Reinforced 3D Composite Structures of γ-, α-Al2O3 with Carbon Nanotubes and Reduced GO Ribbons Printed from Boehmite Gels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Processing
2.2. Characterization
3. Results and Discussion
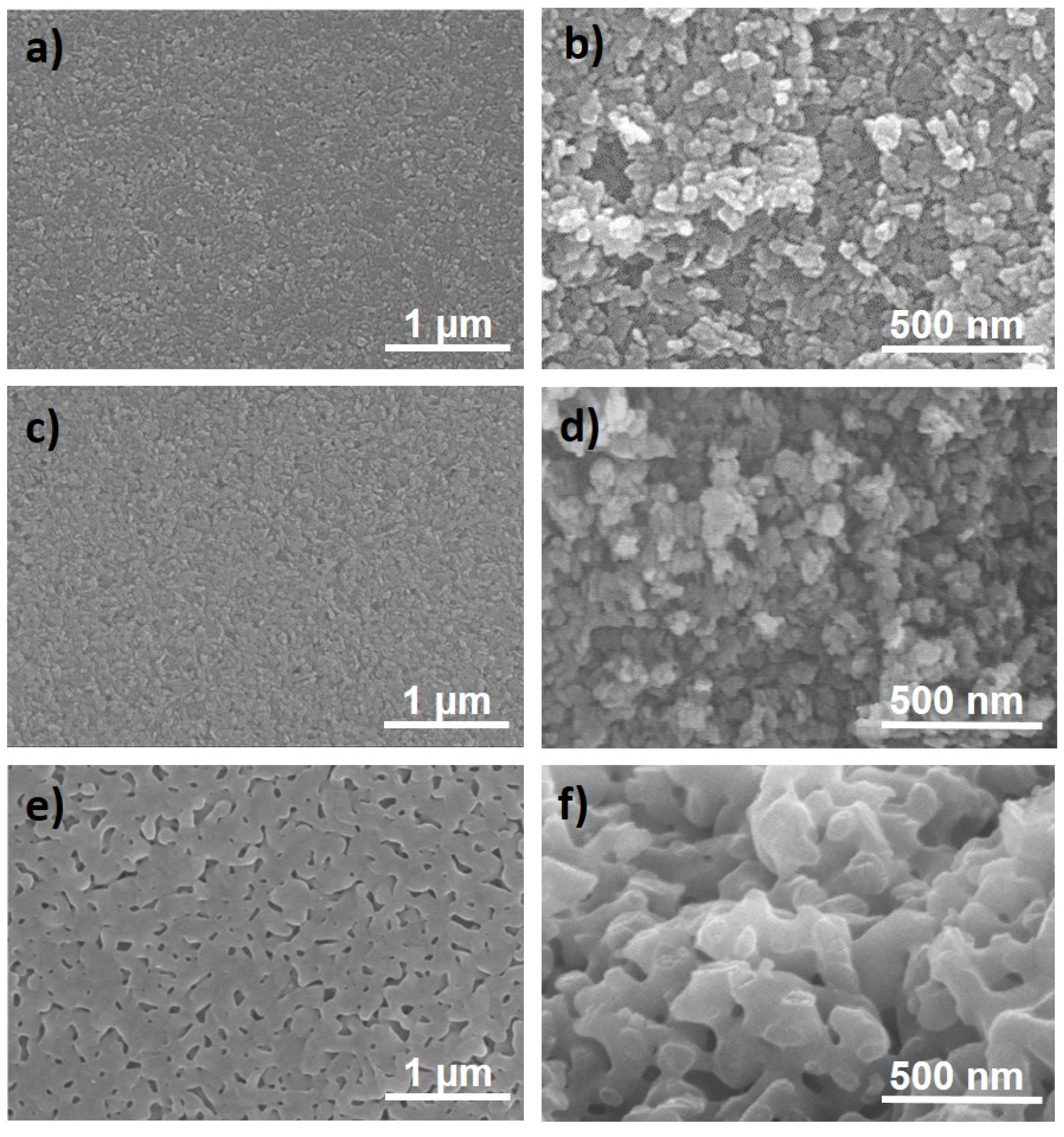
3.1. Boehmite and Al2O3 Structures
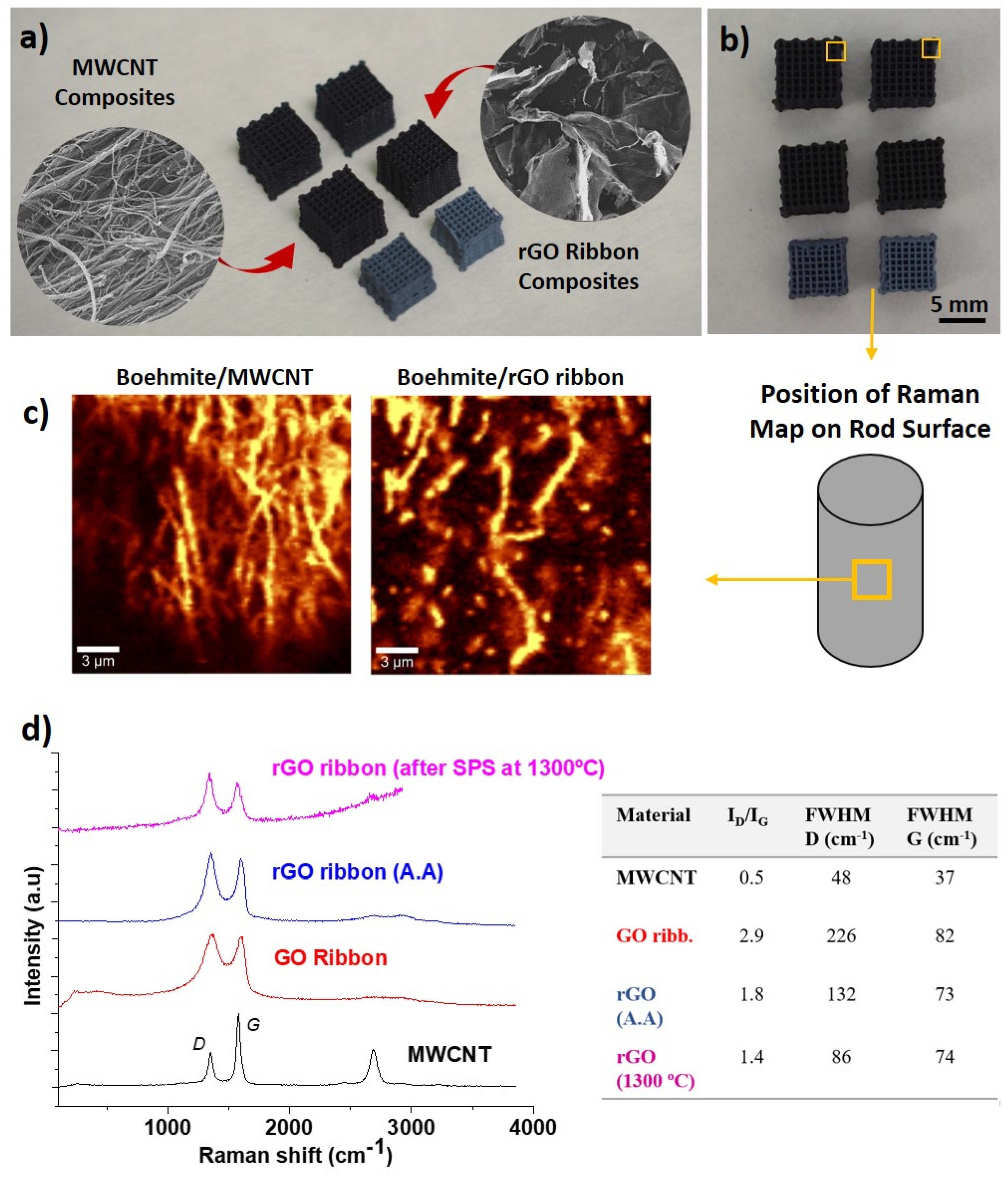
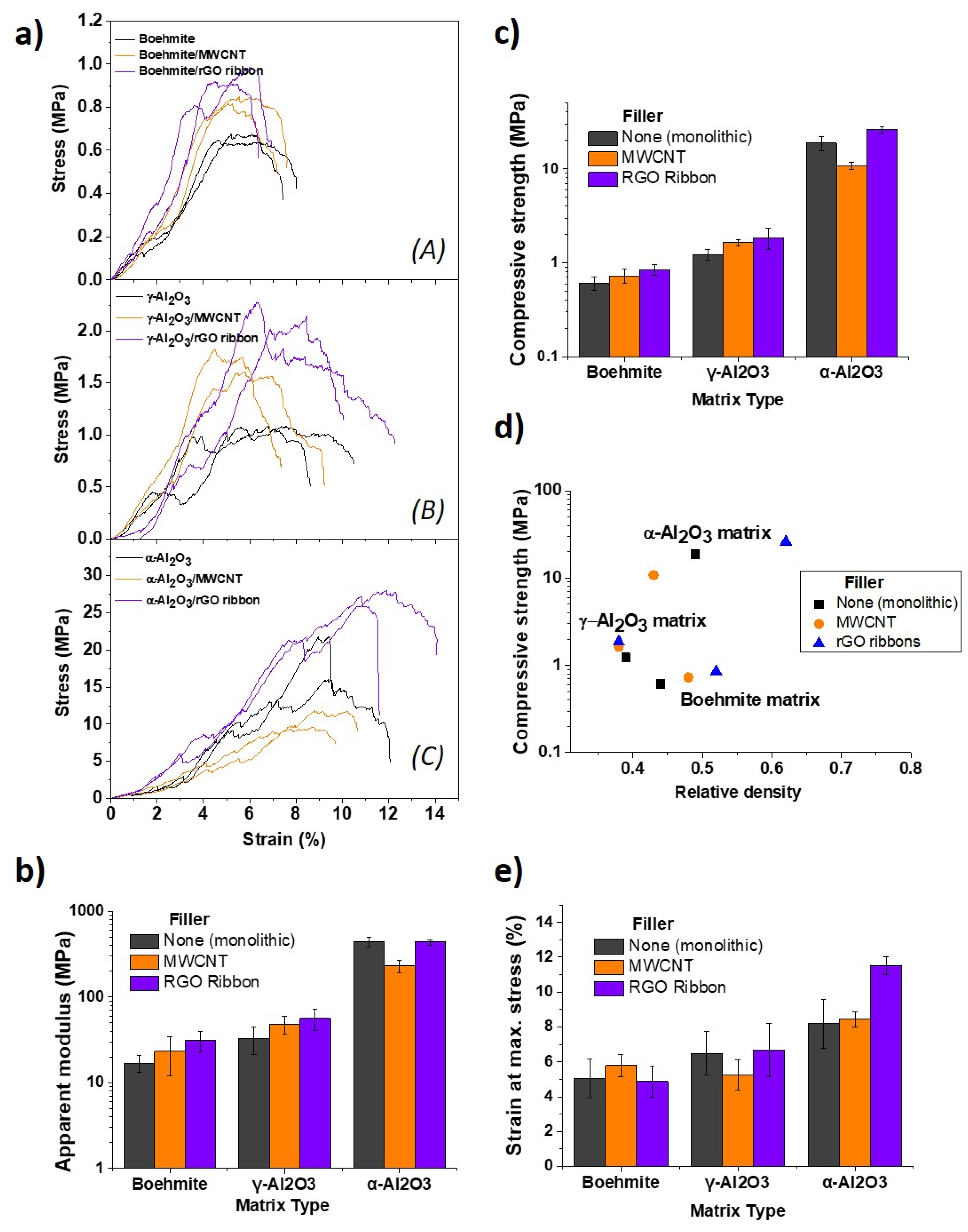
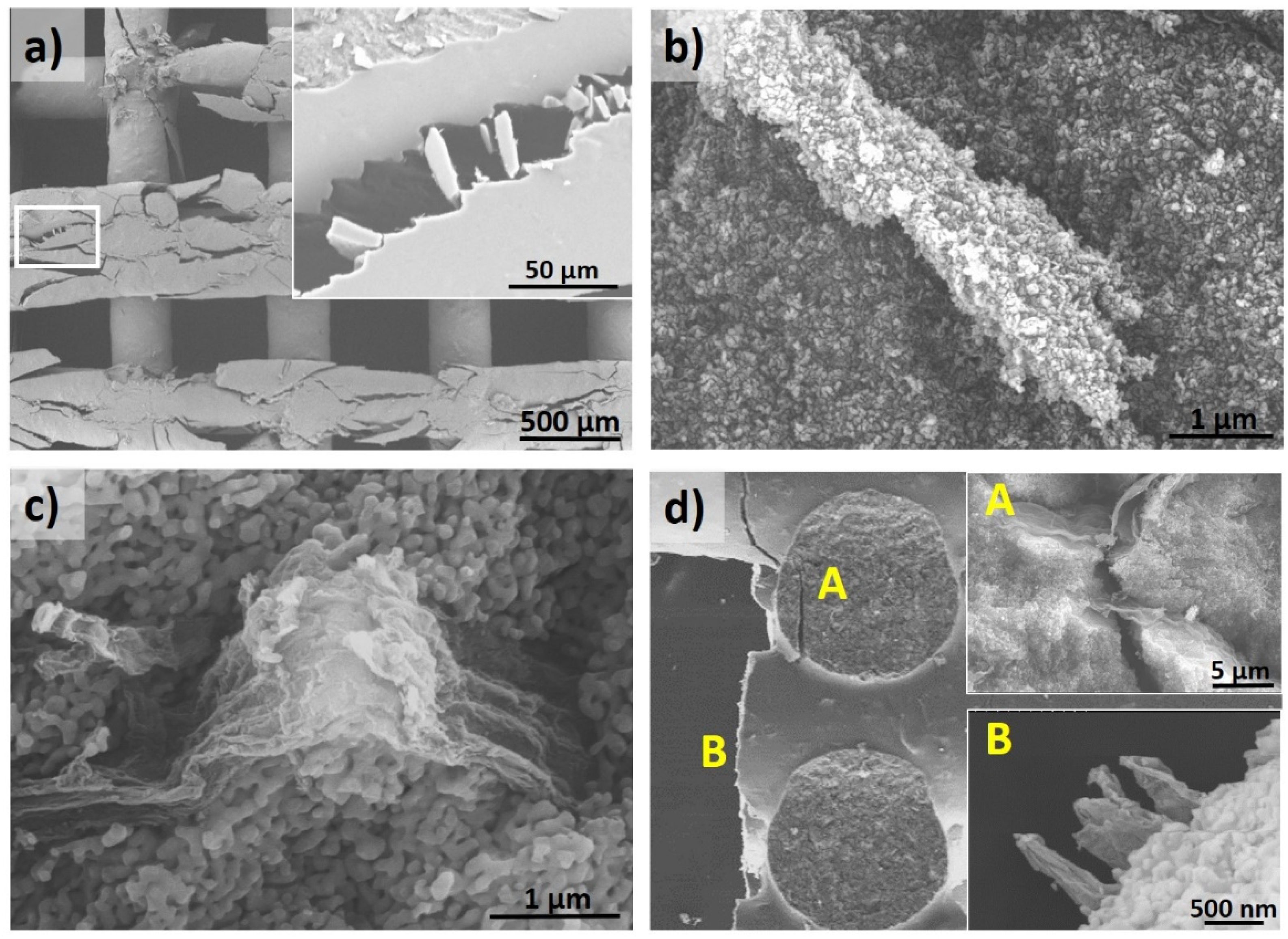
3.2. Composites with Carbon Nanostructures and their Reinforcing Capabilities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, S.J. Phase tranformations and development of microstructure in boehmite-derived transition aluminas. Proc. Br. Ceram. Soc. 1979, 28, 281–294. [Google Scholar]
- Euzen, C.F.P.; Raybaud, P.; Krokidis, X.; Toulhoat, H.; le Loarer, J.; Jolivet, J.P. Alumina. In Handbook of Porous Solids; Schüth, J.W.F., Sing, K., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; pp. 1591–1677. [Google Scholar]
- AlSairafi, S.H.; AlNajdi, N.; AlSheeha, H.; Rana, M.S. Synthesis of alumina support and effect of its properties on thiophene hydrodesulfurization. React. Kinet. Mech. Catal. 2020, 129, 297–313. [Google Scholar] [CrossRef]
- Baudín, C. Processing of alumina and corresponding composites. In Comprehensive Hard Materials; Sarin, D.M.V.K., Llanes, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 31–72. [Google Scholar]
- Yoshizawa, Y.; Hirao, K.; Kanzaki, S. Fabrication of low cost fine-grained alumina powders by seeding for high performance sintered bodies. J. Eur. Ceram. Soc. 2004, 24, 325–330. [Google Scholar] [CrossRef]
- Solovev, Y.V.; Prilepskii, A.Y.; Krivoshapkina, E.F.; Fakhardo, A.F.; Bryushkova, E.A.; Kalikina, P.A.; Koshel, E.I.; Vinogradov, V.V. Sol-gel derived boehmite nanostructures is a versatile nanoplatform for biomedical applications. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Menon, A.R.R.; Prabhakaran, K.; Warrier, K.G.K. Rheology and packing characteristics of alumina extrusion using boehmite gel as a binder. Ceram. Int. 2001, 27, 231–237. [Google Scholar] [CrossRef]
- Marchi, C.S.; Kouzeli, M.; Rao, R.; Lewis, J.A.; Dunand, D.C. Alumina-aluminum interpenetrating-phase composites with three-dimensional periodic architecture. Scr. Mater. 2003, 49, 861–866. [Google Scholar] [CrossRef]
- Rueschhoff, L.; Costakis, W.; Michie, M.; Youngblood, J.; Trice, R. Additive Manufacturing of Dense Ceramic Parts via Direct Ink Writing of Aqueous Alumina Suspensions. Int. J. Appl. Ceram. Technol. 2016, 13, 821–830. [Google Scholar] [CrossRef]
- M’Barki, A.; Bocquet, L.; Stevenson, A. Linking Rheology and Printability for Dense and Strong Ceramics by Direct Ink Writing. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, W.; Liu, J.; Zhang, Y.; Zhang, S.; Yang, J. 3D printing boehmite gel foams into lightweight porous ceramics with hierarchical pore structure. J. Eur. Ceram. Soc. 2020, 40, 930–934. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, X.; You, J.; Li, T.; Hou, Q. Hierarchical porous ceramics with multiple open pores from boehmite gel emulsions. J. Am. Ceram. Soc. 2021, 104, 1902–1907. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Rodriguez, J.N.; Zhu, C.; Worsley, M.A.; Wu, A.S.; Kanarska, Y.; Horn, J.D.; Duoss, E.B.; Ortega, J.M.; Elmer, W.; et al. 3D-Printing of Meso-structurally Ordered Carbon Fiber/Polymer Composites with Unprecedented Orthotropic Physical Properties. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Blanco, I. The Use of Composite Materials in 3D Printing. J. Compos. Sci. 2020, 4, 42. [Google Scholar] [CrossRef]
- Zhu, W.; Fu, H.; Xu, Z.; Liu, R.; Jiang, P.; Shao, X.; Shi, Y.; Yan, C. Fabrication and characterization of carbon fiber reinforced SiC ceramic matrix composites based on 3D printing technology. J. Eur. Ceram. Soc. 2018, 38, 4604–4613. [Google Scholar] [CrossRef]
- Gao, C.; Feng, P.; Peng, S.; Shuai, C. Carbon nanotube, graphene and boron nitride nanotube reinforced bioactive ceramics for bone repair. Acta Biomater. 2017, 61, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yunus, D.E.; He, R.; Shi, W.; Kaya, O.; Liu, Y. Short fiber reinforced 3d printed ceramic composite with shear induced alignment. Ceram. Int. 2017, 43, 11766–11772. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, P.; Sun, M.; Liu, W.; Li, D.; Wu, W.; Yan, W.; Shang, J.; Yu, J. Fabricating Mechanically Robust Binder-Free Structured Zeolites by 3D Printing Coupled with Zeolite Soldering: A Superior Configuration for CO2 Capture. Adv. Sci. 2019, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.t.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Goncalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef]
- Zhong, J.; Zhou, G.X.; He, P.G.; Yang, Z.H.; Jia, D.C. 3D printing strong and conductive geo-polymer nanocomposite structures modified by graphene oxide. Carbon 2017, 117, 421–426. [Google Scholar] [CrossRef]
- Ramirez, C.; Osendi, M.I.; Miranzo, P.; Belmonte, M.; Figueiredo, F.; Castro-Beltrán, A.; Terrones, M. Graphene nanoribbon ceramic composites. Carbon 2015, 90, 207–214. [Google Scholar] [CrossRef]
- de Silva, K.K.H.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Román-Manso, B.; Figueiredo, F.M.; Achiaga, B.; Barea, R.; Perez-Coll, D.; Morelos-Gomez, A.; Terrones, M.; Osendi, M.I.; Belmonte, M.; Miranzo, P. Electrically functional 3D-architectured graphene/SiC composites. Carbon 2016, 100, 318–328. [Google Scholar] [CrossRef]
- Alphonse, P.; Courty, M. Structure and thermal behavior of nanocrystalline boehmite. Thermochim. Acta 2005, 425, 75–89. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hart, R.D.; Winter, K.; Studer, A.J.; Hunter, B.A.; Hanna, J.V. Boehmite Derived γ-Alumina System. 1. Structural Evolution with Temperature, with the Identification and Structural Determination of a New Transition Phase, γ′-Alumina. Chem. Mater. 2004, 16, 220–236. [Google Scholar] [CrossRef]
- Krokidis, X.; Raybaud, P.; Gobichon, A.E.; Rebours, B.; Euzen, P.; Toulhoat, H. Theoretical study of the dehydration process of boehmite to γ-alumina. J. Phys. Chem. B 2001, 105, 5121–5130. [Google Scholar] [CrossRef]
- Tsuchida, T.; Furuichi, R.; Ishii, T. Kinetics of Dehydration of Boehmites Prepared under Different Hydrothermal Conditions. Thermochim. Acta 1980, 39, 103–115. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Udovic, T.J.; Rohl, A.L.; Jones, F.; Maitland, C.F.; Connolly, J. Boehmite-derived γ-alumina system. 2. Consideration of hydrogen and surface effects. Chem. Mater. 2004, 16, 1914–1923. [Google Scholar] [CrossRef]
- Pille, A.; Amamra, M.; Kanaev, A.; Schoenstein, F. Microstructure and optical properties of alumina sintered from various phases. Ceram. Int. 2019, 45, 9625–9630. [Google Scholar] [CrossRef]
- Zaman, A.C.; Üstündaĝ, C.B.; Çelik, A.; Kara, A.; Kaya, F.; Kaya, C. Carbon nanotube/boehmite-derived alumina ceramics obtained by hydrothermal synthesis and spark plasma sintering (SPS). J. Eur. Ceram. Soc. 2010, 30, 3351–3356. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J. Carbon nanotubes reinforced alumina matrix nanocomposites for conductive ceramics by additive manufacturing. Procedia Manuf. 2020, 48, 763–769. [Google Scholar] [CrossRef]
- Padture, N.P. Multifunctional composites of ceramics and single-walled carbon nanotubes. Adv. Mater. 2009, 21, 1767–1770. [Google Scholar] [CrossRef]
- Ramírez, C.; Vega-Diaz, S.M.; Morelos-Gómez, A.; Figueiredo, F.M.; Terrones, M.; Osendi, M.I.; Belmonte, M.; Miranzo, P. Synthesis of conducting graphene/Si3N4 composites by spark plasma sintering. Carbon 2013, 57, 425–432. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Campbell, P.G.; Baumann, T.F.; Worsley, M.A. Carbon aerogel evolution: Allotrope, graphene-inspired, and 3D-printed aerogels. J. Mater. Res. 2017, 32, 4166–4185. [Google Scholar] [CrossRef]
- Saud, M.N. Synthesis of gamma alumina for catalyst support using yeast cell as pore forming agent using regression model. J. Eng. Appl. Sci. 2018, 13, 9558–9563. [Google Scholar]
- Zhang, M.; Li, X.; Zhang, M.; Xiu, Z.; Li, J.G.; Li, J.; Xie, M.; Chen, J.; Sun, X. High-strength macro-porous alumina ceramics with regularly arranged pores produced by gel-casting and sacrificial template methods. J. Mater. Sci. 2019, 54, 10119–10129. [Google Scholar] [CrossRef]
- Polzin, C.; Günther, D.; Seitz, H. 3D printing of porous Al2O3 and SiC ceramics. J. Ceram. Sci. Technol. 2015, 6, 141–146. [Google Scholar] [CrossRef]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, Bio-Inspired Hybrid Materials. Science 2008, 322, 1516–1521. [Google Scholar] [CrossRef] [PubMed]


| 3D-Structure | ρ (g·cm−3) | Total Porosity (%) | Rod Porosity (%) | Vol. Shrinkage (%) | Specific Surface Area (m2·g−1) |
|---|---|---|---|---|---|
| Boehmite | 1.35 | 82 | 55 | - | 101 |
| γ-Al2O3 | 1.43 | 85 | 60 | 9 | 126 |
| α-Al2O3 | 1.95 | 80 | 50 | 41 | 9 |
| 3D-Structure | ρ (g·cm−3) | Total Porosity (%) | Rod Porosity (%) | Vol. Shrinkage (%) | Specific Surface Area (m2·g−1) |
|---|---|---|---|---|---|
| Boehmite/MWCNT | 1.45 | 83 | 51 | - | 77 |
| γ-Al2O3/MWCNT | 1.36 | 87 | 62 | 2 | 136 |
| α-Al2O3/MWCNT | 1.67 | 84 | 57 | 34 | 17 |
| Boehmite/rGO ribbon | 1.58 | 81 | 47 | - | 83 |
| γ-Al2O3/rGO ribbon | 1.37 | 85 | 62 | 9 | 137 |
| α-Al2O3/rGO ribbon | 2.42 | 80 | 38 | 39 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, C.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Reinforced 3D Composite Structures of γ-, α-Al2O3 with Carbon Nanotubes and Reduced GO Ribbons Printed from Boehmite Gels. Materials 2021, 14, 2111. https://doi.org/10.3390/ma14092111
Ramírez C, Belmonte M, Miranzo P, Osendi MI. Reinforced 3D Composite Structures of γ-, α-Al2O3 with Carbon Nanotubes and Reduced GO Ribbons Printed from Boehmite Gels. Materials. 2021; 14(9):2111. https://doi.org/10.3390/ma14092111
Chicago/Turabian StyleRamírez, Cristina, Manuel Belmonte, Pilar Miranzo, and Maria Isabel Osendi. 2021. "Reinforced 3D Composite Structures of γ-, α-Al2O3 with Carbon Nanotubes and Reduced GO Ribbons Printed from Boehmite Gels" Materials 14, no. 9: 2111. https://doi.org/10.3390/ma14092111
APA StyleRamírez, C., Belmonte, M., Miranzo, P., & Osendi, M. I. (2021). Reinforced 3D Composite Structures of γ-, α-Al2O3 with Carbon Nanotubes and Reduced GO Ribbons Printed from Boehmite Gels. Materials, 14(9), 2111. https://doi.org/10.3390/ma14092111









