Organobentonites Modified with Poly(Acrylic Acid) and Its Sodium Salt for Foundry Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Materials
2.2.1. Obtaining SN/PAA Composites
2.2.2. Obtaining SN/PAA/Na Composites
2.2.3. Obtaining SN-Na/PAA Composites
2.3. Characterization Methods
3. Results and Discussion
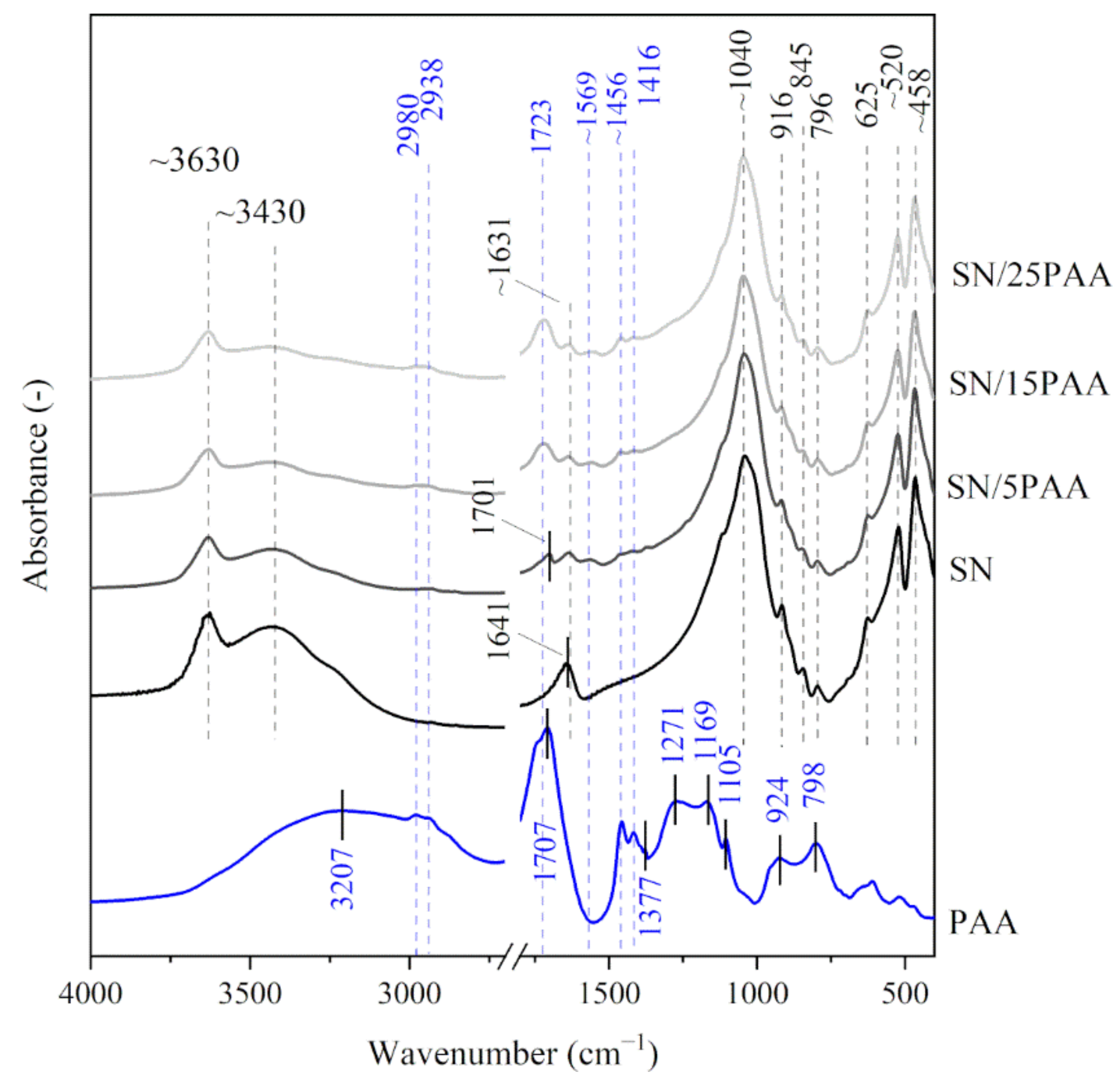
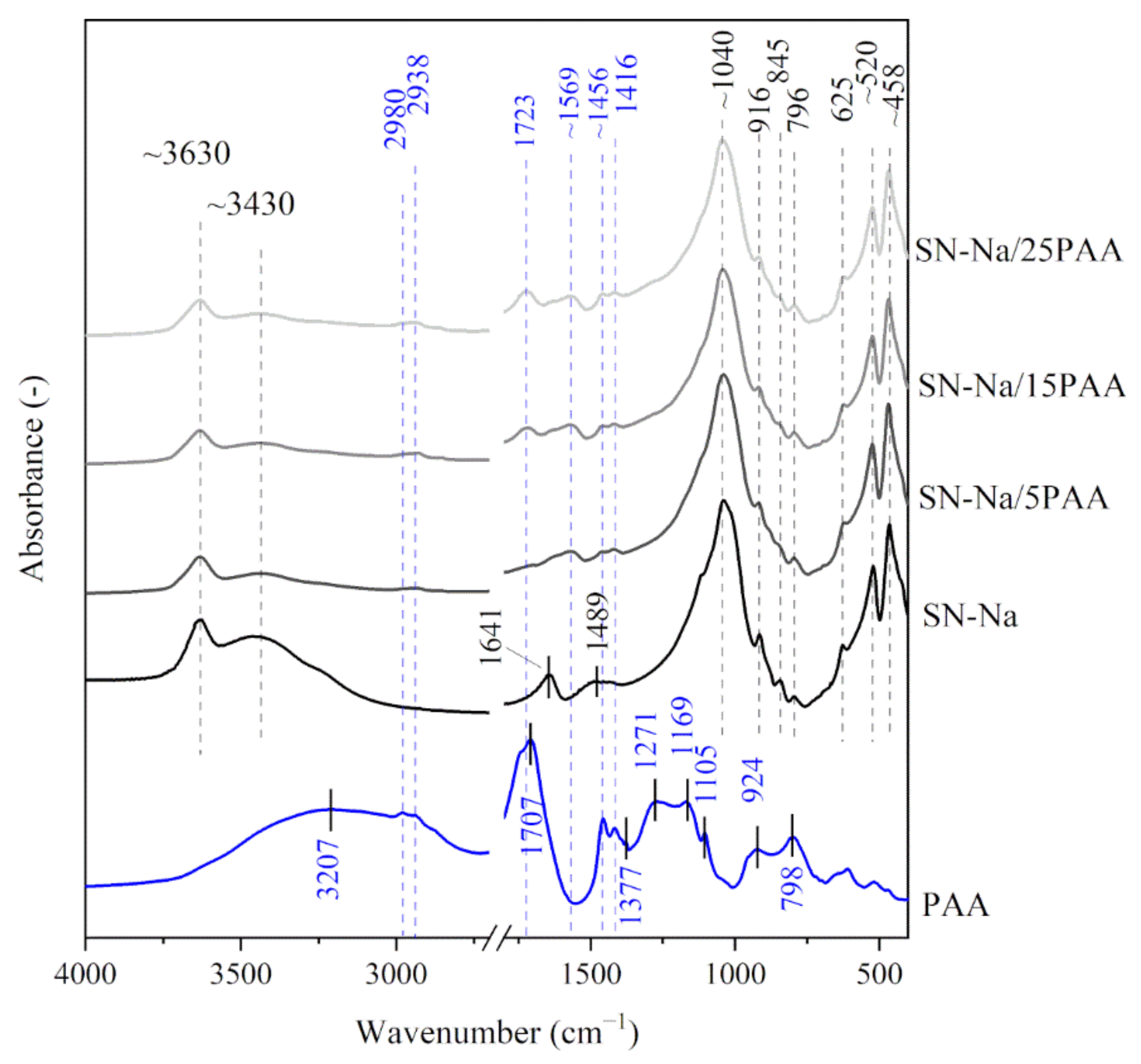
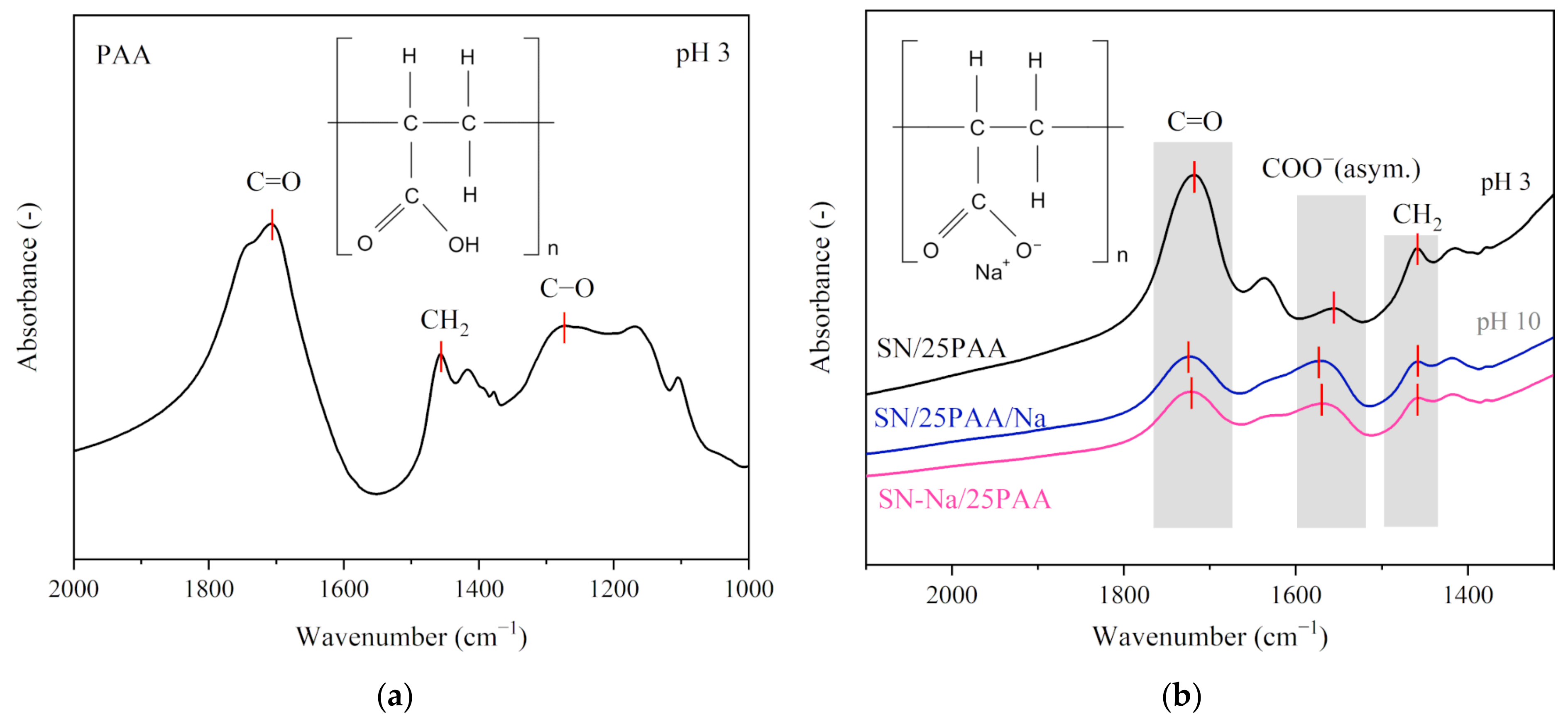
3.1. Fourier Transform-Infrared Spectroscopy Analysis
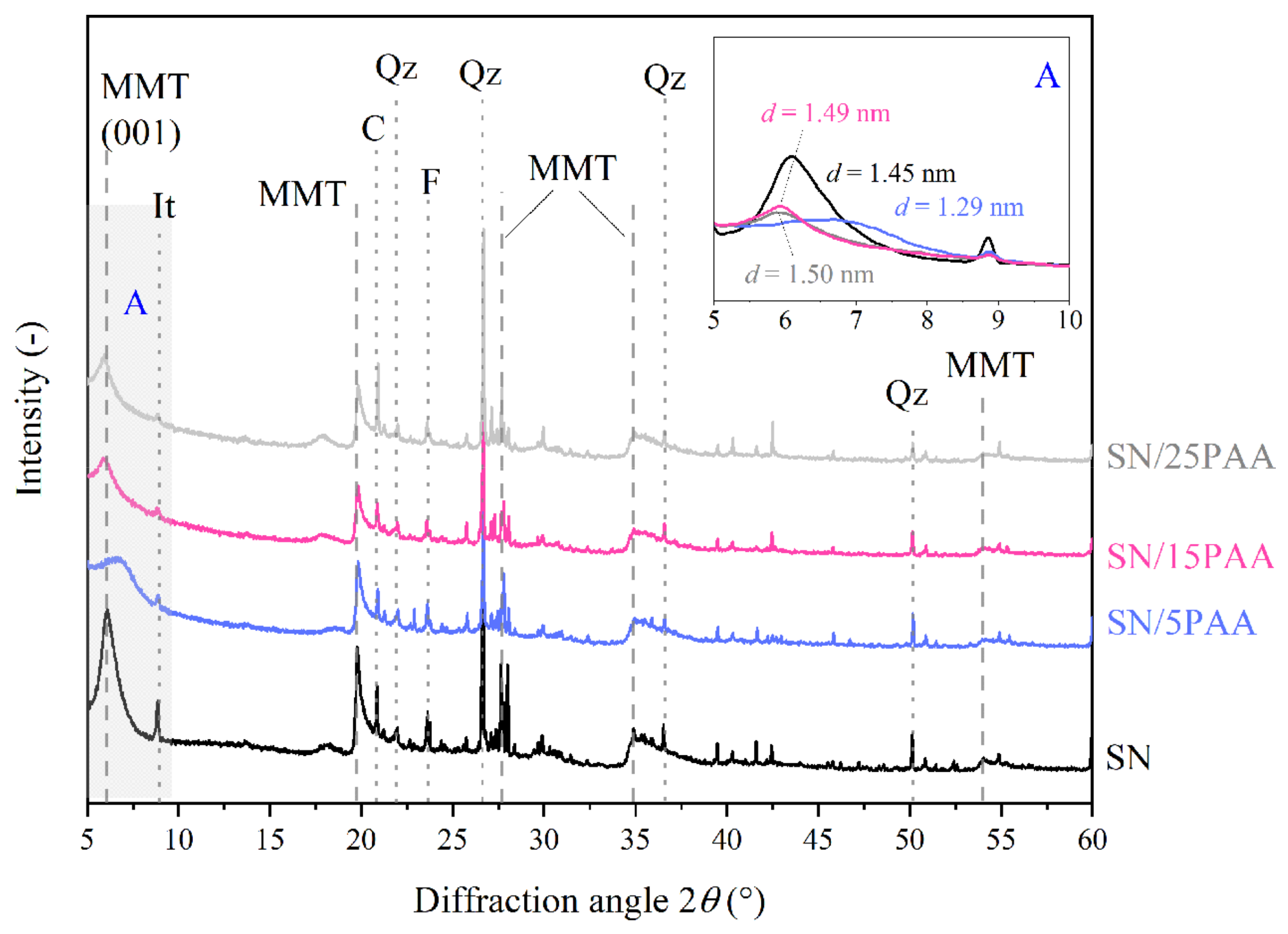
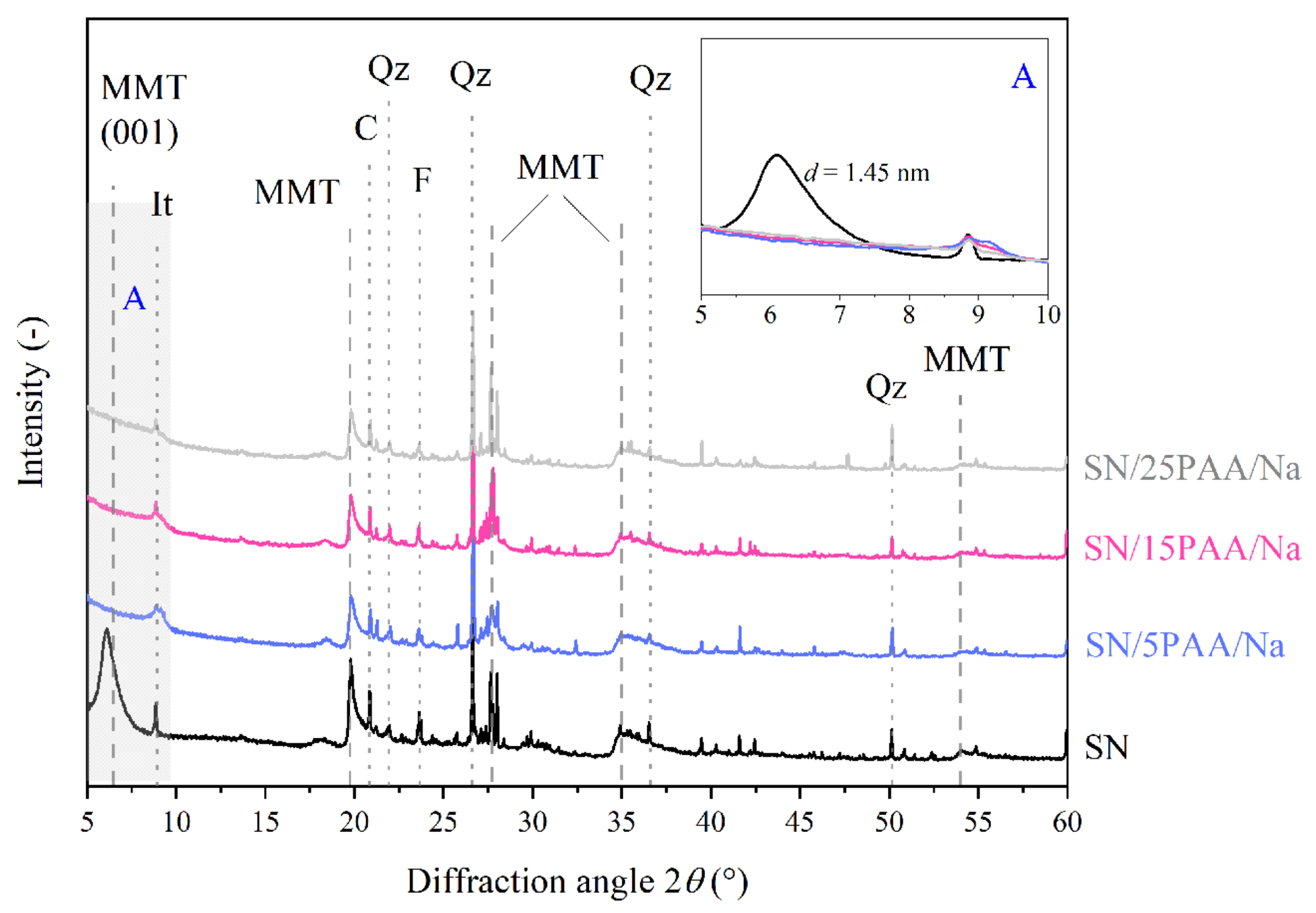
3.2. X-ray Diffraction Analysis
3.3. BET Surface Area Analysis
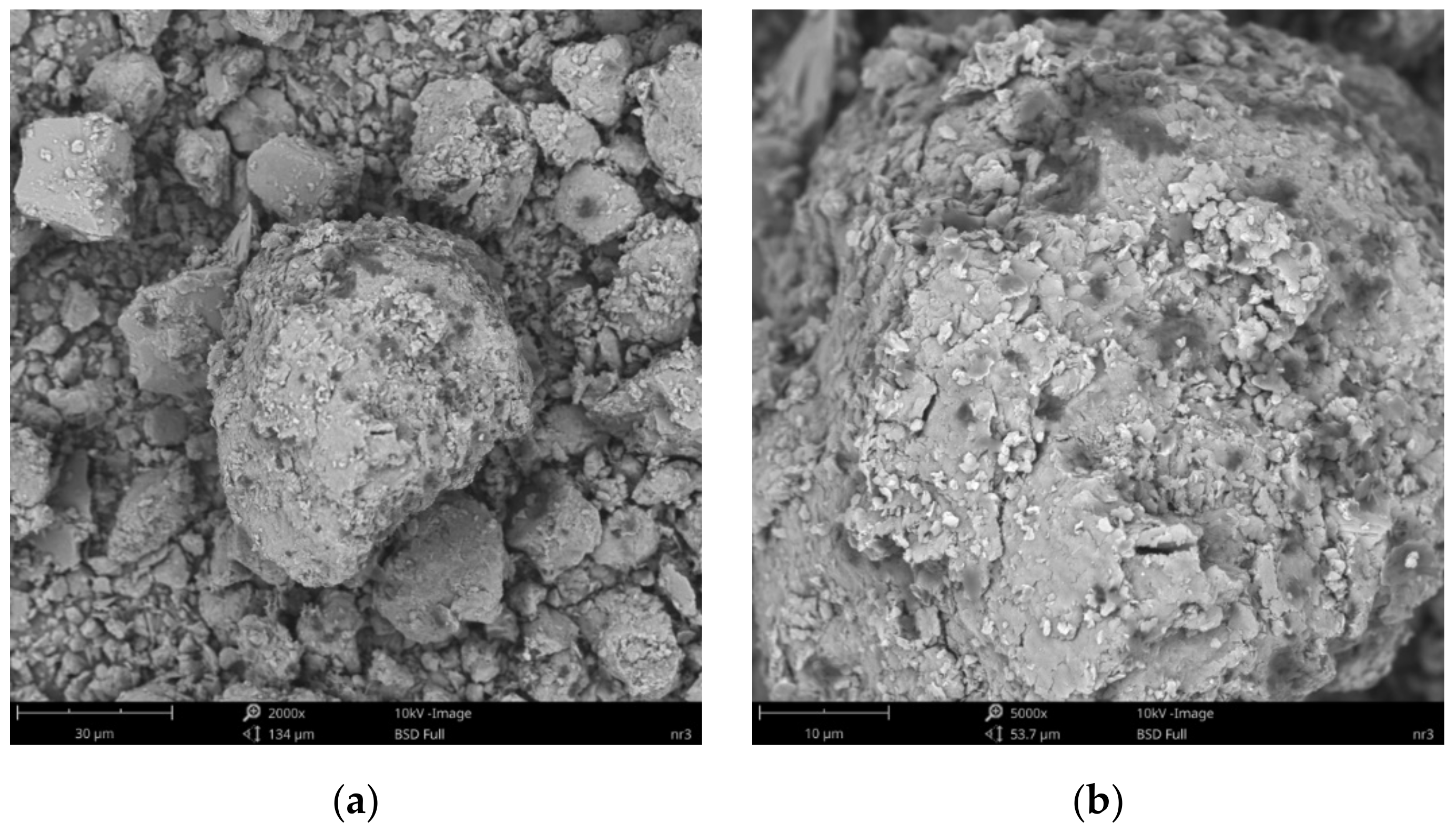
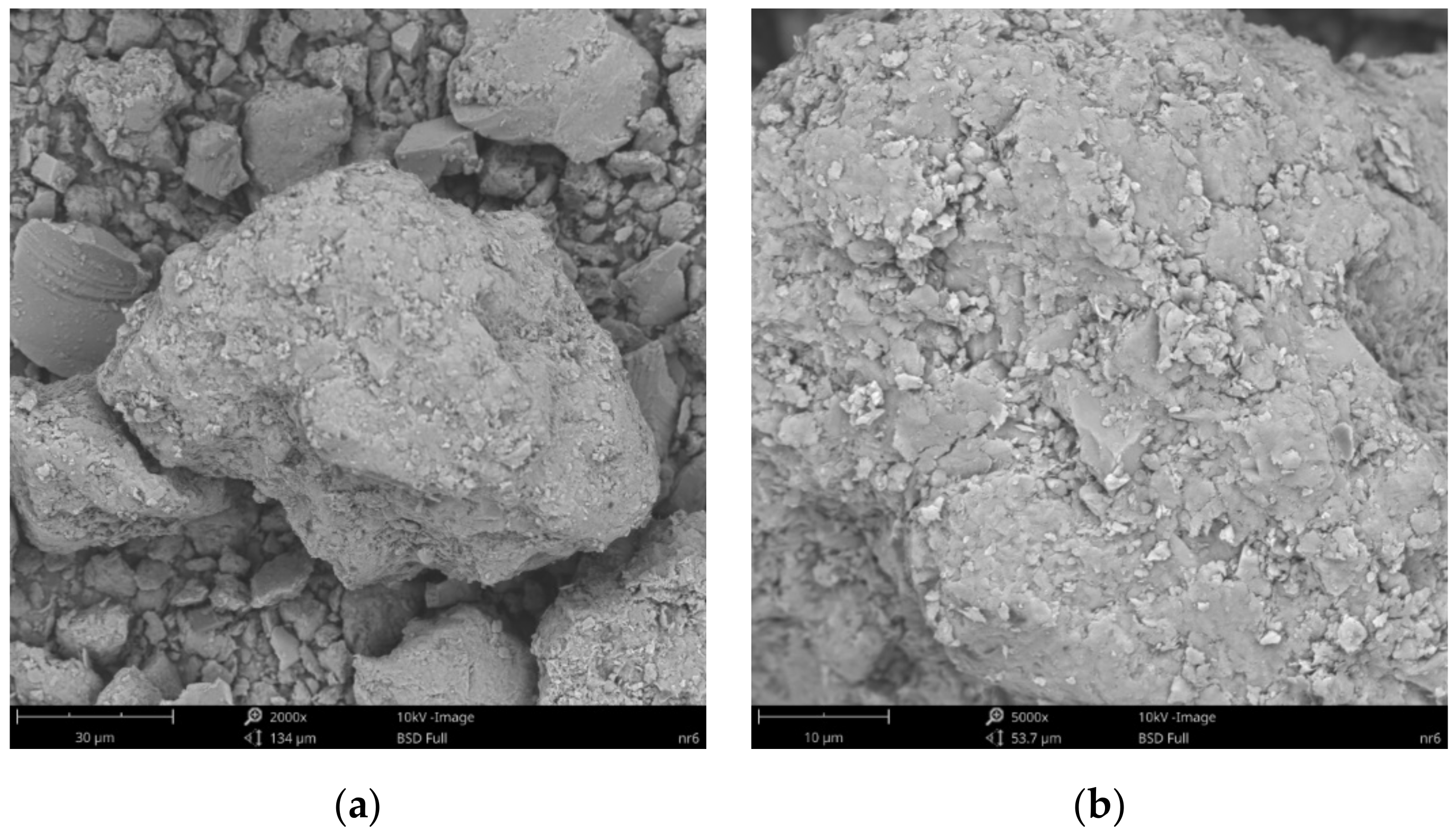
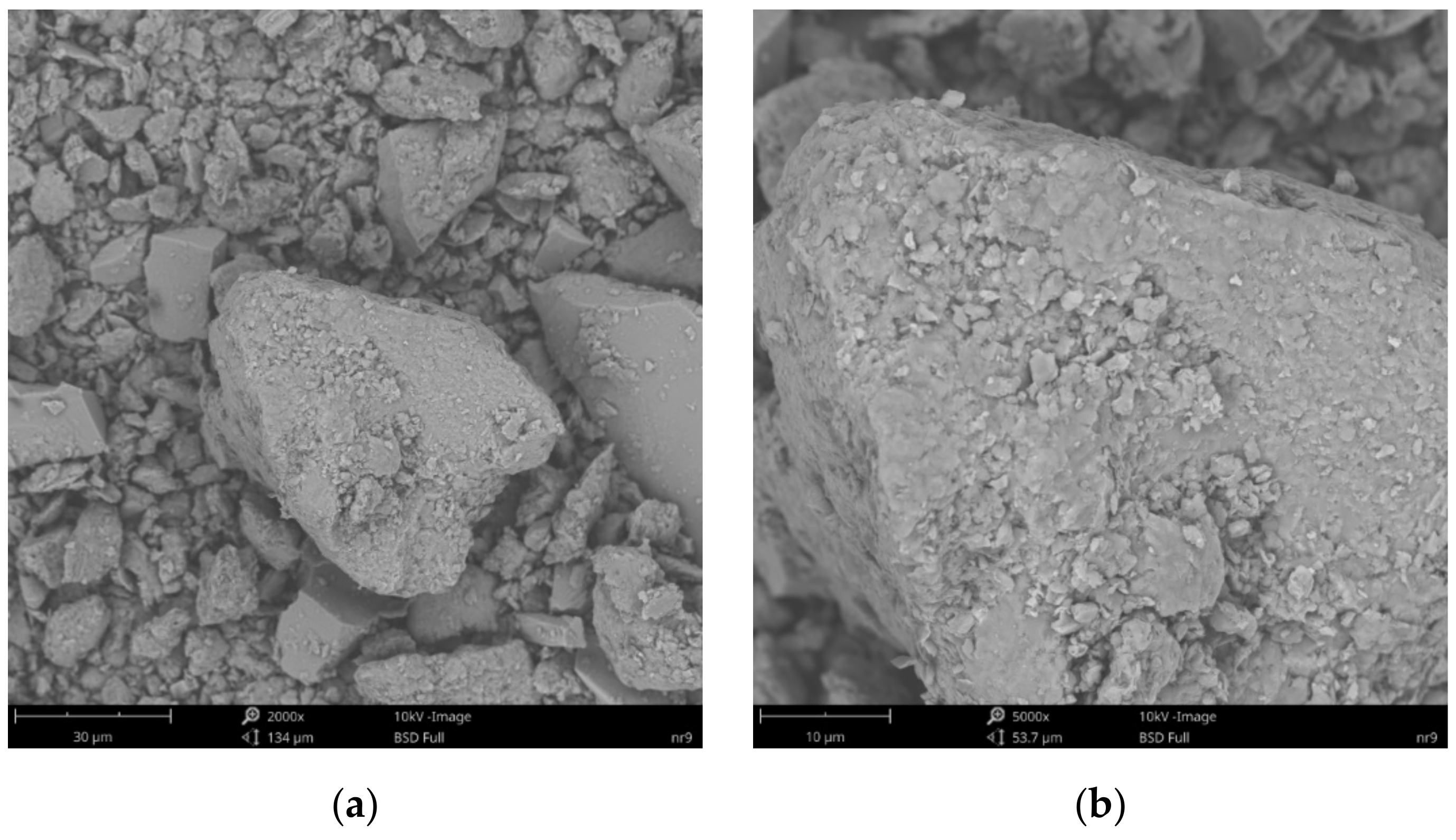
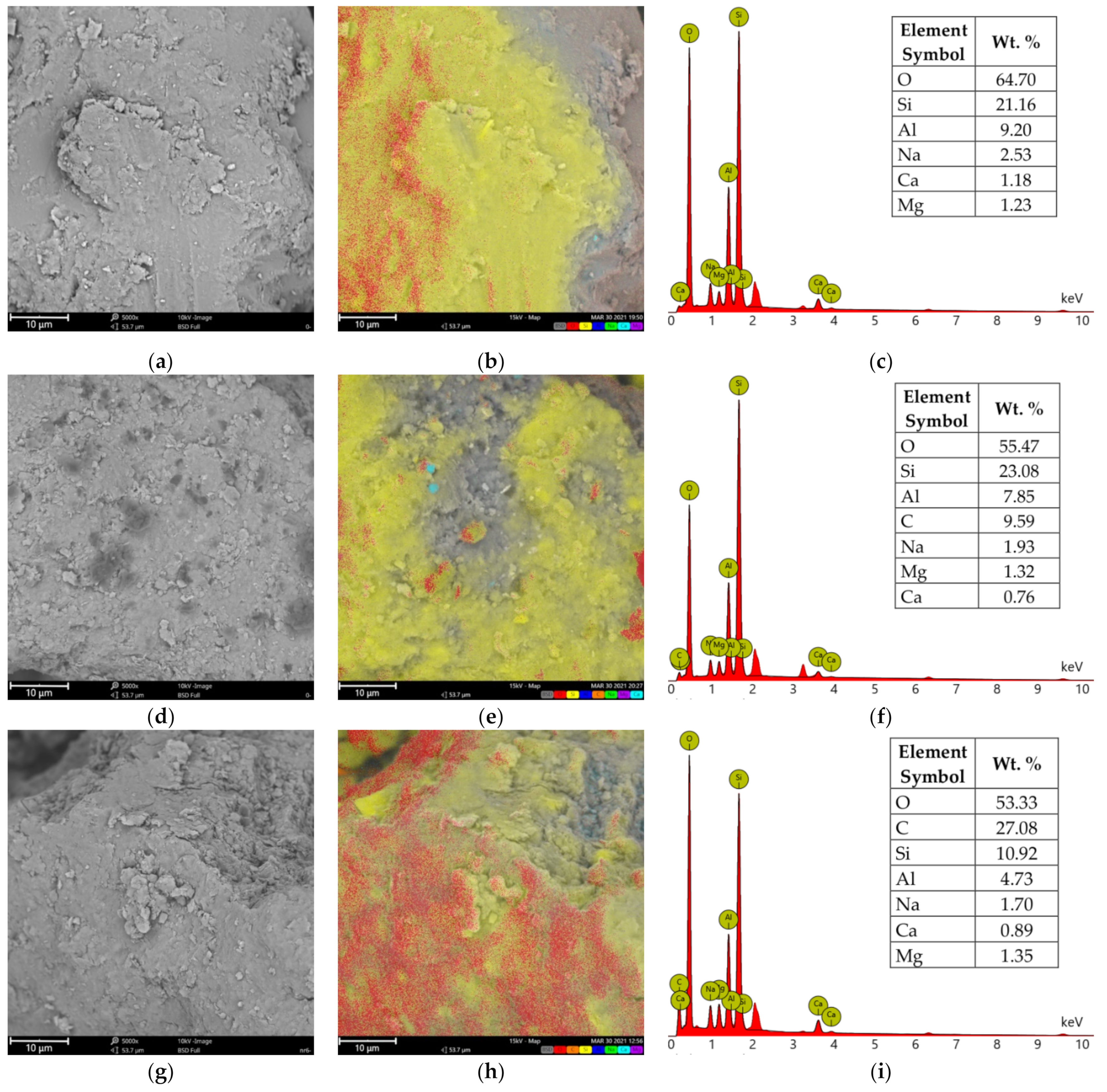
3.4. Scanning Electron Microscopy and X-ray Microanalysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Guégan, R. Organoclay applications and limits in the environment. C. R. Chim. 2018, 22, 132–141. [Google Scholar] [CrossRef]
- He, W.T.; Liao, S.T.; Xiang, Y.S.; Long, L.J.; Qin, S.H.; Yu, J. Structure and properties study of PA6 nanocomposites flame retarded by aluminium salt of diisobutylphosphinic acid and different organic montmorillonites. Polymers 2018, 10, 312. [Google Scholar] [CrossRef]
- Sonawane, S.H.; Chaudhari, P.L.; Ghodke, S.A.; Parande, M.G.; Bhandari, V.M.; Mishra, S.; Kulkarni, R.D. Ultrasound assisted synthesis of polyacrylic acid-nanoclay nanocomposite and its application in sonosorption studies of malachite green dye. Ultrason. Sonochem. 2009, 16, 351–355. [Google Scholar] [CrossRef]
- Li, P.; Khan, M.A.; Xia, M.; Lei, W.; Zhu, S.; Wang, F. Efficient preparation and molecular dynamic (MD) simulations of Gemini surfactant modified layered montmorillonite to potentially remove emerging organic contaminants from wastewater. Ceram. Int. 2019, 45, 10782–10791. [Google Scholar] [CrossRef]
- Pagacz, J.; Pielichowski, K. Modification of layered silicates for applications in nanotechnology. Tech. Trans. 2007, 8, 133–147. [Google Scholar]
- Lewandowski, L. Mould. Core Sands, 1st ed.; Scientific Publishing of PWN: Warsaw, Poland, 1991. [Google Scholar]
- Holtzer, M.; Grabowska, B.; Żymankowska-Kumon, S.; Kwaśniewska-Królikowska, D.; Dańko, R.; Solarski, W.; Bobrowski, A. Harmfulness of moulding sands with bentonite and lustrous carbon carriers. Metalurgija 2012, 51, 437–440. [Google Scholar]
- Lewandowski, J.L.; Solarski, W.; Zawada, J. Toxicity of moulding sands with bentonite and coal dust. Solidif. Met. Alloy 1998, 35, 67–76. [Google Scholar]
- Holtzer, M.; Kmita, A.; Roczniak, A. Processes of pyrolysis and their effect on cast quality and working conditions. Trans. Foundry Res. Inst. 2016, 56, 175–192. [Google Scholar]
- Bobrowski, A.; Holtzer, M. Assessment of environmental influence of bentonite and lustrous carbon carrier—In an aspect of gases emission. Arch. Foundry Eng. 2009, 9, 21–24. [Google Scholar]
- Vasková, I.; Hrubovčáková, M. Ecological binding material of first generation. Arch. Foundry Eng. 2014, 14, 123–128. [Google Scholar] [CrossRef]
- Landis, C.R. Activated Carbon Foundry Sand Additives and Method of Casting Metal for Reduced VOC Emissions. U.S. Patent 5,688,313A, 18 November 1997. [Google Scholar]
- Jelínek, P.; Beňo, J. Morphological forms of carbon and their utilizations at formation of iron casting surfaces. Arch. Foundry Eng. 2008, 8, 67–70. [Google Scholar]
- Grefhorst, C. Modern bentonite bonded molds. Arch. Foundry 2006, 6, 51–57. [Google Scholar]
- Grefhorst, C.; Senden, W.; Ilman, R.; Podobed, O.; Lafay, V.; Tilch, W. Reduction of greensand emissions by minimum 25%—case study. China Foundry 2010, 7, 419–424. [Google Scholar]
- Kumara, P.; Bhat, V.; Ranganatha; Banagara, A. Effect of wood flours on moulding sand properties. J. Mech. Civil. Eng. 2016, 2, 1–6. [Google Scholar]
- Holtzer, M.; Bobrowski, A.; Grabowska, B.; Eichholz, S.; Hodor, K. Investigation of carriers of lustrous carbon at high temperatures by.infrared spectroscopy (FTIR). Arch. Foundry Eng. 2015, 10, 61–68. [Google Scholar]
- De Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral organic interactions. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; Volume 1, pp. 309–377. [Google Scholar]
- Lagaly, G.; Barrer, R.M.; Goulding, K. Clay-organic interactions. Phil. Trans. R. Soc. Lond. A 1984, 311, 315–332. [Google Scholar]
- Bhattacharya, S.S.; Sen, K.K.; Sen, S.O.; Banerjee, S.; Kaity, S.; Ghosh, A.K.; Ghosh, A. Synthesis and characterization of poly(acrylic acid)/modified bentonite superabsorbent polymer. Int. J. Polym. Mater. 2011, 60, 1015–1025. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter. 2016, 12, 2542–2549. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Urban, T.; Grządka, E.; Zarko, V.I.; Gun’ko, V.M. Comparison of adsorption affinity of polyacrylic acid for surfaces of mixed silica-alumina. Colloid Polym. Sci. 2014, 292, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Terao, K. Poly(acrylic acid) (PAA). In Encyclopedia of Polymeric Nanomaterials, 1st ed.; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin, Germany, 2014; Volume 1, pp. 1654–1658. [Google Scholar]
- Elliott, J.E.; MacDonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Fuhrer, L.M.; Sun, S.; Boyko, V.; Kellermeier, M.; Cölfen, H. Tuning the properties of hydrogels made from poly(acrylic acid) and calcium salts. Phys. Chem. Chem. Phys. 2020, 22, 18631–18638. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Slaný, M.; Wang, X.; Chen, G.; Zhang, J. The inhibition property and mechanism of a novel low molecular weight zwitterionic copolymer for improving wellbore stability. Polymers 2020, 12, 708. [Google Scholar] [CrossRef]
- Grabowska, B.; Sitarz, M.; Olejnik, E.; Kaczmarska, K.; Tyliszczak, B. FT-IR and FT-Raman studies of cross-linking processes with Ca2+ ions, glutaraldehyde and microwave radiation for polymer composition of poly(acrylic acid)/sodium salt of carboxymethyl starch—in moulding sands, part II. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 27–33. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Hage, A.P.; Zhou, M.; Sonawane, S.H.; Ashokkumar, M. Ultrasound assisted preparation of nanoclay bentonite-FeCo nanocomposite hybrid hydrogel: A potential responsive sorbent for removal of organic pollutant from water. Desalination 2011, 281, 429–437. [Google Scholar] [CrossRef]
- Harasowski, J.; Paczek, H. Method of Activating Bentonite Clays. U.S. Patent 3,240,616, 15 March 1966. [Google Scholar]
- Silva-Valenzuela, M.G.; Hui, W.S.; Valenzuela-Díaz, F.R. FTIR spectroscopy of some Brazilian clays. In Characterization of Minerals, Metals, and Materials 2016, 1st ed.; Ikhmayies, S.J., Li, B., Carpenter, J.S., Hwang, J.-Y., Monteiro, S., Li, J., Firrao, D., Zhang, M., Peng, Z., Escobedo-Diaz, J.P., et al., Eds.; Springer: Cham, Switzerland, 2016; pp. 227–234. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W. Identification of silicooxygen rings in SiO2 based on IR spectra. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2000, 56, 1819–1823. [Google Scholar] [CrossRef]
- Sitarz, M. The structure of simple silicate glasses in the light of middle infrared spectroscopy studies. J. Non-Cryst. Solids 2011, 357, 1603–1608. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Zhang, H.; Lu, S.; Chen, L.; Yu, X. Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J. Hazard. Mater. 2010, 183, 632–640. [Google Scholar] [CrossRef]
- Natkański, P.; Kuśtrowski, P.; Białas, A.; Surman, J. Effect of Fe3+ ions present in the structure of poly(acrylic acid)/montmorillonite composites on their thermal decomposition. J. Therm. Anal. Calorim. 2013, 113, 335–342. [Google Scholar] [CrossRef]
- El Bouraie, M.; Masoud, A.A. Adsorption of phosphate ions from aqueous solution by modified bentonite with magnesium hydroxide Mg(OH)2. Appl. Clay Sci. 2017, 140, 157–164. [Google Scholar] [CrossRef]
- Hayati-Ashtiani, M. Use of FTIR spectroscopy in the characterization of natural and treated nanostructured bentonites (montmorillonites). Part. Sci. Technol. 2012, 30, 553–564. [Google Scholar] [CrossRef]
- Ravindra Reddy, T.; Kaneko, S.; Endo, T.; Lakshmi Reddy, S. Spectroscopic Characterization of Bentonite. J. Laser. Opt. Photonics 2017, 4, 171–174. [Google Scholar] [CrossRef]
- Grabowska, B.; Kurleto-Kozioł, Ż. Spectral study (FTIR, UV-Vis) of montmorillonite modified by ultrasound and potassium cations. Trans. Foundry Res. Inst. 2017, 57, 125–136. [Google Scholar]
- Chen, S.; Wu, G.; Liu, Y.; Long, D. Preparation of poly(acrylic acid) grafted multiwalled carbon nanotubes by a two-step irradiation technique. Macromolecules 2006, 39, 330–334. [Google Scholar] [CrossRef]
- Grabowska, B.; Sitarz, M.; Olejnik, E.; Kaczmarska, K. FT-IR and FT-Raman studies of cross-linking processes with Ca2+ ions, glutaraldehyde and microwave radiation for polymer composition of poly(acrylic acid)/sodium salt of carboxymethyl starch—part I. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 529–535. [Google Scholar] [CrossRef]
- Solhi, L.; Atai, M.; Nodehi, A.; Imani, M.; Ghaemi, A.; Khosravi, K. Poly(acrylic acid) grafted montmorillonite as novel fillers for dental adhesives: Synthesis, characterization and properties of the adhesive. Dent. Mater. 2012, 28, 369–377. [Google Scholar] [CrossRef]
- Tran, N.H.; Dennis, G.R.; Milev, A.S.; Kannangara, G.S.K.; Wilson, M.A.; Lamb, R.N. Interactions of sodium montmorillonite with poly(acrylic acid). J. Colloid Interface Sci. 2005, 290, 392–396. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; Van Bronswijk, W. In situ FTIR-ATR examination of poly(acrylic acid) adsorbed onto hematite at low pH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Kam, W.; Liew, C.W.; Lim, J.Y.; Ramesh, S. Electrical, structural, and thermal studies of antimony trioxide-doped poly(acrylic acid)-based composite polymer electrolytes. Ionics 2014, 20, 665–674. [Google Scholar] [CrossRef]
- Schroeder, P.A. Infrared spectroscopy in clay science. In CMS Workshop Lectures; Rule, A., Guggenheim, S., Eds.; The Clay Minerals Society: Aurora, CO, USA, 2002; Volume 11, pp. 181–206. [Google Scholar]
- Abdel Zaher, M.S.; Abdel Wahab, S.M.; Taha, M.H.; Masoud, A.M. Sorption characteristics of iron, fluoride and phosphate from wastewater of phosphate fertilizer plant using natural sodium bentonite. J. Membr. Sci. Technol. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Calabria-Holley, J.; Papatzani, S.; Naden, B.; Mitchels, J.; Paine, K. Tailored montmorillonite nanoparticles and their behaviour in the alkaline cement environment. Appl. Clay Sci. 2017, 143, 67–75. [Google Scholar] [CrossRef]
- Chang, T.P.; Shih, J.Y.; Yang, K.M.; Hsiao, T.C. Material properties of portland cement paste with nano-montmorillonite. J. Mater. Sci. 2007, 42, 7478–7487. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Klinkenberg, M.; Siegesmund, S.; Ufer, K. N2-BET specific surface area of bentonites. J. Colloid Interface Sci. 2010, 349, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.W.; Chiou, C.T.; Eberl, D.D. Effects of exchanged cation on the microporosity of montmorillonite. Clays Clay Miner. 1997, 45, 534–543. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Chai, P.; Bian, J.; Wang, X.; Liu, Y.; Yin, X.; Pan, S.; Pan, Z. Pore characterization of different clay minerals and its impact on methane adsorption capacity. Energy Fuels 2020, 34, 12204–12214. [Google Scholar] [CrossRef]
- Michot, L.J.; Villieras, F. Surface Area and Porosity. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 2013; Volume 5, pp. 319–332. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Effects of anionic additives on the rheological behavior of aqueous calcium montmorillonite suspensions. Rheol. Acta 2006, 45, 425–434. [Google Scholar] [CrossRef]
- Su, W.-F. Polymer size and polymer solutions. In Principles of Polymer Design and Synthesis; Carpenter, B., Ceroni, P., Kirchner, B., Koskinen, A., Landfester, K., Leszczynski, J., Luh, T.-Y., Mahlke, C., Polfer, N.C., Salzer, R., Eds.; Springer: Berlin, Germany, 2013; Volume 82, pp. 9–26. [Google Scholar] [CrossRef]
- Lagaly, G.; Ziesmer, S. Colloid chemistry of clay minerals: The coagulation of montmorillonite dispersions. Adv. Colloid Interface Sci. 2003, 100–102, 105–128. [Google Scholar] [CrossRef]
- Krupskaya, V.; Novikova, L.; Tyupina, E.; Belousov, P.; Dorzhieva, O.; Zakusin, S.; Kim, K.; Roessner, F.; Badetti, E.; Brunelli, A.; et al. The influence of acid modification on the structure of montmorillonites and surface properties of bentonites. Appl. Clay Sci. 2019, 172, 1–10. [Google Scholar] [CrossRef]





| Composite | SABET (m2·g−1) | Composite | SABET (m2·g−1) | Composite | SABET (m2·g−1) |
|---|---|---|---|---|---|
| SN/5PAA | 7.71 | SN/5PAA/Na | 8.78 | SN-Na/5PAA | 7.35 |
| SN/15PAA | 5.06 | SN/15PAA/Na | 6.37 | SN-Na/15PAA | 4.49 |
| SN/25PAA | 2.71 | SN/25PAA/Na | 2.45 | SN-Na/25PAA | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cukrowicz, S.; Sitarz, M.; Kornaus, K.; Kaczmarska, K.; Bobrowski, A.; Gubernat, A.; Grabowska, B. Organobentonites Modified with Poly(Acrylic Acid) and Its Sodium Salt for Foundry Applications. Materials 2021, 14, 1947. https://doi.org/10.3390/ma14081947
Cukrowicz S, Sitarz M, Kornaus K, Kaczmarska K, Bobrowski A, Gubernat A, Grabowska B. Organobentonites Modified with Poly(Acrylic Acid) and Its Sodium Salt for Foundry Applications. Materials. 2021; 14(8):1947. https://doi.org/10.3390/ma14081947
Chicago/Turabian StyleCukrowicz, Sylwia, Maciej Sitarz, Kamil Kornaus, Karolina Kaczmarska, Artur Bobrowski, Agnieszka Gubernat, and Beata Grabowska. 2021. "Organobentonites Modified with Poly(Acrylic Acid) and Its Sodium Salt for Foundry Applications" Materials 14, no. 8: 1947. https://doi.org/10.3390/ma14081947
APA StyleCukrowicz, S., Sitarz, M., Kornaus, K., Kaczmarska, K., Bobrowski, A., Gubernat, A., & Grabowska, B. (2021). Organobentonites Modified with Poly(Acrylic Acid) and Its Sodium Salt for Foundry Applications. Materials, 14(8), 1947. https://doi.org/10.3390/ma14081947







