Influence of Fluorine-Containing Monomer Content on the Hydrophobic and Transparent Properties of Nanohybrid Silica Polyacrylate Coating Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Organic–Inorganic Grafting Silica Polyacrylate Material
2.3. Preparation of the Silica-Polyacrylate Coating Film
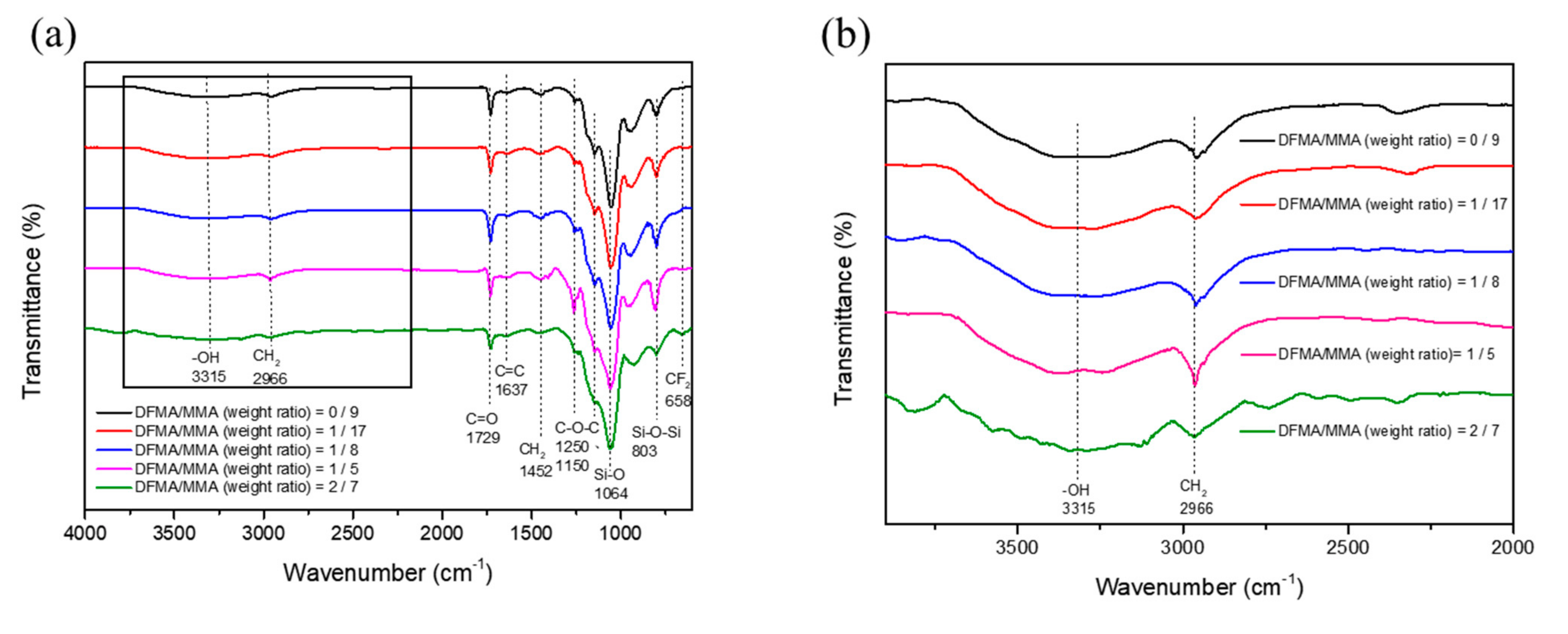
2.4. Characterization
3. Results and Discussion
3.1. Preparation of Nanohybrid Silica Polyacrylate Coating Materials
3.2. Physical Properties of the As-Prepared Nanohybrid Silica Polyacrylate Material
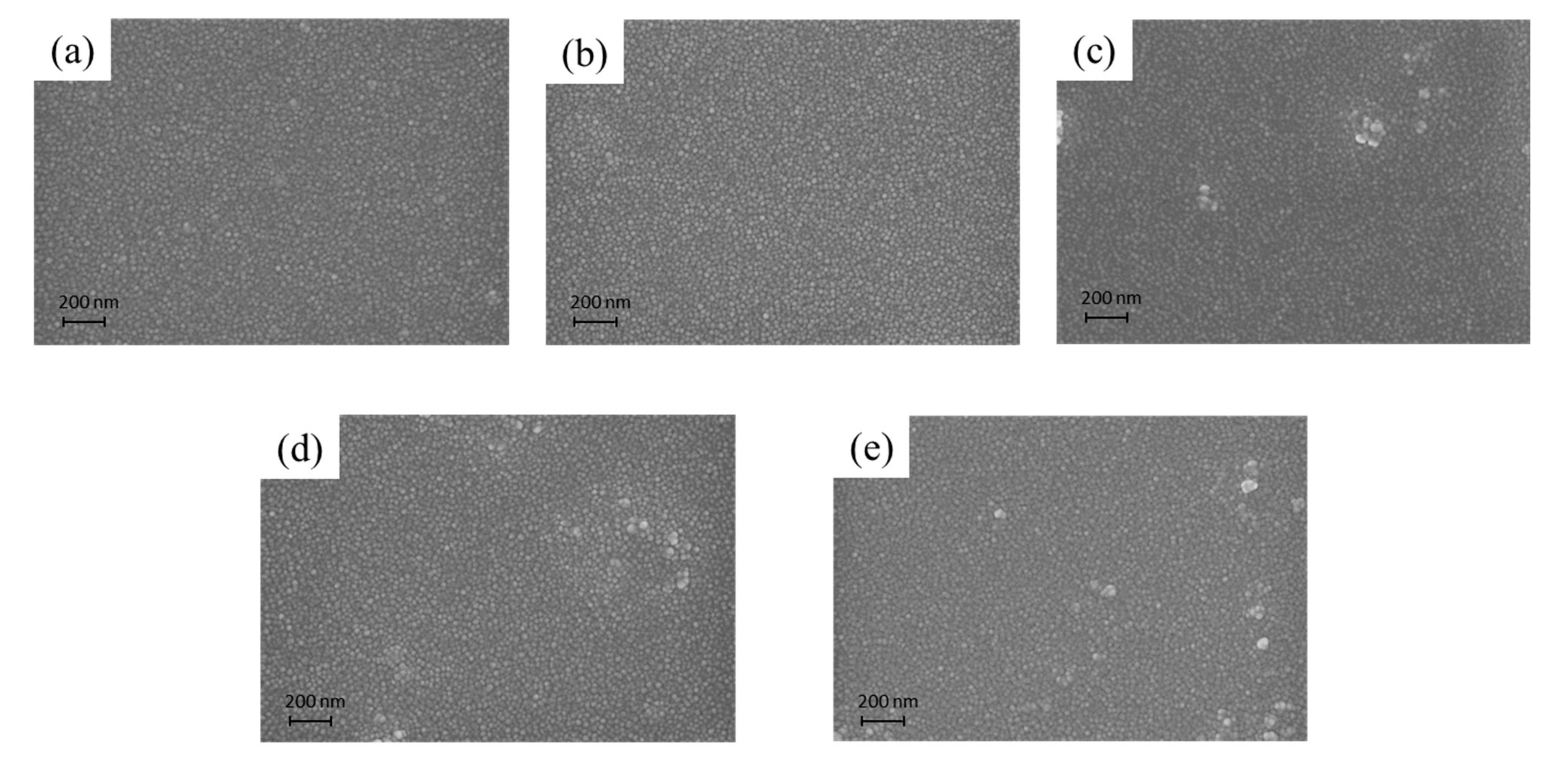
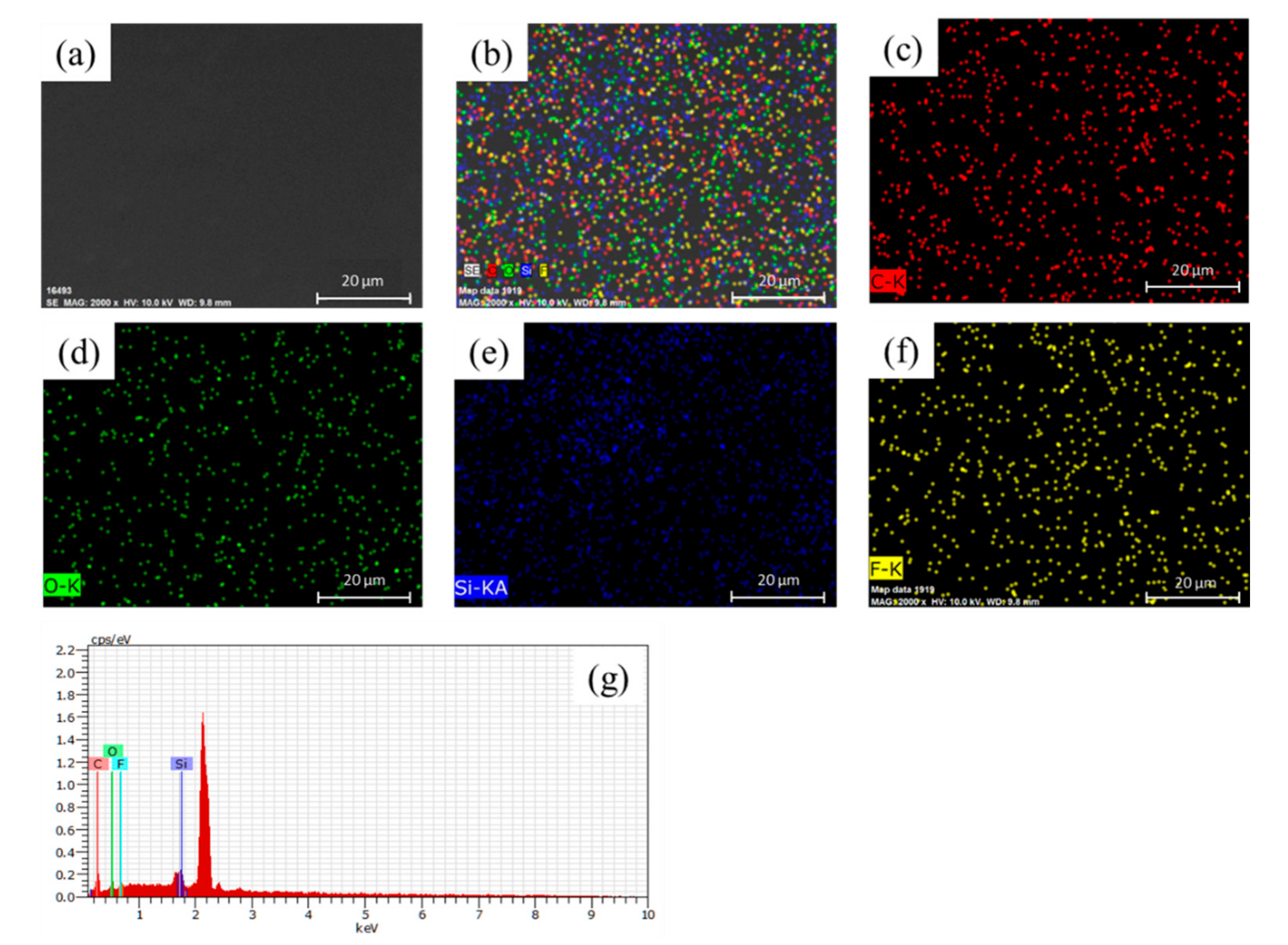
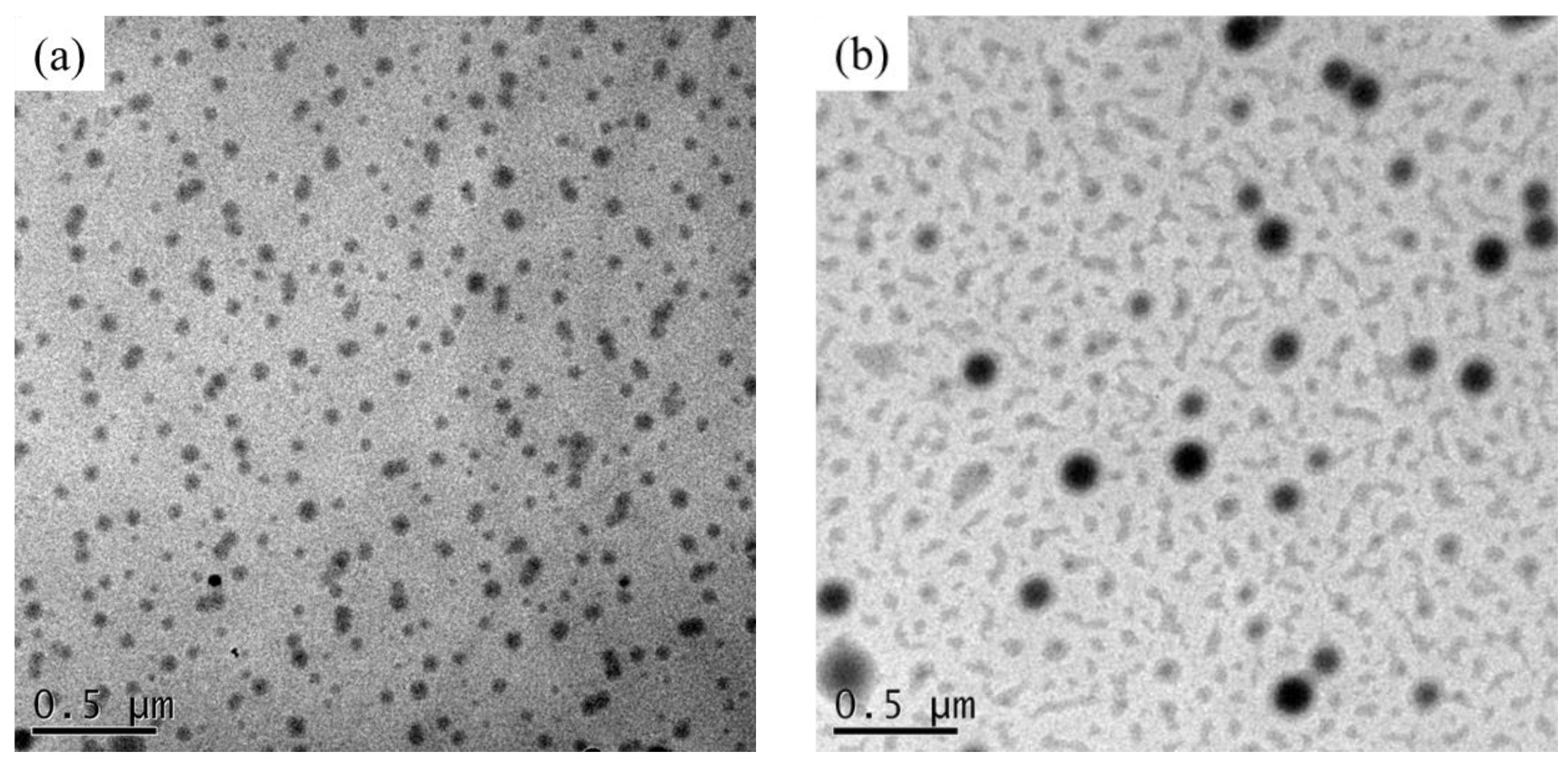
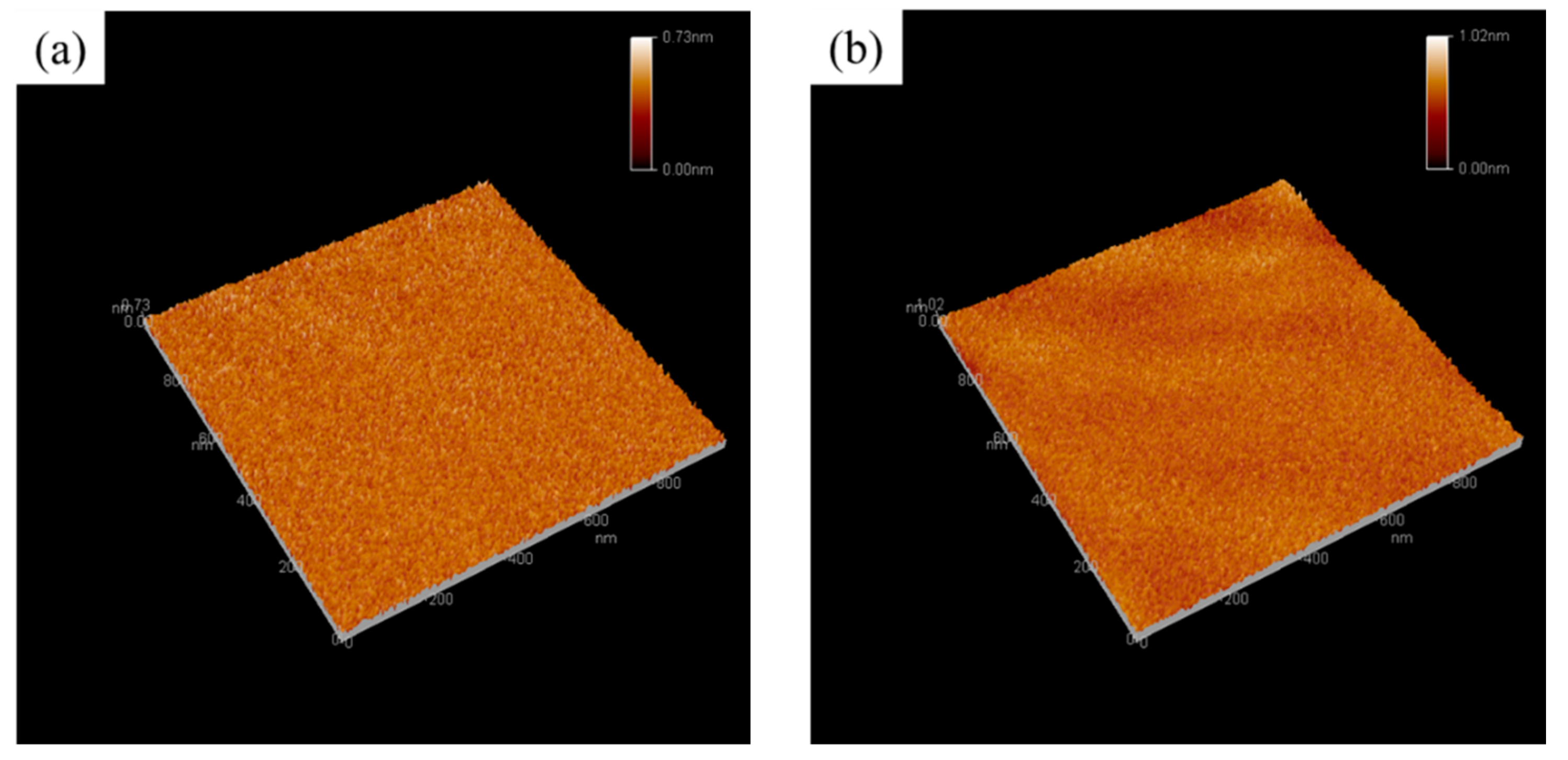
3.3. Morphology Analyses of the As-Prepared Nanohybrid Silica Polyacrylate Film
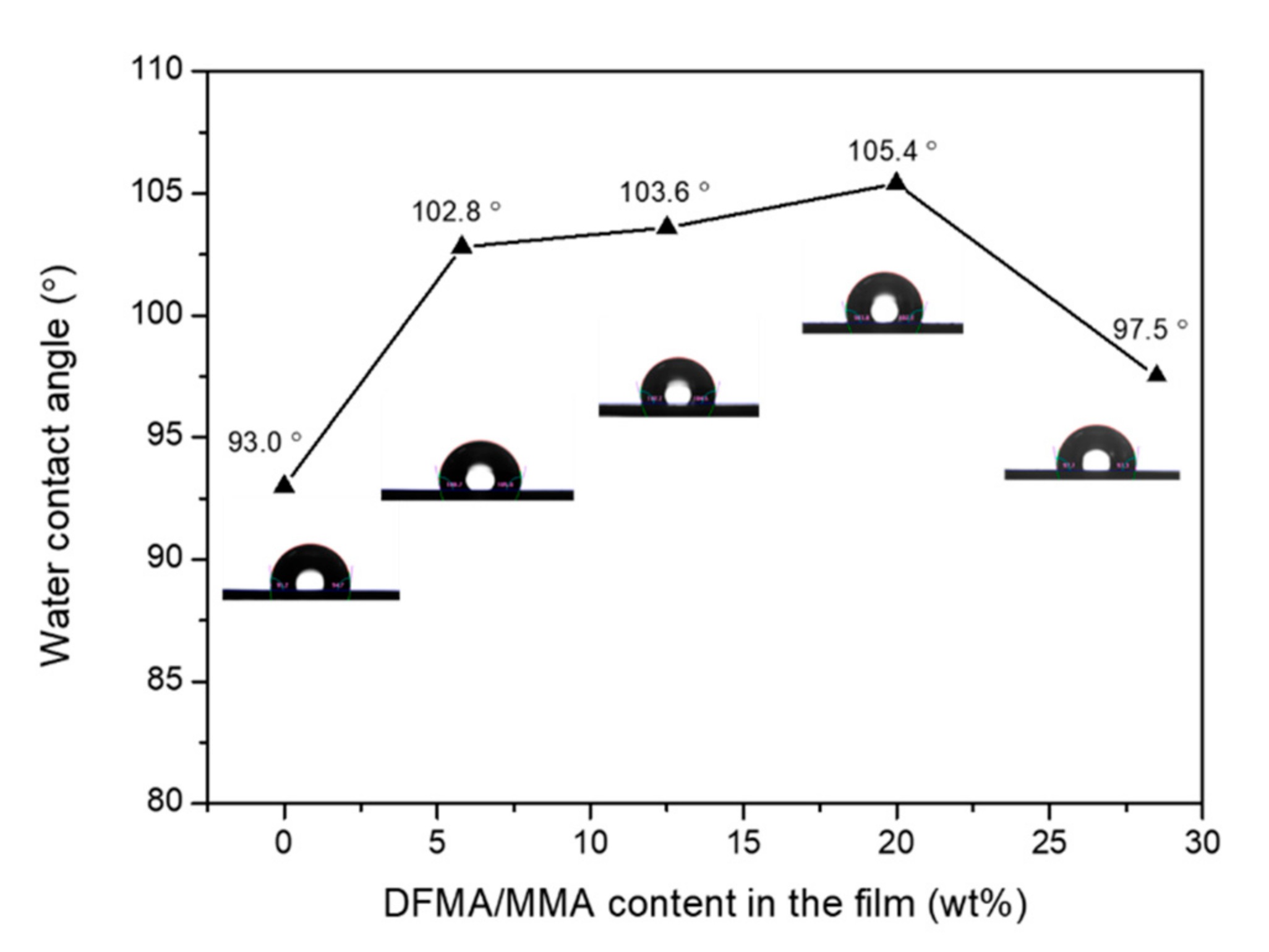
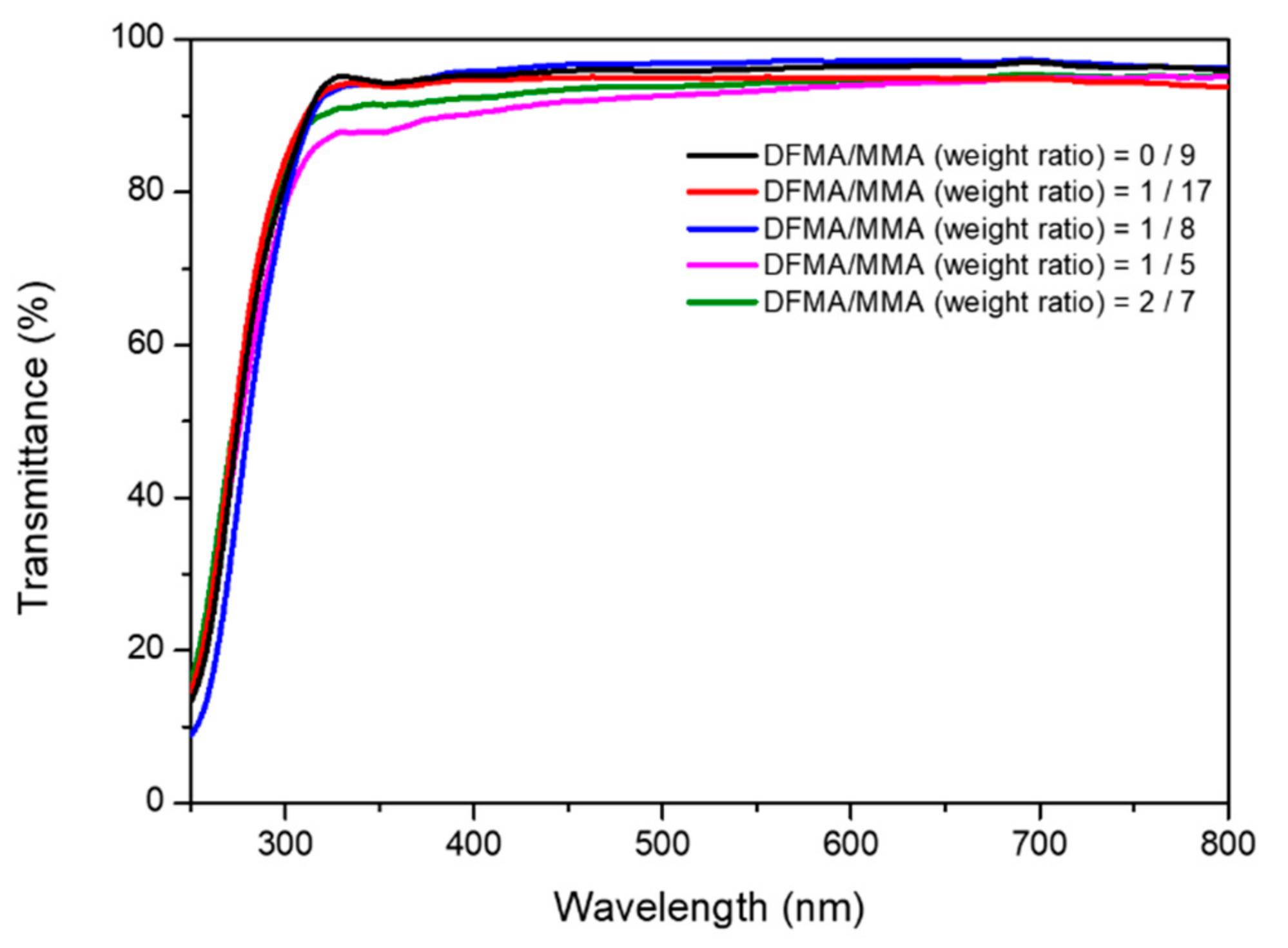
3.4. Hydrophobicity and Transparent Properties of Nanohybrid Silica Polyacrylate Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, B.; Deng, X.; Cao, S.; Hou, X.; Chen, H. A novel approach for the preparation of organic-siloxane oligomers and the creation of hydrophobic surface. Appl. Surf. Sci. 2007, 254, 452–458. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.J.; Feng, X.Y.; Li, N.; Luo, C.W.; Chao, Z.S. Super-hydrophobic Co3O4-loaded nickel foam with corrosion-resistant property prepared by combination of hydrothermal synthesis and PFAS modification. Surf. Coat. Technol. 2017, 309, 1111–1118. [Google Scholar] [CrossRef]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant surfaces using deep reactive ion etching on PDMS substrates. J. Colloid Interface Sci. 2016, 481, 82–90. [Google Scholar] [CrossRef]
- Pintossia, D.; Colombob, A.; Levia, M.; Dragonettib, C.; Turria, S.; Griffinia, G. UV-curable fluoropolymers crosslinked with functional fluorescent dyes: The way to multifunctional thin-film luminescent solar concentrators. J. Mater. Chem. A 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Choi, D.; Yoo, J.; Park, S.M.; Kim, D.S. Facile and cost-effective fabrication of patternable superhydrophobic surfaces via salt dissolution assisted etching. Appl. Surf. Sci. 2017, 393, 449–456. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.-J.; Jeon, S.B.; Seol, M.L.; Choi, Y.K. A triboelectric sponge fabricated from a cube sugar template by 3D soft lithography for superhydrophobicity and elasticity. Adv. Electron. Mater. 2016, 2, 1500331. [Google Scholar] [CrossRef]
- Kothary, P.; Dou, X.; Fang, Y.; Gu, Z.; Leo, S.Y.; Jiang, P. Superhydrophobic hierarchical arrays fabricated by a scalable colloidal lithography approach. J. Colloid Interface Sci. 2017, 487, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, A.R.; Maurya, R.; Balani, K. Superhydrophobic self-floating carbon nanofiber coating for efficient gravity-directed oil/water separation. J. Mater. Chem. A 2017, 5, 2936–2946. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Yan, Q. One-step electrochemical deposition to achieve superhydrophobic cobalt incorporated amorphous carbon-based film with self-cleaning and anti-corrosion. Surf. Interface Anal. 2017, 50, 1–7. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, R.; Zhang, W.; Zhang, M.; Wang, C. Superhydrophobic–superhydrophilic switchable wettability via TiO2 photoinduction electrochemical deposition on cellulose substrate. Chem. Eng. J. 2016, 289, 99–105. [Google Scholar] [CrossRef]
- Li, S.Y.; Xiang, X.G.; Ma, B.H.; Meng, X.D. Facile preparation of diverse alumina surface structures by anodization and superhydrophobic surfaces with tunable water droplet adhesion. J. Alloys Compd. 2019, 779, 219–228. [Google Scholar] [CrossRef]
- Vengatesh, P.; Kulandainathan, M.A. Hierarchically ordered self-lubricating superhydrophobic anodized aluminum surfaces with enhanced corrosion resistance. ACS Appl. Mater. Interfaces 2015, 7, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Pershin, L.; Mostaghimi, J.; Coyle, T.W. Efficient one-step fabrication of ceramic superhydrophobic coatings by solution precursor plasma spray. Mater. Lett. 2018, 211, 24–27. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Gogolides, E. Hydrophobic and superhydrophobic surfaces fabricated using atmospheric pressure cold plasma technology: A review. Adv. Colloid Interface Sci. 2018, 254, 1–21. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, K.; Park, J.Y.; Hwang, B.G.; Ko, Y.C.; Kim, H.J.; Han, J.S.; Seo, E.R.; Park, Y.J.; Lee, S.J. Nearly perfect durable superhydrophobic surfaces fabricated by a simple one-step plasma treatment. Sci. Rep. 2017, 7, 1981. [Google Scholar] [CrossRef] [Green Version]
- Fleming, R.A.; Zou, M. Silica nanoparticle-based films on titanium substrates with long-term superhydrophilic and superhydrophobic stability. Appl. Surf. Sci. 2013, 280, 820–827. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Wang, L.; Zheng, N.; Li, W.; Xue, H.; Li, R.K.Y.; Mai, Y.W. Super-hydrophobic coatings based on non-solvent induced phase separation during electro-spraying. J. Colloid Interface Sci. 2017, 506, 603–612. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakaib, M.; Fujishima, A. Development of sol–gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Gurav, A.B.; Latthe, S.S.; Vhatkar, R.S. Sol-gel-processed porous water-repellent silica microbowls. Surf. Innov. 2013, 1, 157–161. [Google Scholar] [CrossRef]
- Guo, X.J.; Xue, C.H.; Li, M.; Lic, X.; Ma, J.Z. Fabrication of robust, superhydrophobic, electrically conductive and UV-blocking fabrics via layer-by-layer assembly of carbon nanotubes. RSC Adv. 2017, 7, 25560–25565. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Meng, Y.; Li, H. Facile fabrication of superhydrophobic paper with improved physical strength by a novel layer-by-layer assembly of polyelectrolytes and lignosulfonates-amine. Cellulose 2016, 23, 2073–2085. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Hsieh, C.C.; Lin, Y.M.; Li, A.K. Preparation of transparent silica-PMMA nanocomposite hard coatings. Prog. Org. Coat. 2008, 62, 436–439. [Google Scholar] [CrossRef]
- Motamedi, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. One-pot synthesis and construction of a high performance metal-organic structured nano pigment based on nanoceria decorated cerium (III)-17 imidazole network (NC/CIN) for effective epoxy composite coating anti-corrosion and thermomechanical properties improvement. Chem. Eng. J. 2020, 382, 122820. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, Y.; Zhang, T.; He, M. Preparation and characterization of acrylate copolymers modified by fluorine and silicon for application in release films. Polym. Plast. Technol. 2014, 53, 531–538. [Google Scholar] [CrossRef]
- Zhi, D.F.; Lu, Y.; Sathasivam, S.; Parkin, I.P.; Zhang, X. Large-scale fabrication of translucent and repairable superhydrophobic spray coatings with remarkable mechanical, chemical durability and UV resistance. J. Mater. Chem. A 2017, 5, 10622–10631. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Liu, Y.; Wu, D. Synthesis and characterization of polyacrylate modified by polysiloxane latexes and films. Prog. Org. Coat. 2014, 77, 1774–1779. [Google Scholar] [CrossRef]
- Liu, X.; Cui, X.; Zhang, C.; Zhang, X.; Wu, G. Effects of different silanization followed via the sol-gel growing of silica nanoparticles onto carbon fiber on interfacial strength of silicone resin composites. Chem. Phys. Lett. 2018, 707, 1–7. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Weng, C.-J.; Liao, W.-J.; Mau, Y.-W. Anticorrosively enhanced PMMA–SiO2 hybrid coatings prepared from the sol–gel approach with MSMA as the coupling agent. Surf. Coat. Technol. 2006, 201, 1788–1795. [Google Scholar] [CrossRef]
- Arai, K.; Mizutani, T.; Miyamoto, M.; Kimura, Y.; Aoki, T. Colloidal silica bearing thin polyacrylate coat: A facile inorganic modifier of acrylic emulsions for fabricating hybrid films with least aggregation of silica nanoparticles. Prog. Org. Coat. 2019, 128, 11–20. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Trzaskowska, J.; Domanowski, W.; Kieplin, A.; Ofat-Kawalec, I.; Przybylski, J.; Woźniak, M.; Witwicki, D.; Sylwestrzak, K. Studies on synthesis and characterization of aqueous hybrid silicone-acrylic and acrylic-silicone dispersions and coatings: Part II. Prog. Org. Coat. 2020, 138, 105297. [Google Scholar] [CrossRef]
- Xiao, Z.; Guo, P.; Sun, N. Preparation, thermostability, and hydrophobic properties of TiO2/poly(dodecafluoroheptyl methacrylate) nanocomposites. J. Appl. Polym. Sci. 2016, 134, 44377. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Xue, F.; Jing, X. Water-based acrylate copolymer/silica hybrids for facile preparation of robust and durable superhydrophobic coatings. Appl. Surf. Sci. 2018, 447, 489–499. [Google Scholar] [CrossRef]
- Xu, W.; Wang, S.; Hao, L.; Wang, X. Preparation and characterization of trilayer core–shell polysilsesquioxane–fluoroacrylate copolymer composite emulsion particles. J. Appl. Polym. Sci. 2017, 134, 44845. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, D.; Jing, X.; Xue, B. Synthesis and characterization of fluorinated organic–inorganic hybrid coatings on tinplate. J. Appl. Polym. Sci. 2015, 132, 42428. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, H. Poly(vinylidene fluoride) grafted with 3-trimethoxysilylpropyl methacrylate for silyl functional membranes. React. Funct. Polym. 2008, 68, 1499–1506. [Google Scholar] [CrossRef]
- Tung, Y.C.; Chen, W.C. Poly[2,7-(9,9-dihexylfluorene)-block-poly[3-(trimethoxysilyl)propyl methacrylate (PF-b-PTMSPMA) rod-coil block copolymers: Synthesis, morphology and photophysical properties in mixed solvents. React. Funct. Polym. 2009, 69, 507–518. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Li, Z.; Liu, H.; He, P.; Li, J. Preparation of porous aminopropylsilsesquioxane by a nonhydrolytic sol-gel method in ionic liquid solvent. Langmuir 2005, 21, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; She, W.; Shi, D.; Jiang, T.; Hao, T.H.; Liu, J.; Zhang, Q.C.; You, J.; Li, R.Y. An extremely chemical and mechanically durable siloxane bearing copolymer coating with self-crosslinkable and anti-icing properties. Compos. Part B 2020, 195, 108031. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic Transparent and Superhydrophobic Coatings: From Nature and beyond Nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Z.; Liu, J.; Yang, Y.; Meng, Y.; Yu, F.; Jiang, L.; Wei, G.; Zhang, Z. Preparation of fluorinated/silanized polyacrylates amphiphilic polymers and their anticorrosion and antifouling performance. Prog. Org. Coat. 2020, 140, 105510. [Google Scholar] [CrossRef]
- Yu, F.; Gao, J.; Liu, C.; Chen, Y.; Zhong, G.; Hodges, C.; Chen, M.; Zhang, H. Preparation and UV aging of nano-SiO2/fluorinated polyacrylate polyurethane hydrophobic composite coating. Prog. Org. Coat. 2020, 141, 105556. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhao, J.; Li, Y.; Lu, K. Synthesis of cellulose nanocrystals-armored fluorinated polyacrylate latexes via Pickering emulsion polymerization and their film properties. Colloids Surf. B 2020, 192, 111071. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Wang, Y.; Ban, T.; Zhang, Y.; Zhang, J.; Zhu, X. Preparation and characteristics of crosslinked fluorinated acrylate modified waterborne polyurethane for metal protection coating. Prog. Org. Coat. 2021, 158, 106371. [Google Scholar] [CrossRef]
- Lyu, B.; Li, X.; Liu, H.; Gao, D.; Ma, J.; Zhang, M. Preparation of an amphiphilic Janus SiO2/fluorinated polyacrylate latex film and its application as a hydrophobic fabric agent. J. Colloid Interface Sci. 2021, 599, 88–99. [Google Scholar] [CrossRef]
- Shao, T.; Chen, X.; Chen, L. Preparation and characterization of self-cross-linking long fluorocarbon polyacrylate latex modified by silane cross-linker. Pigment Resin Technol. 2020. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Shin, W.; Singh, P.K. Progress in ionic organic-inorganic composite membranes for fuel cell applications. Polym. Chem. 2010, 1, 388–408. [Google Scholar] [CrossRef]
- Xu, S.; Liu, W. Synthesis and surface characterization of an amphiphilic fluorinated copolymer via emulsifier-free emulsion polymerization of RAFT. J. Fluor. Chem. 2008, 129, 125–130. [Google Scholar] [CrossRef]
- Fang, C.; Huang, X.; Ge, T.; Li, Y.; Cao, Y.; Zhu, X.; Dong, X. Effect of dodecafluoroheptyl methacrylate (DFMA) on the comprehensive properties of acrylate emulsion pressure sensitive adhesives. Int. J. Adhes. Adhes. 2020, 101, 102634. [Google Scholar] [CrossRef]
- Ni, H.; Wang, X.; Zhang, W.; Wang, X.; Shen, Z. Stable hydrophobic surfaces created by self-assembly of poly (methyl methacrylate) end-capped with 2-perfluorooctylethyl methacrylate units. Surf. Sci. 2007, 601, 3632–3639. [Google Scholar] [CrossRef]
- Lü, T.; Qi, D.; Zhang, D.; Liu, Q.; Zhao, H. Fabrication of self-cross-linking fluorinated polyacrylate latex particles with core-shell structure and film properties. React. Funct. Polym. 2016, 104, 9–14. [Google Scholar] [CrossRef]
- Wang, Y.; Long, J.; Bai, Y.; Zhang, C.; Cheng, B.; Shao, L.; Qi, S. Preparation and characterization of fluorinated acrylic pressure sensitive adhesives for low surface energy substrates. J. Fluorine Chem. 2015, 180, 103–109. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.; Hao, L.; Zhang, D.; Zhang, M. Synthesis and characterization of self-crosslinking fluorinated polyacrylate soap-free latices with core–shell structure. Appl. Surf. Sci. 2013, 268, 373–380. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhan, X.; Chen, F.; Shi, Y.; Wang, Q. Block copolymers of dodecafluoroheptyl methacrylate and butyl methacrylate by RAFT miniemulsion polymerization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1585–1594. [Google Scholar] [CrossRef]
- Manring, L.E. Thermal degradation of poly(methyl methacrylate). 2. Vinyl-terminated polymer. Macromolecules 1989, 22, 2673–2677. [Google Scholar] [CrossRef]
- Zhou, J.; Ghen, X.; Duan, H.; Ma, J. Synthesis and characterization of organic fluorine and nano-SiO2 modified polyacrylate emulsifier-free latex. Prog. Org. Coat. 2015, 89, 192–198. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, L.; Zhang, Y.; Chen, Y.; Bao, B.; Xu, J.; Zhou, W. Surface properties and self-cleaning ability of the fluorinated acrylate coatings modified with dodecafluoroheptyl methacrylate through two adding ways. Appl. Surf. Sci. 2014, 295, 44–49. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Duan, H.; Ma, J.; Ma, Y. Synthesis and Characterization of nano-SiO2 modified fluorine-containing polyacrylate emulsifier-free emulsion. Appl. Surf. Sci. 2015, 331, 504–511. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Ma, J. Fluorinated polyacrylate emulsifier-free emulsion mediated by poly(acrylic acid)-b-poly(hexafluorobutyl acrylate) trithiocarbonate via ab initio RAFT emulsion polymerization. Chem. Eng. J. 2013, 223, 8–17. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Li, W.; Wang, F.; Ou, J.; Amirfazli, A. One-step fabrication of transparent superhydrophobic surface. Appl. Surf. Sci. 2021, 542, 148534. [Google Scholar] [CrossRef]
- Zhang, C.; Kalulu, M.; Sun, S.; Jiang, P.; Zhou, X.; Wei, Y.; Jiang, Y. Environmentally safe, durable and transparent superhydrophobic coating prepared by one-step spraying. Colloids Surf. A 2019, 570, 147–155. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, Y.; Du, F. Rational design of superhydrophobic, transparent hybrid coating with superior durability. Prog. Org. Coat. 2021, 157, 106294. [Google Scholar] [CrossRef]
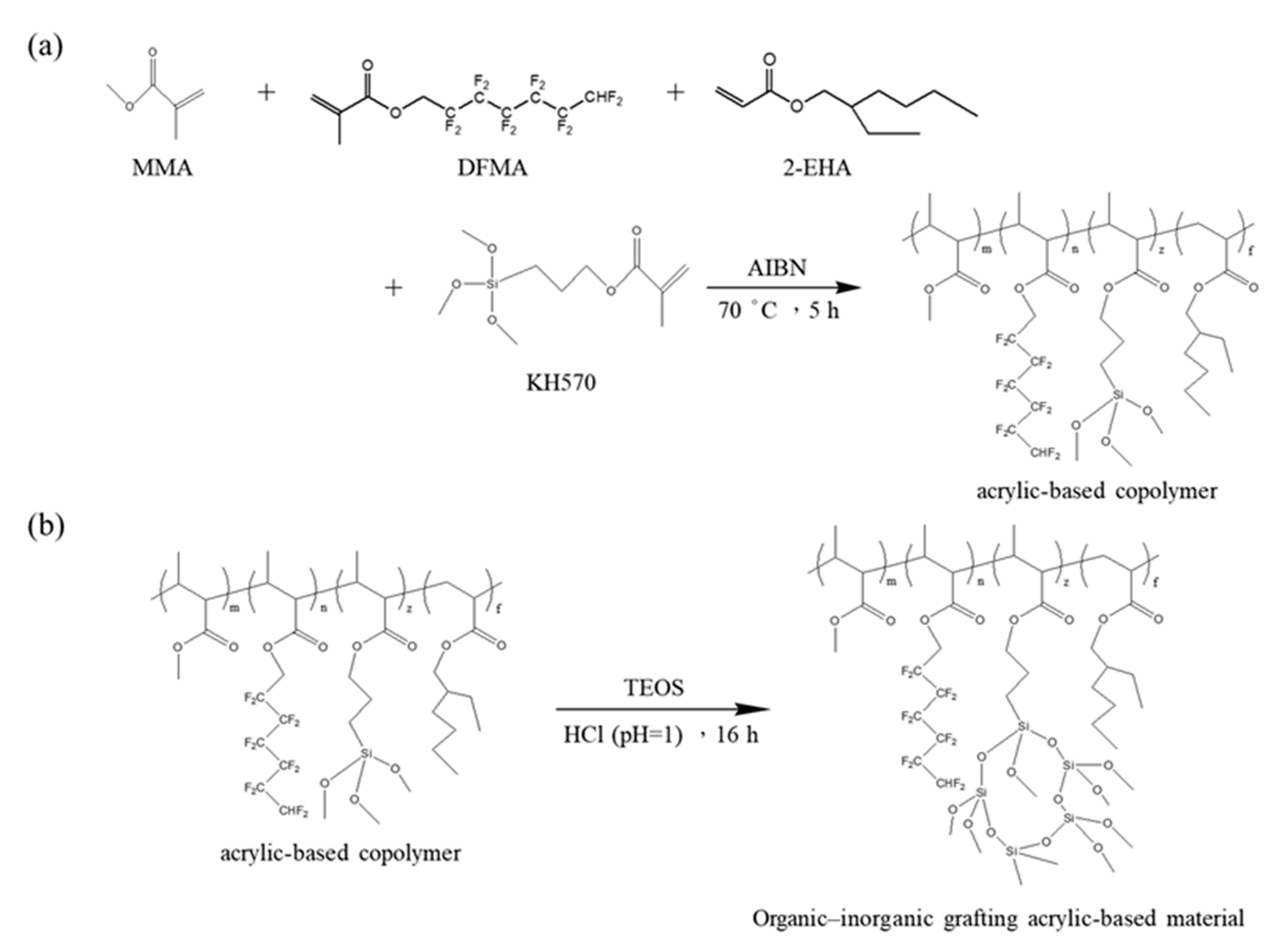


| First Stage (Free Radical Polymerization) | Second Stage (Sol–Gel Route) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DFMA (g) | MMA (g) | DFMA/MMA (Weight Ratio) | DFMA/MMA (Molar Ratio) | 2-EHA (g) | KH-570 (g) | THF (g) | AIBN (g) | TEOS (g) | HCl(aq) (g) | THF (g) |
| 0 | 9.0 | 0/9 | - | 1.0 | 0.05 | 40 | 0.02 | 1.0 | 1.5 | 5.0 |
| 0.5 | 8.5 | 1/17 | 1/68 | 1.0 | 0.05 | 40 | 0.02 | 1.0 | 1.5 | 5.0 |
| 1.0 | 8.0 | 1/8 | 1/32 | 1.0 | 0.05 | 40 | 0.02 | 1.0 | 1.5 | 5.0 |
| 1.5 | 7.5 | 1/5 | 1/20 | 1.0 | 0.05 | 40 | 0.02 | 1.0 | 1.5 | 5.0 |
| 2.0 | 7.0 | 2/7 | 1/14 | 1.0 | 0.05 | 40 | 0.02 | 1.0 | 1.5 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-M.; Wang, H.-Y.; Fang, S.-Y.; Yang, W.-D. Influence of Fluorine-Containing Monomer Content on the Hydrophobic and Transparent Properties of Nanohybrid Silica Polyacrylate Coating Materials. Materials 2021, 14, 4261. https://doi.org/10.3390/ma14154261
Huang C-M, Wang H-Y, Fang S-Y, Yang W-D. Influence of Fluorine-Containing Monomer Content on the Hydrophobic and Transparent Properties of Nanohybrid Silica Polyacrylate Coating Materials. Materials. 2021; 14(15):4261. https://doi.org/10.3390/ma14154261
Chicago/Turabian StyleHuang, Chih-Ming, Her-Yung Wang, Sing-Yuan Fang, and Wein-Duo Yang. 2021. "Influence of Fluorine-Containing Monomer Content on the Hydrophobic and Transparent Properties of Nanohybrid Silica Polyacrylate Coating Materials" Materials 14, no. 15: 4261. https://doi.org/10.3390/ma14154261
APA StyleHuang, C.-M., Wang, H.-Y., Fang, S.-Y., & Yang, W.-D. (2021). Influence of Fluorine-Containing Monomer Content on the Hydrophobic and Transparent Properties of Nanohybrid Silica Polyacrylate Coating Materials. Materials, 14(15), 4261. https://doi.org/10.3390/ma14154261





