Photopatterning of PDMS Films: Challenging the Reaction between Benzophenone and Silicone Functional Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Photopatterning of Silicone Formulation
2.4. Irradiation of Model Molecules
3. Results
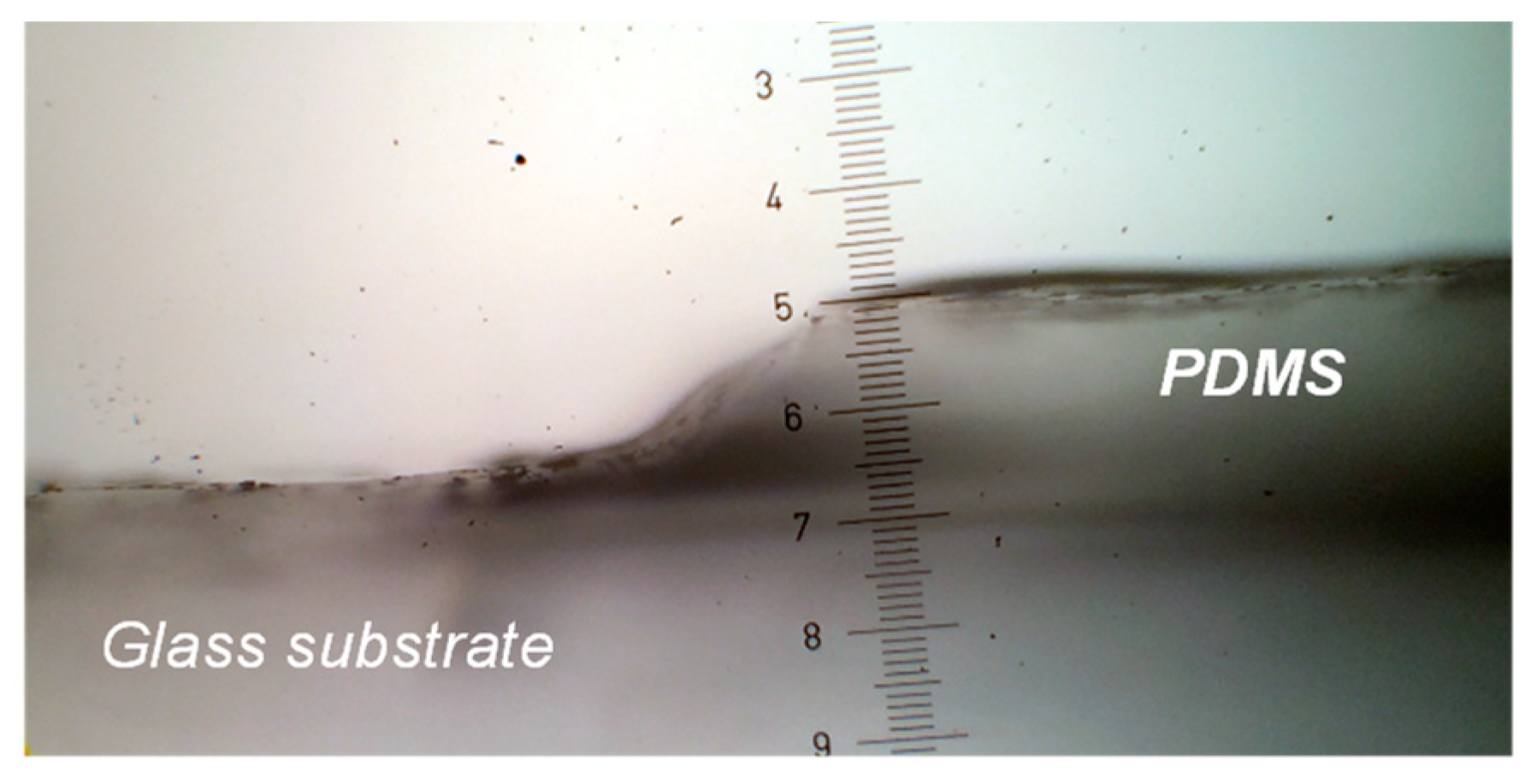
3.1. Photopatterning Reproduction
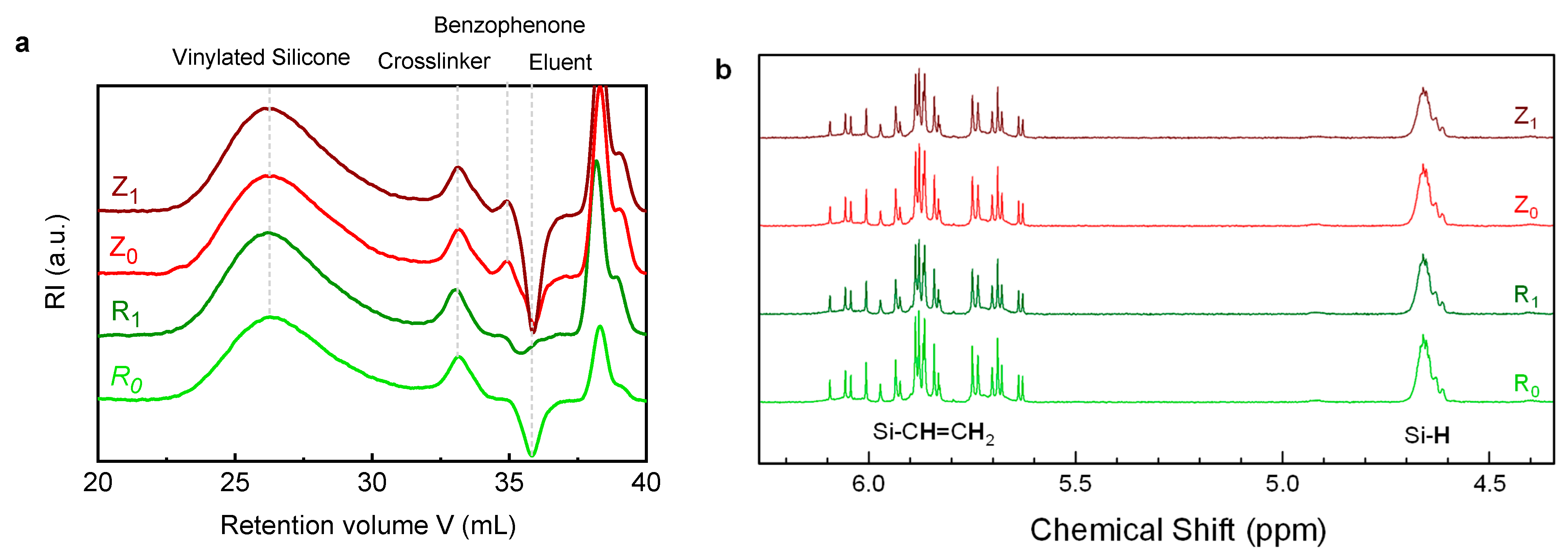
3.2. Analyses on LSR Formulations
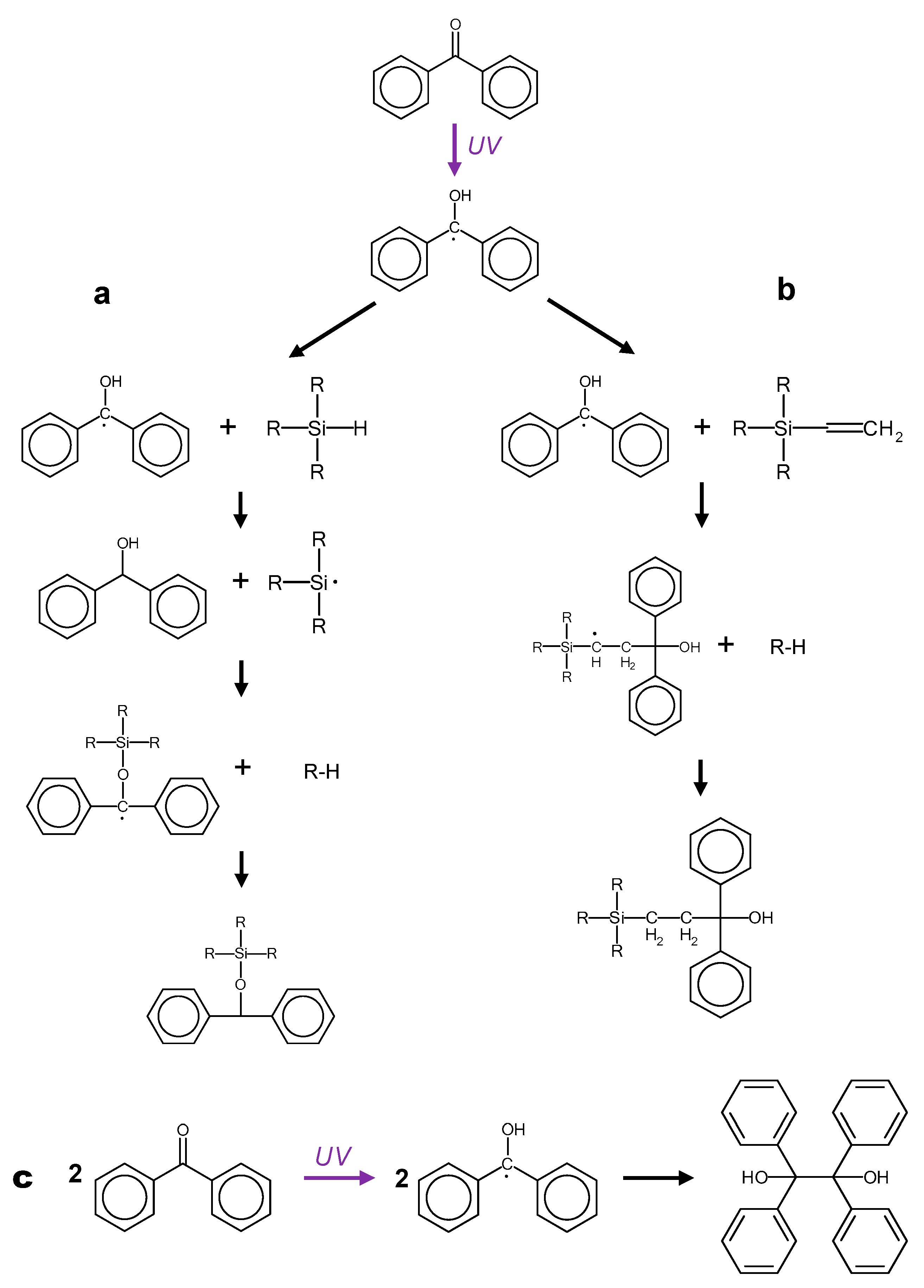
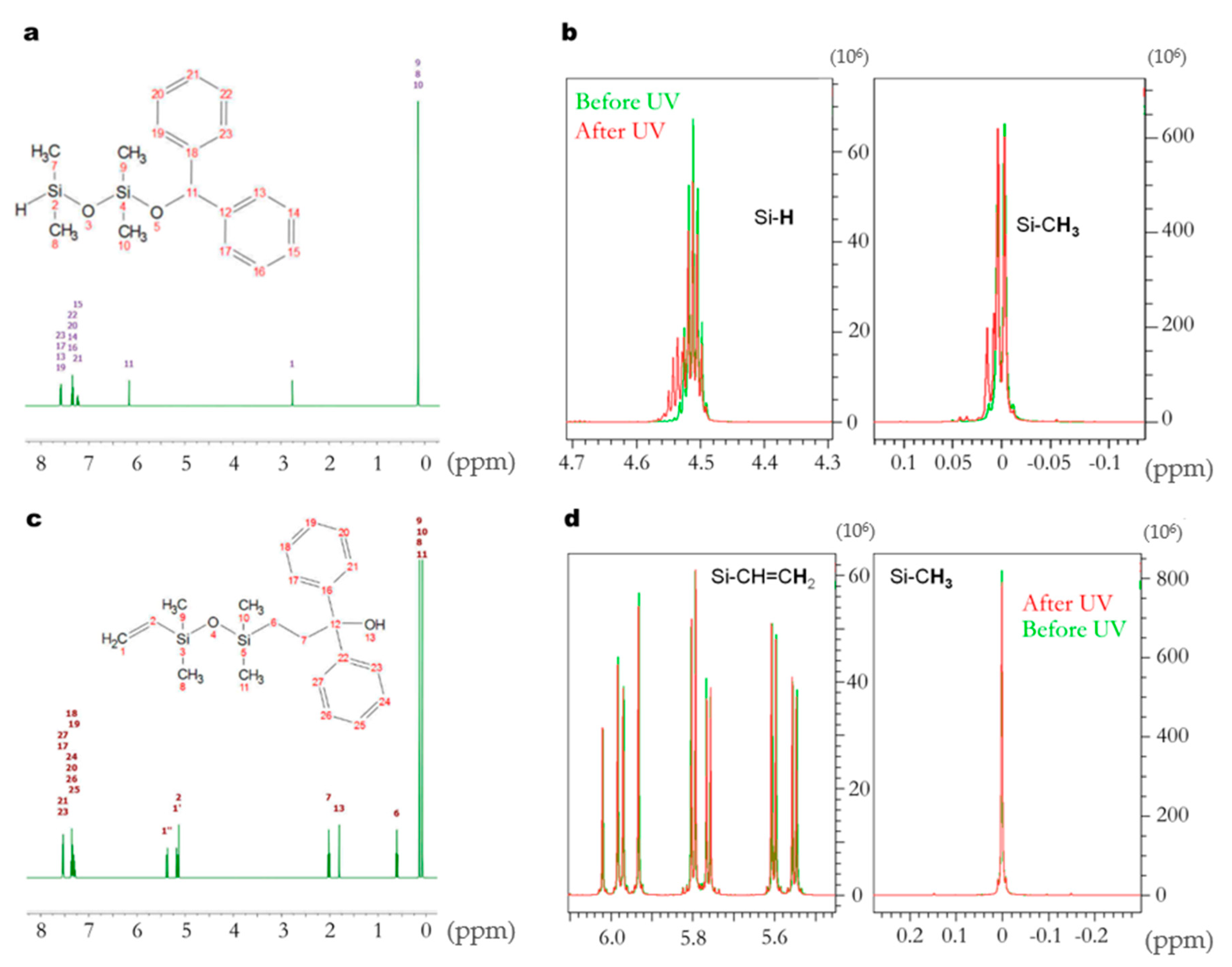
3.3. Study on Model Molecules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: Advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Scaiano, J.; Ingold, K.U. Absolute rate constants for the reactions of tert-butoxyl radicals and some ketone triplets with silanes. Organometallics 1982, 1, 466–469. [Google Scholar] [CrossRef]
- Moore, W.M.; Hammond, G.S.; Foss, R.P. Mechanisms of Photoreactions in Solutions. I. Reduction of Benzophenone by Benzhydrol. J. Am. Chem. Soc. 1961, 1068, 2789–2794. [Google Scholar] [CrossRef]
- Hammond, G.S.; Baker, W.P.; Moore, W.M. Mechanisms of Photoreactions in Solution. II. Reduction of Benzophenone by Toluene and Cumene. J. Am. Chem. Soc. 1961, 83, 2795–2799. [Google Scholar] [CrossRef]
- Weizmann, C.; Bergmann, E.; Hirsberg, Y. Photochemical Interaction between Ketones and Alcohols. J. Am. Chem. Soc. 1938, 60, 1530–1533. [Google Scholar] [CrossRef]
- DeSmet, N.; Rymarczyk-Machal, M.; Schacht, E. Modification of polydimethylsiloxane surfaces using benzophenone. J. Biomat. Sci. Polym. Ed. 2009, 20, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hou, Z. Homogenous Grafted Poly(acrylic acid) Brushes on Ultra-Flat Polydimethylsiloxane (PDMS) Films by UV Irradiation. NanoBiomed. Eng. 2011, 3, 42–46. [Google Scholar]
- Zhou, B.; Gao, X.; Wang, C.; Ye, Z.; Gao, Y.; Xie, J.; Wu, X.; Wen, W. Functionalized PDMS with Versatile and Scalable Surface Roughness Gradients for Cell Culture. ACS Appl. Mater. Interfaces 2015, 7, 17181–17187. [Google Scholar] [CrossRef]
- Kuliasha, C.A.; Fedderwitz, R.L.; Calvo, P.R.; Sumerlin, B.S.; Brennan, A.B. Engineering the Surface Properties of Poly(dimethylsiloxane) Utilizing Aqueous RAFT Photografting of Acrylate/Methacrylate Monomers. Macromolecules 2018, 51, 306–317. [Google Scholar] [CrossRef]
- Kuliasha, C.A.; Fedderwitz, R.L.; Finlay, J.A.; Franco, S.C.; Clare, A.S.; Brennan, A.B. Engineered Chemical Nanotopographies: Reversible Addition–Fragmentation Chain-Transfer Mediated Grafting of Anisotropic Poly(acrylamide) Patterns on Poly(dimethylsiloxane) To Modulate Marine Biofouling. Langmuir 2020, 36, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Jothimuthu, P.; Papautsky, I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on-a-chip prototyping. LabChip 2007, 7, 1192–1197. [Google Scholar] [CrossRef]
- Jothimuthu, P.; Carroll, A.; Bhagat, A.A.S.; Lin, G.; Mark, J.E.; Papautsky, I. Photodefinable PDMS thin films or microfabrication applications. J. Micromech. Microeng. 2009, 19, 045024. [Google Scholar] [CrossRef]
- Cong, H.; Pan, T. Photopatternable Conductive PDMS Materials for Microfabrication. Adv. Funct. Mater. 2008, 18, 1912–1921. [Google Scholar] [CrossRef]
- Cotton, D.P.J.; Popel, A.; Graz, I.M.; Lacour, S.P. Photopatterning the mechanical properties of polydimethylsiloxane films. J. Appl. Phys. 2011, 109, 054905. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.T.; Okada, R.; Fu, J. UV-Modulated Substrate Rigidity for Multiscale Study of Mechanoresponsive Cellular Behaviors. Langmuir 2012, 28, 10789–10796. [Google Scholar] [CrossRef][Green Version]
- Prauzner-Bechcicki, S.; Raczkowska, J.; Madej, E.; Pabijan, J.; Lukes, J.; Sepitka, J.; Rysz, J.; Awsiuk, K.; Bernasik, A.; Budkowski, A.; et al. PDMS substrate stiffness affects the morphology and growth profiles of cancerous prostate and melanoma cells. J. Mech. Behav. Biomed. Mater. 2015, 41, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Lacour, S.P. Concurrent photopatterning of elastic modulus and structures in photosensitive silicone elastomers. Extrem. Mech. Lett. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Gao, S.; Tung, W.-T.; Siu-HongWong, D.; Bian, L.; Zhang, A.P. Direct optical micropatterning of poly(dimethylsiloxane) for microfluidic devices. J. Micromech. Microeng. 2018, 28, 095011. [Google Scholar] [CrossRef]
- Cho, D.; Park, J.; Kim, T.; Jeon, S. Recent advances in lithographic fabrication of micro-/nanostructured polydimethylsiloxanes and their soft electronic applications. J. Semicond. 2019, 40, 111605. [Google Scholar] [CrossRef]
- LeeSmith, A. The Analytical Chemistry of Silicones; Wiley Interscience: New York, NY, USA, 1991. [Google Scholar]
- Delebecq, E.; Ganachaud, F. Looking over Liquid Silicone Rubbers: (1) Network Topology vs. Chemical Formulations. ACS Appl. Mater. Interfaces 2012, 4, 3340–3352. [Google Scholar] [CrossRef]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry; Wiley Interscience: New York, NY, USA, 1999. [Google Scholar]
- Abdellah, L.; Boutevin, B.; Youssef, B. Synthesis and applications of photocrosslinkable poly(siloxanes). Prog. Org. Coat. 1994, 23, 201–236. [Google Scholar] [CrossRef]
- Stricher, A.M.; Rinaldi, R.G.; Barrès, C.; Ganachaud, F.; Chazeau, L. How I met your elastomers: From network topology to mechanical behaviours of conventional silicone materials. RSC Adv. 2015, 5, 53713–53725. [Google Scholar] [CrossRef]
- Hitchcock, P.; Lappert, M.; Warhurst, N.J.W. Synthesis and Structure of a rac-Tris(divinyldisiloxane)diplatinum(0) Complex and its Reaction with Maleic Anhydride. Angew. Chem. Int. Ed. 1991, 30, 438–440. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Saion, E. Influence of Dose on Particle Size and Optical Properties of Colloidal Platinum Nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, C.Y.; Zhu, Y.R.; Chen, Z.Y. A Novel Ultraviolet Irradiation Technique for Shape-Controlled Synthesis of Gold Nanoparticles at Room Temperature. Chem. Mater. 1999, 11, 2310–2312. [Google Scholar] [CrossRef]
- Stricher, A.; Rinaldi, R.G.; Machado, G.; Chagnon, G.; Favier, D.; Chazeau, L.; Ganachaud, F. Light-Induced Bulk Architecturation of PDMS Membranes. Macromol. Mater. Eng. 2016, 301, 1151–1157. [Google Scholar] [CrossRef]
- Gallo, M.; Rinaldi, R.G. The effect of pre-curing UV-irradiation on the crosslinking of silicone rubber. J. Appl. Polym. Sci. 2021, 138, e49807. [Google Scholar] [CrossRef]
- Gallo, M.; Chesnais, C.; Ege, K.; Leclere, Q.; Totaro, N.; Rinaldi, R.G. Spatial Patterning of the Viscoelastic Core Layer of a Hybrid Sandwich Composite Material to Trigger Its Vibro-Acoustic Performances; SAE Technical Paper 2018-01-1500; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
| Samples | SEC | NMR | |||||
|---|---|---|---|---|---|---|---|
| Crosslinker + Oils | Vinylated Chains | nSiVi a | nSiH a | nSiVi/nSiH a | |||
| Mw (g·mol−1) | Đ | Mw (g·mol−1) | Đ | ||||
| R0 | 1570 | 1.4 | 134,000 | 2.3 | 4.26 | 7.72 | 1.81 |
| R1 | 1900 | 1.3 | 139,000 | 2.3 | 4.24 | 7.63 | 1.80 |
| Z0 | 1640 | 1.4 | 138,000 | 2.3 | 4.27 | 7.72 | 1.81 |
| Z1 | 1750 | 1.4 | 136,000 | 2.3 | 4.23 | 7.40 | 1.75 |
| UV | NMR | ||||
|---|---|---|---|---|---|
| nSiMe a | nreactive moieties a | rcalculated b | rtheoretical b | ||
| M2H + Benzophenone | 0 | 4 | 1.85 | 2.16 | 2 |
| 1 | 4 | 1.67 | 2.39 | 2 | |
| M2Vi + Benzophenone | 0 | 4 | 1.87 | 2.14 | 2 |
| 1 | 4 | 1.88 | 2.13 | 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stricher, A.; Rinaldi, R.G.; Chazeau, L.; Ganachaud, F. Photopatterning of PDMS Films: Challenging the Reaction between Benzophenone and Silicone Functional Groups. Materials 2021, 14, 2027. https://doi.org/10.3390/ma14082027
Stricher A, Rinaldi RG, Chazeau L, Ganachaud F. Photopatterning of PDMS Films: Challenging the Reaction between Benzophenone and Silicone Functional Groups. Materials. 2021; 14(8):2027. https://doi.org/10.3390/ma14082027
Chicago/Turabian StyleStricher, Arthur, Renaud G. Rinaldi, Laurent Chazeau, and François Ganachaud. 2021. "Photopatterning of PDMS Films: Challenging the Reaction between Benzophenone and Silicone Functional Groups" Materials 14, no. 8: 2027. https://doi.org/10.3390/ma14082027
APA StyleStricher, A., Rinaldi, R. G., Chazeau, L., & Ganachaud, F. (2021). Photopatterning of PDMS Films: Challenging the Reaction between Benzophenone and Silicone Functional Groups. Materials, 14(8), 2027. https://doi.org/10.3390/ma14082027







