Hardening Slurries with Fluidized-Bed Combustion By-Products and Their Potential Significance in Terms of Circular Economy
Abstract
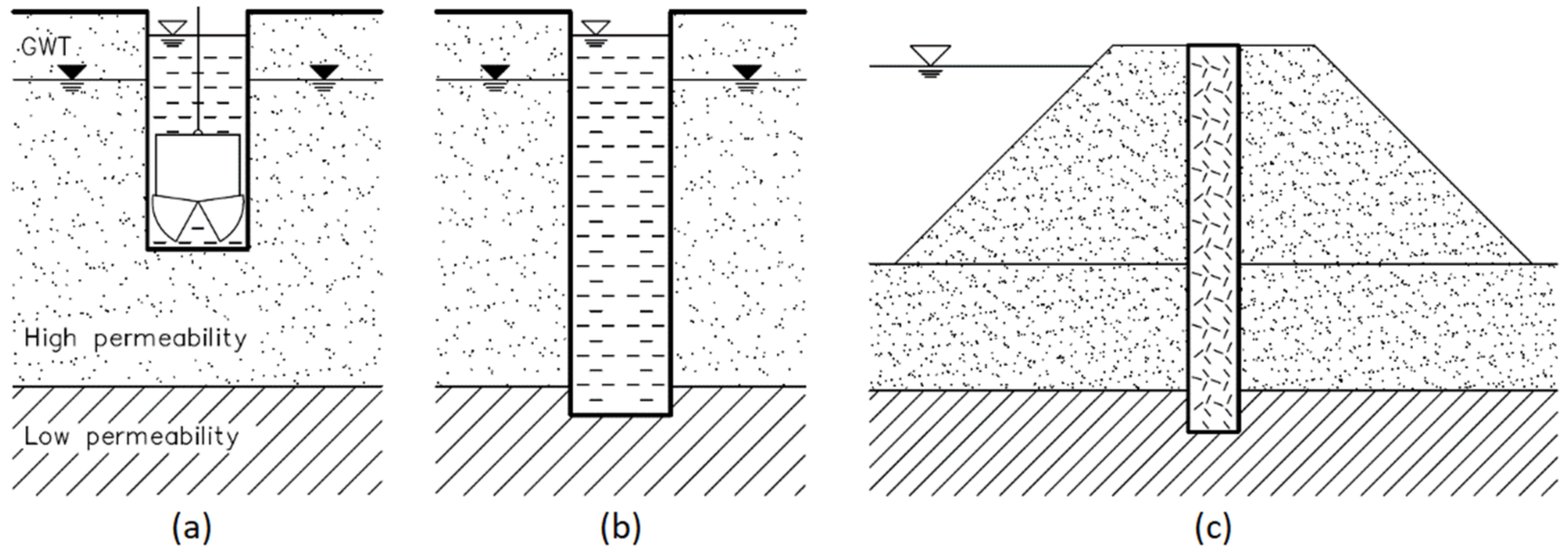
1. Introduction
- bulk density: determines the pressure supporting excavation walls and displacement potential in the two-phase method (1.15–1.55 g/cm3);
- viscosity: important in the stages of slurry production, pumping, and trench execution and determines the susceptibility of the slurry to penetration into the ground and to displacement in the two-phase method (35–70 s);
- water bleed: measure of slurry stability and its segregation tendency (0–6%);
- structural (gel) strength: associated with slurry thixotropy, which is the sole ability to turn into gel while resting. This gel exhibits a certain shear strength, referred to as “structural”, which limits the sedimentation of slurry components and excavated soil (from 5 to over 30 Pa).
- hydraulic conductivity (filtration coefficient k): a sufficiently low conductivity is the most important feature of a material in a finished cut-off wall (<10−8 m/s after 28 days of curing) [10];
- compressive strength: important in terms of material durability and maintaining cut-off wall integrity under earth and groundwater pressure conditions. Slurries typically achieve a uniaxial compressive strength of no more than several MPa (above 0.5–1.0 MPa) [11];
- corrosion resistance: the filtering water might contain pollutants that can react with the components of a hardened slurry. The available test methods involve slurry specimen storage in aggressive solutions or filtration through the specimen using aggressive solutions. Symptoms evidencing susceptibility to aggression are mass loss, strength reduction, and an increasing filtration coefficient [4]. A review by Huang et al. [1] claims that poor resistance to sulfates and acids is a major problem for many common slurry mixes;
- ability to immobilize heavy metals: the use of industrial waste in the composition of a hardening slurry poses a risk of pollutants (e.g., heavy metals) contained therein being released to groundwater. Immobilization testing involves, e.g., measuring heavy metal content in filtrates originating from the hardening slurry specimen filtration testing [12].
2. Materials and Methods
2.1. Recipes of Tested Slurries
2.2. Fluidized-Bed Combustion Fly-Ash
2.3. Ash from the Thermal Treatment of Municipal Sewage Sludge
2.4. Blast Furnace Slag
2.5. Research Scope and Methodology
- bulk density using a Baroid scale, a simple measurement involving filling a container of known volume with the slurry and balancing the scale [36];
- relative viscosity using a flow viscometer, measured as the outflow time for a specified volume of slurry from a standard vessel (Marsh funnel) [36]—the longer the time, the higher the viscosity;
- daily water bleed, the relative volume of water released from the slurry spontaneously after being left to rest for 24 h and observed in a transparent graduated cylinder [37];
- structural (gel) strength, measured using a shearometer and based on the sinking depth of a hollow tin cylinder under its own weight in the slurry, after letting the slurry rest for 10 min [36].
- compressive strength via an unconfined compression test in a hydraulic press, as per [38], conducted on cylinder specimens (d = 8 cm, h = 8 cm);
- hydraulic conductivity (filtration coefficient k10, i.e., at a temperature of +10 °C), with a variable hydraulic gradient [10]. The method consists of determining, in established times, the values of water pressure in a supply tube of a certain cross-section area during the liquid’s flow through the specimen of a certain height;
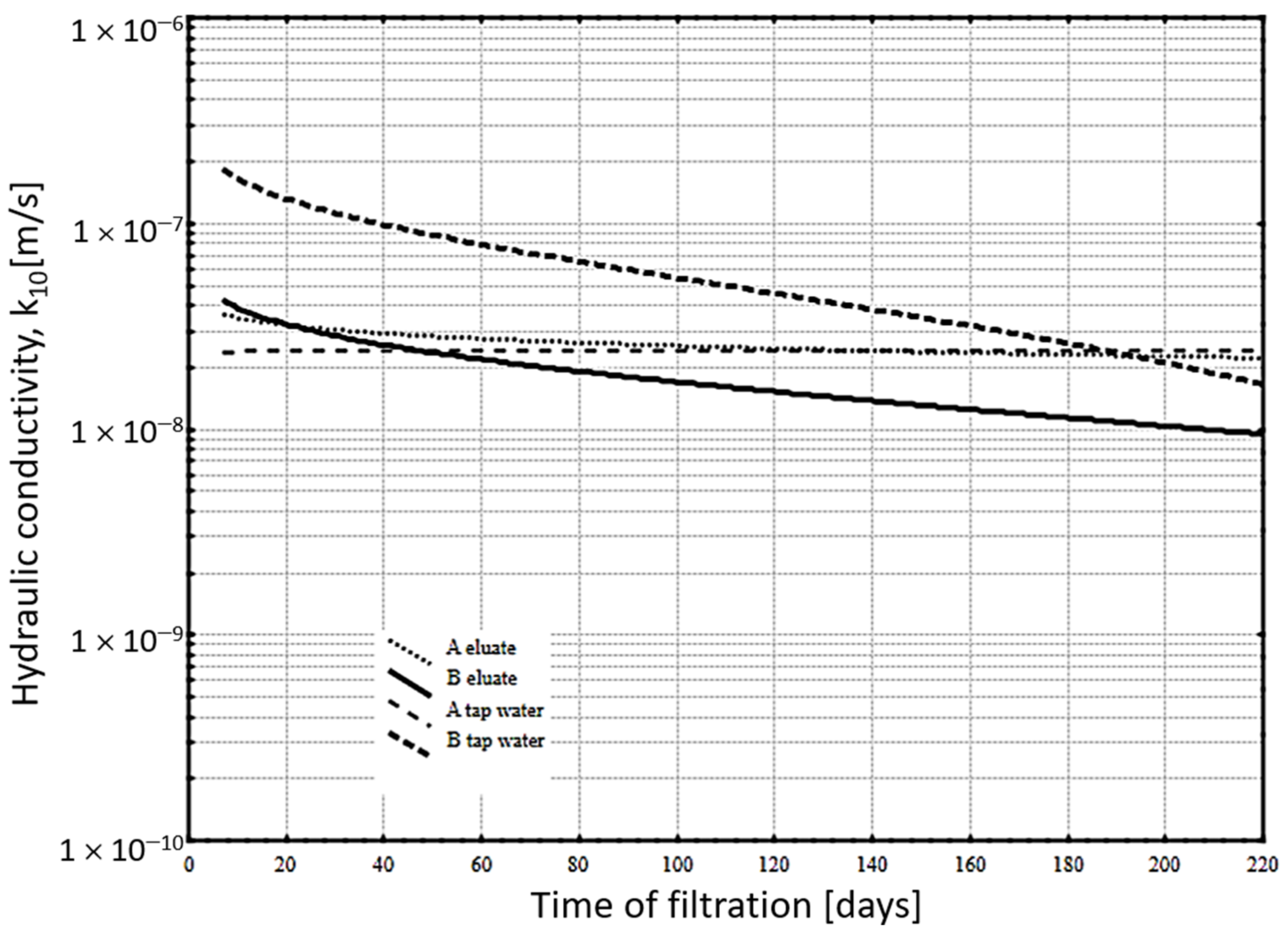
- corrosion resistance (only slurries A and B) using the so-called adequacy test, which means a long-term hydraulic permeability test of the slurry under conditions of filtration flow of eluates from a specific landfill (Table 3) that is to be surrounded by a cut-off wall. The test, involving eluates with a complex chemical composition, does not only verify the sealing properties of a cut-off wall material—rather, it is a prognosis of its durability under conditions of complex corrosive aggressiveness. The italics in Column 5 of Table 3 mark values that exceed the permissible—in this case, those determined in the perspective of domestic regulations on protecting waters against pollutants. Among these highlighted values, a bold font additionally indicates the content of ammonia nitrogen—since the XA3 exposure class limit value according to [39], which is a requirement specified from the perspective of ammonia aggressiveness of cement-based materials—has been exceeded almost sevenfold;
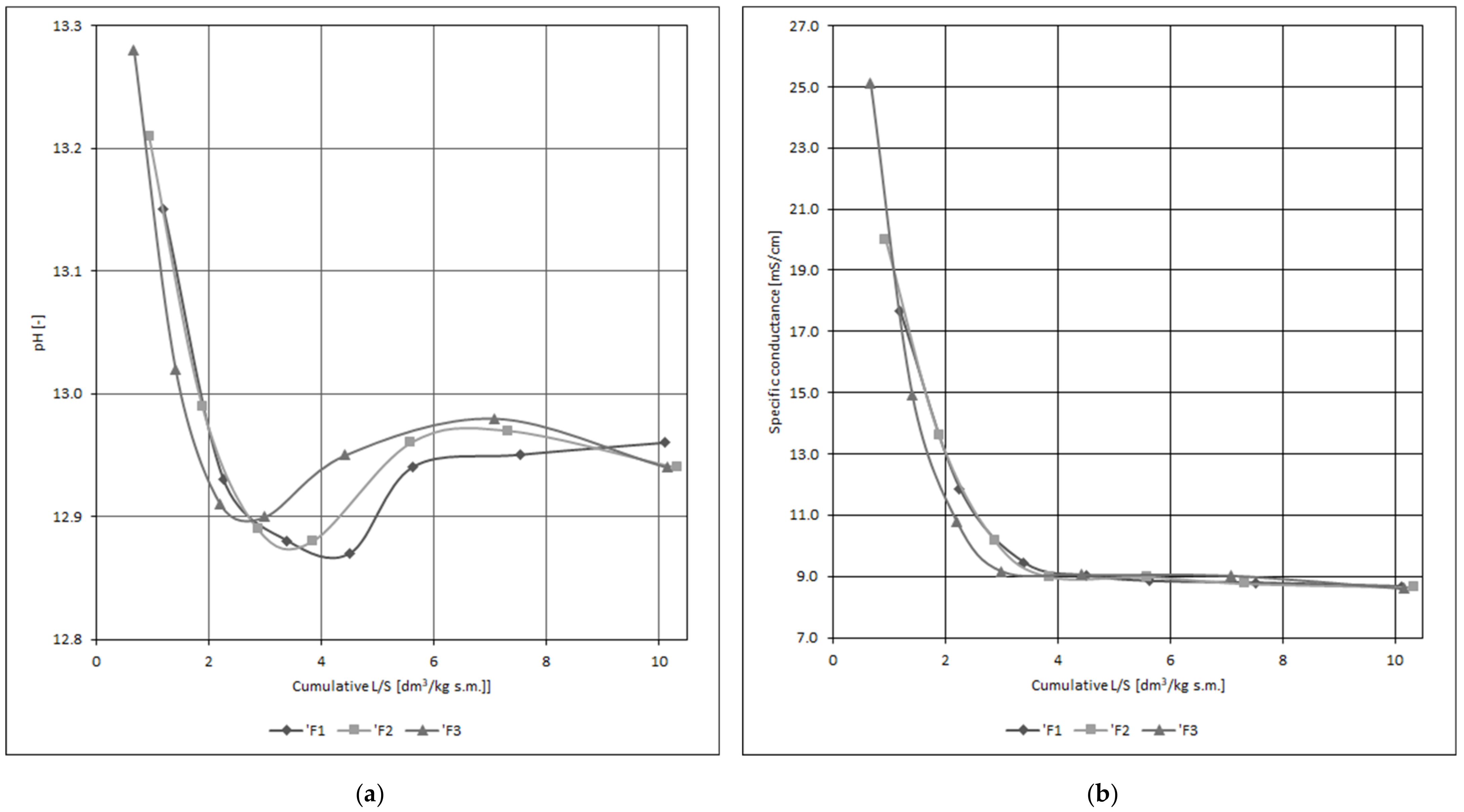
- heavy metal leachability (only slurry C) for this purpose, hardening slurry specimens after 28 days of curing in tap water were filtered with distilled water (simulation of slurry operation in the ground). The medium flow was laminar and a quasi-constant hydraulic gradient was applied. The eluate (specimen filtrate) was collected in 7 fractions with increasing volume (associated with specimen dry mass), until the obtained L/S (liquid to solid) ratio = 10 dm3/kg d.m. The reaction and specific conductivity were determined in eluates obtained this way, and after they were preserved with nitric acid (V), the heavy metal content was then determined utilizing the flame atomic absorption spectroscopy (FAAS) method. The release intensity for the elements in question was calculated similarly to eluate sampling within the percolation method [40];
- porosity (only slurries A and B) with the mercury porosimetry method;
- phase composition with methods such as X-ray diffraction analysis (XRD, Bruker, Karlsruhe, Germany)—employing a Bruker D8 Advance device equipped with a LYNXEYE position-sensitive detector that operates within the Bragg-Brentano geometry using CuKα (λ = 0.15418 nm) radiation with a nickel filter—and scanning electron microscopy (SEM, Oxford Instruments Ltd., Abingdon, UK) with a ZEISS LEO 1430 scanning electron microscope equipped with an Oxford ISIS 300 energy-dispersive detector (EDS, Oxford Instruments, Abingdon, UK) at the Institute of High Pressure of the Polish Academy of Sciences (PAN) in Warsaw.
3. Results and Analysis
3.1. Slurry A and B
3.2. Slurry C
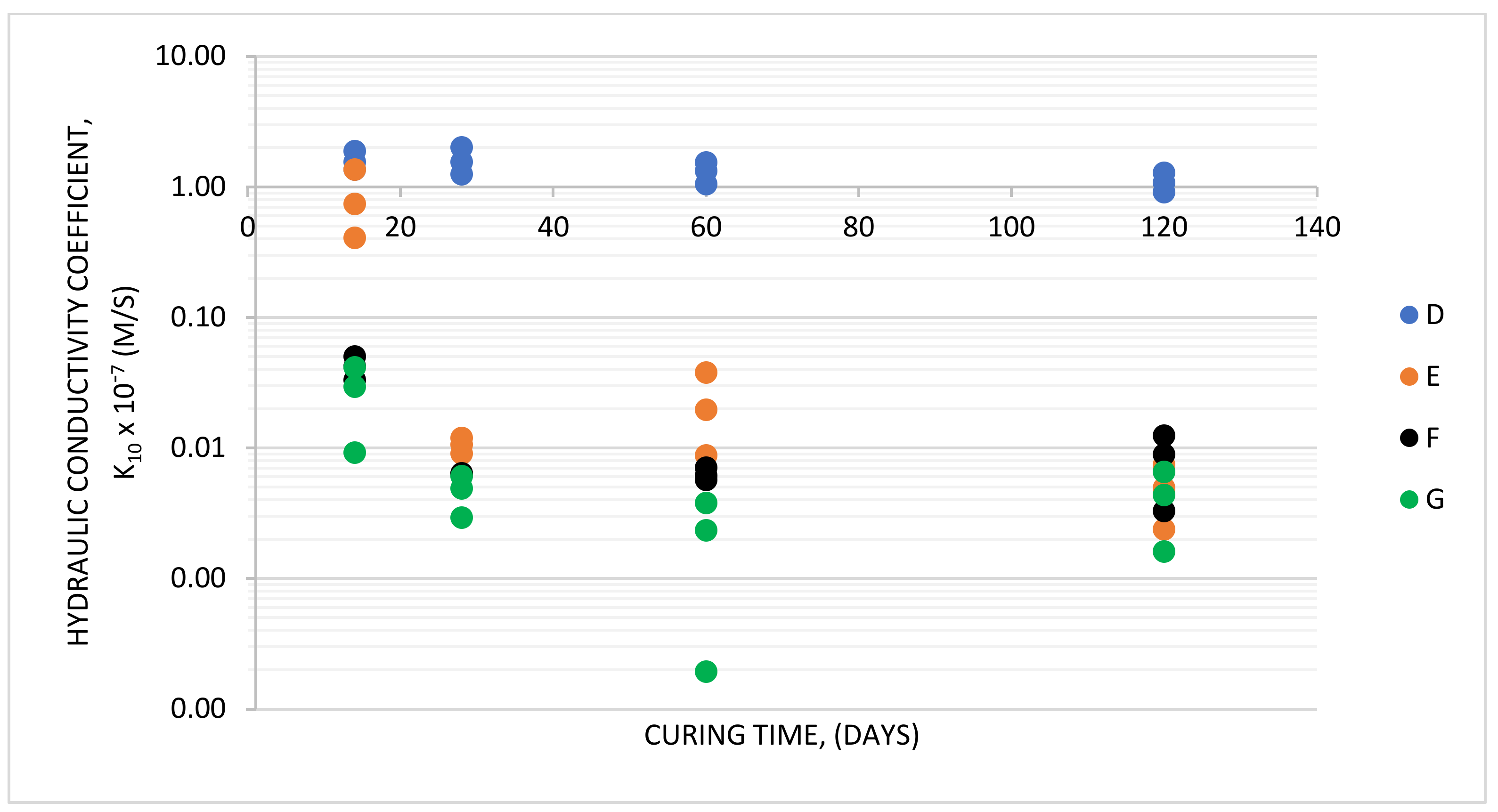
3.3. Slurries D–G
4. Potential Significance of Hardening Slurries in Cut-Off Walls as a Deposit for Combustion By-Products in Terms of Circular Economy
5. Conclusions
- Cement-bentonite-ash-aqueous slurries subjected to landfill eluate filtration did not undergo corrosive destruction. Instead, they exhibited an increasing tightness (reduced hydraulic conductivity coefficient) compared to the permeability tests utilizing tap water. The test results confirm the high resistance of slurries with fluidized-bed fly ash additives (both hard and brown coal) to an aggressive environment of municipal landfill eluates.
- It is possible to produce a hardening slurry with desired technological and performance properties using fly ash from Thermal Treatment of Municipal Sewage Sludge (TTMSS).
- The hardening slurry with TTMSS fly ash was characterized by high immobilization in respect of tested heavy metals.
- The most leachable element was lead. The concentrations of other studied elements (zinc, copper, cadmium, and mercury) in eluates were low (below the determination limit).
- It is possible to produce cement-free bentonite-aqueous hardening slurries with blast furnace slag activated by the addition of specific fluidized-bed brown coal combustion fly ash as the binder. The progress of binder hydration over time is evidenced by the changes ongoing in the course of slurry curing. They include increasing compressive strength and declining hydraulic conductivity, and result in a tightening of the slurry structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Li, J.; Xue, Q.; Chen, Z.; Du, Y.; Wan, Y.; Liu, L.; Poon, C.S. Use of self-hardening slurry for trench cutoff wall: A review. Constr. Build. Mater. 2021, 286, 122959. [Google Scholar] [CrossRef]
- Jefferis, S. Cement-bentonite slurry systems. In Proceedings of the Fourth International Conference on Grouting and Deep Mixing, New Orleans, LA, USA, 15–18 February 2012; pp. 1–24. [Google Scholar] [CrossRef]
- Jefferis, S.A. Grouts and slurries. In Construction Materials Reference Book, 2nd ed.; Doran, D., Cather, B., Eds.; Routledge: Abingdon, UK, 2013; pp. 165–194. [Google Scholar]
- Kledyński, Z.; Rafalski, L. Zawiesiny Twardniejące; Komitet Inżynierii Lądowej i Wodnej PAN: Warsaw, Poland, 2009. [Google Scholar]
- Ruffing, D.; Evans, J.C. Soil Mixing and Slurry Trench Cutoff Walls for Coal Combustion Residue Sites. In Proceedings of the 2019 World of Coal Ash, St. Louis, MO, USA, 13–16 May 2019. [Google Scholar]
- Bruce, D.A.; di Cervia, A.R.; Amos-Venti, J. Seepage remediation by positive cut-off walls: A compendium and analysis of North American case histories. In Proceedings of the ASDSO Annual Conference, Boston, MA, USA, 10–14 September 2006. [Google Scholar]
- Falaciński, P.; Szarek, Ł. Possible applications of hardening slurries with fly ash from thermal treatment of municipal sewage sludge in environmental protection structures. Arch. Hydro-Eng. Environ. Mech. 2016, 63, 47–61. [Google Scholar] [CrossRef]
- Falaciński, P. Influence of fly ash from the thermal treatment of municipal sewage sludge on chosen properties of hardening slurries. Tech. Trans. 2017, 114, 125–138. [Google Scholar] [CrossRef]
- Kledyński, Z.; Machowska, A. Stan i perspektywy wykorzystania materiałów mineralnych w konstrukcji przesłon przeciwfiltracyjnych. Mater. Bud. 2005, 2, 71–74. [Google Scholar]
- Falaciński, P. Leak tightness of hardening slurries with fluidal fly ashes in chemically aggressive environments. Arch. Environ. Prot. 2011, 37, 115–134. [Google Scholar]
- Kledyński, Z.; Falaciński, P.; Machowska, A.; Dyczek, J. Utilisation of CFBC fly ash in hardening slurries for flood-protecting dikes. Arch. Civ. Eng. 2016, 62, 75–88. [Google Scholar] [CrossRef]
- Szarek, Ł.; Falaciński, P.; Wojtkowska, M. Immobilization of selected heavy metals from fly ash from thermal treatment of municipal sewage sludge in hardening slurries. Arch. Civ. Eng. 2018, 64. [Google Scholar] [CrossRef]
- Statistics Poland. Ochrona środowiska 2019; Statistics Poland: Warsaw, Poland, 2019.
- Rajczyk, K. Popioły Lotne z Kotłów Fluidalnych i Możliwości ich Uszlachetniania; Wydawnictwo Instytut Śląski: Opole, Poland, 2012. [Google Scholar]
- Szarek, Ł. The influence of addition fly ash from thermal treatment of municipal sewage sludge on selected hardening slurries properties. In Monitoring and Safety of Hydrotechnical Constructions, Proceedings of the XVIII Technical Dam Control International Conference, Biała Woda, Poland, September 10–13 2019; IMGW: Warsaw, Poland, 2019; pp. 329–340. [Google Scholar]
- Czarnecki, L. Would recycled plastics be a driving force in concrete technology? J. Zhejiang Univ. A Appl. Phys. Eng. 2019, 20, 384–388. [Google Scholar] [CrossRef]
- Kledyński, Z.; Bogdan, A.; Jackiewicz-Rek, W.; Lelicińska-Serafin, K.; Machowska, A.; Manczarski, P.; Masłowska, D.; Rolewicz-Kalińska, A.; Rucińska, J.; Szczygielski, T. Condition of circular economy in Poland. Arch. Civ. Eng. 2020, 66. [Google Scholar] [CrossRef]
- Mixing Water for Concrete—Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, as Mixing Water for Concrete; PN-EN 1008:2004; Polish Committee for Standardization: Warsaw, Poland, 2004.
- Czarnecki, L.; Broniewski, T.; Henning, O. Chemia w Budownictwie, 1st ed.; Arkady: Warsaw, Poland, 1994. [Google Scholar]
- Fusova, L.; Cechlova, K.; Cablik, V. Badanie procesu sorpcji jonów Pb i Cu na bentonicie. Inżynieria Miner. 2011, 12, 11–18. [Google Scholar]
- Kledyński, Z. Odporność korozyjna zawiesin twardniejących w obiektach ochrony środowiska. Pr. Nauk. Politech. Warsz. Inżynieria Środowiska 2000, 33, 3–101. [Google Scholar]
- Kledyński, Z. Influence of fly ashes on hardening slurries resistance to sulphate attack. Arch. Hydro-Eng. Environ. Mech. 2004, 51, 119–133. [Google Scholar]
- Giergiczny, Z. Rola popiołów lotnych wapniowych i krzemionkowych w kształtowaniu właściwości współczesnych spoiw budowlanych i tworzyw cementowych. Monografia 325, Seria Inżynieria Lądowa; Cracow University of Technology: Cracow, Poland, 2006. [Google Scholar]
- Brandt, A.M.; Jóźwiak-Niedźwiedzka, D.; Małolepszy, J.; Marks, M.; Śliwiński, J.; Kasperkiewicz, J. Zastosowanie popiołów lotnych z kotłów fluidalnych w betonach konstrukcyjnych. Stud. Z Zakr. Inżynierii 2010, 72, 339–351. [Google Scholar]
- Zapotoczna-Sytek, G.; Łaskawiec, K.; Gębarowski, P.; Małolepszy, J.; Szymczak, J. Popioły Lotne nowej Generacji do Produkcji Autoklawizowanego Betonu Komórkowego—Monografia ICiMB; Wydawnictwo Instytut Śląski: Opole, Poland, 2013. [Google Scholar]
- Łukawska, M. Speciation analysis of phosphorus in sewage sludge after thermal utilization of sludge. Inżynieria Ochr. Środowiska 2014, 17, 433–439. [Google Scholar]
- Wzorek, Z. Odzysk Związków Fosforu z Termicznie Przetworzonych Odpadów i ich Zastosowanie Jako Substytutu Naturalnych Surowców Fosforowych; Politechnika Krakowska: Warsaw, Poland, 2008. [Google Scholar]
- Szarek, Ł. Leaching of heavy metals from thermal treatment municipal sewage sludge fly ashes. Arch. Environ. Prot. 2020, 49–59. [Google Scholar] [CrossRef]
- Szarek, Ł.; Wojtkowska, M. Properties of fly ash from thermal treatment of municipal sewage sludge in terms of EN 450-1. Arch. Environ. Prot. 2018, 44. [Google Scholar] [CrossRef]
- Franz, M. Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 2008, 28, 1809–1818. [Google Scholar] [CrossRef]
- Białowiec, A.; Janczukowicz, W.; Krzemieniewski, M. Possibilities of management of waste fly ashes from sewage sludge thermal treatment in the aspect of legal regulations. Rocz. Ochr. Sr. 2009, 11, 959–971. [Google Scholar]
- Lam, C.H.; Barford, J.P.; McKay, G. Utilization of incineration waste ash residues in Portland cement clinker. Chem. Eng. 2010, 21, 757–762. [Google Scholar] [CrossRef]
- Rutkowska, G.; Fronczyk, J.; Wichowski, P. Research on the Possibility of Using Fly Ashes from Combustion of Municipal Sewage Sludge on Properties of Ordinary Concretes. Rocz. Ochr. Środowiska 2018, 20, 1113–1128. [Google Scholar]
- Lin, D.F.; Chang, W.C.; Yuan, C.; Luo, H.L. Production and characterization of glazed tiles containing incinerated sewage sludge. Waste Manag. 2008, 28, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Borowski, B. Processing of ashes from sewage sludge combustion for building material. Inżynieria Ekol. 2011, 251–258. [Google Scholar]
- Płuczka Wiertnicza. Metody Badań w Warunkach Polowych; BN-90/1785-01:1990 (Polish Standard); Wydawnictwa Normalizacyjne “Alfa”: Warsaw, Poland, 1990.
- Wiertnictwo. Cementy i Zaczyny Cementowe do Cementowania w Otworach Wiertniczych; PN-85/G-02320:1985 (Polish Standard); Polish Committee for Standardization: Warsaw, Poland, 1985.
- Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens; PN-EN 12390-3:2011; Polish Committee for Standardization: Warsaw, Poland, 2011.
- Concrete—Specification, Performance, Production and Conformity; PN-EN 206+A1:2016-12; Polish Committee for Standardization: Warsaw, Poland, 2016.
- Characterization of Waste—Leaching Behaviour Tests—Up-Flow Percolation Test (Under Specified Conditions); CEN/TS 14405:2004; European Committee for Standardization: Brussels, Belgium, 2004.
- Regulation of the Minister of the Environment of Poland of 18 November 2014 on Conditions to Be Met when Discharging Waste Water into Waters or onto the Ground and on Substances that are Particularly Harmful to the Aquatic Environment; Ministry of the Environment of Poland: Warsaw, Poland, 2014.
- Szarek, Ł. Wymywalność metali ciężkich z zawiesin twardniejących z dodatkiem popiołów z termicznego przekształcania komunalnych osadów ściekowych. Ph. D. Thesis, Warsaw University of Technology, Warsaw, Poland, 2019. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Li, J.; Xue, Q.; Fang, L.; Poon, C.S. Characteristics and metal leachability of incinerated sewage sludge ash and air pollution control residues from Hong Kong evaluated by different methods. Waste Manag. 2017, 64, 161–170. [Google Scholar] [CrossRef]
- Chen, M.; Blanc, D.; Gautier, M.; Mehu, J.; Gourdon, R. Environmental and technical assessments of the potential utilization of sewage sludge ashes (SSAs) as secondary raw materials in construction. Waste Manag. 2013, 33, 1268–1275. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Escadeillas, G. Use of metakaolin to stabilize sewage sludge ash and municipal solid waste incineration fly ash in cement-based materials. J. Hazard. Mater. 2012, 243, 193–203. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. EU landfill waste acceptance criteria and EU Hazardous Waste Directive compliance testing of incinerated sewage sludge ash. Waste Manag. 2010, 30, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bilim, C.; Atiş, C.D. Alkali activation of mortars containing different replacement levels of ground granulated blast furnace slag. Constr. Build. Mater. 2012, 28, 708–712. [Google Scholar] [CrossRef]




| Component | Content of Component [kg/m3] in the Slurry: | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |
| Tap water | 841 | 842 | 841 | 879 | 929 | 869 | 807 |
| Sodium bentonite | 34 | 25 | 21 | 35 | 56 | 35 | 48 |
| CEM I 32.5 R cement | 137 | 143 | 380 | - | - | - | - |
| Ground blast furnace slag | - | - | - | 171 | 181 | 413 | 383 |
| Hard coal fly ash-fluidized bed | 272 | - | - | - | - | - | - |
| Brown coal fly ash-fluidized bed | - | 275 | - | 154 | 14 | 13 | 141 |
| TTMSS fly ash 1 | - | - | 84 | - | - | - | - |
| No. | Chemical Composition/Physical Properties | Ingredient Content [mass %] in: | ||||
|---|---|---|---|---|---|---|
| Fluidized-Bed Hard Coal Fly Ash | Fluidized-Bed Brown Coal Fly Ash 1 | TTMSS Fly Ash | Ground Granular Blast Furnace Slag | |||
| in Slurry A | in Slurry B | in Slurries D–G | in Slurry C | Slurries D–G | ||
| 1 | Cl | 0.070 | 0.015 | 0.040 | 0.038 | 0.110 |
| 2 | SO3 | 6.19 | 3.33 | 5.66 | 2.78 | 0.67 |
| 3 | CaO | 12.52 | 17.38 | 21.10 | 13.2 | 45.4 |
| 4 | CaOfree | 3.89 | 6.27 | 7.00 | 0.12 | 0.80 |
| 5 | SiO2 | 35.73 | 36.51 | 34.87 | 36.4 | 36.5 |
| 6 | Al2O3 | 19.89 | 27.10 | 23.09 | 18.1 | 9.20 |
| 7 | Fe2O3 | 6.30 | 5.91 | 4.82 | 5.7 | 1.56 |
| 8 | Na2O | 0.82 | 1.58 | 0.02 | 2.26 | 0.01 |
| 9 | K2O | 1.88 | 0.74 | 0.01 | 2.95 | 0.05 |
| 10 | MgO | 2.20 | 2.31 | 1.69 | 4.15 | 5.89 |
| 11 | Loss on ignition | 13.65 | 3.35 | 3.15 | 2.09 | 0.01 |
| No. | Index | Unit | Permissible Index Value | Index Value in Eluate |
|---|---|---|---|---|
| 1 | pH | - | 6.5–9.0 | 7.71 |
| 2 | BOD5 | mg O2/dm3 | 25–50 | 4850 |
| 3 | COD | mg O2/dm3 | 125–250 | 13,136 |
| 4 | Total nitrogen (N) | mg N/dm3 | 30 | 884 |
| 5 | Ammonium nitrogen (NH4) | mg NH4/dm3 | 10–30 | 763 |
| 6 | Total phosphorus (p) | mg p/dm3 | 2–10 | 78.4 |
| 7 | Chlorides (Cl) | mg/dm3 | 1000 | 1597 |
| 8 | Sulphates (SO4) | mg/dm3 | 500 | 37.5 |
| 9 | ∑ Cl, SO4 | mg/dm3 | 1500 | 1634.5 |
| 10 | Lead (Pb) | mg Pb/dm3 | 0.1–0.5 | 4.25 |
| 11 | Copper (Cu) | mg Cu/dm3 | 0.1–0.5 | 0.336 |
| 12 | Zinc (Zn) | mg Zn/dm3 | 2.0 | 0.808 |
| 13 | Nickel (Ni) | mg Ni/dm3 | 0.1–0.5 | 0.394 |
| 14 | Total suspended solids | mg/dm3 | 35–70 | 6774 |
| Property or Parameter | Slurry Designation: | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |
| w/c 1 | 6.13 | 5.88 | 2.21 | - | - | - | - |
| w/d 2 | 1.90 | 1.90 | 1.73 | 14.6 | 26.1 | 4.4 | 3.9 |
| Density [kg/m3] | 1.29 | 1.30 | 1.33 | 1.24 | 1.18 | 1.33 | 1.38 |
| Relative viscosity [s] | 45 | 39 | 50 | 38 | 40 | 37 | 46 |
| Structural strength of gel after 10 min of standing time [Pa] | - | - | 5.2 | 3.0 | 8.0 | 3.8 | 40.0 |
| Daily water bleed [%] | 3.0 | 5.0 | 3.9 | 8.5 | 1.5 | 4.0 | 2.0 |
| Compressive strength [MPa] after: –14 days | - | - | - | 0.25 | 0.34 | 0.66 | 1.77 |
| –28 days | 1.21 | 1.38 | 1.80 | 0.37 | 0.52 | 1.62 | 2.45 |
| –60 days | - | - | - | 0.40 | 0.57 | 2.14 | 3.28 |
| –120 days | - | - | - | 0.48 | 0.75 | 2.64 | 3.47 |
| Hydraulic conductivity k10 [m/s] after: –14 days | - | - | - | 1.56 × 10−7 | 7.48 × 10−8 | 4.23 × 10−9 | 2.98 × 10−9 |
| –28 days | 3.5 × 10−8 | 2.5 × 10−8 | 9.6 × 10−9 | 1.56 × 10−7 | 1.07 × 10−9 | 6.35 × 10−10 | 4.95 × 10−10 |
| –60 days | - | - | - | 1.34 × 10−7 | 1.98 × 10−9 | 6.19 × 10−10 | 2.35 × 10−10 |
| –120 days | - | - | - | 1.09 × 10−7 | 4.99 × 10−10 | 9.00 × 10−10 | 4.40 × 10−10 |
| No. | Type of Solution | A | B | A | B | A | B | A | B | A | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ap [m2/g] | vp > 0.2 μm | vp < 0.2 μm | Pc [%] | dmax [μm] | |||||||
| 1 | Tap water | 99.54 | 100.91 | 0.89 | 0.99 | 0.46 | 0.44 | 76.22 | 75.90 | 4.0 | 7.0 |
| 2 | Eluate | 94.45 | 85.76 | 0.79 | 0.82 | 0.32 | 0.39 | 73.88 | 72.13 | 3.0 | 4.0 |
| 3 | 2:1 [%] | 94.9 | 85.0 | 88.8 | 82.8 | 69.6 | 88.6 | 96.9 | 95.0 | 75.0 | 57.1 |
| No. | Metal | TTMSS Fly Ash | CEM I 32.5 R | Hardening Slurry |
|---|---|---|---|---|
| Value [mg/kg d.m.] | ||||
| 1 | Zinc (Zn) | 3290 ± 83 | 804 ± 24 | 1057 ± 66 |
| 2 | Copper (Cu) | 808 ± 24 | 120 ± 4 | 206 ± 14 |
| 3 | Lead (Pb) | 83.0 ± 6.5 | 104 ± 7 | 84.5 ± 8.8 |
| 4 | Cadmium (Cd) | 14.0 ± 0.7 | 10.8 ± 0.6 | 9.6 ± 0.9 |
| 5 | Chromium (Cr) | 179 ± 9 | 64.4 ± 4.2 | 71.8 ± 6.8 |
| Name | Eluate | Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zinc | Copper | Lead | Cadmium | Chromium | |||||||
| Ci [mg/dm3] | Δ [%] | Ci [mg/dm3] | Δ [%] | Ci [mg/dm3] | Δ [%] | Ci [mg/dm3] | Δ [%] | Ci [mg/dm3] | Δ [%] | ||
| F1 | 1 | 0.022 | 29 | <0.02 | - | 0.132 | 24.2 | <0.01 | - | 0.046 | 27 |
| 2 | <0.01 | - | <0.02 | - | 0.121 | 14.5 | <0.01 | - | 0.046 | 17.7 | |
| 3 | 0.02 | 50 | <0.02 | - | 0.166 | 15.5 | <0.01 | - | 0.04 | 2.9 | |
| 4 | <0.01 | - | <0.02 | - | 0.244 | 1.8 | <0.01 | - | 0.04 | 9.1 | |
| 5 | <0.01 | - | <0.02 | - | 0.12 | 17.8 | <0.01 | - | 0.088 | 14.4 | |
| 6 | <0.01 | - | <0.02 | - | 0.123 | 10.5 | <0.01 | - | 0.04 | 25.8 | |
| 7 | <0.01 | - | <0.02 | - | 0.122 | 14.1 | <0.01 | - | 0.052 | 6 | |
| F2 | 1 | <0.01 | - | <0.02 | - | 0.133 | 12.5 | <0.01 | - | 0.064 | 5.1 |
| 2 | 0.021 | 39 | <0.02 | - | 0.149 | 8.2 | <0.01 | - | 0.044 | 16.6 | |
| 3 | 0.038 | 36 | <0.02 | - | 0.193 | 4.1 | <0.01 | - | 0.045 | 33 | |
| 4 | <0.01 | - | <0.02 | - | 0.103 | 13.3 | <0.01 | - | 0.052 | 19.4 | |
| 5 | <0.01 | - | <0.02 | - | 0.129 | 6.6 | 0.014 | 29.1 | 0.068 | 13.9 | |
| 6 | <0.01 | - | <0.02 | - | 0.129 | 14.5 | <0.01 | - | <0.03 | - | |
| 7 | <0.01 | - | <0.02 | - | 0.119 | 1.9 | <0.01 | - | 0.056 | 12.7 | |
| F3 | 1 | <0.01 | - | <0.02 | - | 0.143 | 10.6 | <0.01 | - | 0.085 | 3.5 |
| 2 | 0.016 | 119 | <0.02 | - | 0.099 | 11.3 | <0.01 | - | 0.052 | 9.7 | |
| 3 | 0.014 | 51 | <0.02 | - | 0.196 | 15.3 | <0.01 | - | 0.042 | 19.2 | |
| 4 | <0.01 | - | <0.02 | - | 0.104 | 7.3 | <0.01 | - | 0.031 | 25.6 | |
| 5 | <0.01 | - | <0.02 | - | 0.122 | 7.7 | 0.011 | 37.2 | <0.03 | - | |
| 6 | <0.01 | - | <0.02 | - | 0.118 | 20.5 | <0.01 | - | <0.03 | - | |
| 7 | <0.01 | - | <0.02 | - | 0.1 | 19.1 | <0.01 | - | 0.035 | 11 | |
| Sample Name | Zinc [%] | Copper [%] | Lead [%] | Cadmium [%] | Chromium [%] |
|---|---|---|---|---|---|
| F1 | >99.99 | >99.90 | 98.31 | >98.95 | 99.30 |
| F2 | >99.99 | >99.90 | 98.38 | >98.85 | >99.25 |
| F3 | >99.99 | >99.90 | 98.57 | >98.93 | >99.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kledyński, Z.; Falaciński, P.; Machowska, A.; Szarek, Ł.; Krysiak, Ł. Hardening Slurries with Fluidized-Bed Combustion By-Products and Their Potential Significance in Terms of Circular Economy. Materials 2021, 14, 2104. https://doi.org/10.3390/ma14092104
Kledyński Z, Falaciński P, Machowska A, Szarek Ł, Krysiak Ł. Hardening Slurries with Fluidized-Bed Combustion By-Products and Their Potential Significance in Terms of Circular Economy. Materials. 2021; 14(9):2104. https://doi.org/10.3390/ma14092104
Chicago/Turabian StyleKledyński, Zbigniew, Paweł Falaciński, Agnieszka Machowska, Łukasz Szarek, and Łukasz Krysiak. 2021. "Hardening Slurries with Fluidized-Bed Combustion By-Products and Their Potential Significance in Terms of Circular Economy" Materials 14, no. 9: 2104. https://doi.org/10.3390/ma14092104
APA StyleKledyński, Z., Falaciński, P., Machowska, A., Szarek, Ł., & Krysiak, Ł. (2021). Hardening Slurries with Fluidized-Bed Combustion By-Products and Their Potential Significance in Terms of Circular Economy. Materials, 14(9), 2104. https://doi.org/10.3390/ma14092104








