zhao, D.; Katsouras, I.; Li, M.; Asadi, K.; Tsurumi, J.; Glasser, G.; Takeya, J.; Blom, P.W.M.; De Leeuw, D.M. Polarization fatigue of organic ferroelectric capacitors. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Seol, W.; Anoop, G.; Samanta, S.; Unithrattil, S.; Ahn, D.; Kim, W.; Jung, G.; Jo, J. Stabilization of Ferroelectric Phase in Highly Oriented Quinuclidinium Perrhenate (HQReO4) Thin Films. Materials 2021, 14, 2126. https://doi.org/10.3390/ma14092126
Lee J, Seol W, Anoop G, Samanta S, Unithrattil S, Ahn D, Kim W, Jung G, Jo J. Stabilization of Ferroelectric Phase in Highly Oriented Quinuclidinium Perrhenate (HQReO4) Thin Films. Materials. 2021; 14(9):2126. https://doi.org/10.3390/ma14092126
Chicago/Turabian StyleLee, Junyoung, Woojun Seol, Gopinathan Anoop, Shibnath Samanta, Sanjith Unithrattil, Dante Ahn, Woochul Kim, Gunyoung Jung, and Jiyoung Jo. 2021. "Stabilization of Ferroelectric Phase in Highly Oriented Quinuclidinium Perrhenate (HQReO4) Thin Films" Materials 14, no. 9: 2126. https://doi.org/10.3390/ma14092126
APA StyleLee, J., Seol, W., Anoop, G., Samanta, S., Unithrattil, S., Ahn, D., Kim, W., Jung, G., & Jo, J. (2021). Stabilization of Ferroelectric Phase in Highly Oriented Quinuclidinium Perrhenate (HQReO4) Thin Films. Materials, 14(9), 2126. https://doi.org/10.3390/ma14092126






