The Effect of Temperature and Sputtered Particles on the Wettability of Al/Al2O3
Abstract
:1. Introduction
2. Materials and Methods
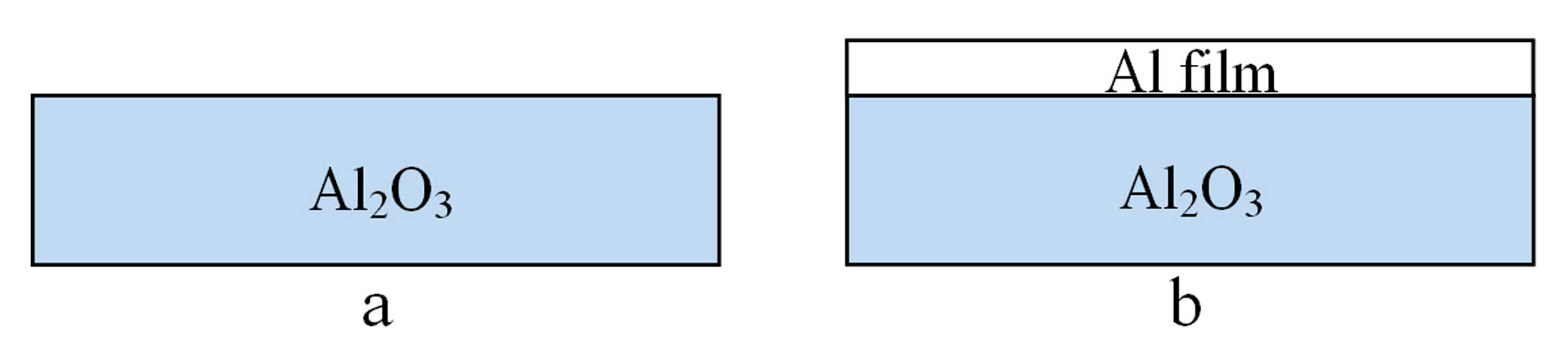
2.1. Sample Design
2.2. Thin Film Deposition
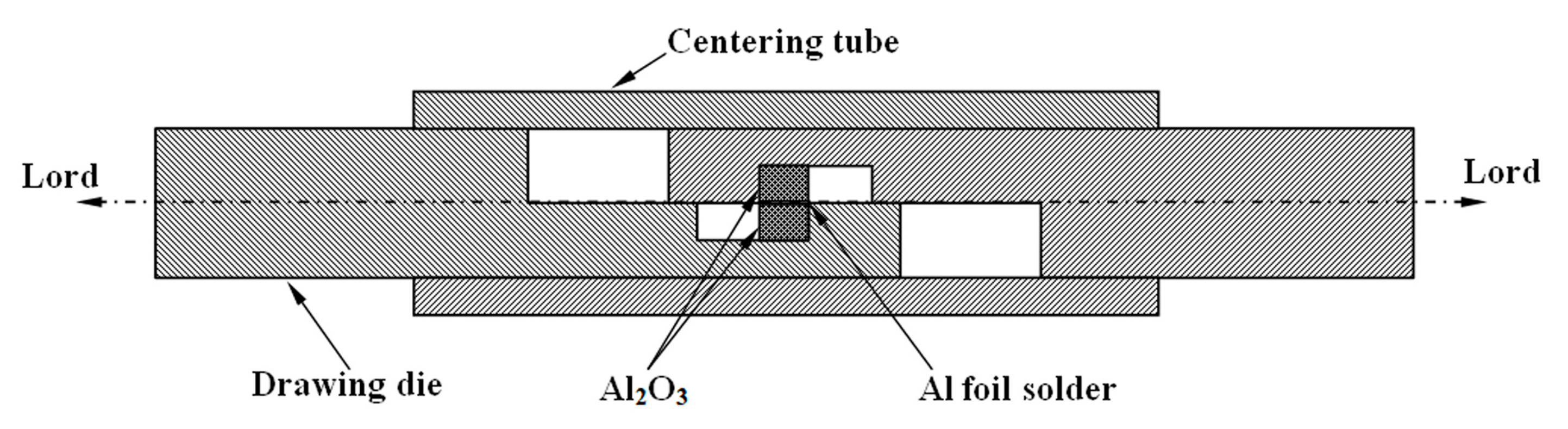
2.3. Wetting Experiment
2.4. Interface Strength Test
3. Results
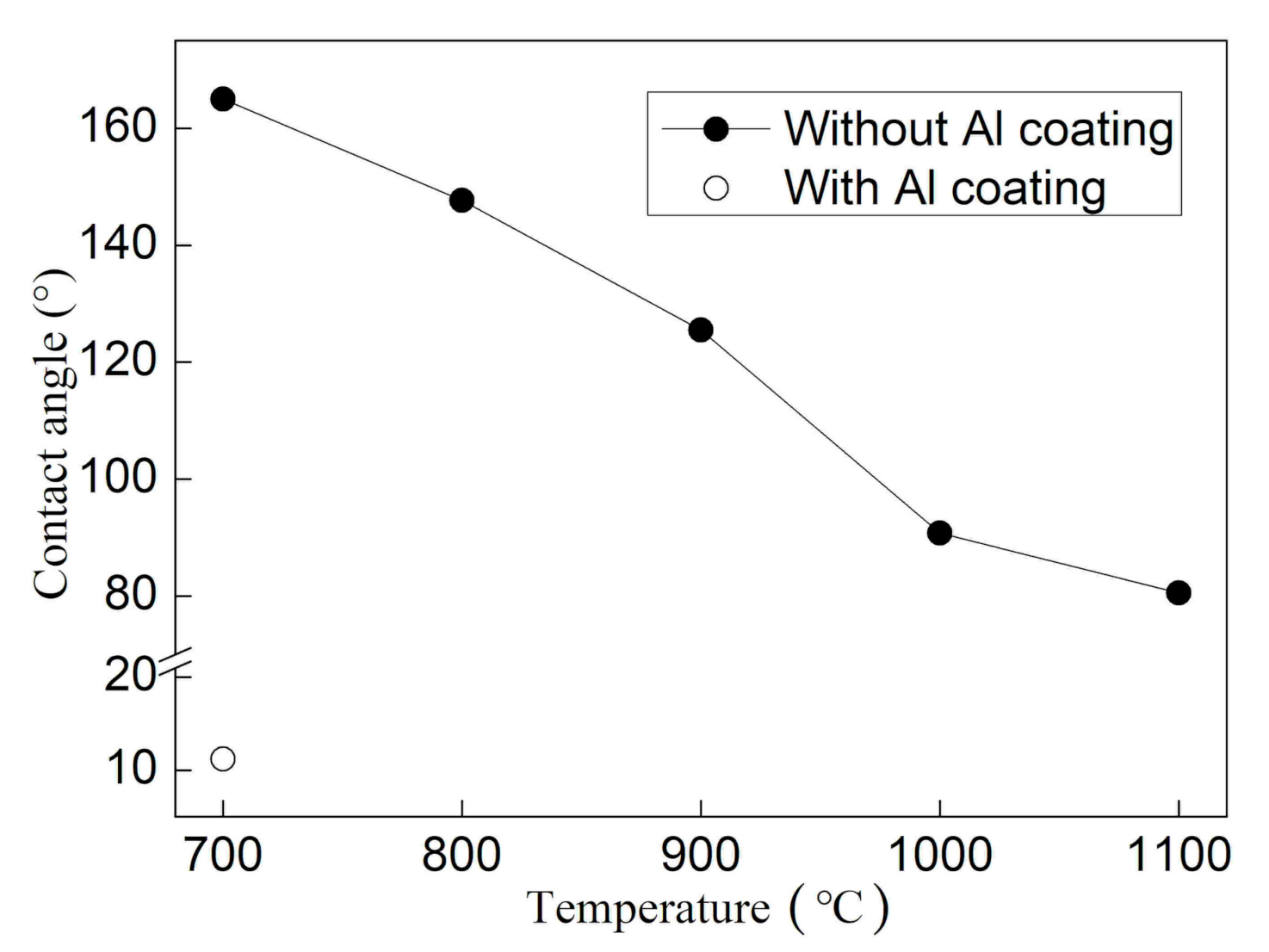
3.1. Wetting Behavior
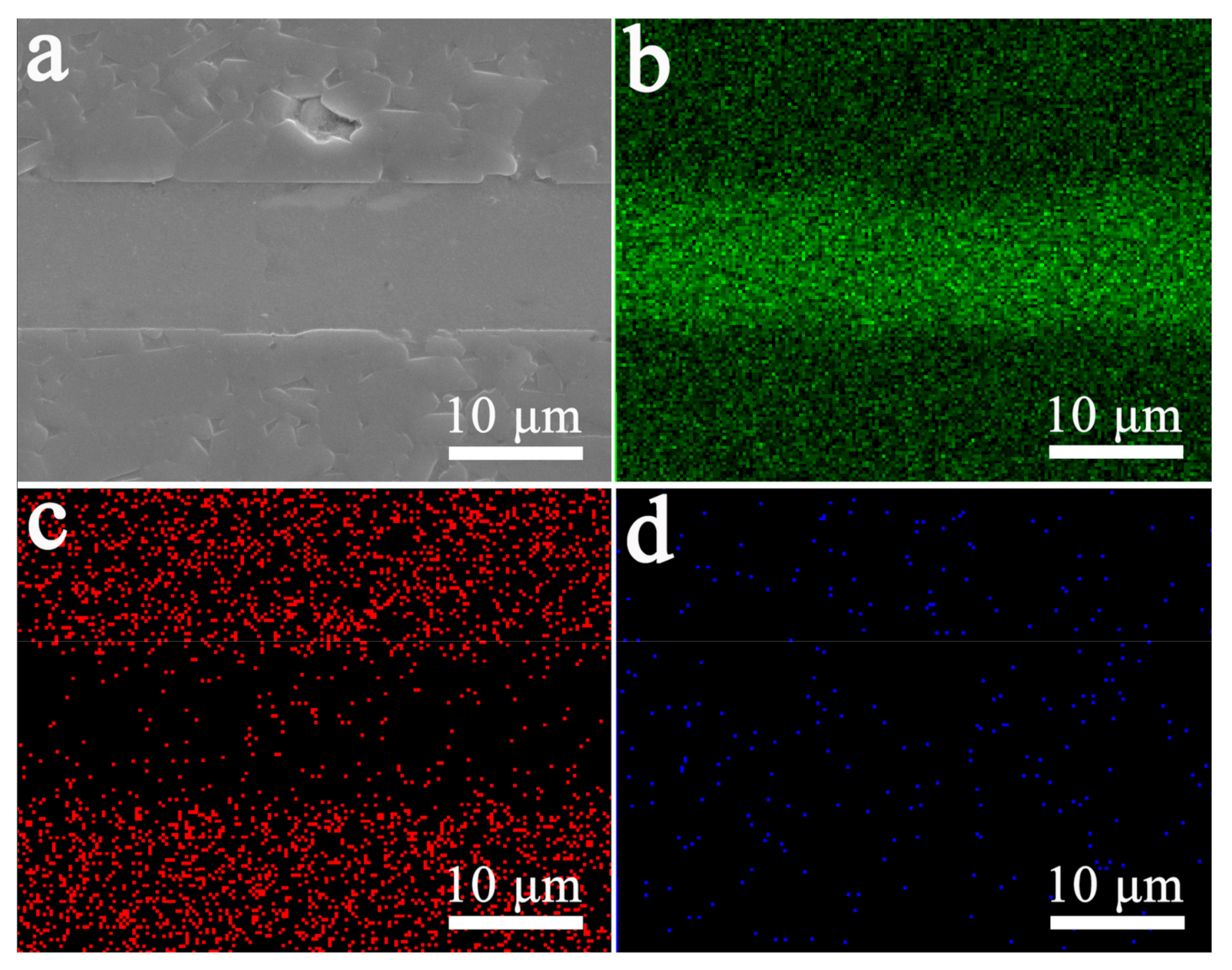
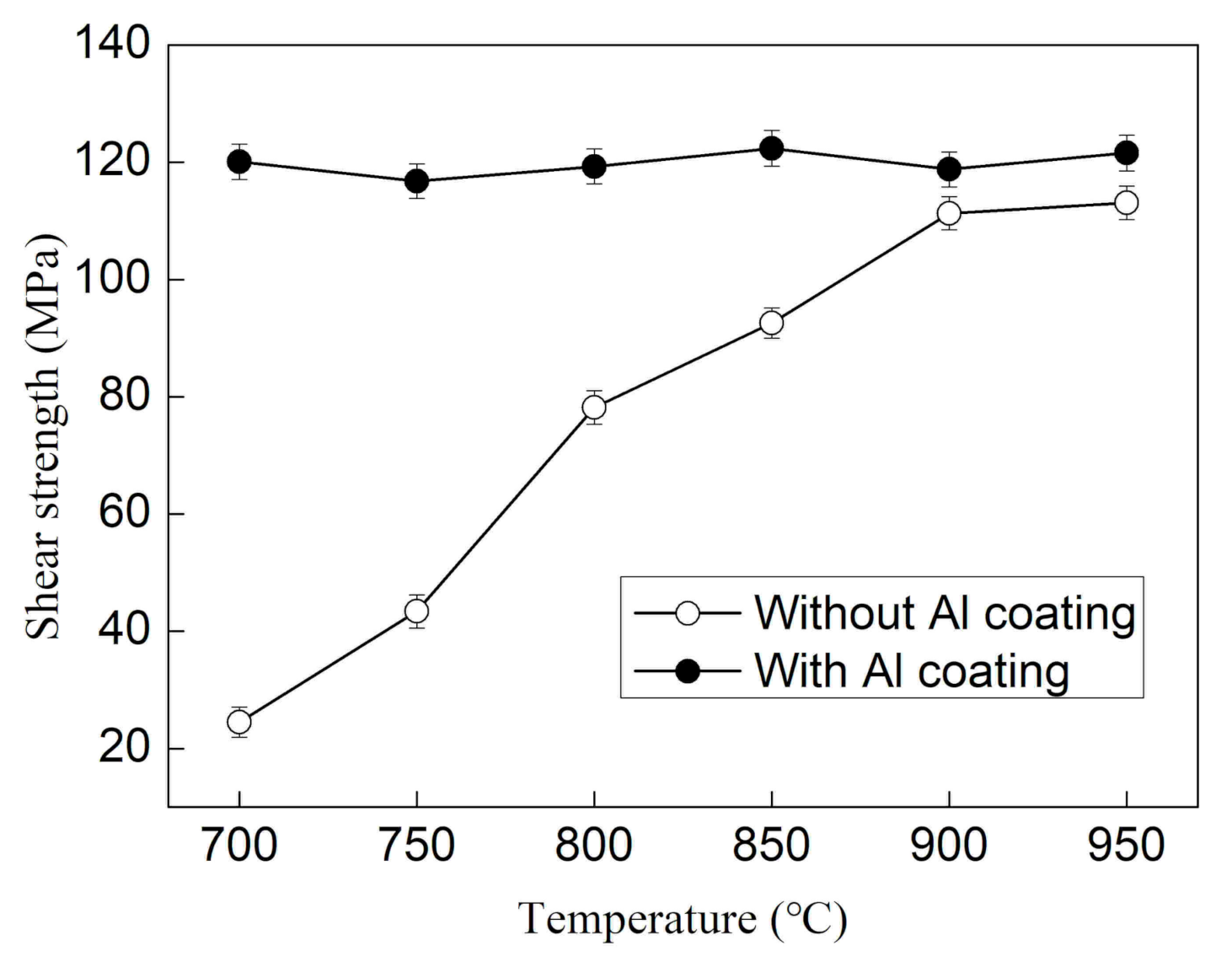
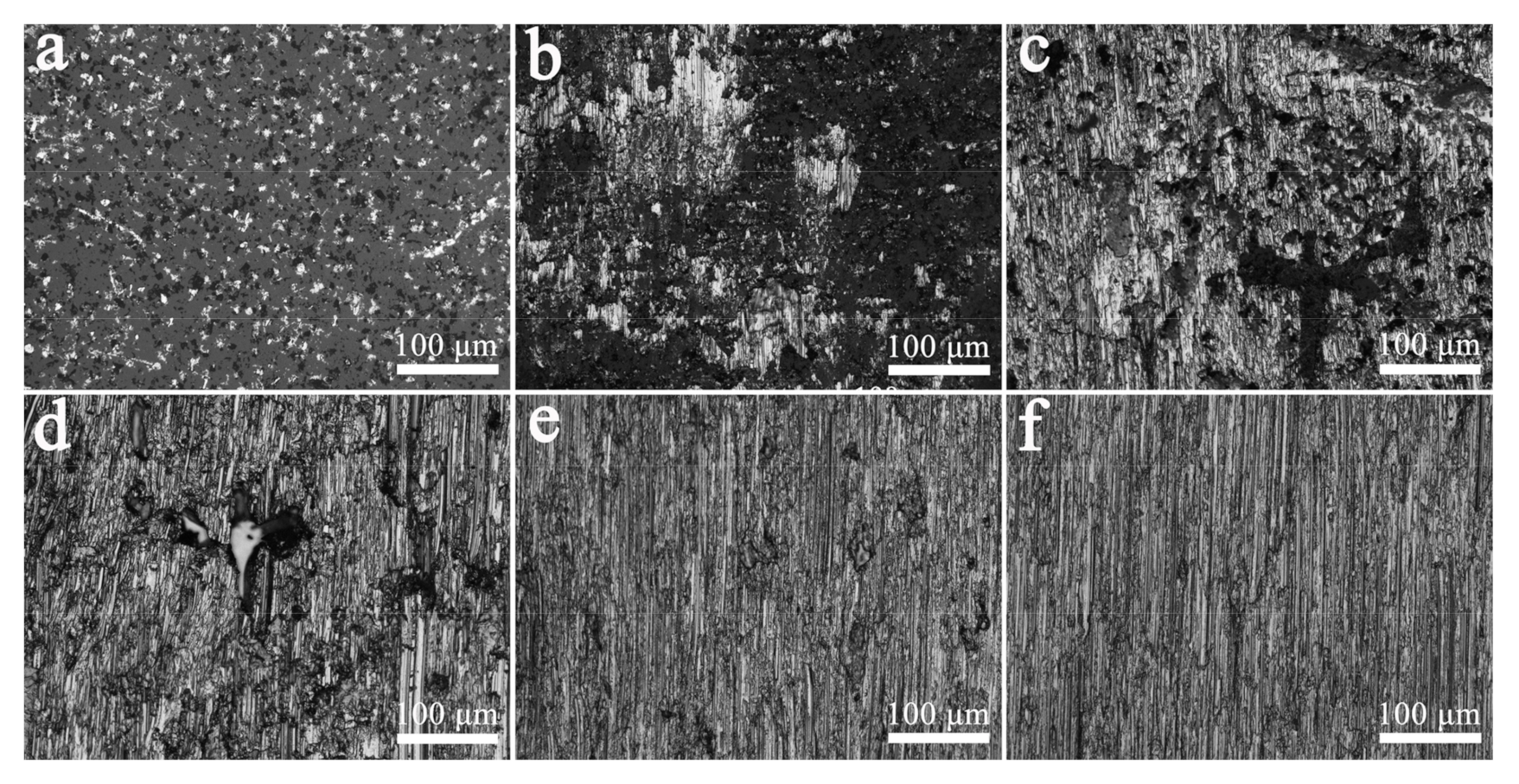
3.2. Interface Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Yan, R.; Zong, N.; Davidchack, R.L.; Dong, H. Unveiling the influence of interfacial bonding and dynamics on solid/liquid interfacial structures: An ab initio molecular dynamics study of (0001) sapphire-liquid Al interfaces. Phys. Rev. Mater. 2020, 4, 023401. [Google Scholar] [CrossRef]
- Fang, C.; Yasmin, S.; Fan, Z. Interfacial interaction and pre-nucleation at liquid-Al/γ-Al2O3{1 1 1} interfaces. J. Phys. Commun. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Voigt, C.; Ditscherlein, L.; Werzner, E.; Zienert, T.; Nowak, R.; Peuker, U.; Sobczak, N.; Aneziris, C.G. Wettability of AlSi7Mg alloy on alumina, spinel, mullite and rutile and its influence on the aluminum melt filtration efficiency. Mater. Des. 2018, 150, 75–85. [Google Scholar] [CrossRef]
- Schneider, G.; Marta, F.; Weber, L.; Mortensen, A. Kinetic processes in the high-temperature pressure-infiltration of Al into Al2O3. Acta Mater. 2020, 189, 105–117. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, R.; Dandapat, N.; Pal, N.S.; Datta, S.; Asu, D.B. Characterization of alumina–alumina/graphite/monel superalloy brazed joints. Ceram. Int. 2012, 38, 663–670. [Google Scholar] [CrossRef]
- Sangghaleh, A.; Halali, M. An investigation on the wetting of polycrystalline alumina by aluminium. J. Mater. Process. Technol. 2008, 197, 156–160. [Google Scholar] [CrossRef]
- Xia, H.; Wu, A.; Fan, F.; Zou, G.; Ren, J. Effects of ion implantation on the brazing properties of high purity alumina. Surf. Coat. Technol. 2012, 206, 2098–2104. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, Y.M. Direct observation of in-plane ordering in the liquid at a liquid Al/α-Al2O3 interface. Acta Mater. 2011, 59, 1383–1388. [Google Scholar] [CrossRef]
- Yang, J.; Bao, S.; Akhtar, S.; Shen, P.; Li, Y. Influence of grain refiners on the wettability of Al2O3 substrate by aluminum melt. Metall. Mater. Trans. B 2020, 52, 382–392. [Google Scholar] [CrossRef]
- Zhang, Q.; Cagin, T.; Duin, A.V.; Iii, W.A.G.; Yue, Q.; Hector, L.G., Jr. Adhesion and nonwetting-wetting transition in the Al/α-Al2O3 interface. Phys. Rev. B 2004, 69, 045423. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Matsumoto, T.; Nogi, K. The influence of surface structure on wetting of α-Al2O3 by aluminum in a reduced atmosphere. Acta Mater. 2003, 51, 4897–4906. [Google Scholar] [CrossRef]
- Aguilar-Santillan, J. Wetting of Al/sapphire (0001) system: Measurement effect and affecting factors. Metall. Mater. Trans. B 2009, 40, 376–387. [Google Scholar] [CrossRef]
- Klinter, A.J.; Suarez, G.M.; Drew, R.A.L. Wetting of pure aluminum and selected alloys on polycrystalline alumina and sapphire. Mater. Sci. Eng. A 2008, 459, 147–152. [Google Scholar] [CrossRef]
- Levi, G.; Kaplan, W.D. Oxygen induced interfacial phenomena during wetting of alumina by liquid aluminium. Acta Mater. 2002, 50, 75–88. [Google Scholar] [CrossRef]
- Brennan, J.J.; Pask, J.A. Effect of nature of surfaces on wetting of sapphire by liquid aluminum. J. Am. Ceram. Soc. 1968, 51, 569–573. [Google Scholar] [CrossRef]
- Jung, W.; Song, H.; Sang, W.P.; Kim, D.Y. Variation of contact angles with temperature and time in the Al-Al2O3 system. Metall. Mater. Trans. B 1996, 27, 51–55. [Google Scholar] [CrossRef]
- Naidich, Y.V.; Chubashov, Y.N.; Ishchuk, N.F.; Krasovskii, V.P. Wetting of some nonmetallic materials by aluminum. Powder Metal. Met. Ceram. 1983, 22, 481–483. [Google Scholar] [CrossRef]
- Ping, S.; Fujii, H.; Matsumoto, T.; Nogi, K. Critical factors affecting the wettability of α-Alumina by molten aluminum. J. Am. Ceram. Soc. 2004, 87, 2151–2159. [Google Scholar]
- Bao, S.; Tang, K.; Kvithyld, A.; Tangstad, M.; Engh, T.A. Wettability of aluminum on alumina. Metall. Mater. Trans. B 2011, 42, 1358–1366. [Google Scholar] [CrossRef]
- Zheng, W.T. Physical methods of film preparation. In Thin Film Materials and Thin Film Technology, 2nd ed.; Ding, S.L., Ed.; Chemical Industry Press: Beijing, China, 2004; p. 75. [Google Scholar]
- Zhang, X.Y.; Ma, B.Y.; Li, R.B.; Li, G.Y. Brazing of coated Al foil filler to AlN ceramic. Acta Metall. Sin. 2018, 54, 575–580. [Google Scholar]
- Sun, S.Y.; Xu, P.P.; Ma, B.Y.; Shang, H.L.; Li, G.Y. Effects of temperature and O partial pressure on the atomic structure of Al2O3 (0001) surface. Comput. Mater. Sci. 2019, 157, 37–42. [Google Scholar] [CrossRef]
- Pilania, G.; Thijsse, B.J.; Hoagland, R.G.; Lazic, I.; Liu, X.Y. Revisiting the Al/Al2O3 interface: Coherent interfaces and misfit accommodation. Sci. Rep. 2014, 4, 1–9. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Shang, H.; Ma, B.; Guo, X.; Li, R.; Li, G. The Effect of Temperature and Sputtered Particles on the Wettability of Al/Al2O3. Materials 2021, 14, 2110. https://doi.org/10.3390/ma14092110
Li Y, Shang H, Ma B, Guo X, Li R, Li G. The Effect of Temperature and Sputtered Particles on the Wettability of Al/Al2O3. Materials. 2021; 14(9):2110. https://doi.org/10.3390/ma14092110
Chicago/Turabian StyleLi, Yang, Hailong Shang, Bingyang Ma, Xuqiang Guo, Rongbin Li, and Geyang Li. 2021. "The Effect of Temperature and Sputtered Particles on the Wettability of Al/Al2O3" Materials 14, no. 9: 2110. https://doi.org/10.3390/ma14092110
APA StyleLi, Y., Shang, H., Ma, B., Guo, X., Li, R., & Li, G. (2021). The Effect of Temperature and Sputtered Particles on the Wettability of Al/Al2O3. Materials, 14(9), 2110. https://doi.org/10.3390/ma14092110






