Electrical Properties of Textiles Treated with Graphene Oxide Suspension
Abstract
1. Introduction
2. Materials and Methods
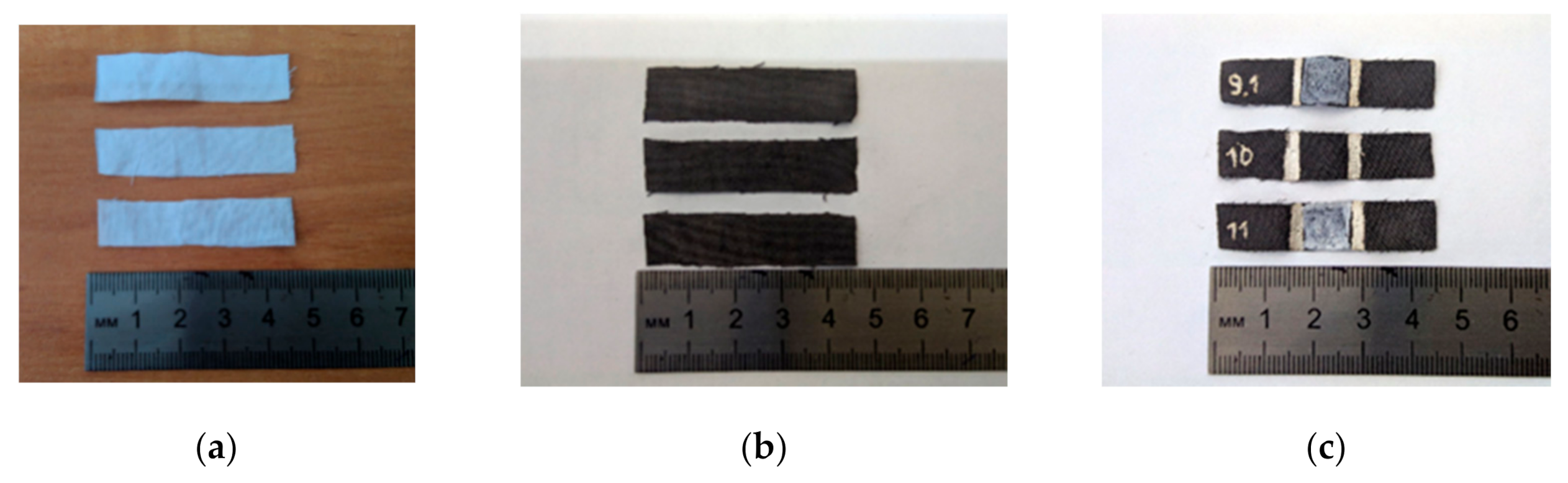
- Nylon and cotton samples with a size of 1 cm × 4.5 cm were dipped in an acidified BSA solution and dried at room temperature. 5 mL of BSA solution was used for 14 samples.
- The nylon and cotton samples with BSA layer were immersed into the 1 and 3 mg/mL GO suspensions under stirring and dried at room temperature. The GO concentration was determined by weighing the dry residue. 5 mL of the GO suspension was used for 14 samples.
- Obtained fabrics with dry GO layer were treated with hydrazine vapor on a water bath for one hour at a temperature of 60 °C in a sealed container.
- Some of the samples were covered with a thin layer of BF-6 glue from both sided and dried at room temperature.
- The samples were washed according to standard washing process GOST 9733.4–83. The washing solution consisted of 0.5 g of soap and 0.2 g of soda for every 100 mL of water. The fabrics in the washing solution were stirred using a magnetic stirrer at a temperature of 60 °C for 30 min.
- For sheet resistance measurement, silver paste contacts were applied. The distance between the contacts was 10 mm for tensile testing and 15 mm for bending testing. After application, the contacts were dried for 24 h at room temperature.
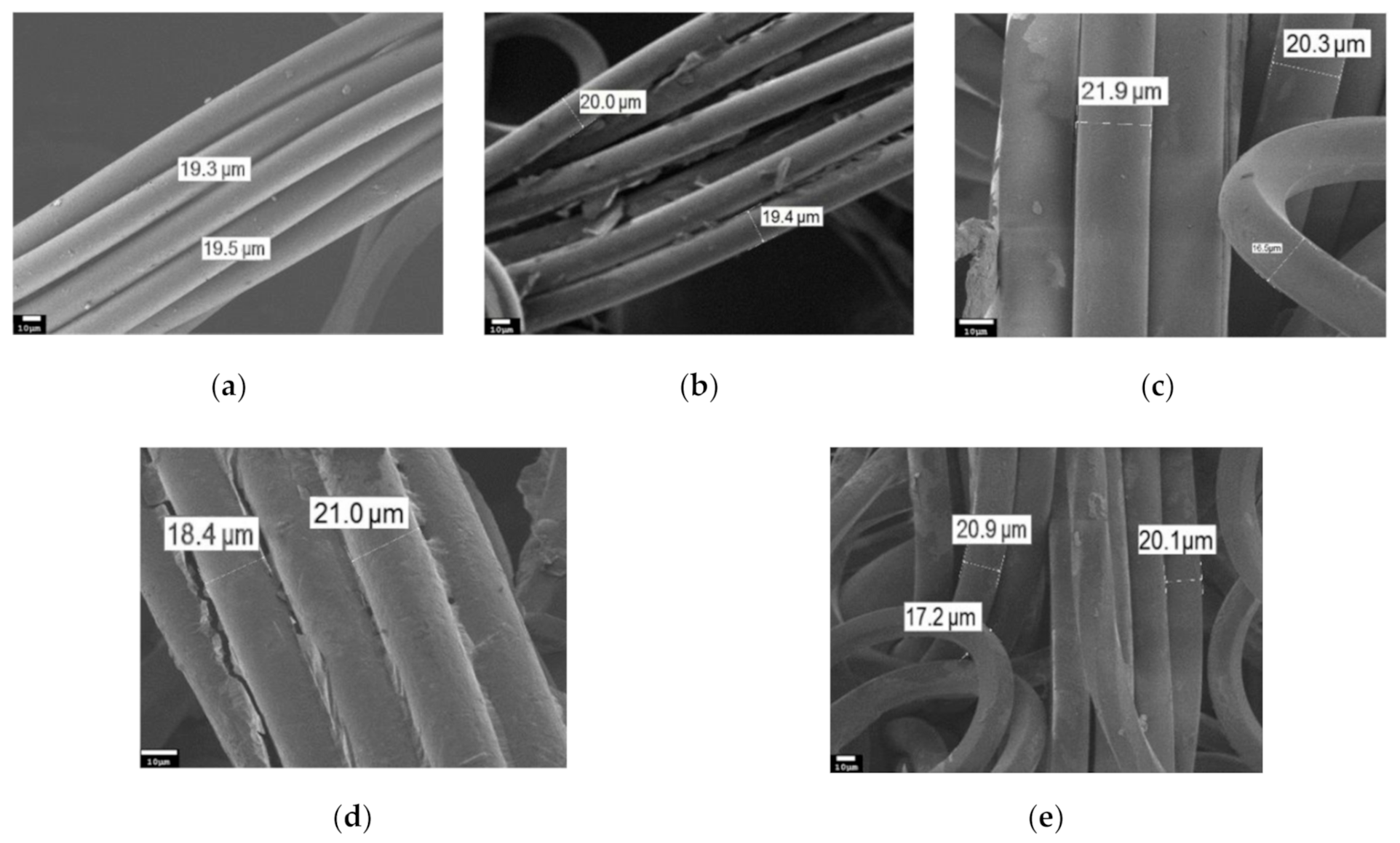
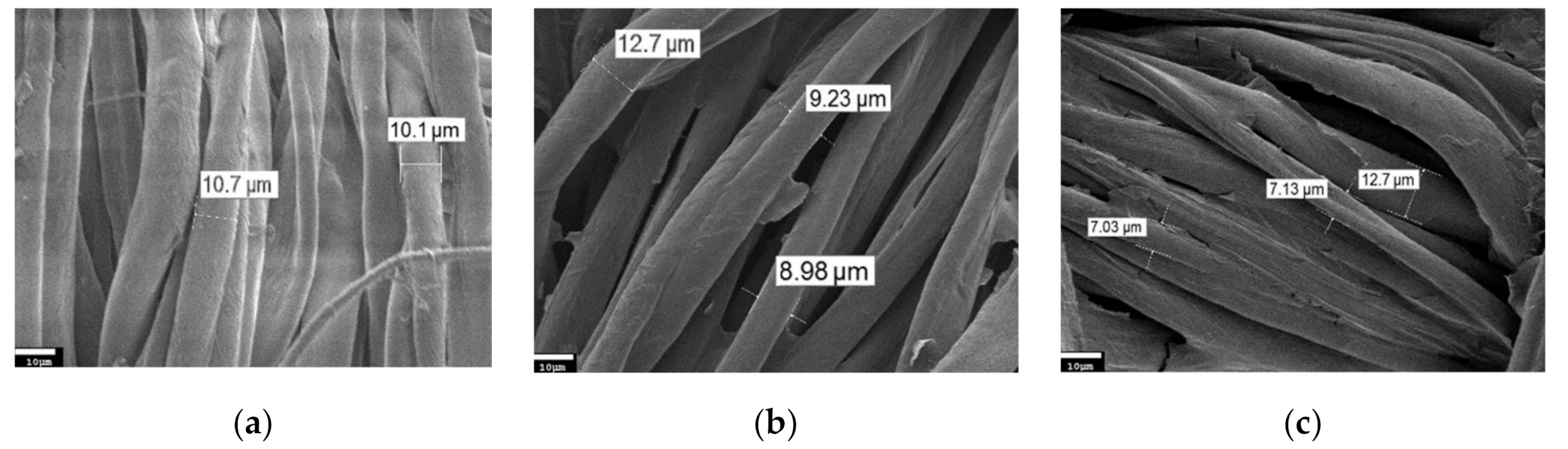
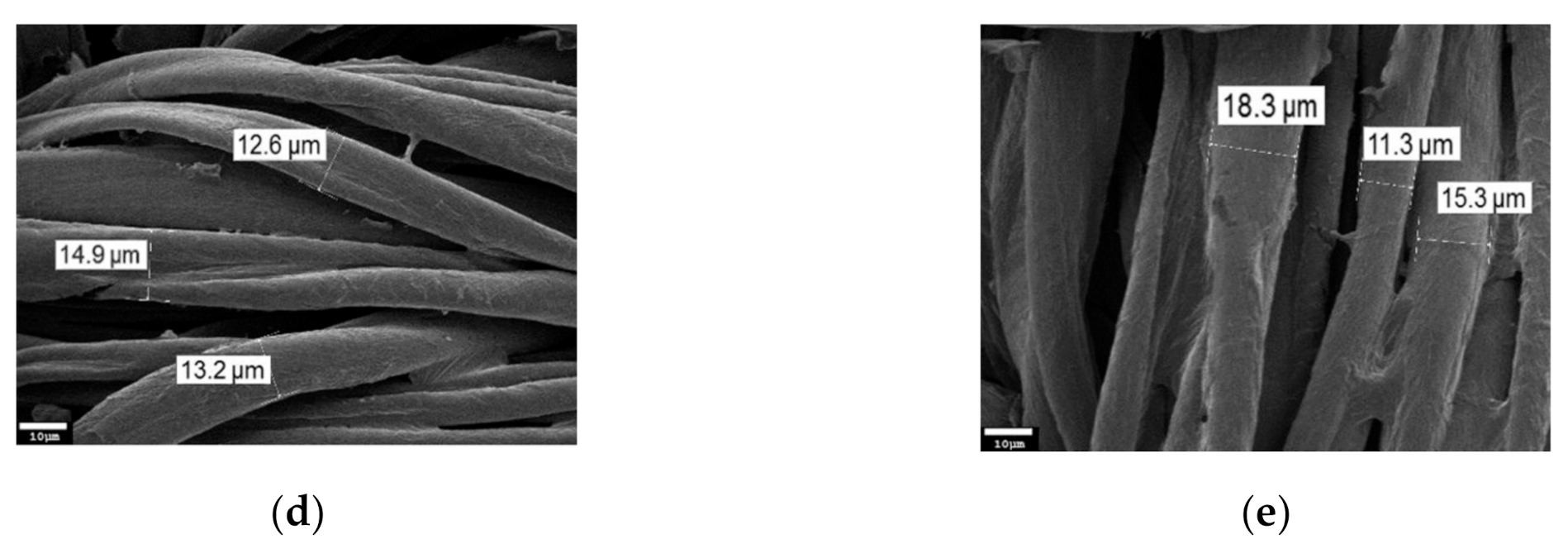
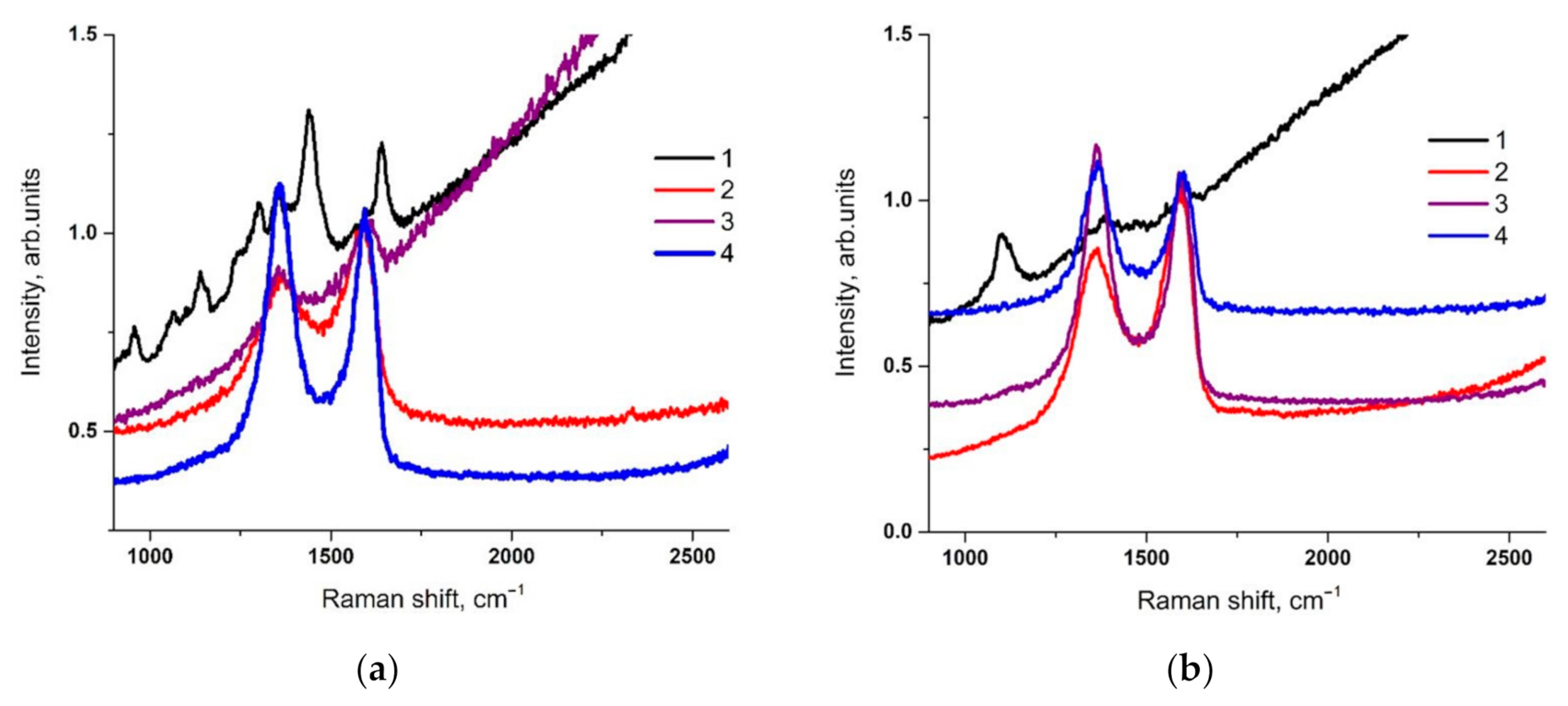
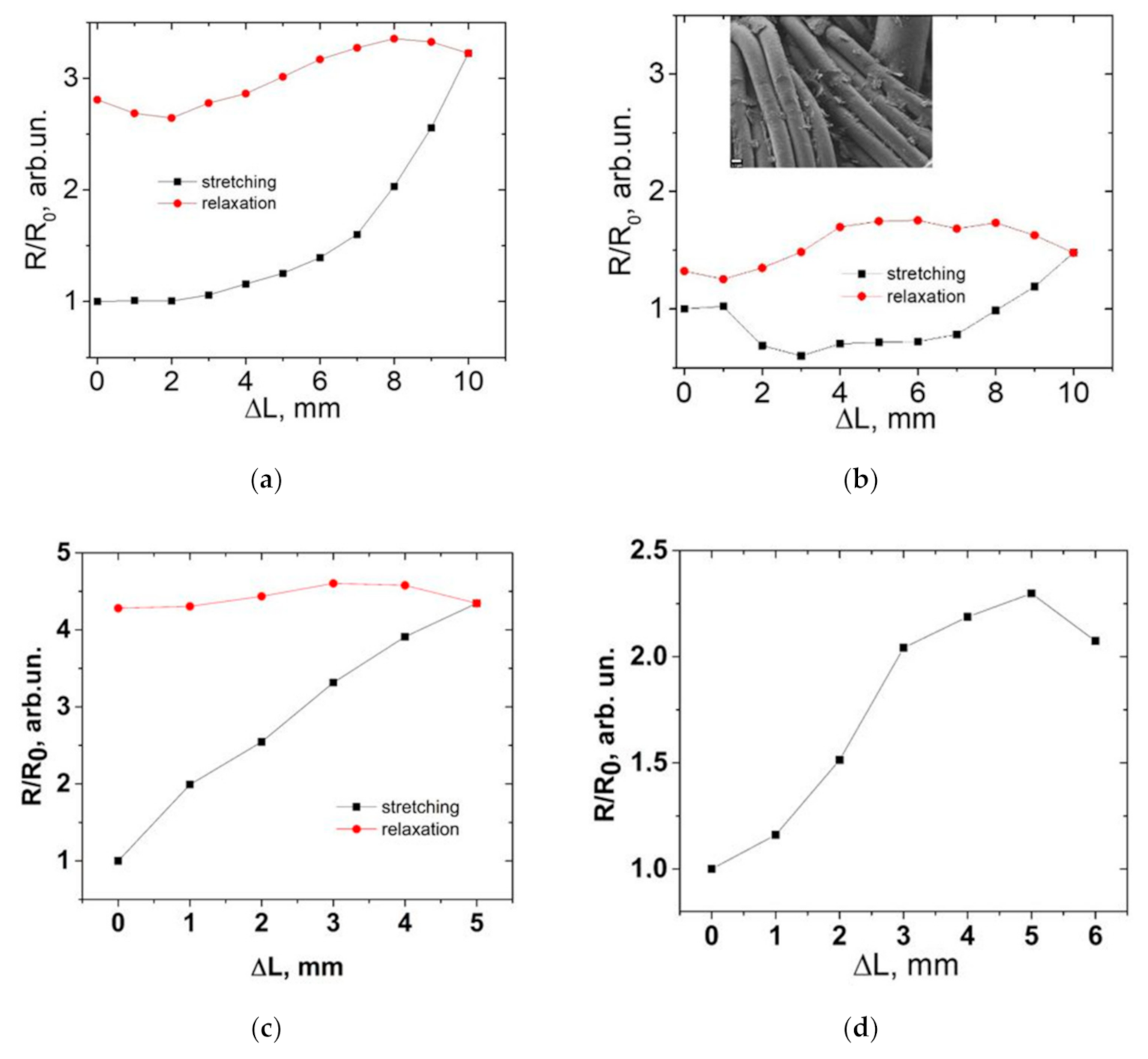
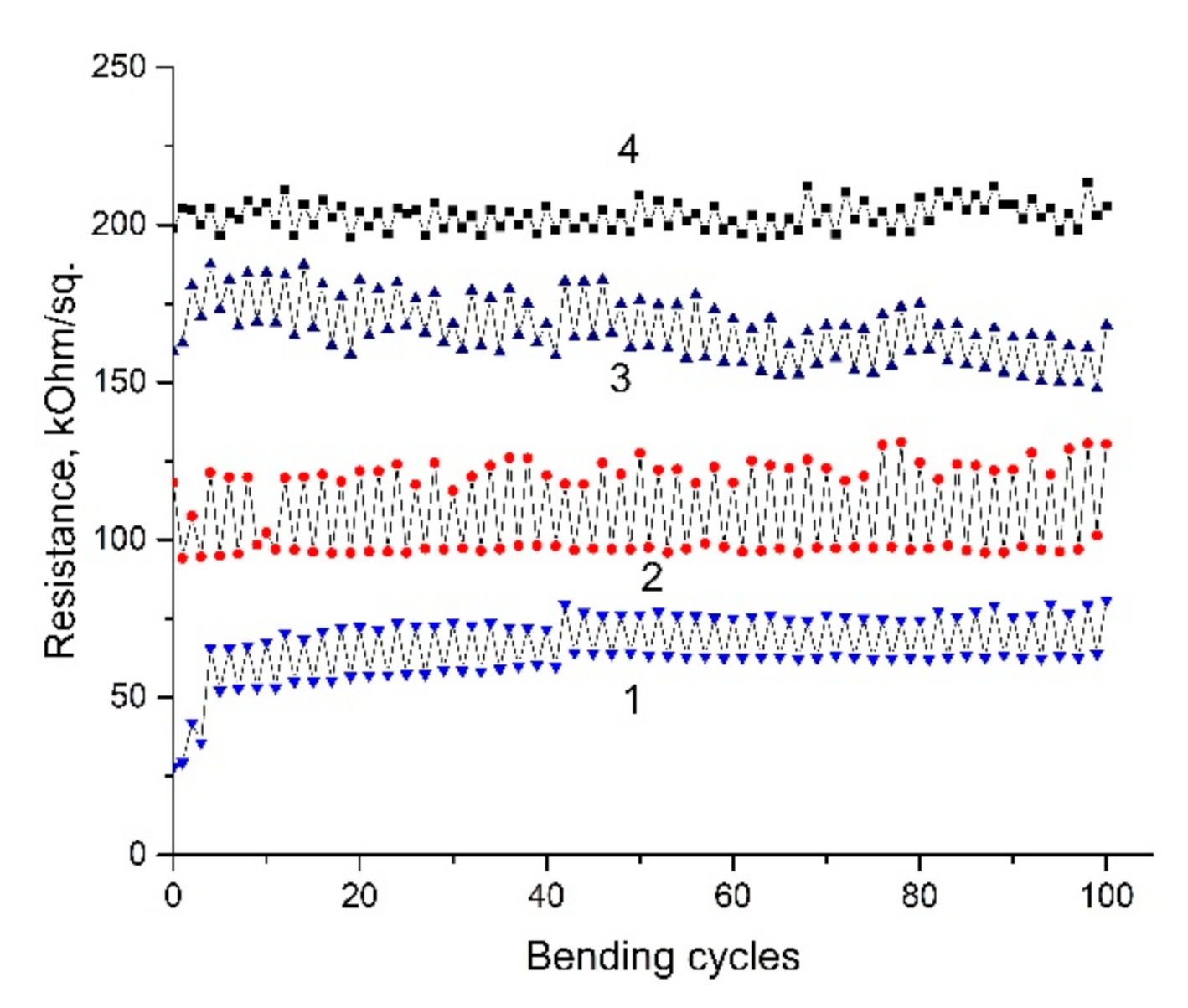
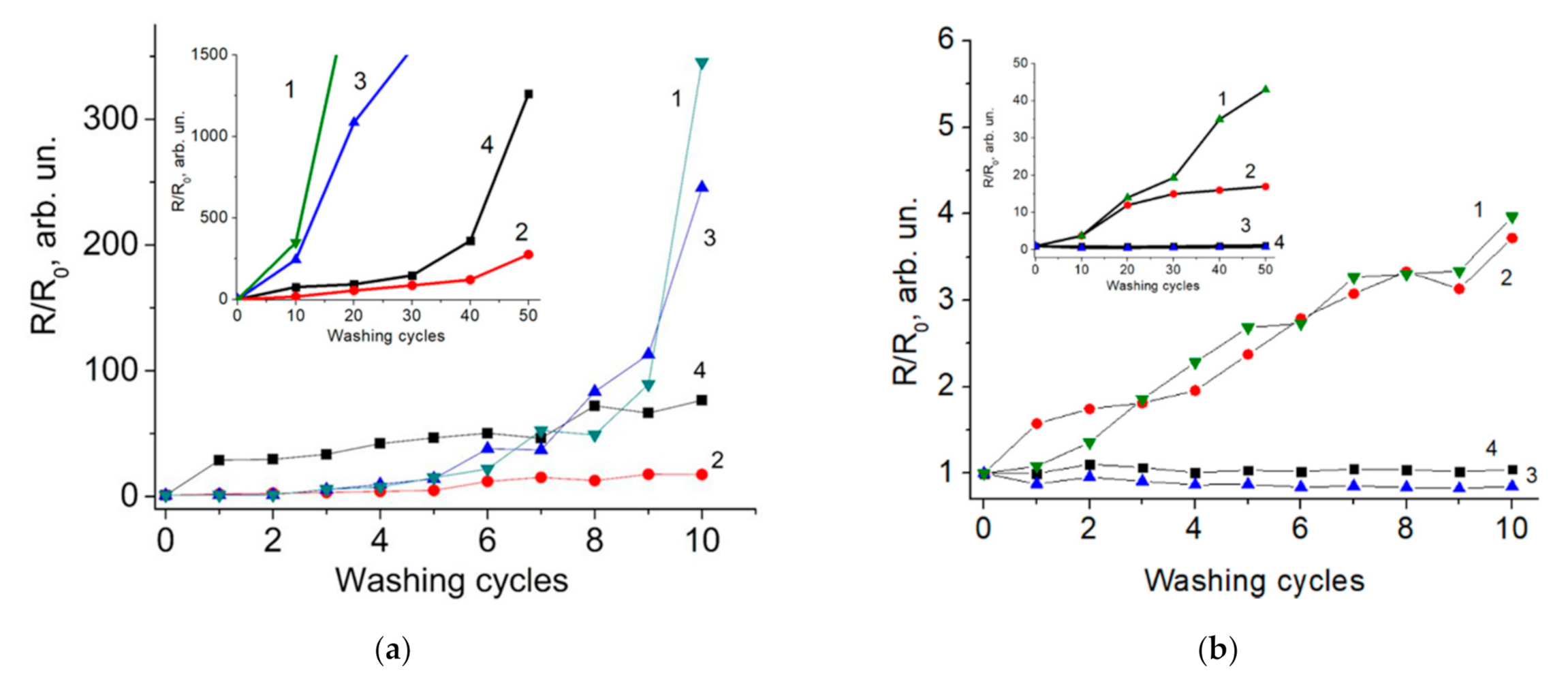
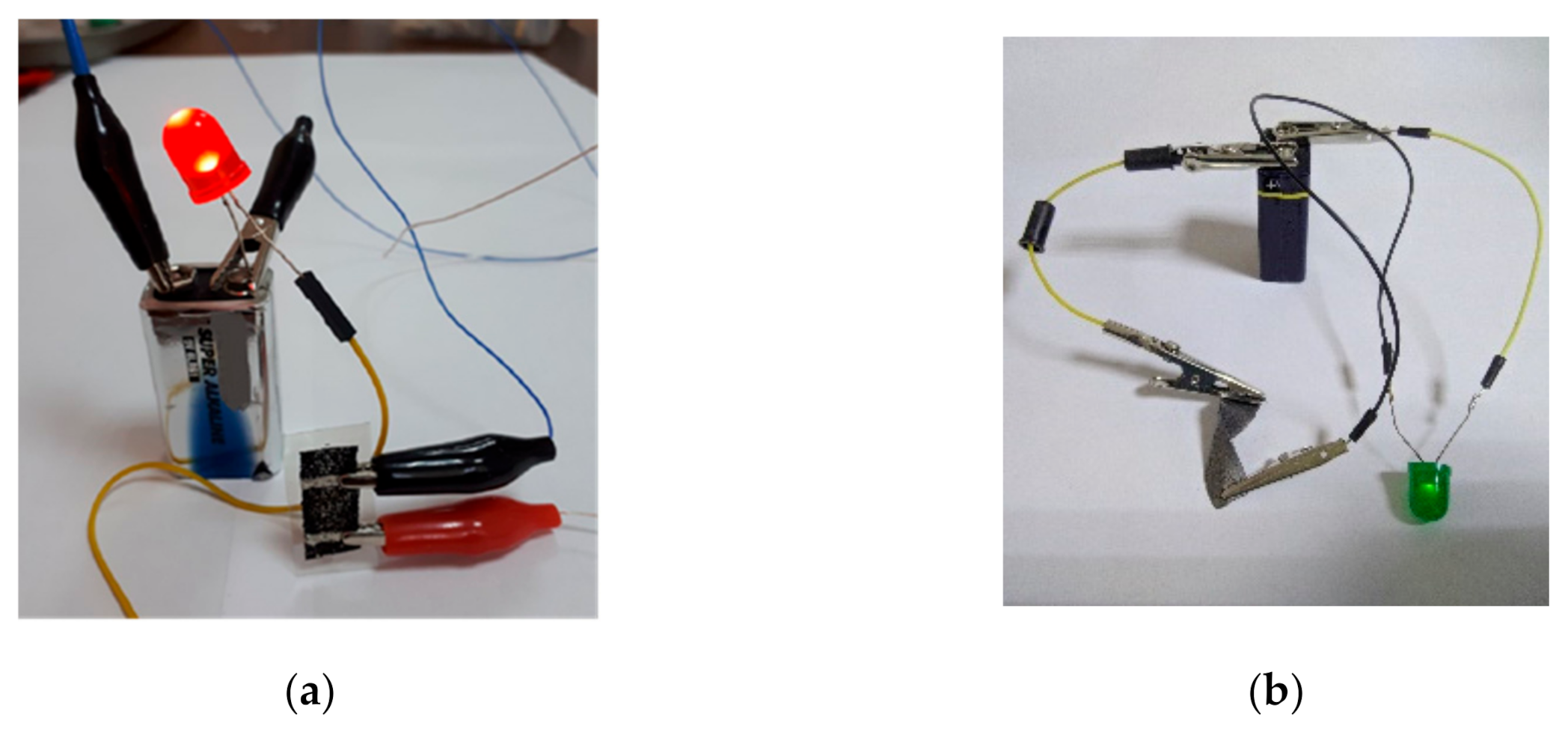
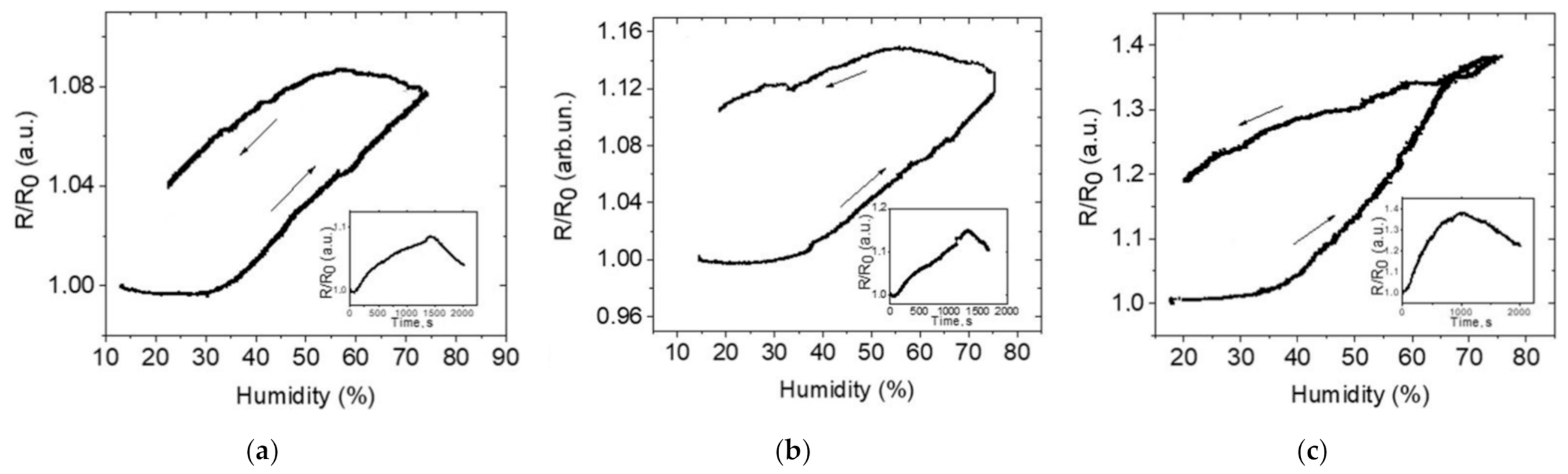
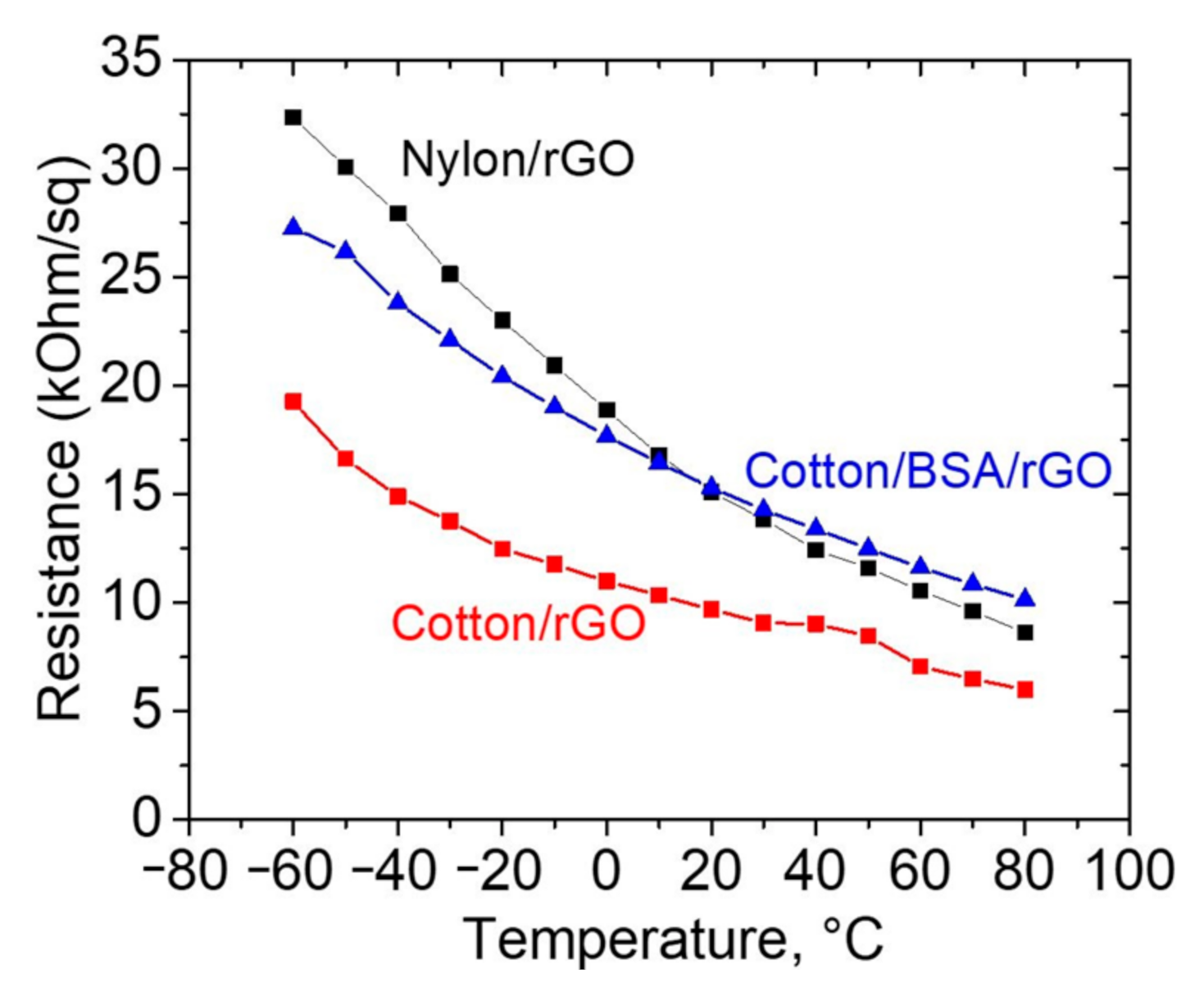
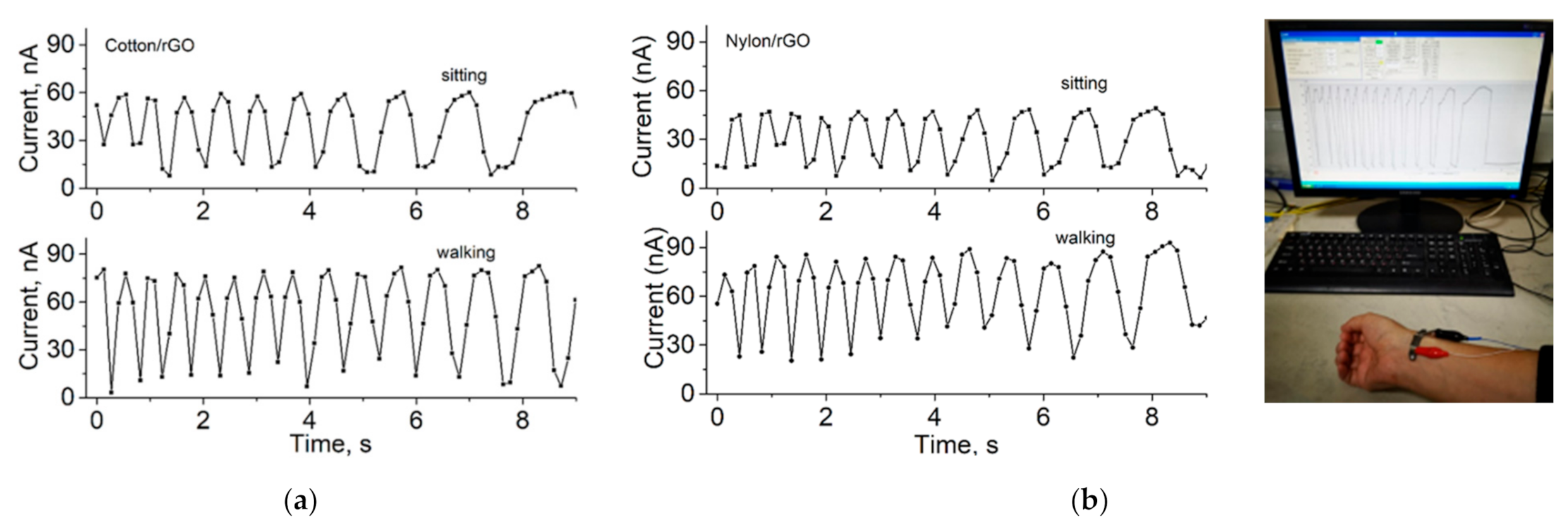
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Zhang, T.; Chen, H.; Feng Wang, Y.P.; Li, S. Mini Review on Flexible and Wearable Electronics for Monitoring Human Health Information. Nanoscale Res. Lett. 2019, 14, 263. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Kumar, J.; Pandit, P. Recent Advancements in Wearable & Smart Textiles: An Overview. Mater. Today-Proc. 2019, 16, 1518–1523. [Google Scholar]
- Yetisen, A.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.; Hinestroza, J.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef]
- Di, J.; Zhang, X.; Yong, Z.; Zhang, Y.; Li, D.; Li, R.; Li, Q. Carbon-Nanotube Fibers for Wearable Devices and Smart Textiles. Adv. Mater. 2016, 28, 10529–10538. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Tabor, J.; Ghosh, T.K. Electrically Conductive Coatings for Fiber-Based E-Textiles. Fibers 2019, 7, 51. [Google Scholar] [CrossRef]
- Gurarslan, A.; Özdemir, B.; Halil Bayat, İ.; Berke Yelten, M.; Karabulut Kurt, G. Silver nanowire coated knitted wool fabrics for wearable electronic applications. J. Eng. Fiber Fabr. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Ashayer-Soltani, R.; Hunt, C.; Thomas, O. Fabrication of highly conductive stretchable textile with silver nanoparticles. Text. Res. J. 2015, 86, 1041–1049. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Tan, S.; He, P.; Fernando, A.; Carr, C.; Novoselov, K.S. Scalable Production of Graphene-Based Wearable E-Textiles. ACS Nano 2017, 11, 12266–12275. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- An, H.J.; Sarkheil, M.; Park, H.S.; Yu, I.J.; Johari, S.A. Comparative toxicity of silver nanoparticles (AgNPs) and silver nanowires (AgNWs) on saltwater microcrustacean, Artemia salina. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 218, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Afroj, S.; Karim, N.; Wang, Z.; Tan, S.; He, P.; Holwill, M.; Ghazaryan, D.; Fernando, A.; Novoselov, K.S. Engineering Graphene Flakes for Wearable Textile Sensors via Highly Scalable and Ultrafast Yarn Dyeing Technique. ACS Nano 2019, 13, 3847–3857. [Google Scholar] [CrossRef]
- Thekkekara, L.V.; Gu, M. Large-scale waterproof and stretchable textile-integrated laser- printed graphene energy storages. Sci. Rep. 2019, 9, 11822. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Mohanan, P.V. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int. J. Biol. Macromol. 2016, 86, 546–555. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Lithium Aluminum Hydride as Reducing Agent for Chemically Reduced Graphene Oxides. Chem. Mater. 2012, 24, 2292–2298. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Zhu, S.; Wan, J.; Liu, Z.; Vaaland, O.; Lacey, S.; Fang, Z.; Dai, H.; Li, T.; et al. Hybridizing wood cellulose and graphene oxide toward high-performance fibers. NPG Asia Mater. 2015, 7, e150. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Kim, W.-J.; Jun, Y.; Kim, B.H. A Novel Method for Applying Reduced Graphene Oxide Directly to Electronic Textiles from Yarns to Fabrics. Adv. Mater. 2013, 25, 5701–5705. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, S. Highly conductive and ultra-durable electronic textiles by covalent immobilization of carbon nanomaterials on cotton fabrics. J. Mater. Chem. C. 2018, 6, 12273. [Google Scholar] [CrossRef]
- Fugetsu, B.; Sano, E.; Yu, H.; Mori, K.; Tanaka, T. Graphene oxide as dyestuffs for the creation of electrically conductive fabrics. Carbon 2010, 48, 3340–3345. [Google Scholar] [CrossRef]
- Cai, G.; Yang, M.; Xu, Z.; Liu, J.; Tang, B.; Wang, X. Flexible and wearable strain sensing fabrics. Chem. Eng. J. 2017, 325, 396–403. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Fabricating electroconductive cotton textiles using graphene. Carbohydr. Polym. 2013, 96, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sahito, I.A.; Sun, K.C.; Arbab, A.A.; Qadir, M.B.; Jeong, S.H. Integrating high electrical conductivity and photocatalytic activity in cotton fabric by cationizing for enriched coating of negatively charged graphene oxide. Carbohydr. Polym. 2015, 130, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Lee, G.; Choi, J.; Lee, D.J. Studies on composites based on HTV and RTV silicone rubber and carbon nanotubes for sensors and actuators. Polymer 2020, 190, 122221. [Google Scholar] [CrossRef]
- Kumar, V.; Alam, M.N.; Manikkavel, A.; Choi, J.; Lee, D.J. Investigation of silicone rubber composites reinforced with carbon nanotube, nanographite, their hybrid, and applications for flexible devices. J. Vinyl Addit. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Ouadil, B.; Cherkaoui, O.; Safi, M.; Zahouily, M. Surface modification of knit polyester fabric for mechanical, electrical and UV protection properties by coating with graphene oxide, graphene and graphene/silver nanocomposites. Appl. Surf. Sci. 2017, 414, 292–302. [Google Scholar] [CrossRef]
- Shemetov, A.A.; Nabiev, I.; Sukhanova, A. Molecular Interaction of Proteins and Peptides with Nanoparticles. ACS Nano 2012, 6, 4585–4602. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, S.; Yuan, B.; Li, Y.; Deng, Z. Toward a Universal “Adhesive Nanosheet” for the Assembly of Multiple Nanoparticles Based on a Protein-Induced Reduction/Decoration of Graphene Oxide. J. Am. Chem. Soc. 2010, 132, 7279–7281. [Google Scholar] [CrossRef]
- Xu, L.; Zhai, H.; Chen, X.; Liu, Y.; Wang, M.; Liu, Z.; Terry, T.Y. Coolmax/graphene-oxide functionalized textile humidity sensor with ultrafast response for human activities monitoring. Chem. Eng. J. 2021, 412, 128639. [Google Scholar] [CrossRef]
- Aleksandrov, G.N.; Smagulova, S.A.; Kapitonov, A.N.; Vasil’eva, F.D.; Kurkina, I.I.; Vinokurov, P.V.; Antonova, I.V. Thin partially reduced oxide-graphene films: Structural, optical and electrical properties. Nanotechnol. Russ. 2014, 9, 18–22. [Google Scholar] [CrossRef]
- Ren, J.; Wang, C.; Zhang, X.; Carey, T.; Chen, K.; Yin, Y.; Torrisi, F. Environmentally-friendly conductive cotton fabric as flexible strain sensor based on hot press reduced graphene oxide. Carbon 2017, 111, 622–630. [Google Scholar] [CrossRef]
- Cai, G.; Xu, Z.; Yang, M.; Tang, B.; Wang, X. Functionalization of cotton fabrics through thermal reduction of graphene oxide. Appl. Surf. Sci. 2017, 393, 441–448. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- He, S.; Xin, B.; Chen, Z.; Liu, Y. Functionalization of cotton by reduced graphene oxide for improved electrical conductivity. Text. Res. J. 2019, 89, 1038–1050. [Google Scholar] [CrossRef]
- Chatterjee, A.; Nivas Kumar, M.; Maity, S. Influence of graphene oxide concentration and dipping cycles on electrical conductivity of coated cotton textiles. J. Text. Inst. 2017, 108, 1910–1916. [Google Scholar] [CrossRef]
- Reddy, K.R.; Gandla, S.; Gupta, D. Highly sensitive, rugged, and wearable fabric strain sensor based on graphene clad polyester knitted elastic band for human motion monitoring. Adv. Mater. Interfaces 2019, 6, 1900409. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaev, D.V.; Evseev, Z.I.; Smagulova, S.A.; Antonova, I.V. Electrical Properties of Textiles Treated with Graphene Oxide Suspension. Materials 2021, 14, 1999. https://doi.org/10.3390/ma14081999
Nikolaev DV, Evseev ZI, Smagulova SA, Antonova IV. Electrical Properties of Textiles Treated with Graphene Oxide Suspension. Materials. 2021; 14(8):1999. https://doi.org/10.3390/ma14081999
Chicago/Turabian StyleNikolaev, Danil Valeriyevich, Zakhar Ivanovich Evseev, Svetlana Afanasyevna Smagulova, and Irina Veniaminovna Antonova. 2021. "Electrical Properties of Textiles Treated with Graphene Oxide Suspension" Materials 14, no. 8: 1999. https://doi.org/10.3390/ma14081999
APA StyleNikolaev, D. V., Evseev, Z. I., Smagulova, S. A., & Antonova, I. V. (2021). Electrical Properties of Textiles Treated with Graphene Oxide Suspension. Materials, 14(8), 1999. https://doi.org/10.3390/ma14081999






