Induced Biological Response in Contact with Ag-and Cu-Doped Carbon Coatings for Potential Orthopedic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Coatings Deposition
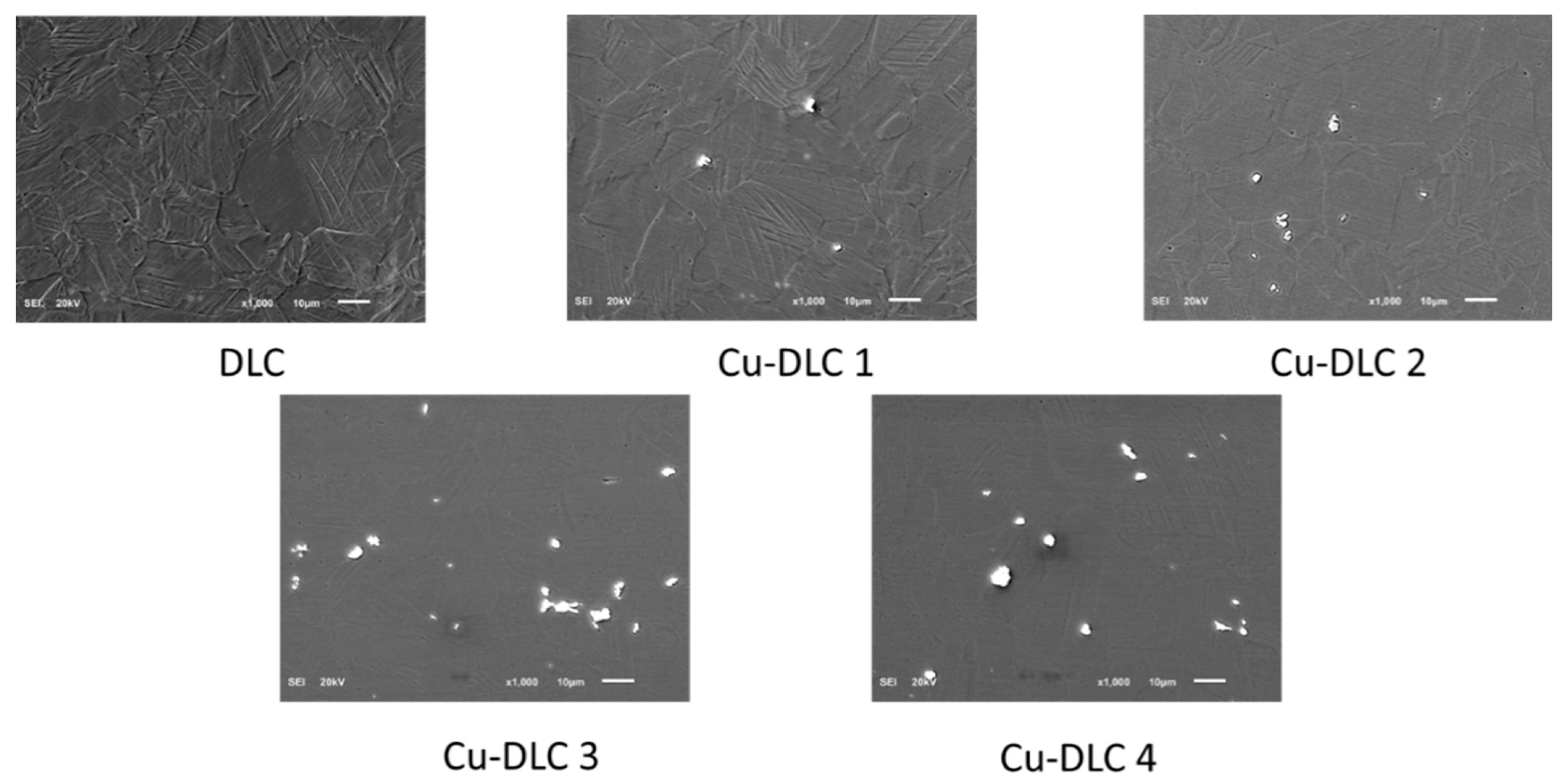
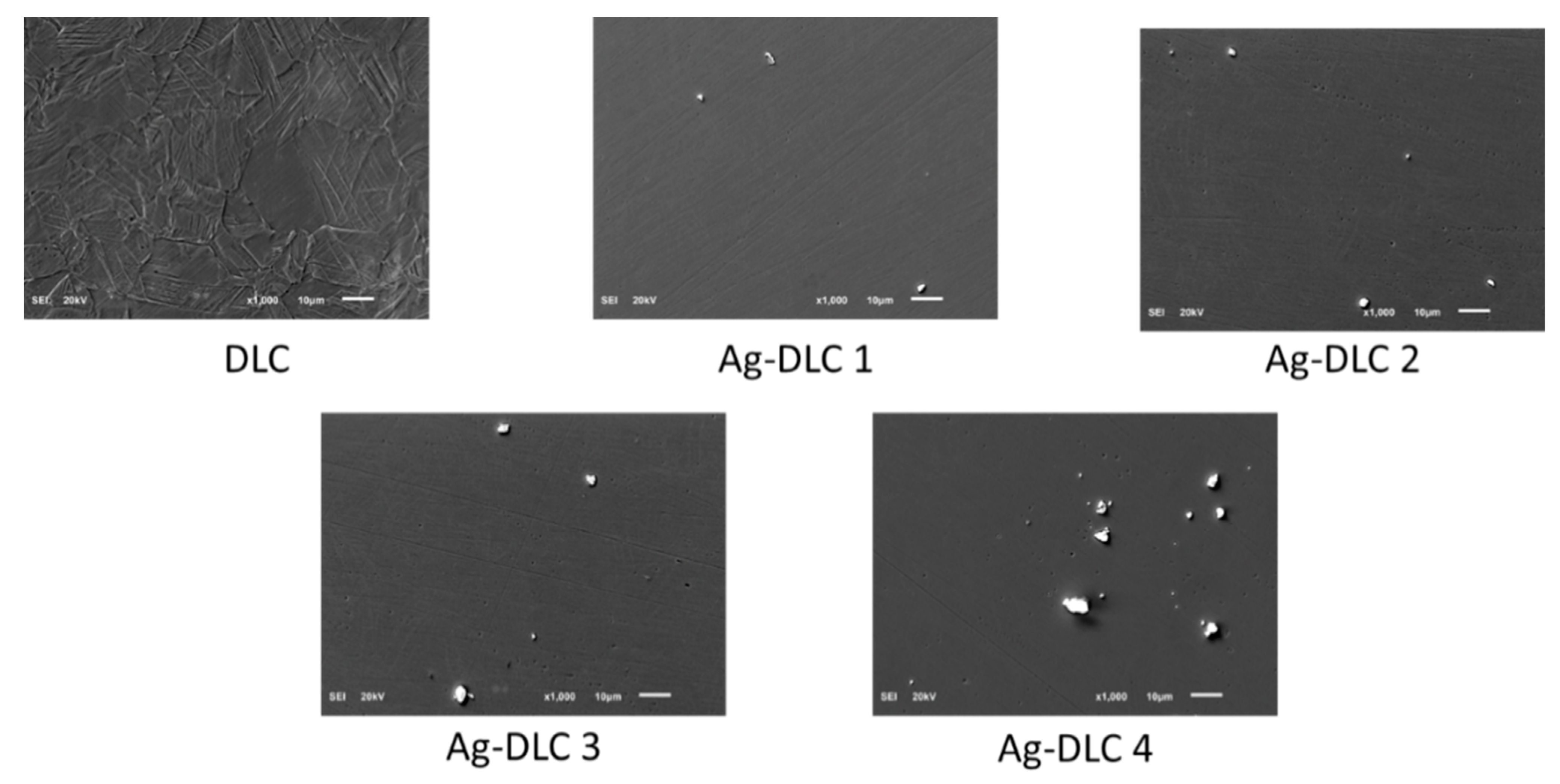
2.2. Scanning Electron Microscopy (SEM)
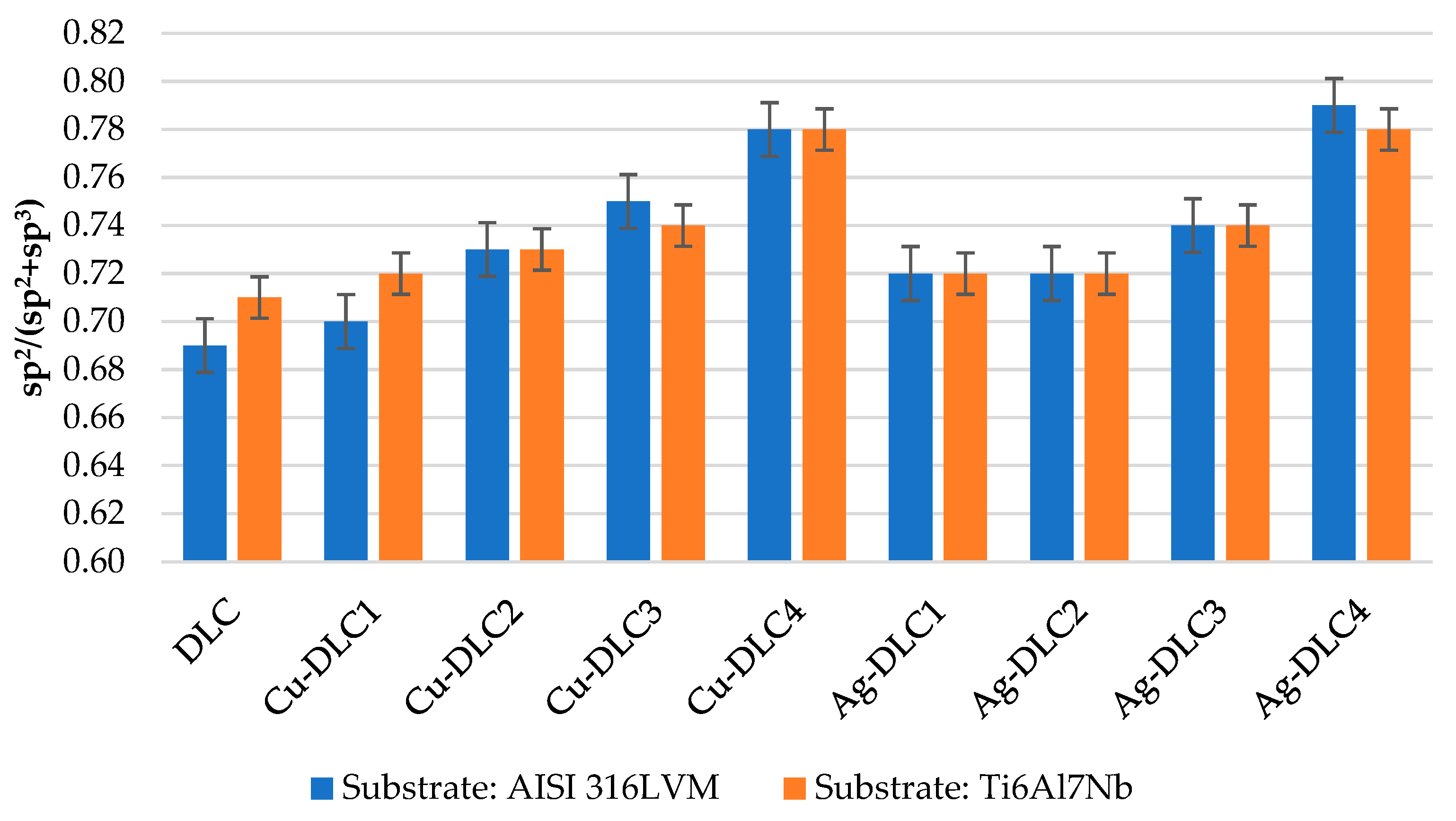
2.3. X-ray Photoelectron Spectroscopy (XPS)
- C1S peak corresponding to the energy of 284.6 eV (deconvolution was performed using Gauss–Lorentz curves corresponding to the peaks characteristic for sp2 C=C (284.5 eV) and sp3 C–C (285.3 eV) bonds, as well as C–O (286.1 eV) and C=O (288 eV) bonds,
- Cu2p peak (922–970 eV) corresponding to the region characteristic (922–970 eV) for copper admixture,
- Ag3d (922–970 eV) corresponding to the region characteristic for silver admixture.
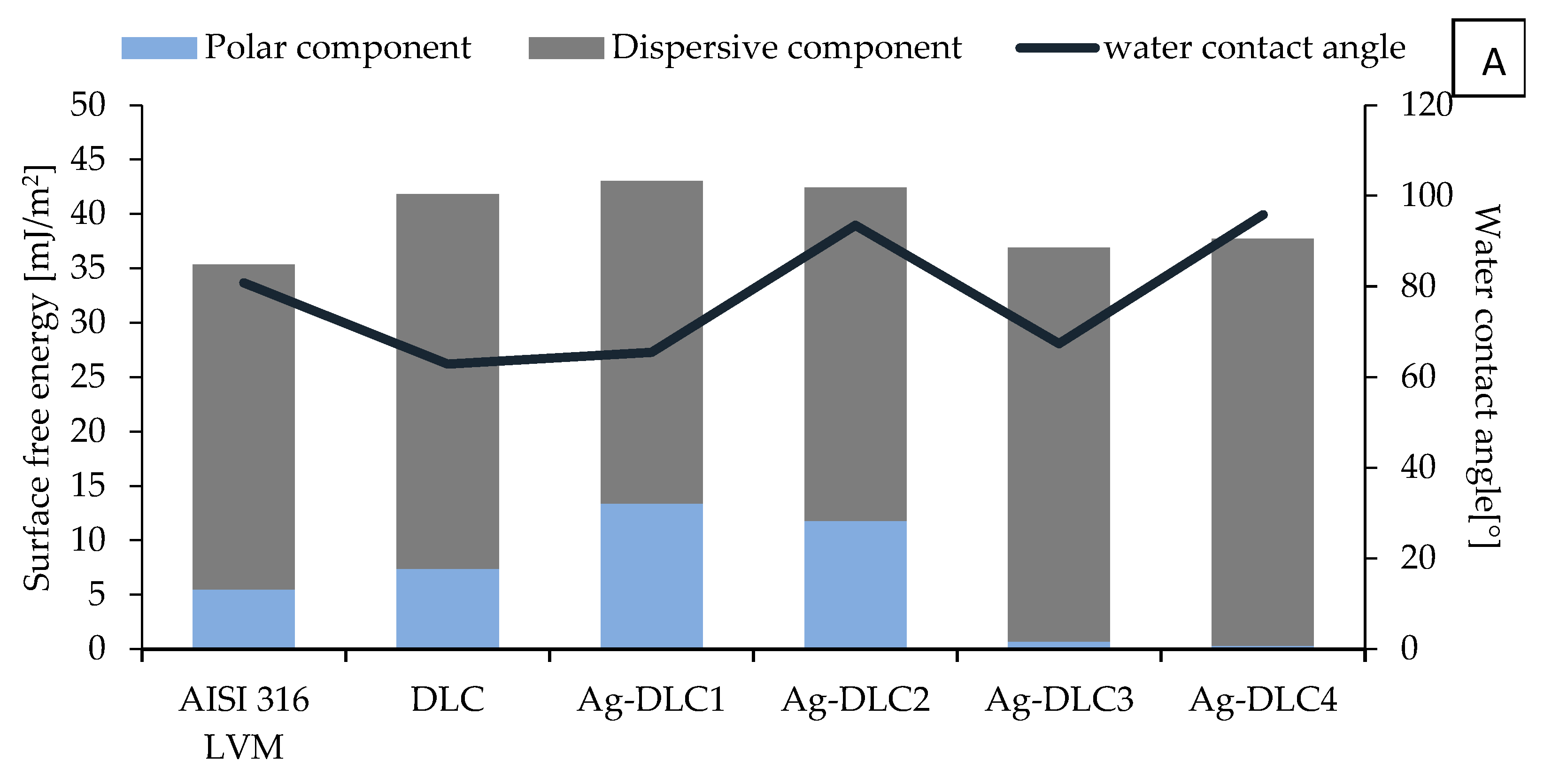
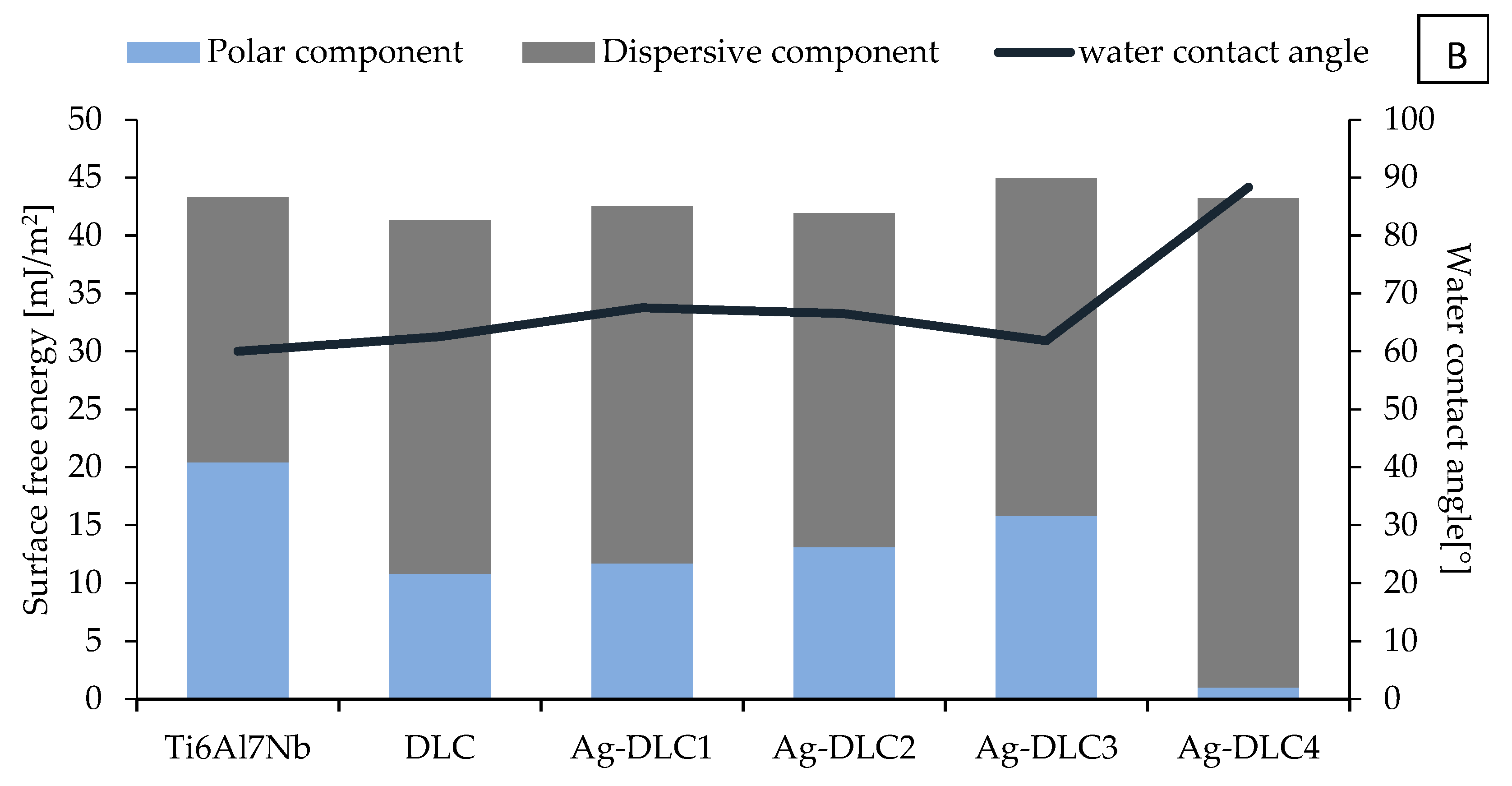
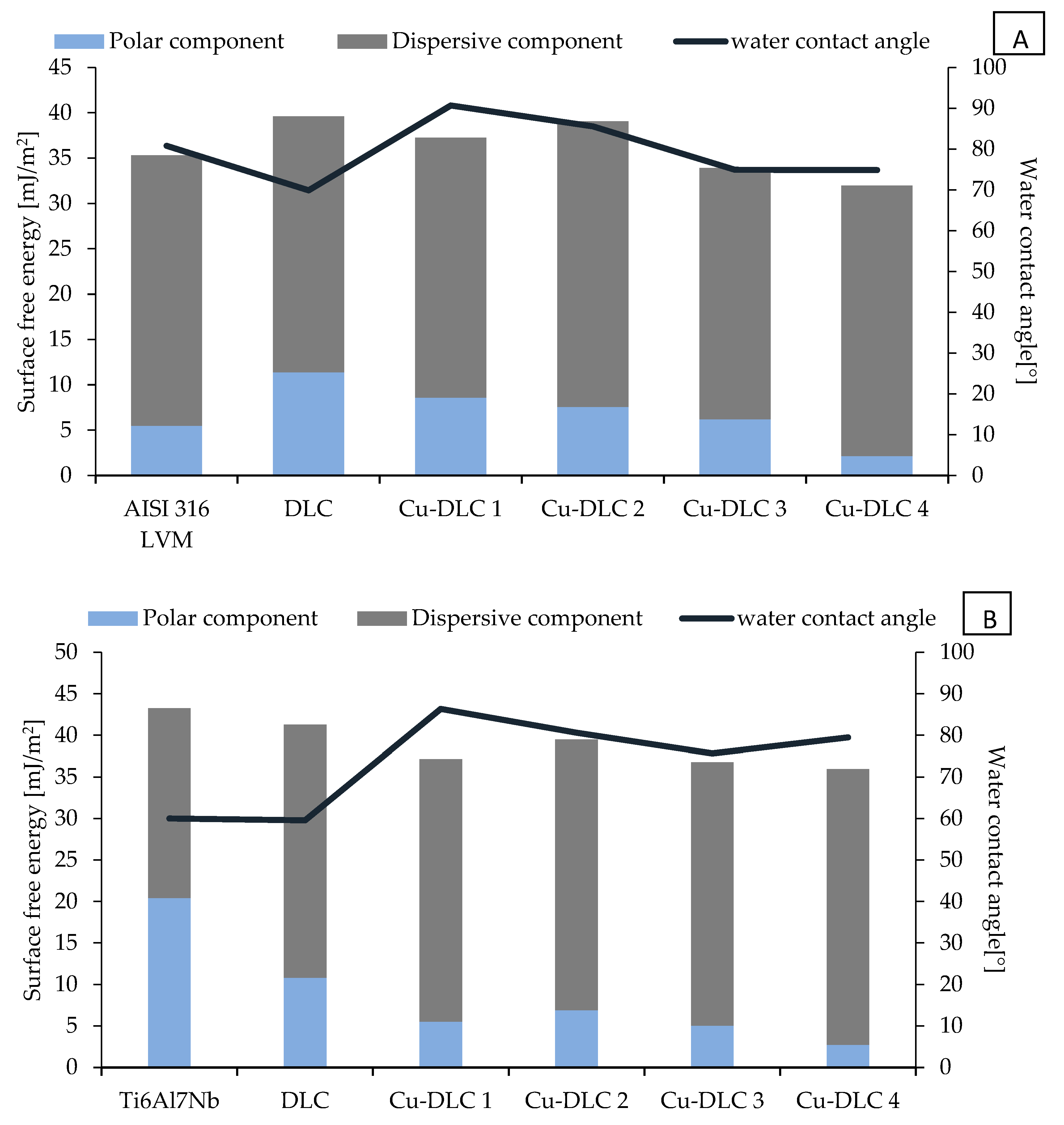
2.4. Wettability and Surface Free Energy (SFE)
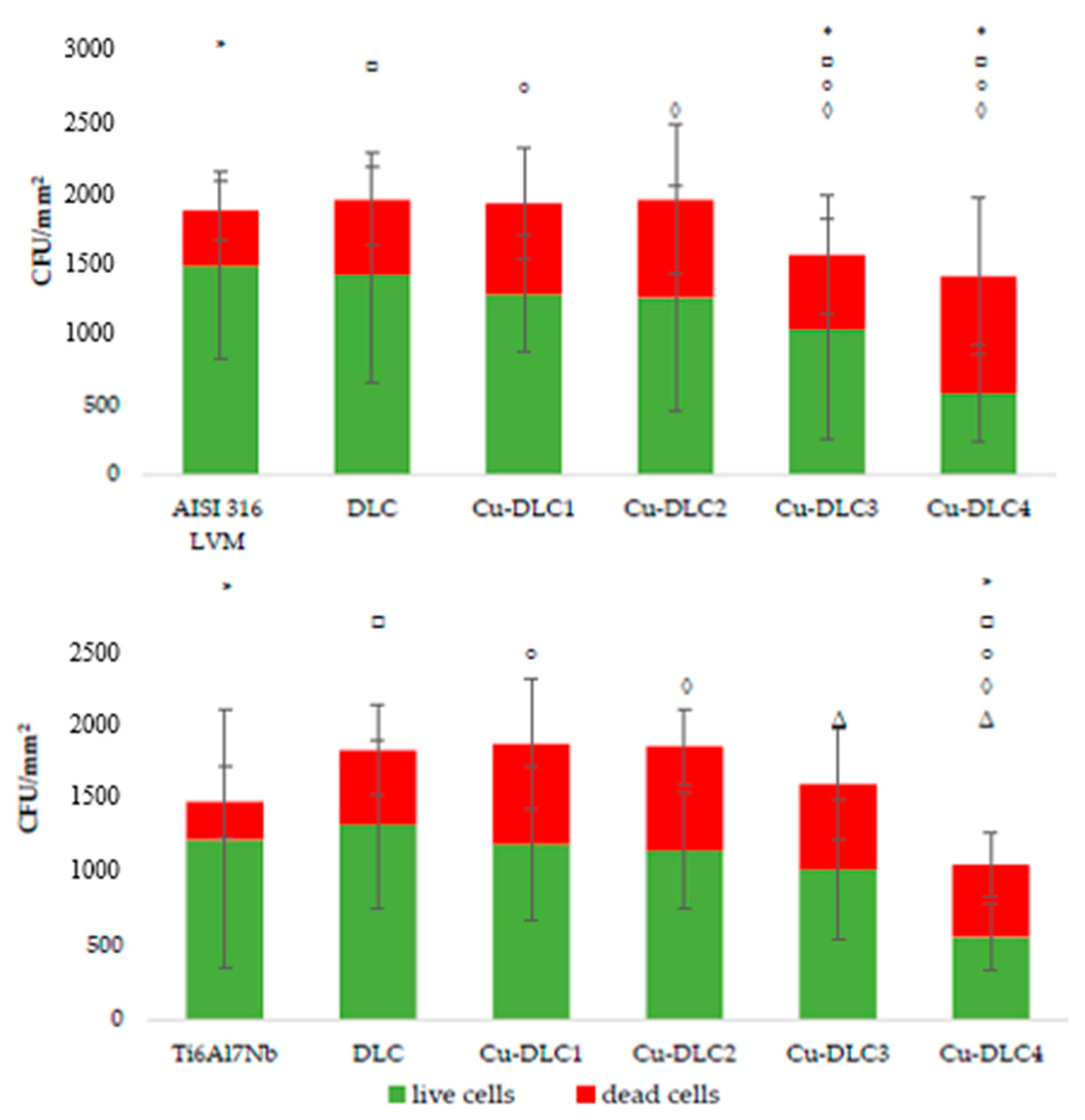
2.5. Assessment of Microbial Colonization
2.6. In Vitro Evaluation with Mammalian Cells
- Saos-2 (ATCC, Manassas, VA, USA)—treated as a stable line of osteoblast-like human cells,
- EA.hy926 (ATCC, Manassas, USA)—hybrid of human endothelial cells isolated from umbilical vein (HUVEC) and human lung cancer cells (A549).
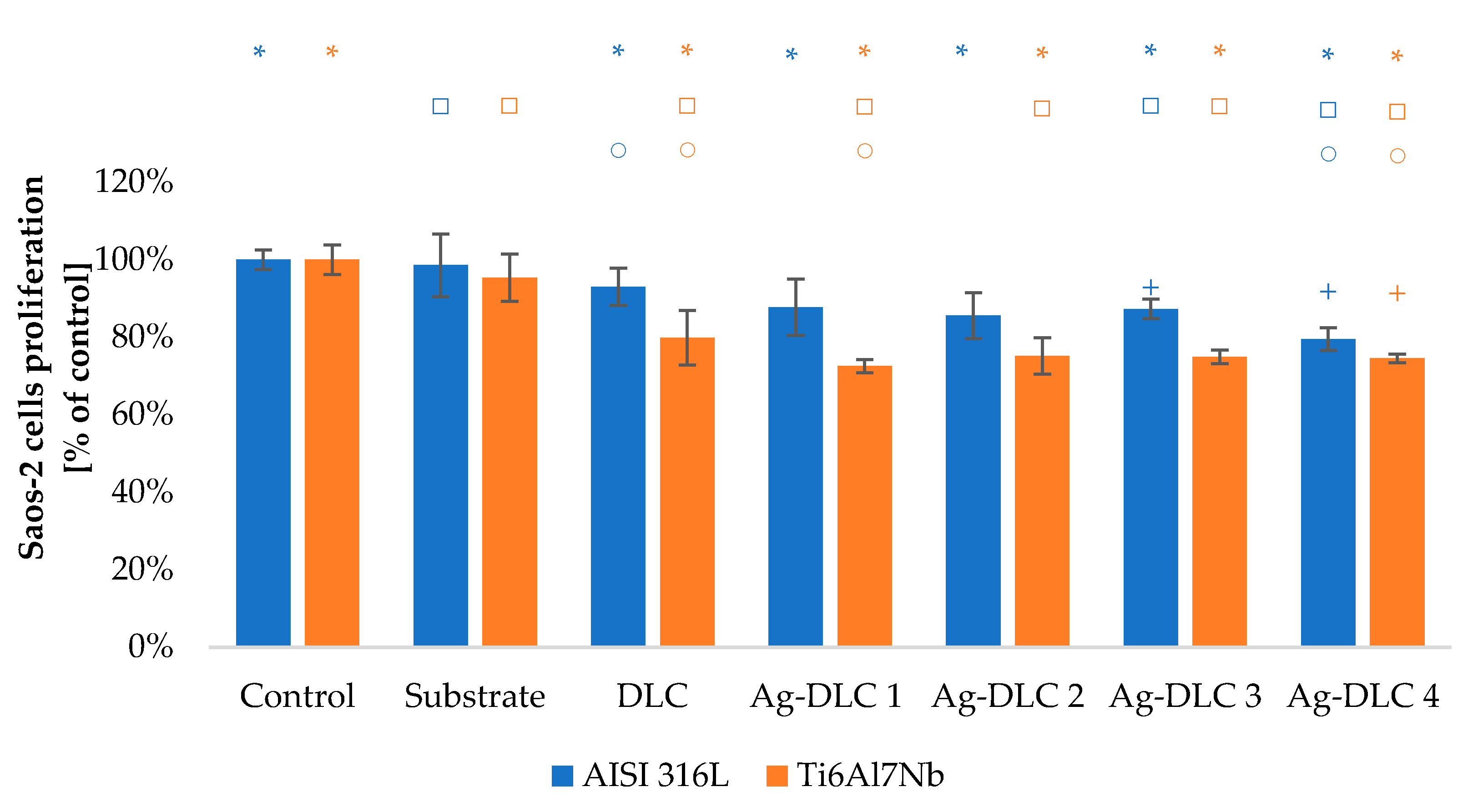
2.6.1. Cells Metabolic Activity
2.6.2. Cell Viability
2.7. Statistical Analysis
3. Results
3.1. Surface Characterization
3.2. Assessment of Microbial Colonization
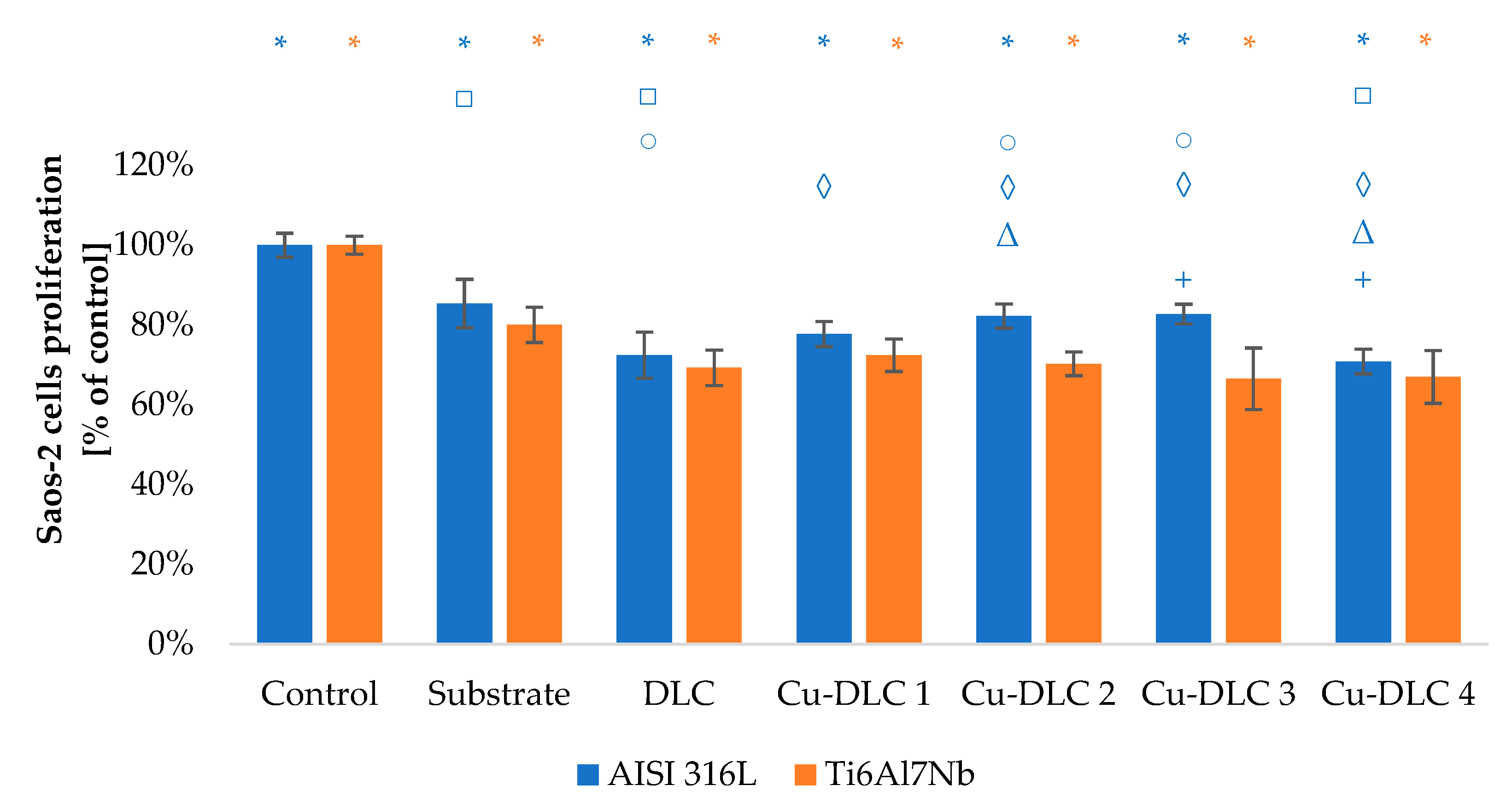
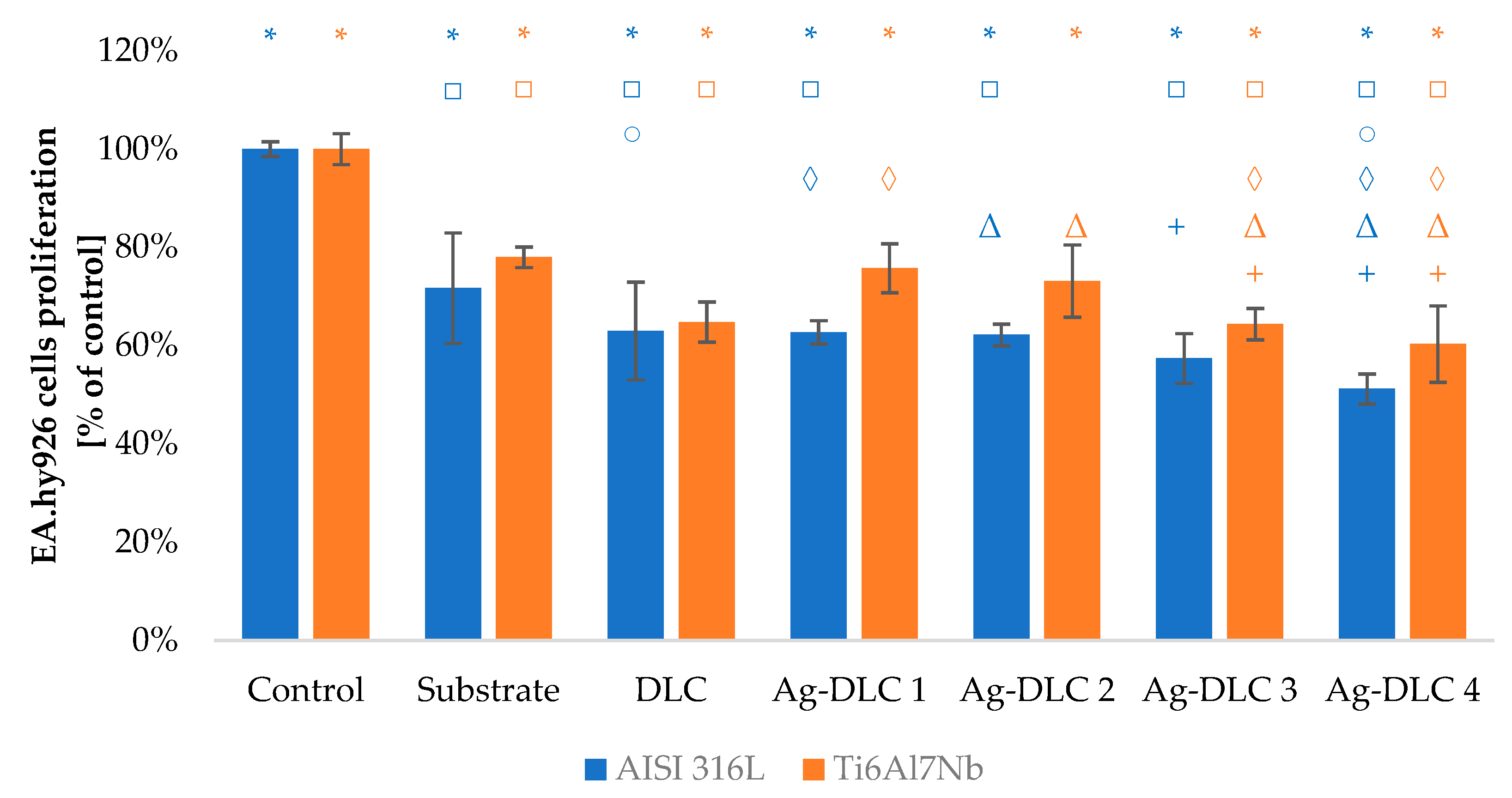
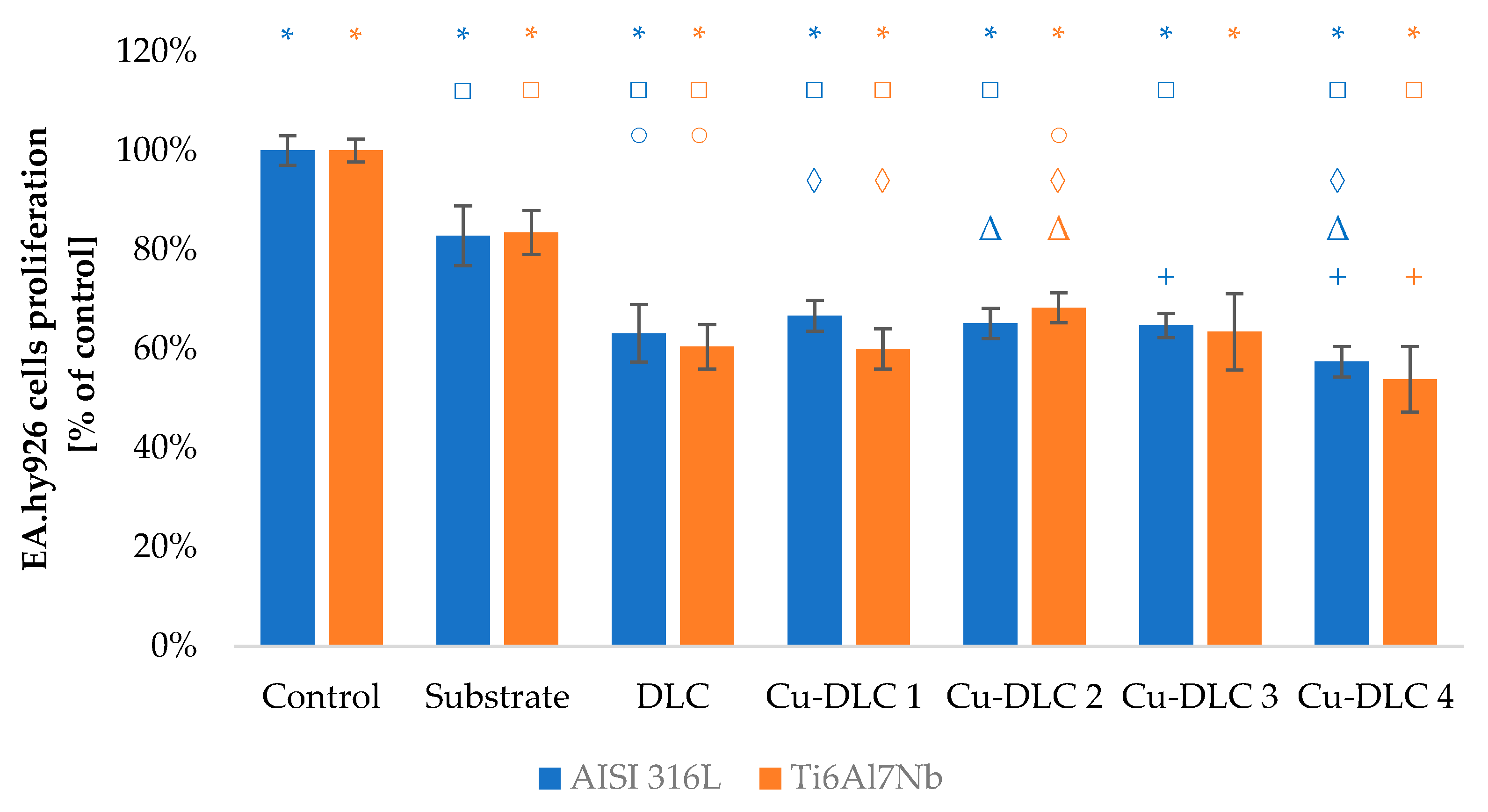
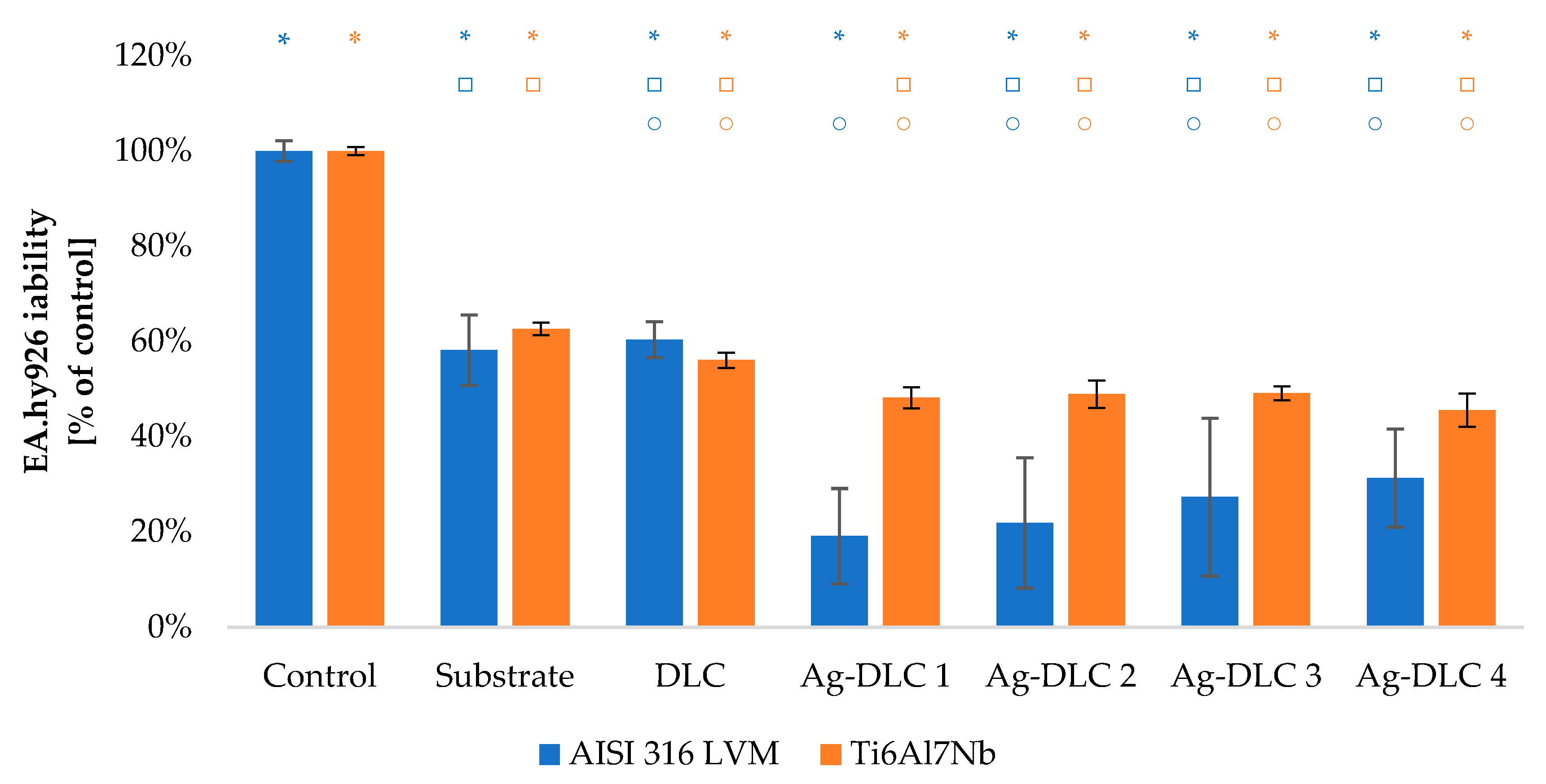
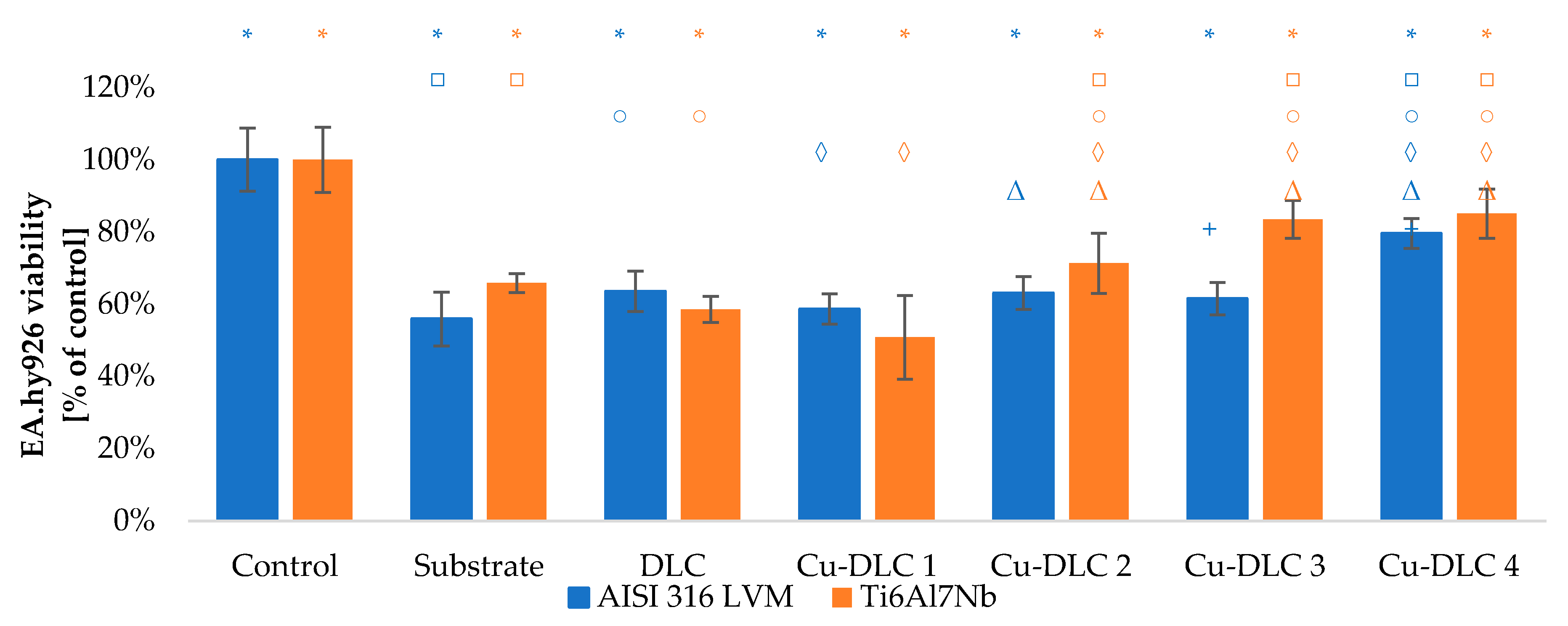
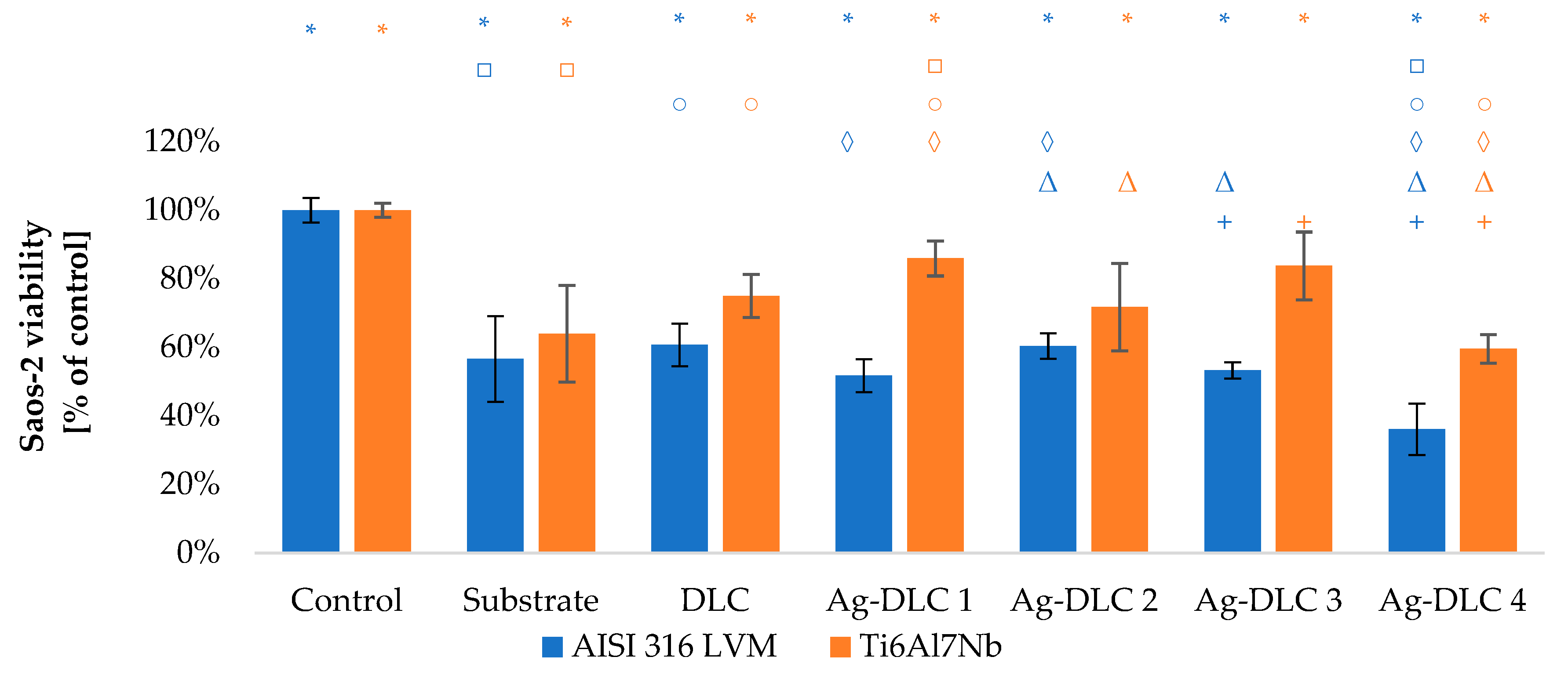
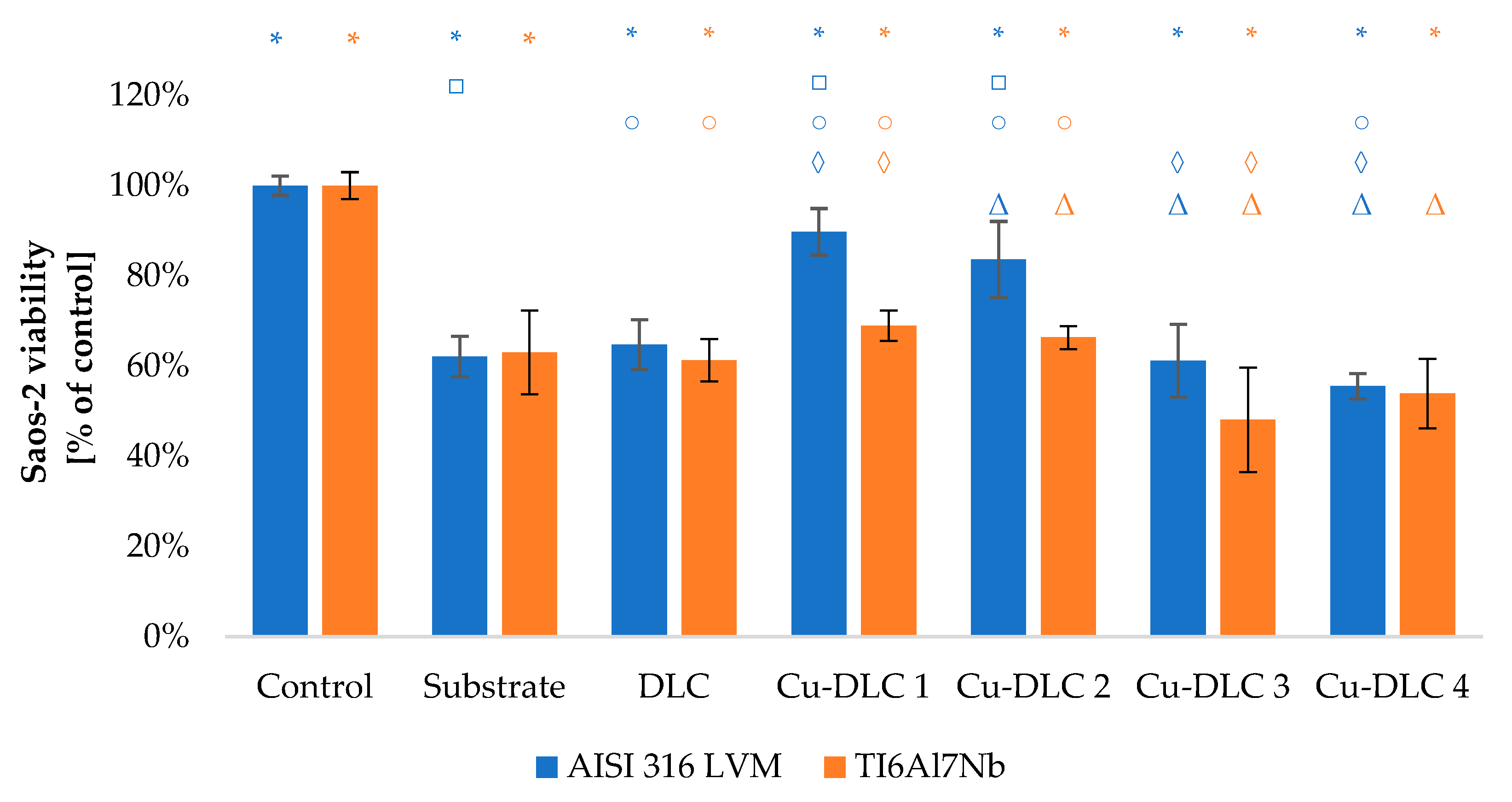
3.3. In Vitro Evaluation with Mammalian Cells (XTT Test Results)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organisation for Economic Co-operation and Development (OECD); European Commission. Hip and Knee Replacement. Health at a Glance: Europe 2019: State of Health in the EU Cycle; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Global Hip and Knee Replacement Market Set to Be Worth $20.4bn by 2028, Says GlobalData. 2018. Available online: https://www.globaldata.com/global-hip-knee-replacement-market-set-worth-20-4bn-2028-says-globaldata/ (accessed on 5 March 2021).
- Annual Report 2016—American Joint Replacement Registry; AAOS: Rosemont, IL, USA, 2016.
- Parisi, T.J.; Konopka, J.F.; Bedair, H.S. What is the long-term economic societal effect of periprosthetic infections after THA? A Markov analysis. Clin. Orthop. Relat. Res. 2017, 475, 1891–1900. [Google Scholar] [CrossRef]
- Brochin, R.L.; Phan, K.; Poeran, J.; Zubizarreta, N.; Galatz, L.M.; Moucha, C.S. Trends in periprosthetic hip infection and associated costs: A population-based study assessing the impact of hospital factors using national data. J. Arthroplast. 2018, 33, S233–S238. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef]
- Romanò, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial coating of implants: Are we missing something? Bone Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef]
- Belanger, M.C.; Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. A 2001, 58, 467–477. [Google Scholar] [CrossRef]
- Park, B.S.; Heo, S.J.; Kim, C.S.; Oh, J.E.; Kim, J.M.; Lee, G.; Park, W.H.; Chung, C.P.; Min, B.M. Effects of adhesion molecules on the behavior of osteoblast-like cells and normal human fibro- blasts on different titanium surfaces. J. Biomed. Mater. Res. A 2005, 74, 640–651. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Gristina, A.G. Implant failure and the immuno-incompetent fibroinflammatory zone. Clin. Orthop. Relat. Res. 1994, 298, 106–118. [Google Scholar]
- Chu, L.; Yang, Y.; Yang, S.; Fan, Q.; Yu, Z.; Hu, X.L.; James, T.D.; He, X.P.; Tang, T. Preferential Colonization of Osteoblasts Over Co-cultured Bacteria on a Bifunctional Biomaterial Surface. Front. Microbiol. 2018, 9, 2219. [Google Scholar] [CrossRef]
- Gotzmann, G.; Beckmann, J.; Wetzel, C.; Scholz, B.; Herrmann, U.; Neunzehn, J. Electron-beam modification of DLC coatings for biomedical applications. Surf. Coat. Technol. 2017, 311, 248–256. [Google Scholar] [CrossRef]
- Ren, D.W.; Zhao, Q.; Bendavid, A. Anti-bacterial property of Si and F doped diamond-like carbon coatings. Surf. Coat. Technol. 2013, 226, 1–6. [Google Scholar] [CrossRef]
- Ishihara, M.; Kosaka, T.; Nakamura, T.; Tsugawa, K.; Koga, Y. Antibacterial activity of fluorine incorporated DLC films. Diam. Relat. Mater. 2006, 15, 1011–1014. [Google Scholar] [CrossRef]
- Robertson, S.N.; Gibson, D.; MacKay, W.G.; Reid, S.; Birney, R. Investigation of the antimicrobial properties of modified multilayer diamond-like carbon coatings on 316 stainless steel. Surf. Coat. Technol. 2017, 314, 72–78. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Li, H.; Ji, L.; Ye, Y.; Zhou, H. Preparation and properties of Ag/DLC nanocomposite films fabricated by unbalanced magnetron sputtering. Appl. Surf. Sci. 2013, 284, 165–170. [Google Scholar] [CrossRef]
- Hussain, S.; Pal, A.K. Synthesis of composite films of mixed Ag–Cu nanocrystallites embedded in DLC matrix and associated surface plasmon properties. Appl. Surf. Sci. 2007, 253, 3649–3657. [Google Scholar] [CrossRef]
- Su, Y.; Wang, K.; Gao, J.; Yang, Y.; Qin, Y.; Zheng, Y.; Zhu, D. Enhance cytocompatibility and antibacterial property of zincphosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-doped nanocomposite carbon coatings (Ag-DLC) for biomedical applications Physiochemical and biological evaluation. Appl. Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- Evrim, B.; Zeynep, B.; Ramazan, E.; Birgül, Y.D. TiO2-NT electrodes modified with Ag and diamond like carbon (DLC) for hydrogen production by alkaline water electrolysis. Appl. Surf. Sci. 2017, 420, 416–428. [Google Scholar]
- Morrison, M.L.; Buchanan, R.A.; Liaw, P.K.; Berry, C.J.; Brigmon, R.L.; Riester, L.; Abernathy, H.; Jin, C.; Narayan, R.J. Electrochemical and antimicrobial properties of diamondlike carbon-metal composite films. Diam. Relat. Mater. 2006, 15, 138–146. [Google Scholar] [CrossRef]
- Constantinou, M.; Pervolaraki, M.; Nikolaou, P.; Prouskas, C.; Patsalas, P.; Kelires, P.; Giapintzakis, J.; Constantinides, G. Microstructure and nanomechanical properties of pulsed excimer laser deposited DLC:Ag films: Enhanced nanotribological response. Surf. Coat. Technol. 2017, 309, 320–330. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W.; Morimura, T. Preparation and antibacterial properties of Ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 2013, 89, 179–184. [Google Scholar] [CrossRef]
- Świdwińska-Gajewska, A.M.; Czerczak, S. Nanosilver harmful effect of biological activity. Med. Pr. 2014, 65, 831–845. [Google Scholar] [PubMed]
- Lansdown, A.B.G.; Anderson, D.; Waters, M.D.; Marrs, T. Silver in Healthcare: Its Antimicrobial Efficacy and Safety in Use. Chapter 8—The Toxicology of Silver; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Sengstock, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2012. 17, 6981–6987. [Google Scholar]
- Liu, Y.; Guo, P.; He, X.; Li, L.; Li, H. Developing transparent copper-doped diamond-like carbon films for marine antifouling applications. Diam. Relat. Mater. 2016, 69, 144–151. [Google Scholar] [CrossRef]
- Jonas, J.; Burns, J.; Abel, E.W.; Cresswell, M.J.; Strain, J.J.; Paterson, C.R. Impaired mechanical strength of bone in experimental copper deficiency. Int. J. Nutr. Metab. 1993, 37, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Käppel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity ofosteoblastic cells. J. Biomed. Mater. Res. 2012, 100, 2392–2400. [Google Scholar]
- Barralet, J.; Gbureck, U.; Habibovic, P.; Vorndran, E.; Gerard, C.; Doillon, C.J. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. Part A 2009, 15, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, G.; Saldamli, B.; Schwarz, F.; Jurgens, P.; Leiggener, C.; Haeberlen, M.; Assmann, W.; Stritzker, B. Biocompatibility of doped diamond-like carbon coatings for medical implants. Plasma Process Polym. 2007, 4, 364–368. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Szymanski, W.; Jedrzejczak, A.; Jastrzebska, A.; Olejnik, A.; Swiatek, L.; Jastrzebski, K. Diamond like carbon coatings doped by Si fabricated by a multi-target DC-RF magnetron sputtering method—Mechanical properties, chemical analysis and biological evaluation. Vacuum 2017, 143, 395–406. [Google Scholar] [CrossRef]
- Ferraria, A.; Carapeto, A.; Rego, A. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Self-accumulated Ag nanoparticles on mesoporous TiO2 thin film with high bactericidal activities. Surf. Coat. Technol. 2010, 204, 3676–3683. [Google Scholar] [CrossRef]
- Castro, C.A.; Jurado, A.; Sissa, D.; Giraldo, S.A. Performance of Ag-TiO2 photocatalysts towards the photocatalytic disinfection of water under interior-lighting and solar-simulated light irradiations. Int. J. Photoenergy 2012, 12, 1–10. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.T.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Ivanov-Omskii, V.I.; Panina, L.K.; Yastrebov, S.G. Amorphous hydrogenated carbon doped with copper as antifungal protective coating. Carbon 2000, 38, 495–499. [Google Scholar] [CrossRef]
- Mazare, A.; Anghel, A.; Surdu-Bob, C.; Totea, G.; Ionita, D. Silver doped diamond-like carbon antibacterial and corrosion resistance coatings on titanium. Thin Solid Films 2018, 657, 16–23. [Google Scholar] [CrossRef]
- Písařík, P.; Jelínek, M.; Remsa, J.; Mikšovský, J.; Šepitka, J. Antibacterial, mechanical and surface properties of Ag-DLC films prepared by dual PLD for medical applications. Mater. Sci. Eng. C 2017, 77, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.C.H.; Ha, P.C.T.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Biocompatibility of calcium and phosphorus doped diamond-like carbon thin films synthesized by plasma immersion ion implantation and deposition. Diam. Relat. Mater. 2006, 15, 893–897. [Google Scholar] [CrossRef]
- Meškinis, Š.; Čiegis, A.; Vasiliauskas, A.; Šlapikas, K.; Tamulevičius, S. Optical properties of diamond-like carbon films containing copper, grown by high power pulsed magnetron sputtering and direct current magnetron sputtering: Structure and composition effects. Thin Solid Films 2015, 58, 48–53. [Google Scholar] [CrossRef]
- Stoian, A.B.; Surdu-Bo, C.; Anghel, A.; Ionita, D.; Demetrescu, I. Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC Coatings on Ti50Zr with Different Ag Amounts. Coatings 2019, 9, 792. [Google Scholar] [CrossRef]
- Rhee, S.K. Critical surface energies of Al2O3 and graphite. J. Am. Ceram. Soc. 2006, 55, 300–303. [Google Scholar] [CrossRef]
- Sun, L.; Guo, P.; Li, X.; Wang, A. Comparative study on structure and wetting properties of diamond-like carbon films by W and Cu doping. Diam. Relat. Mater. 2017, 73, 278–284. [Google Scholar] [CrossRef]
- Micali, G.; Grilli, J.; Marchi, J.; Osella, M.; Cosentino Lagomarsino, M. Dissecting the Control Mechanisms for DNA Replication and Cell Division in E. coli. Cell Rep. 2018, 25, 761–771. [Google Scholar] [CrossRef] [PubMed]
- McClintock, M.K.; Fahnhorst, G.W.; Hoye, T.R.; Zhang, K. Engineering the production of dipicolinic acid in E. coli. Metab. Eng. 2018, 48, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Huang, C.F.; Ou, K.L.; Peng, P.W. Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf. Coat. Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Cremet, L.; Broquet, A.; Brulin, B.; Jacqueline, C.; Dauvergne, S.; Brion, R.; Asehnoune, K.; Corvec, S.; Heymann, D.; Caroff, N. Pathogenic potential of Escherichia coli clinical strains from ortopedic implant infections towards human osteoblastic cells. Pathog. Dis. 2015, 73, 26333570. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Goudouri, O.M.; Kontonasaki, E.; Lohbauer, U.; Boccaccini, A.R. Antibacterial properties of metal and metalloid ions in chronic periodontitis and peri-implantitis therapy. Acta Biomater. 2014, 108, 3795–3810. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.; Jung, F.; Pietzsch, J. Human endothelial cell models in biomaterial research. Trends Biotechnol. 2017, 35, 265–277. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef]
- Burghardt, I.; Lüthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C.; Neumann, H.G.; Rychly, J. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chub, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 82, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.Q.; Gao, A.; Huang, X.B.; Wang, X.G.; Zhang, X.Y.; Qin, L.; Tang, B. Antibacterial activity and cytocompatibility of Cu–Ti–O nanotubes. J. Biomed. Mater. Res. B 2014, 102, 1850–1858. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, W.; Bal, B.S.; Rahaman, M.N. Effect of copper-doped silicate 13–93 bioactive glass scaffolds on the response of MC3T3-E1 cells in vitro and on bone regeneration and angiogenesis in rat calvarial defects in vivo. Mater. Sci. Eng. C 2016, 67, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhang, R.; Yang, X.; Lan, C.; Feng, Q.; Liu, Y. The development of Cu-incorporated micro/nano-topographical bio-ceramic coatings for enhanced osteoblast response. Appl. Surf. Sci. 2019, 465, 575–583. [Google Scholar] [CrossRef]
- G’erard, C.; Bordeleau, L.J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Rafi, A.; Devaki, R.; Sabita, K.; Mohanty, S.; Rao, P. Importance of serum copper and vascular endothelial growth factor vegf-a levels in postmenopausal bleeding. Indian J. Clin. Biochem. 2013, 28, 147–151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Xie, H.; Li, S.; Kang, Y.J. Copper stimulates growth of human umbilical vein endothelial cells in a vascular endothelial growth factor-independent pathway. Exp. Biol. Med. 2012, 237, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Santos, L.V.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Antibacterial activity of DLC and Ag–DLC films produced by PECVD technique. Diam. Relat. Mater. 2009, 18, 1010–1014. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B 2009, 73, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bostancıoğlu, R.B.; Peksen, C.; Genc, H.; Gürbüz, M.; Karel, F.B.; Koparal, A.S.; Dogan, A.; Kose, N.; Koparal, A.T. Analyses of the modulatory effects of antibacterial silver doped calcium phosphate-based ceramic nano-powder on proliferation, survival, and angiogenic capacity of different mammalian cells in vitro. Biomed. Mater. 2015, 10, 045024. [Google Scholar] [CrossRef] [PubMed]


| Coating | Chemical Composition | |
|---|---|---|
| O (at.%) | C (at.%) | |
| DLC substrate: AISI 316LVM | 18.15 ± 0.37 | 81.85 ± 0.40 |
| DLC substrate: Ti6Al7Nb | 18.44 ± 0.11 | 81.56 ± 0.15 |
| Ag-DLC | Cu-DLC | ||
|---|---|---|---|
| Substrate: AISI 316 LVM | Substrate: AISI 316 LVM | ||
| Sample ID | Ag (at.%) | Sample ID | Cu (at.%) |
| DLC | 0.00 | DLC | 0.00 |
| Ag-DLC1 | 0.65 ± 0.10 | Cu-DLC1 | 0.48 ± 0.08 |
| Ag-DLC2 | 0.86 ± 0.09 | Cu-DLC2 | 1.23 ± 0.05 |
| Ag-DLC3 | 6.99 ± 0.70 | Cu-DLC3 | 4.74 ± 0.46 |
| Ag-DLC4 | 9.77 ± 0.98 | Cu-DLC4 | 6.85 ± 0.16 |
| Substrate: Ti6Al7Nb | Substrate: Ti6Al7Nb | ||
| Sample ID | Ag (at.%) | Sample ID | Cu (at.%) |
| DLC | 0.00 | DLC | 0.00 |
| Ag-DLC1 | 0.49 ± 0.07 | Cu-DLC1 | 0.98 ± 0.03 |
| Ag-DLC2 | 0.82 ± 0.07 | Cu-DLC2 | 1.38 ± 0.09 |
| Ag-DLC3 | 2.58 ± 0.23 | Cu-DLC3 | 2.60 ± 0.40 |
| Ag-DLC4 | 13.38 ± 1.20 | Cu-DLC4 | 5.99 ± 0.20 |
| Substrate: AISI 316LVM | Substrate: Ti6Al7Nb | |||||||
|---|---|---|---|---|---|---|---|---|
| sp2 | sp3 | C–O | C=O | sp2 | sp3 | C–O | C=O | |
| DLC | 56.4 ± 1.9 | 24.9 ± 1.1 | 12.5 ± 0.2 | 6.3 ± 0.4 | 58.7 ± 0.7 | 23.3 ± 0.7 | 11.8 ± 0.1 | 6.3 ± 0.1 |
| Cu-DLC1 | 61.7 ± 0.2 | 24.6 ± 1.1 | 7.7 ± 0.8 | 5.9 ± 0.5 | 61.7 ± 1.0 | 24.4 ± 1.0 | 5.6 ± 0.7 | 8.3 ± 0.5 |
| Cu-DLC2 | 62.1 ± 1.0 | 24.6 ± 1.1 | 6.1 ± 0.5 | 7.2 ± 0.2 | 62.5 ± 0.7 | 24.0 ± 1.2 | 6.2 ± 0.5 | 7.2 ± 0.4 |
| Cu-DLC3 | 63.8 ± 1.0 | 23.0 ± 0.6 | 7.1 ± 1.1 | 6.1 ± 1.0 | 63.5 ± 0.8 | 22.8 ± 1.0 | 8.3 ± 0.5 | 5.3 ± 0.3 |
| Cu-DLC4 | 64.1 ± 2.6 | 16.6 ± 2.5 | 7.4 ± 0.5 | 11.9 ± 0.6 | 65.7 ± 0.8 | 18.6 ± 0.8 | 8.2 ± 0.8 | 7.5 ± 0.7 |
| Ag-DLC1 | 60.3 ± 0.4 | 25.7 ± 3.0 | 7.8 ± 2.8 | 6.2 ± 0.4 | 61.4 ± 0.1 | 24.3 ± 0.3 | 7.6 ± 0.3 | 6.8 ± 0.5 |
| Ag-DLC2 | 62.8 ± 1.3 | 23.3 ± 2.8 | 12.4 ± 2.0 | 1.4 ± 3.0 | 62.4 ± 2.3 | 23.6 ± 1.5 | 8.1 ± 0.7 | 5.8 ± 0.6 |
| Ag-DLC3 | 63.6 ± 0.5 | 21.1 ± 0.8 | 9.0 ± 0.4 | 6.2 ± 0.4 | 64.0 ± 0.7 | 23.0 ± 0.4 | 4.6 ± 0.2 | 8.5 ± 0.3 |
| Ag-DLC4 | 69.4 ± 2.1 | 19.0 ± 3.0 | 6.5 ± 1.5 | 5.1 ± 0.9 | 67.7 ± 1.0 | 36.2 ± 1.0 | 5.3 ± 0.6 | 7.5 ± 0.7 |
| Cu 2p3/2 (eV) | Bond Type | AISI 316LVM | Ti6Al7Nb | |
|---|---|---|---|---|
| Share of Bonds | Share of Bonds | |||
| Cu-DLC1 | 932.2 | Cu2O | 0.1% ± 0.1% | 0.4% ± 0.3% |
| 932.6 | Cu0 | 20.3% ± 1.0% | 27.8% ± 0.9% | |
| 933.8 | CuO | 38.8% ± 0.9% | 38.4% ± 1.0% | |
| 934.7 | Cu(OH)2 | 40.8% ± 1.2% | 33.4% ± 1.1% | |
| Cu-DLC2 | 932.2 | Cu2O | 0.1% ± 0.1% | 0.3% ± 0.1% |
| 932.6 | Cu0 | 22.1% ± 0.9% | 24.7% ± 0.5% | |
| 933.8 | CuO | 39.2% ± 1.0% | 37.0% ± 0.6% | |
| 934.7 | Cu(OH)2 | 38.6% ± 0.8% | 38.1% ± 1.0% | |
| Cu-DLC3 | 932.2 | Cu2O | 1.4% ± 0.9% | 0.8% ± 0.9% |
| 932.6 | Cu0 | 23.6% ± 0.7% | 24.9% ± 4.3% | |
| 933.8 | CuO | 39.4% ± 1.1% | 32.8% ± 2.8% | |
| 934.7 | Cu(OH)2 | 35.4% ± 0.9% | 41.5% ± 2.3% | |
| Cu-DLC4 | 932.2 | Cu2O | 0.2% ± 0.1% | 0.3% ± 0.1% |
| 932.6 | Cu0 | 24.5% ± 4.0% | 22.3% ± 0.9% | |
| 933.8 | CuO | 28.0% ± 2.3% | 27.0% ± 0.7% | |
| 934.7 | Cu(OH)2 | 49.3% ± 2.8% | 50.5% ± 0.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrzębski, K.; Białecki, J.; Jastrzębska, A.; Kaczmarek, A.; Para, M.; Niedzielski, P.; Bociaga, D. Induced Biological Response in Contact with Ag-and Cu-Doped Carbon Coatings for Potential Orthopedic Applications. Materials 2021, 14, 1861. https://doi.org/10.3390/ma14081861
Jastrzębski K, Białecki J, Jastrzębska A, Kaczmarek A, Para M, Niedzielski P, Bociaga D. Induced Biological Response in Contact with Ag-and Cu-Doped Carbon Coatings for Potential Orthopedic Applications. Materials. 2021; 14(8):1861. https://doi.org/10.3390/ma14081861
Chicago/Turabian StyleJastrzębski, Krzysztof, Jerzy Białecki, Aleksandra Jastrzębska, Anna Kaczmarek, Marcin Para, Piotr Niedzielski, and Dorota Bociaga. 2021. "Induced Biological Response in Contact with Ag-and Cu-Doped Carbon Coatings for Potential Orthopedic Applications" Materials 14, no. 8: 1861. https://doi.org/10.3390/ma14081861
APA StyleJastrzębski, K., Białecki, J., Jastrzębska, A., Kaczmarek, A., Para, M., Niedzielski, P., & Bociaga, D. (2021). Induced Biological Response in Contact with Ag-and Cu-Doped Carbon Coatings for Potential Orthopedic Applications. Materials, 14(8), 1861. https://doi.org/10.3390/ma14081861







