Carbon Fiber Reinforced Polymer Composites Doped with Graphene Oxide in Light of Spectroscopic Studies
Abstract
1. Introduction
- microwave curing of the composites (instead of typical heating) [12]–improves the wettability of fibers;
2. Materials and Methods
2.1. Materials
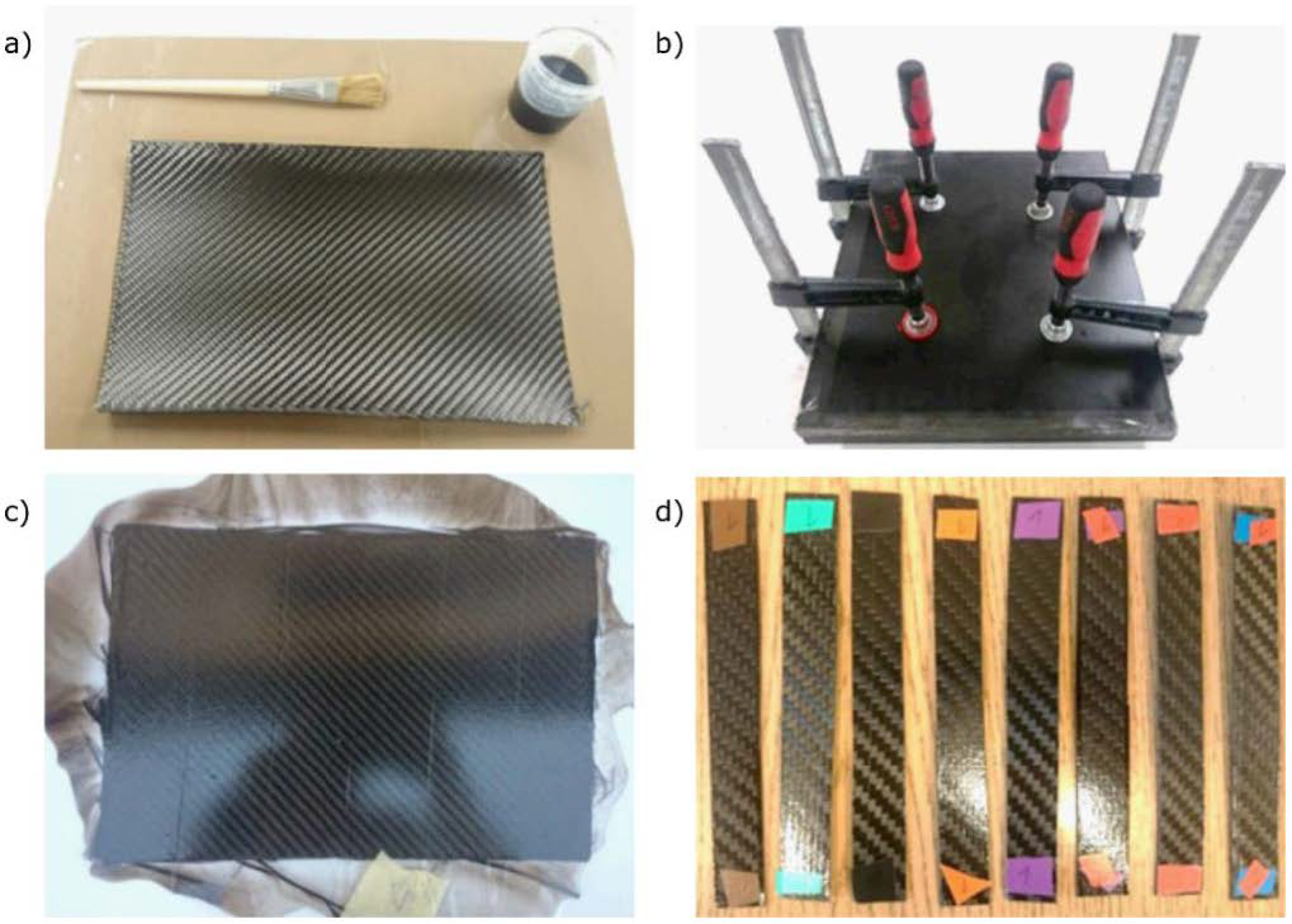
2.2. Samples and Preparation
2.3. Instrumentation
3. Results and Discussions
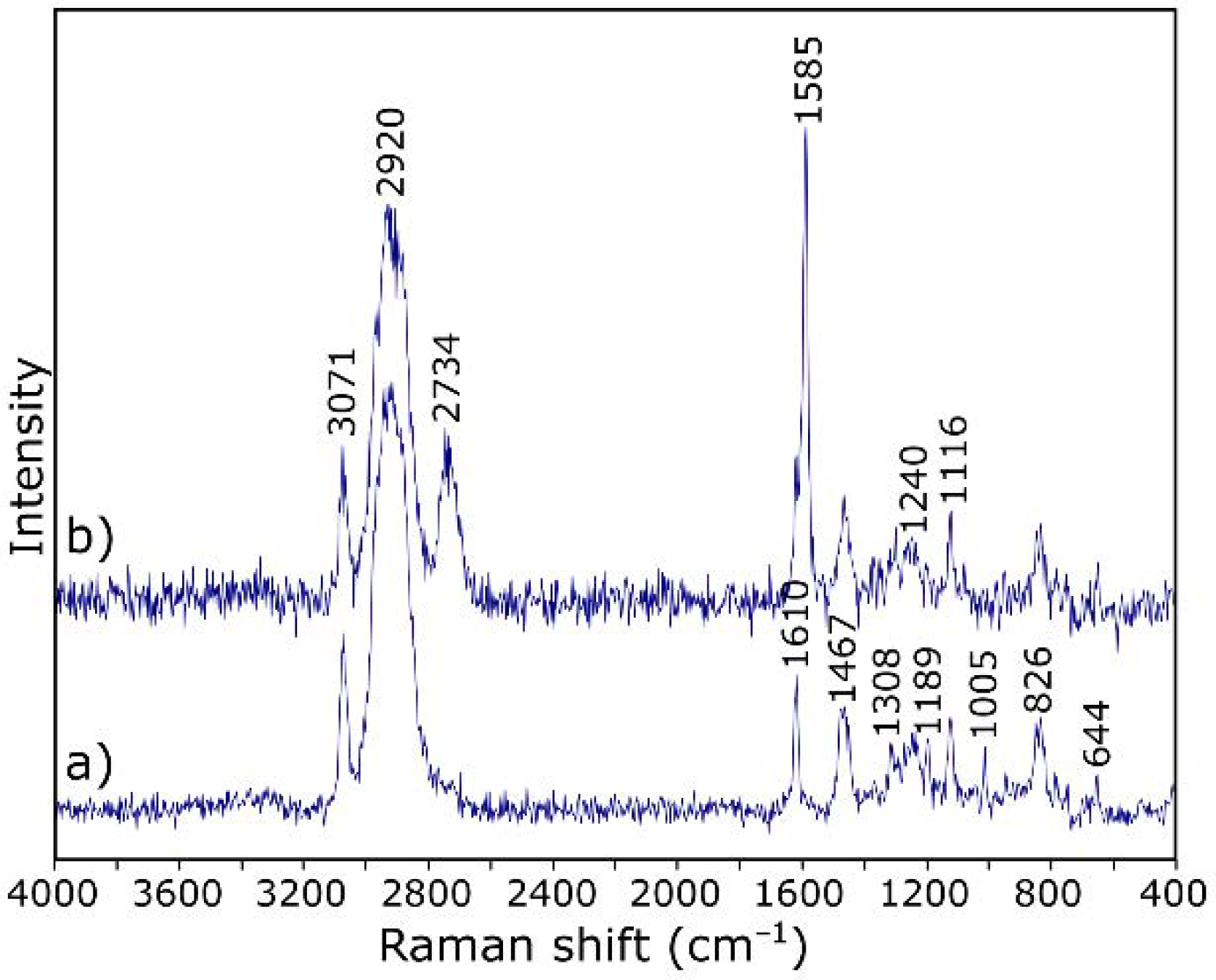
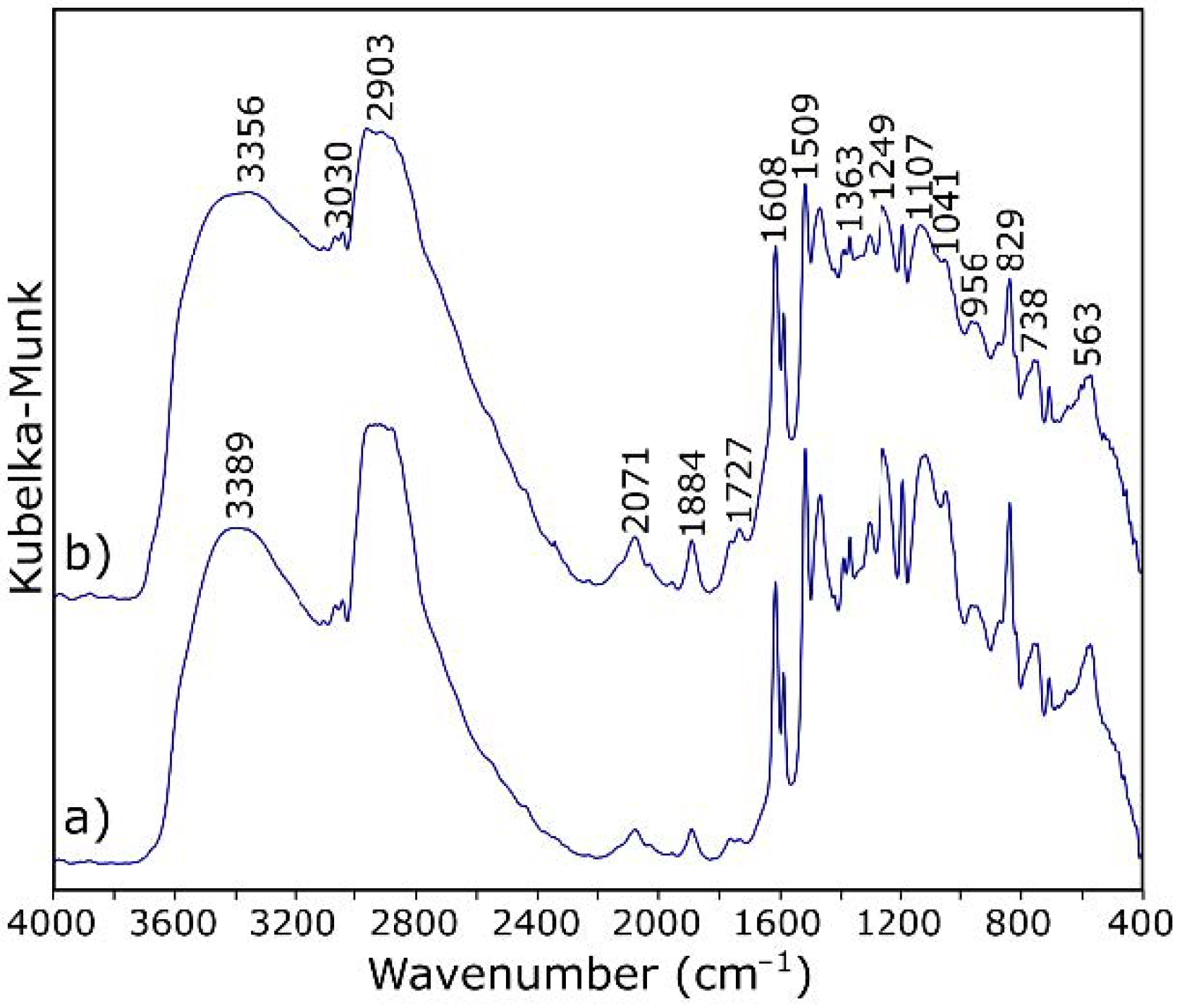
3.1. Characterization of Starting Materials
3.2. Characterization of ER Modified by GO
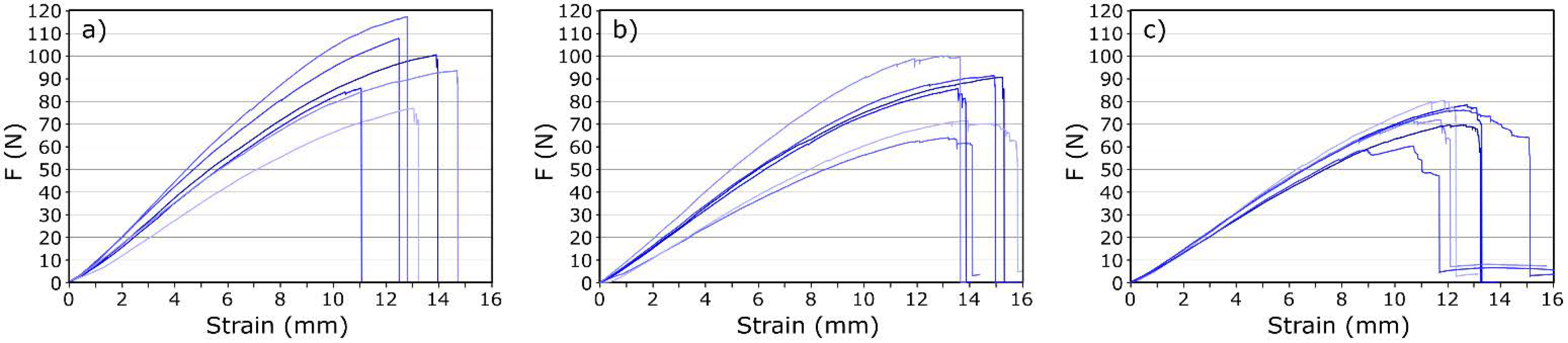
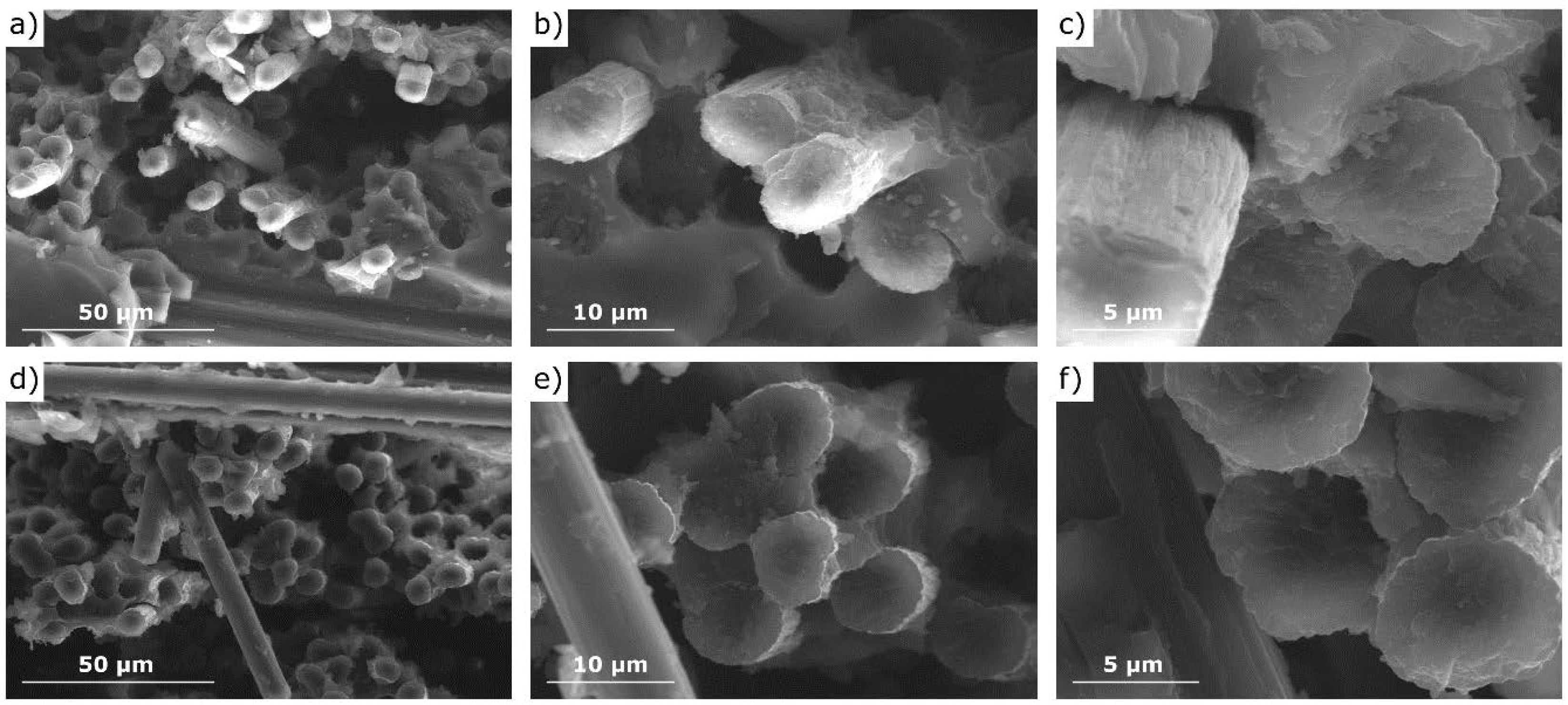
3.3. Properties and Microstructure of CF/GO Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantwell, W.J.; Morton, J. Comparison of the low and high velocity impact response of CFRP. Composites 1989, 20, 545–551. [Google Scholar] [CrossRef]
- Meier, U. Carbon fiber-reinforced polymers: Modern materials in bridge engineering. Struct. Eng. Int. 1992, 2, 7–12. [Google Scholar] [CrossRef]
- Miller, T.C.; Chajes, M.J.; Mertz, D.R.; Hastings, J.N. Strengthening of a steel bridge girder using CFRP plates. J. Bridge Eng. 2001, 6, 514–522. [Google Scholar] [CrossRef]
- Ozkan, D.; Gok, M.S.; Karaoglanli, A.C. Carbon Fiber Reinforced Polymer (CFRP) Composite Materials, Their Characteristic Properties, Industrial Application Areas and Their Machinability. In Engineering Design Applications III; Andreas Öchsner, A., Altenbach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–253. [Google Scholar]
- Morgan, P. Carbon Fibers and Their Composites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Raphael, N.; Namratha, K.; Chandrashekar, B.N.; Sadasivuni, K.K.; Ponnamma, D.; Smitha, A.S.; Byrappa, K. Surface modification and grafting of carbon fibers, A route to better interface. Prog. Cryst. Growth Charact. Mater. 2018, 64, 75–101. [Google Scholar] [CrossRef]
- Park, S.-J.; Meng, L.-Y. Surface Treatment and Sizing of Carbon Fibers. In Carbon Fibers; Park, S.-J., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 101–133. [Google Scholar]
- Severini, F.; Formaro, L.; Pegoraro, M.; Posca, L. Chemical modification of carbon fiber surfaces. Carbon 2002, 40, 735–741. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, X.; Xu, C.; Su, Y.; Zhou, L.; Deng, C. Influence of electrochemical oxidation of carbon fiber on the mechanical properties of carbon fiber/graphene oxide/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 95, 248–256. [Google Scholar] [CrossRef]
- Yu, J.; Meng, L.; Fan, D.; Zhang, C.; Yu, F.; Huang, Y. The oxidation of carbon fibers through K2S2O8/AgNO3 system that preserves fiber tensile strength. Compos. B Eng. 2014, 60, 261–267. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.R.; Cao, F.; Wang, S.Q.; Cao, Y.B.; Chen, B. Surface oxidation of carbon fiber and its influence on the properties of carbon fiber reinforced BN-Si3N4 composites. Key Eng. Mater. 2008, 368–372, 901–904. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Cai, Q.; Wang, X.; Wei, R.; Du, S. Improvement of the compressive strength of carbon fiber/epoxy composites via microwave curing. J. Mater. Sci. Technol. 2016, 32, 226–232. [Google Scholar] [CrossRef]
- Qian, H.; Bismarck, A.; Greenhalgh, E.S.; Kalinka, G.; Shaffer, M.S.P. Hierarchical composites reinforced with carbon nanotube grafted fibers: The potential assessed at the single fiber level. Chem. Mater. 2008, 20, 1862–1869. [Google Scholar] [CrossRef]
- Lavagna, L.; Massella, D.; Pavese, M. Preparation of hierarchical material by chemical grafting of carbon nanotubes onto carbon fibers. Diam. Relat. Mater. 2017, 80, 118–124. [Google Scholar] [CrossRef]
- Dong, J.; Jia, C.; Wang, M.; Fang, X.; Wei, H.; Xie, H.; Zhang, T.; He, J.; Jiang, Z.; Huang, Y. Improved mechanical properties of carbon fiber-reinforced epoxy composites by growing carbon black on carbon fiber surface. Compos. Sci. Technol. 2017, 149, 75–80. [Google Scholar] [CrossRef]
- He, R.; Chang, Q.; Huang, X.; Bo, J. Improved mechanical properties of carbon fiber reinforced PTFE composites by growing graphene oxide on carbon fiber surface. Compos. Interfaces 2018, 25, 995–1004. [Google Scholar] [CrossRef]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fiber surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Bekyarova, E.; Thostenson, E.T.; Yu, A.; Kim, H.; Gao, J.; Tang, J.; Hahn, H.T.; Chou, T.-W.; Itkis, M.E.; Haddon, R.C. Multiscale carbon nanotube−carbon fiber reinforcement for advanced epoxy composites. Langmuir 2007, 23, 3970–3974. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Liu, C.; Hu, Y.; Xu, S.; He, Y.; Zhou, J.; Zhang, Y. Chemically grafting carbon nanotubes onto carbon fibers for enhancing interfacial properties of fiber metal laminate. Materials 2020, 13, 3813. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, H.; Misra, M.; Mohanty, A.K. Mechanical properties of carbon nanotubes and their polymer nanocomposites. J. Nanosci. Nanotechnol. 2005, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Sowers, T.; Bender, M.; Raynaud, D.; Korotkevich, Y.S.; Grootes, P.M.; Steig, E.J.; Morse, D.L. Solution properties of single-walled carbon nanotubes. Science 1998, 282, 95–98. [Google Scholar]
- Hamon, M.A.; Chen, J.; Hu, H.; Chen, Y.; Itkis, M.E.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Dissolution of single-walled carbon nanotubes. Adv. Mater. 1999, 11, 834–840. [Google Scholar] [CrossRef]
- Mickelson, E.T.; Huffman, C.B.; Rinzler, A.G.; Smalley, R.E.; Hauge, R.H.; Margrave, J.L. Fluorination of single-wall carbon nanotubes. Chem. Phys. Lett. 1998, 296, 188–194. [Google Scholar] [CrossRef]
- Hayashi, T.; Terrones, M.; Scheu, C.; Kim, Y.A.; Rühle, M.; Nakajima, T.; Endo, M. NanoTeflons, structure and EELS characterization of fluorinated carbon nanotubes and nanofibers. Nano Lett. 2002, 2, 491–496. [Google Scholar] [CrossRef]
- Desmecht, A.; Sheet, D.; Poleunis, C.; Hermans, S.; Riant, O. Covalent grafting of BPin functions on carbon nanotubes and Chan–Lam–Evans post-functionalization. Chem. A Eur. J. 2019, 25, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, L.; Massella, D.; Pantano, M.F.; Bosia, F.; Pugno, M.M.; Pavese, M. Grafting carbon nanotubes onto carbon fibres doubles their effective strength and the toughness of the composite. Compos. Sci. Technol. 2018, 166, 140–149. [Google Scholar] [CrossRef]
- Islam, M.S.; Deng, Y.; Tong, L.; Faisal, S.N.; Roy, A.K.; Minett, A.I.; Gomes, V.G. Grafting carbon nanotubes directly onto carbon fibers for superior mechanical stability: Towards next generation aerospace composites and energy storage applications. Carbon 2016, 96, 701–710. [Google Scholar] [CrossRef]
- Hung, P.; Lau, K.; Fox, B.; Hameed, N.; Lee, J.H.; Hui, D. Surface modification of carbon fibre using graphene–related materials for multifunctional composites. Compos. Part. B Eng. 2018, 133, 240–257. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Han, X.; Zhao, Y.; Sun, J.M.; Li, Y.; Zhang, J.D.; Hao, Y. Effect of graphene oxide addition on the interlaminar shear property of carbon fiber-reinforced epoxy composites. New Carbon Mater. 2017, 32, 48–55. [Google Scholar] [CrossRef]
- Pathak, A.K.; Borah, M.; Gupta, A.; Yokozeki, T.; Dhakate, S.R. Improved mechanical properties of carbon fiber/graphene oxide-epoxy hybrid composites. Compos. Sci. Technol. 2016, 135, 28–38. [Google Scholar] [CrossRef]
- Hung, K.H.; Kuo, W.S.; Ko, T.H.; Tzeng, S.S.; Yan, C.F. Processing and tensile characterization of composites composed of carbon nanotube-grown carbon fibers. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1299–1304. [Google Scholar] [CrossRef]
- Qian, H.H.; Bismarck, A.; Greenhalgh, E.S.; Shaffer, M.S.P. Carbon nanotube grafted silica fibres: Characterising the interface at the single fibre level. Compos. Sci. Technol. 2010, 70, 393–399. [Google Scholar] [CrossRef]
- Qian, H.; Greenhalgh, E.S.; Shaffer, M.S.P.; Bismarck, A. Carbon nanotube-based hierarchical composites: A review. J. Mater. Chem. 2010, 20, 4751–4762. [Google Scholar] [CrossRef]
- Shaffer, M.M.; Sandler, J. Carbon Nanotube/Nanofibre Polymer Composites. In Processing and Properties of Nanocomposites; Advani, S.G., Ed.; World Scientific Publishing: Hackensack, NJ, USA, 2007; pp. 1–59. [Google Scholar]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Zhang, H.; Hinesa, D.; Akinsa, D.L. Synthesis of a nanocomposite composed of reduced graphene oxide and gold nanoparticles. Dalton Trans. 2014, 43, 2670–2675. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A.; Nguyen, S.T.; Ruoff, R.S. Aqueous suspension and characterization of chemically modified graphene sheets. Chem. Mater. 2008, 20, 6592–6594. [Google Scholar] [CrossRef]
- Gholampour, A.; Kiamahalleh, M.V.; Tran, D.N.H.; Ozbakkaloglu, T.; Losic, D. From graphene oxide to reduced graphene oxide, impact on the physiochemical and mechanical properties of graphene−cement composites. Appl. Mater. Interfaces 2017, 9, 43275–43286. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite, Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Lespade, P.; Marchand, A.; Couzi, M.; Cruege, F. Caracterisation de materiaux carbones par microspectrometrie Raman. Carbon 1984, 2, 375–385. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman spectrum with the chemical composition of graphene oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Miguel, A. Raman spectroscopy study of HM carbon fibres, effect of plasma treatment on the interfacial properties of single fibre/epoxy composites. Carbon 2002, 40, 845–855. [Google Scholar]
- Tang, L.C.; Wan, Y.J.; Yan, D.; Pei, Y.B.; Zhao, L.; Li, Y.B.; Lai, G.Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.J.; Tang, L.C.; Yan, D.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 2013, 82, 60–68. [Google Scholar] [CrossRef]
- Wajid, A.S.; Ahmed, H.S.T.; Das, S.; Irin, F.; Jankowski, A.F.; Green, M.J. High-performance pristine graphene/epoxy composites with enhanced mechanical and electrical properties. Macromol. Mater. Eng. 2013, 298, 339–347. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Perumbilavil, S.; Sankar, P.; Rose, T.P.; Philip, R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced grapheneoxide nanosheets in the 400–700 nm region. Appl. Phys. Lett. 2015, 107, 051104. [Google Scholar] [CrossRef]
- Pathak, A.K.; Garg, H.; Singh, M.; Yokozeki, T.; Dhakate, S.R. Enhanced interfacial properties of graphene oxide incorporated carbon fiber reinforced epoxy nanocomposite, a systematic thermal properties investigation. J. Polym. Res. 2019, 26, 23. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; IntechOpen: London, UK, 2012; pp. 261–284. [Google Scholar]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier Transform IR characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Shen, M.Y.; Chang, T.Y.; Hsieh, T.H.; Li, Y.L.; Chiang, C.L.; Yang, H.; Yip, M.C. Mechanical properties and tensile fatigue of graphene nanoplatelets reinforced polymer nanocomposites. J. Nanomater. 2013, 565401. [Google Scholar] [CrossRef]
- Abdullah, S.I.; Ansari, M.N.M. Mechanical properties of graphene oxide (GO)/epoxy composites. HBRC J. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Chou, C.T.; Gaur, U.; Miller, B. Fracture mechanisms during fiber pull-out for carbon-fiber-reinforced thermosetting composites. Compos. Sci. Technol. 1993, 48, 307–316. [Google Scholar] [CrossRef]
- Vašková, H.; Křesálek, V. Quasi real-time monitoring of epoxy resin crosslinking via Raman microscopy. Int. J. Math. Models Methods Appl. Sci. 2011, 5, 1197–1204. [Google Scholar]
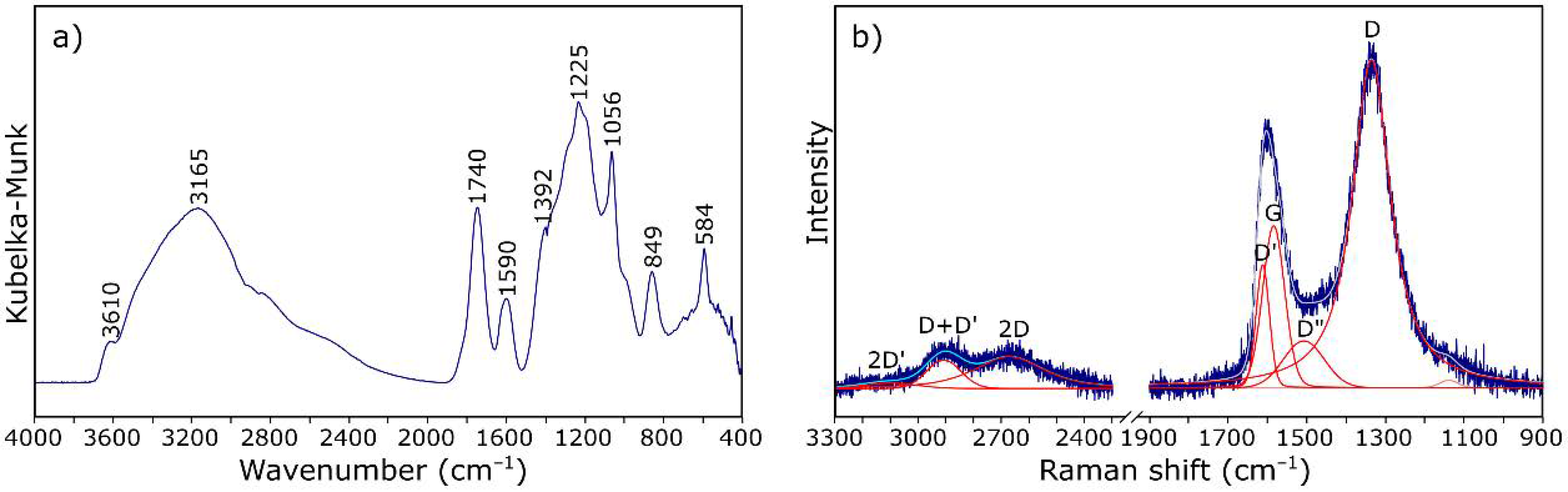
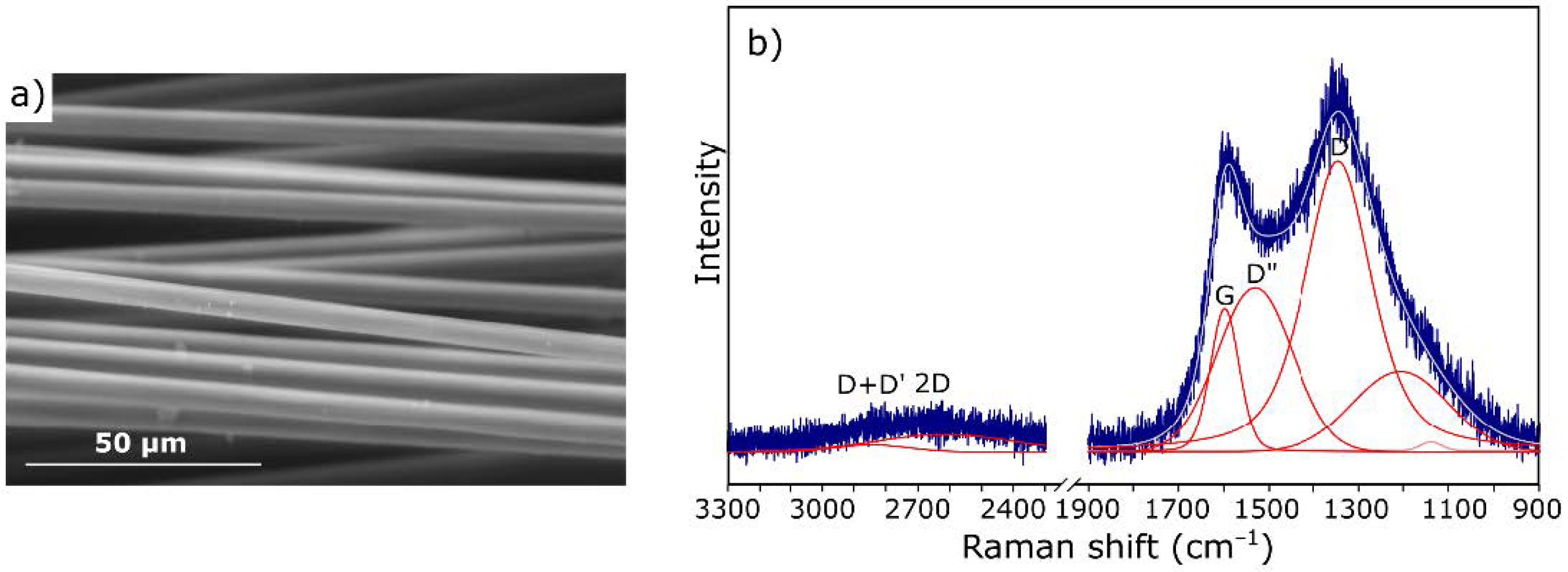
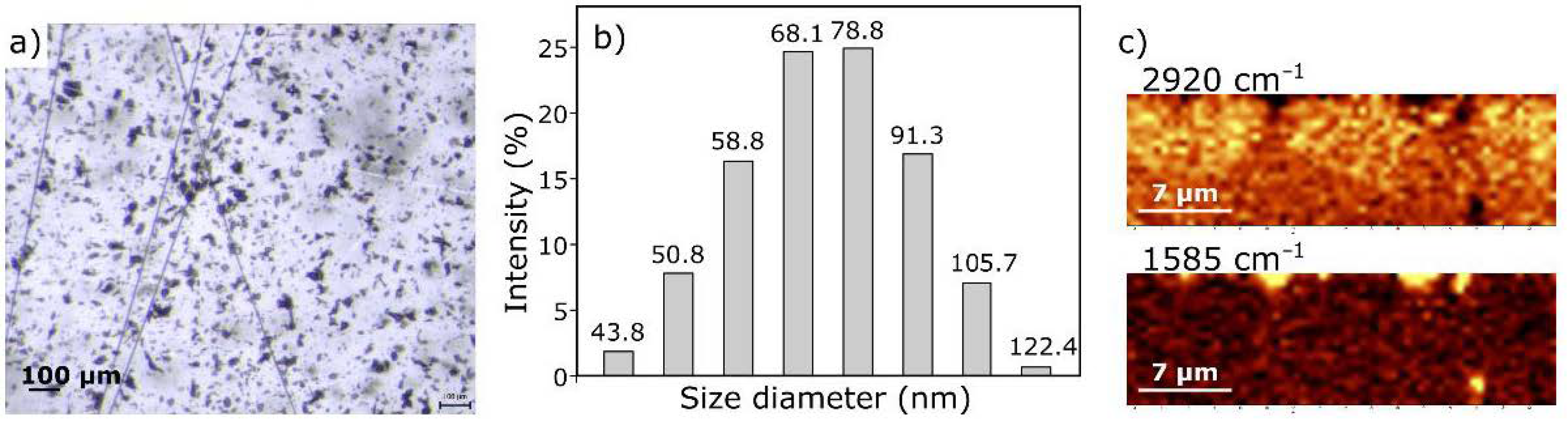
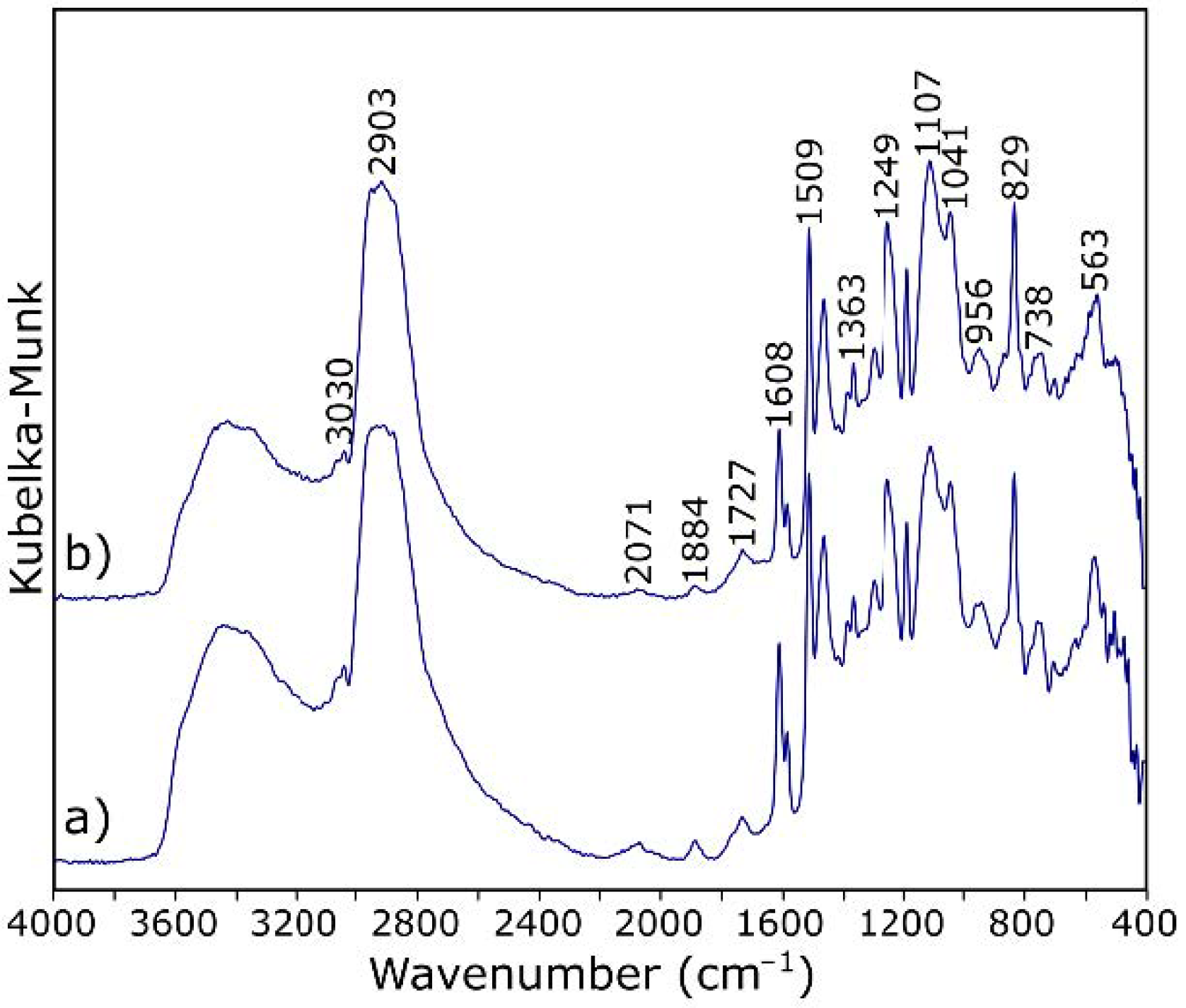
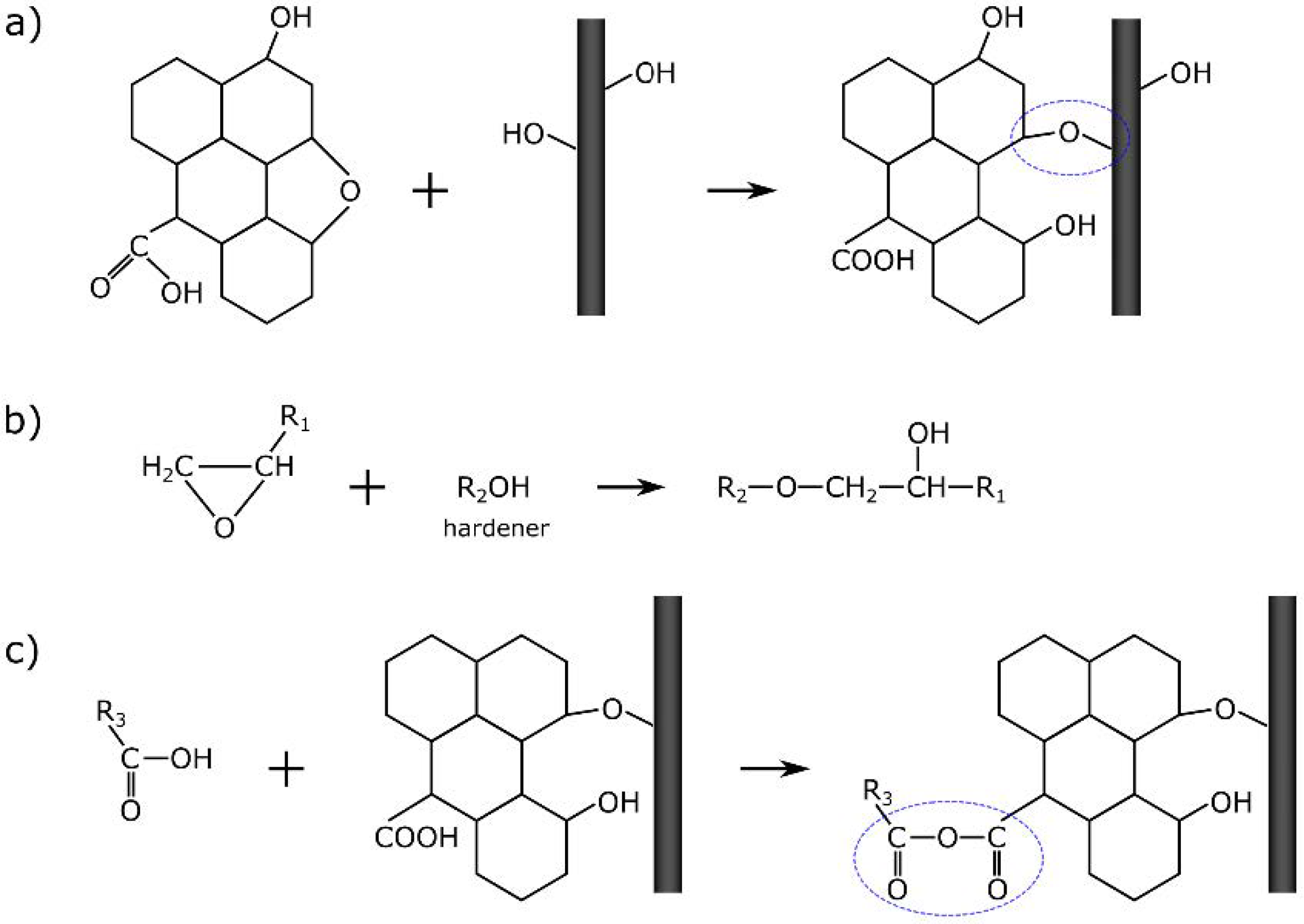
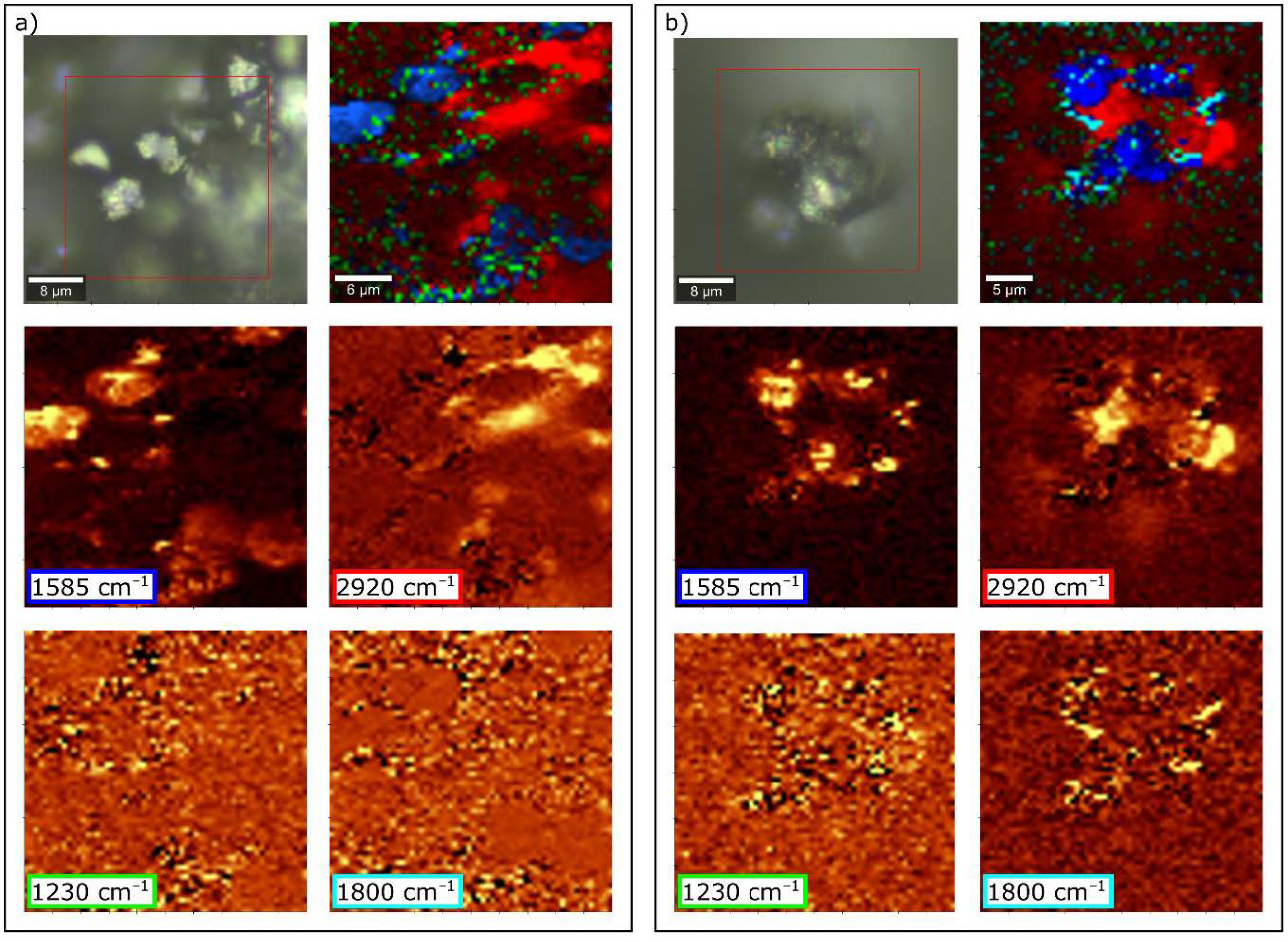
| Band (cm−1) | Assignment [54,55] |
|---|---|
| 3700–3100 | O–H stretching |
| 3030 | stretching of C–H of the epoxy group (oxirane ring) |
| 2965–2873 | stretching C–H of CH2 and CH aromatic and aliphatic |
| 1720 | stretching –C=O of carboxyl group |
| 1608, 1581, 1509 | stretching C=C of aromatic rings |
| 1249 | stretching C–C of aromatic |
| 1041 | stretching C–O–C of ethers |
| 915 | stretching C–O of epoxy group (oxirane ring) |
| 829 | bending out of plane –CH in aromatic |
| 738 | rocking CH2 |
| GO Addition (wt.%) | Fmax (N) | εmax (mm) | σ (MPa) | E (GPa) | Pp (J/m2) |
|---|---|---|---|---|---|
| 0.0 | 93.1 ± 14.8 | 12.7 ± 1.3 | 654.6 ± 58.5 | 44.5 ± 4.3 | 50.4 |
| 0.1 | 86.7 ± 18.7 | 12.4 ± 1.3 | 678.2 ± 65.3 | 50.3 ± 7.5 | 51.9 |
| 0.2 | 80.0 ± 7.3 | 12.8 ± 0.7 | 678.7 ± 30.8 | 51.4 ± 2.1 | 53.6 |
| 0.3 | 84.1 ± 12.6 | 13.9 ± 1.0 | 774.9 ± 55.7 | 60.3 ± 9.2 | 59.9 |
| 0.4 | 86.0 ± 16.9 | 13.0 ± 1.6 | 724.4 ± 74.2 | 54.9 ± 9.4 | 54.9 |
| 0.5 | 72.4 ± 6.9 | 11.7 ± 0.7 | 623.2 ± 56.4 | 51.1 ± 2.7 | 43.4 |
| 1.0 | 73.2 ± 8.2 | 11.6 ± 0.7 | 609.9 ± 80.5 | 48.7 ± 13.5 | 43.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florek, P.; Król, M.; Jeleń, P.; Mozgawa, W. Carbon Fiber Reinforced Polymer Composites Doped with Graphene Oxide in Light of Spectroscopic Studies. Materials 2021, 14, 1835. https://doi.org/10.3390/ma14081835
Florek P, Król M, Jeleń P, Mozgawa W. Carbon Fiber Reinforced Polymer Composites Doped with Graphene Oxide in Light of Spectroscopic Studies. Materials. 2021; 14(8):1835. https://doi.org/10.3390/ma14081835
Chicago/Turabian StyleFlorek, Paulina, Magdalena Król, Piotr Jeleń, and Włodzimierz Mozgawa. 2021. "Carbon Fiber Reinforced Polymer Composites Doped with Graphene Oxide in Light of Spectroscopic Studies" Materials 14, no. 8: 1835. https://doi.org/10.3390/ma14081835
APA StyleFlorek, P., Król, M., Jeleń, P., & Mozgawa, W. (2021). Carbon Fiber Reinforced Polymer Composites Doped with Graphene Oxide in Light of Spectroscopic Studies. Materials, 14(8), 1835. https://doi.org/10.3390/ma14081835








