Effects of Various Types of Expandable Graphite and Blackcurrant Pomace on the Properties of Viscoelastic Polyurethane Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites of Viscoelastic Polyurethane Foams
2.3. Methods
3. Results and Discussion
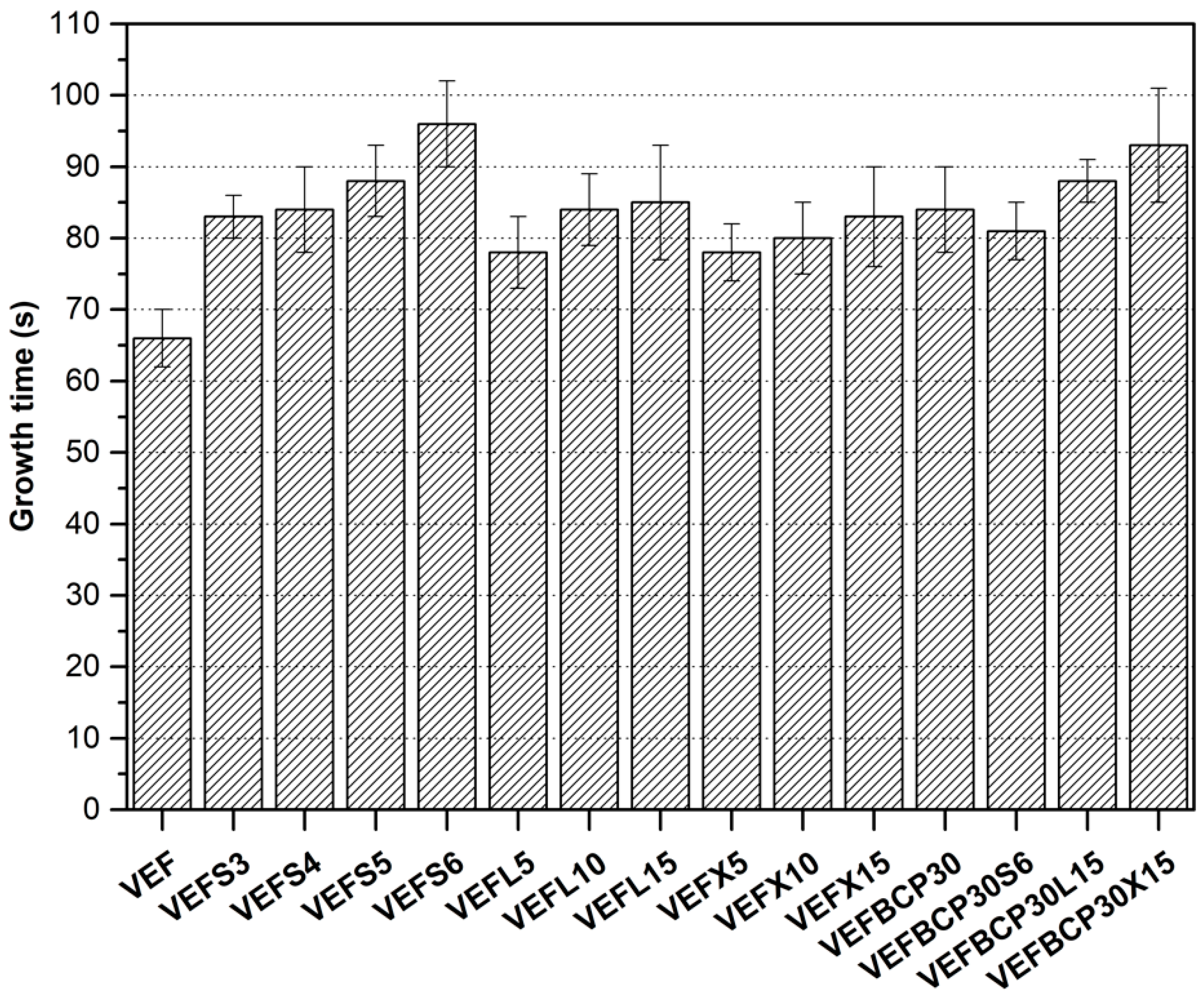
3.1. Physical Properties of Polyurethane Foams
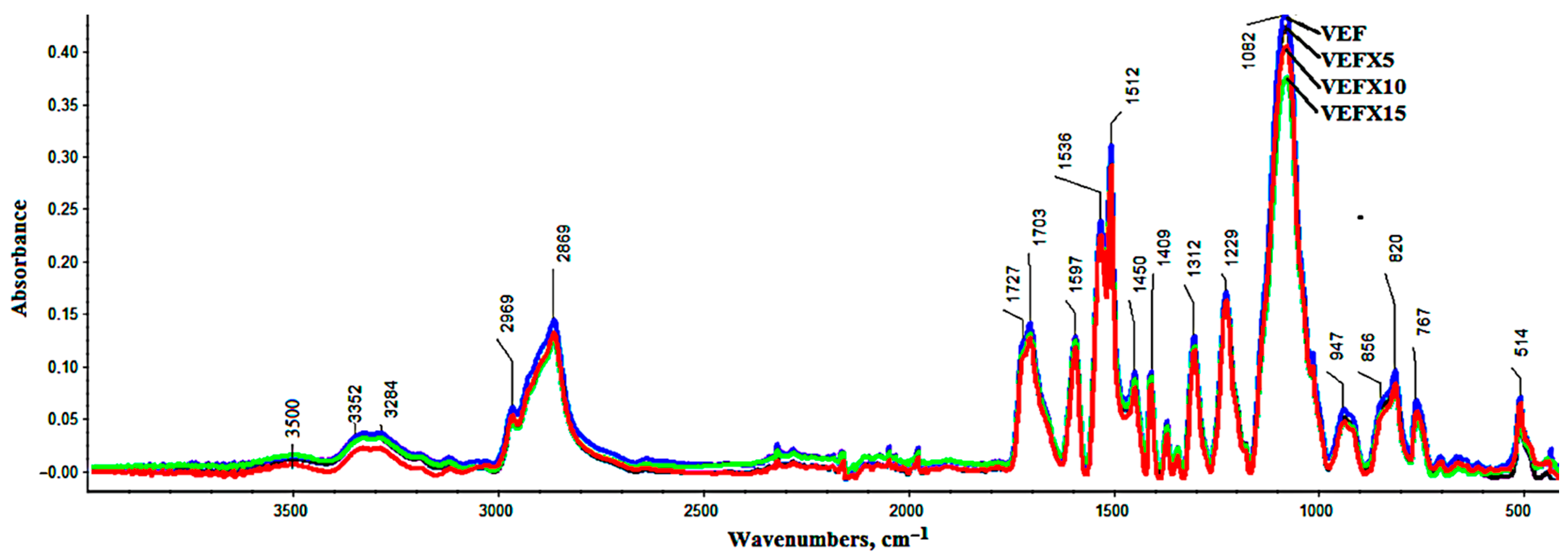
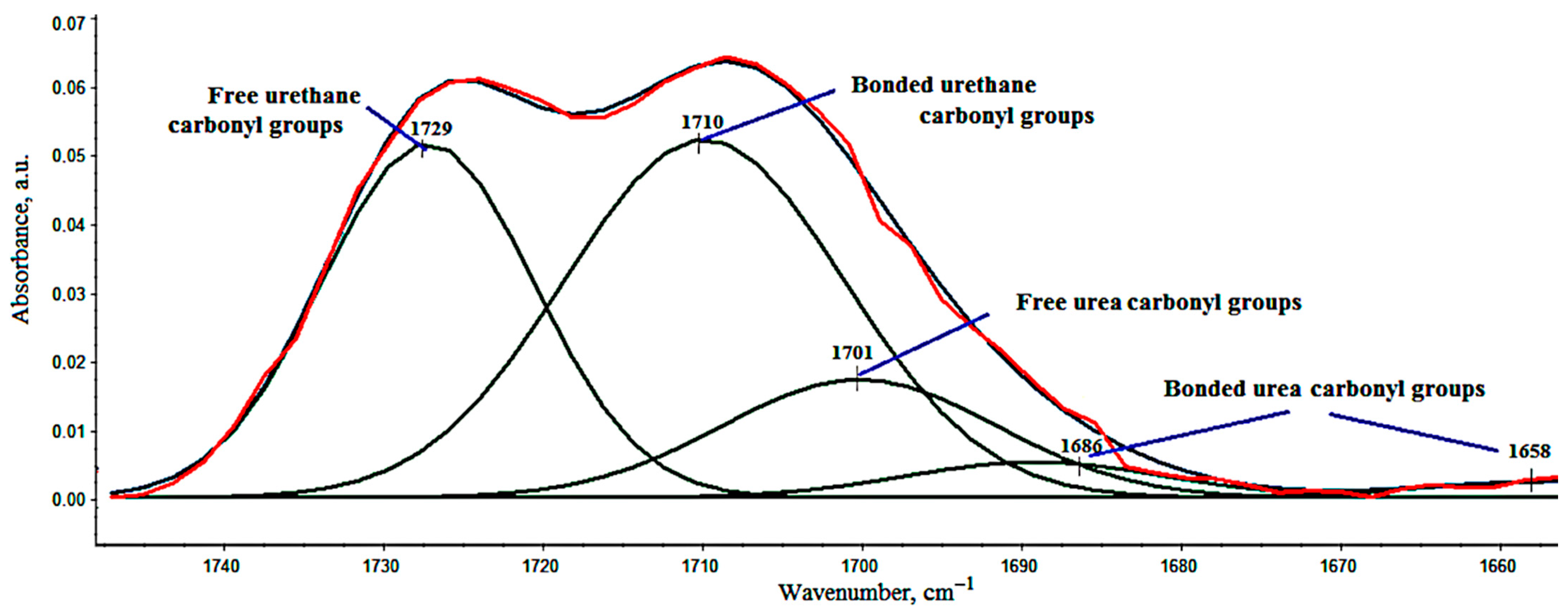
3.2. FT-IR Analysis
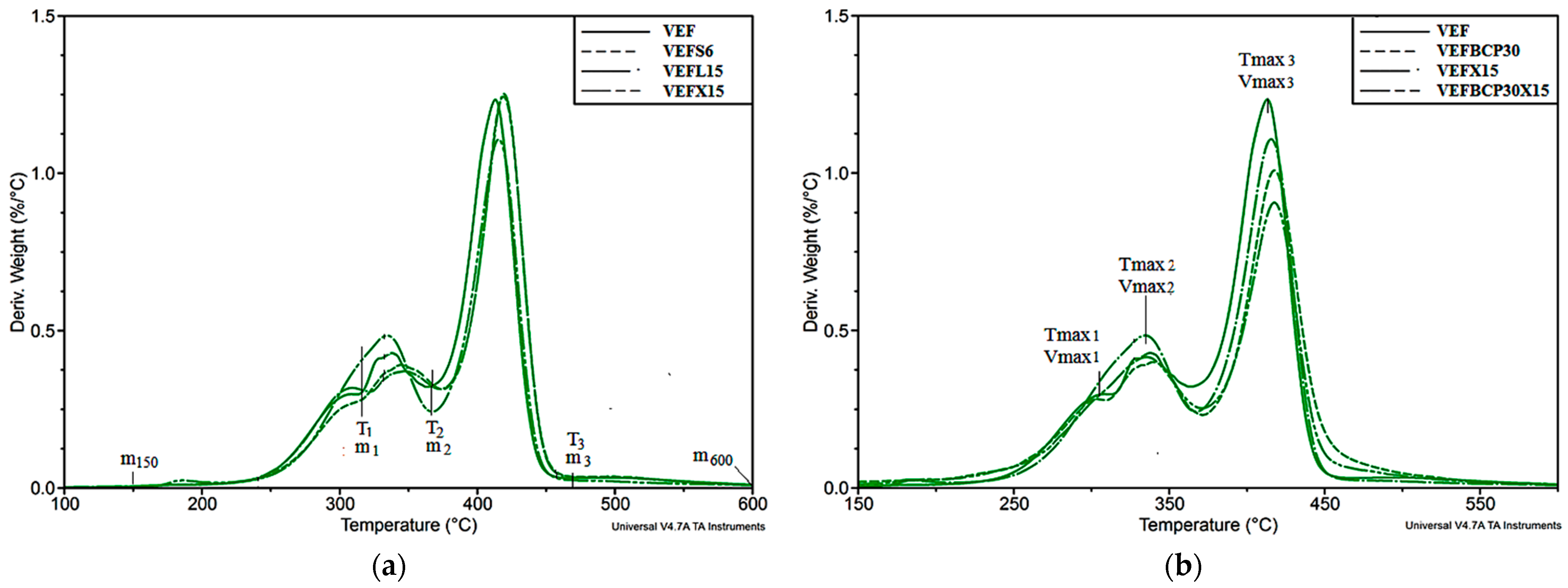
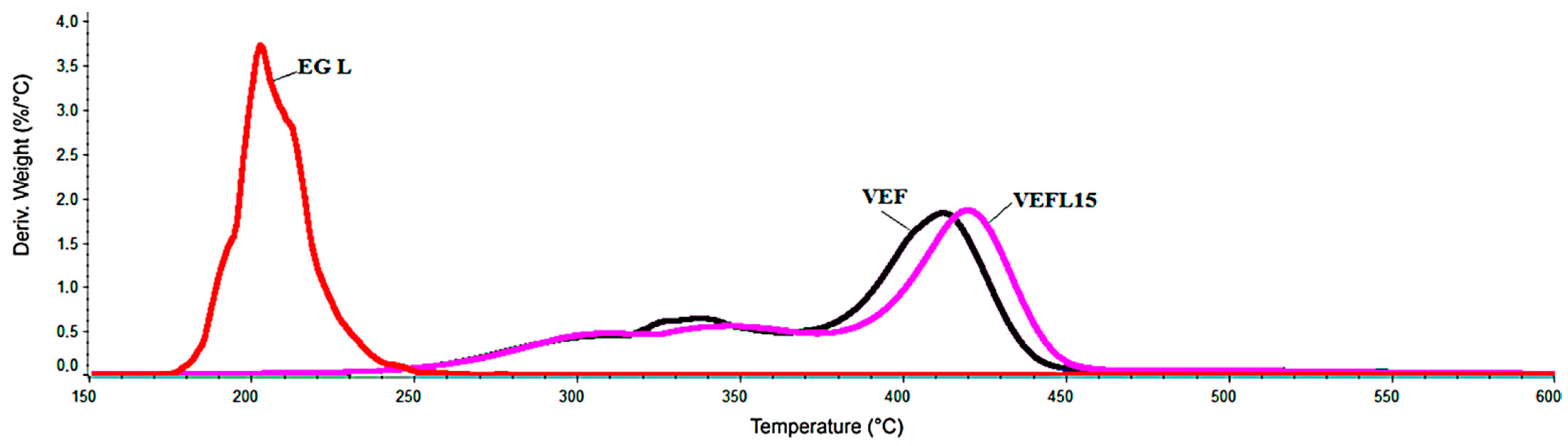
3.3. Thermogravimetric Analysis
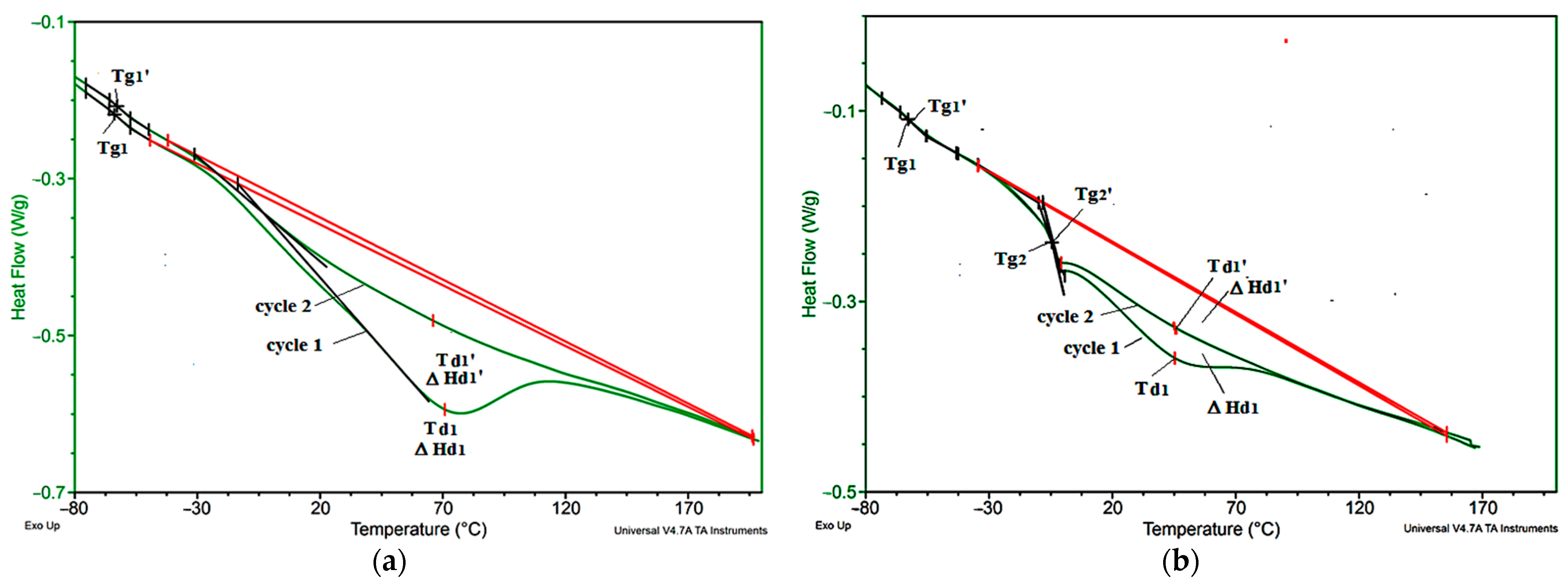
3.4. Thermophysical Properties
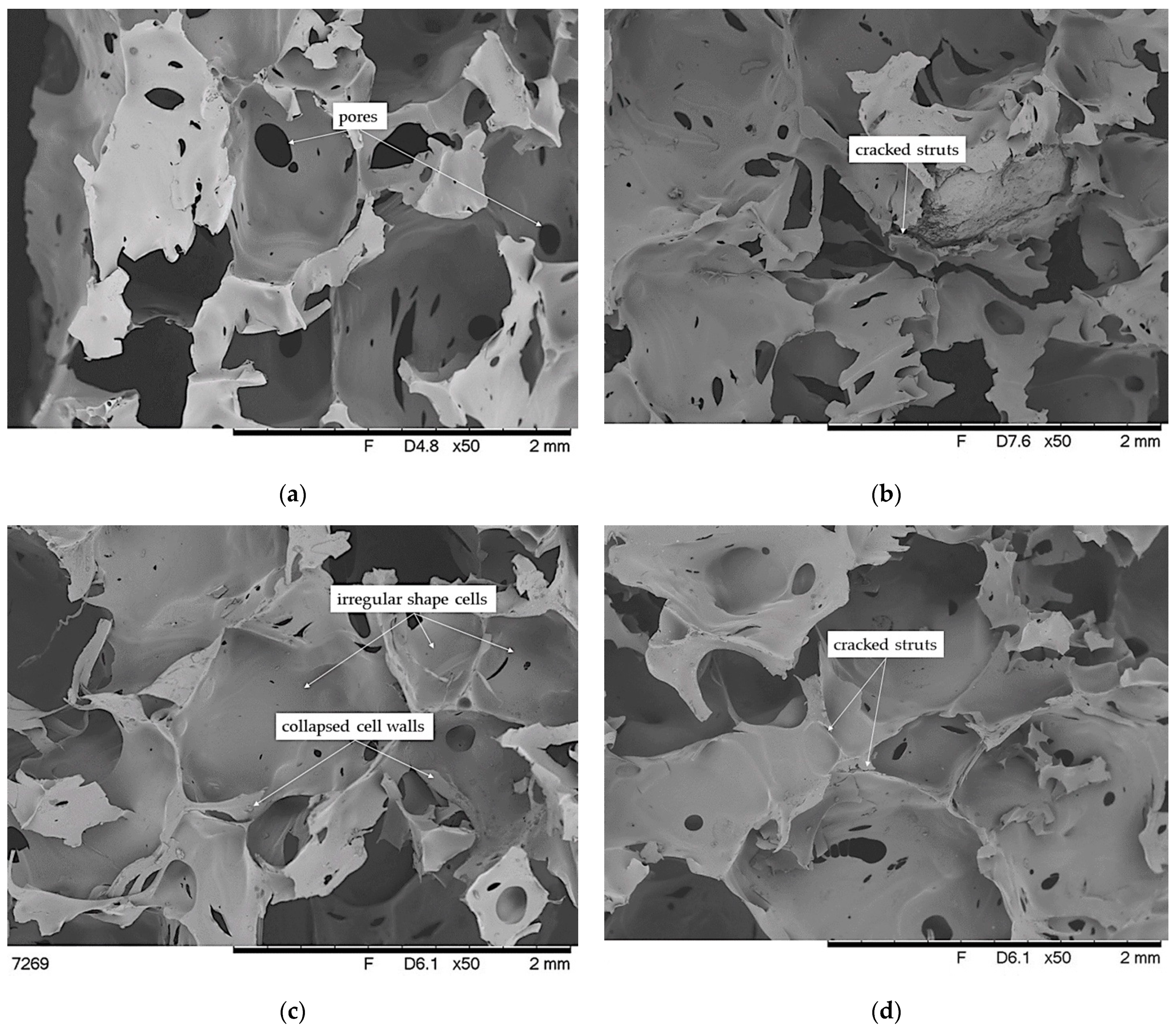
3.5. SEM Analysis of Brittle Fracture Surface of VEF Composites
3.6. Mechanical Properties of VEF Composites
3.7. Flammability of VEF Composites
3.7.1. Limiting Oxygen Index and UL94
3.7.2. MLC Analysis
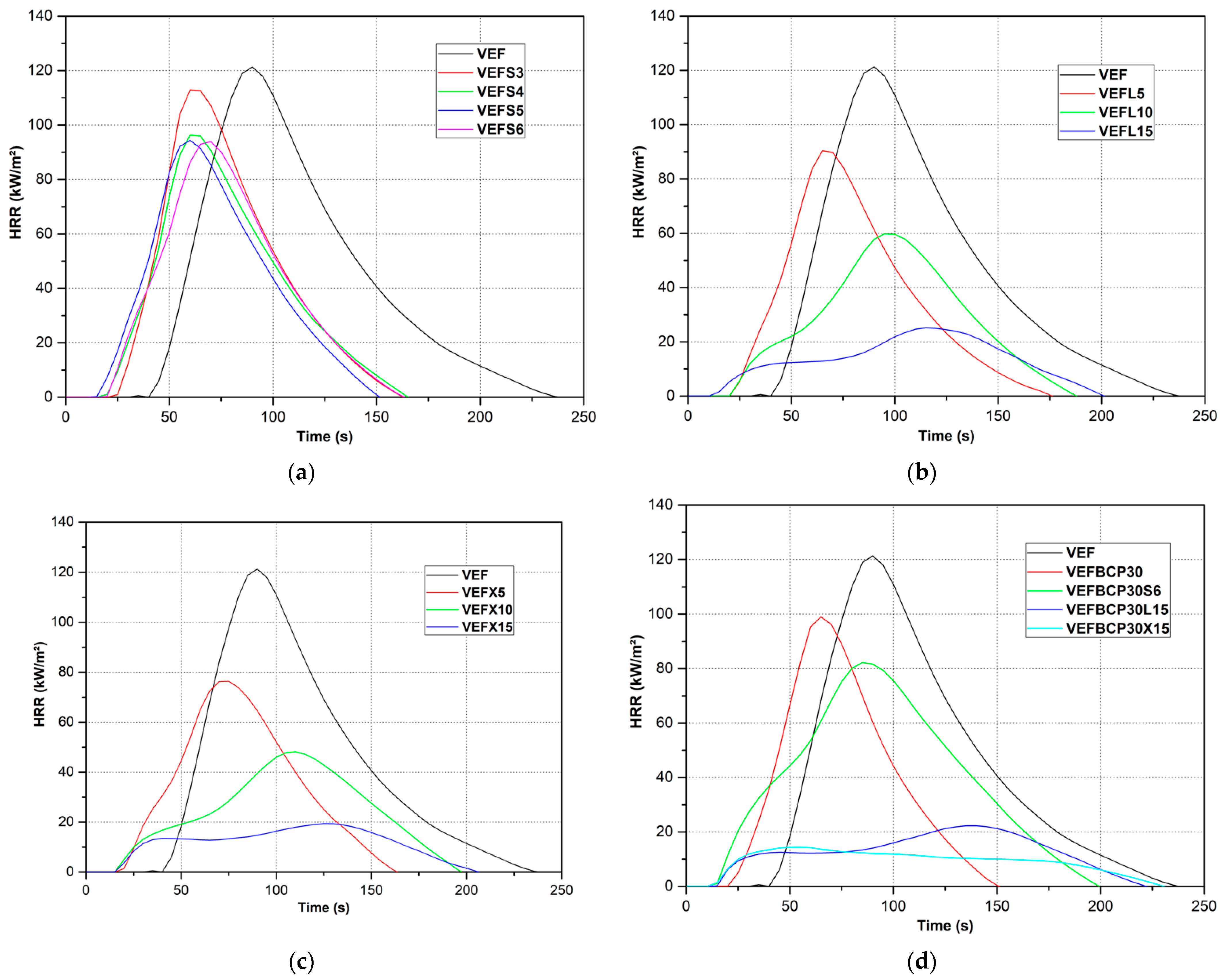
3.7.3. Fire Growth Rate Index
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- McKenna, S.T.; Hull, T.R. The Fire Toxicity of Polyurethane Foams. Fire Sci. Rev. 2016, 5, 1–27. [Google Scholar] [CrossRef]
- Rao, W.-H.; Zhu, Z.-M.; Wang, S.-X.; Wang, T.; Tan, Y.; Liao, W.; Zhao, H.-B.; Wang, Y.-Z. A Reactive Phosphorus-Containing Polyol Incorporated into Flexible Polyurethane Foam: Self-Extinguishing Behavior and Mechanism. Polym. Degrad. Stab. 2018, 153, 192–200. [Google Scholar] [CrossRef]
- Flame Retardants Used in Flexible Polyurethane Foam: An Alternatives Assessment Update; EPA: Washington, DC, USA, 2015; Volumes 1–6, pp. 1–76.
- Gosz, K.; Haponiuk, J.; Piszczyk, Ł. The Influence of Substitution of a Phosphorus-Containing Polyol with the Bio-Polyol on the Properties of Bio-Based PUR/PIR Foams. J. Polym. Environ. 2018, 26, 3877–3888. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The Study of Mechanical Behavior and Flame Retardancy of Castor Oil Phosphate-Based Rigid Polyurethane Foam Composites Containing Expanded Graphite and Triethyl Phosphate. Polym. Degrad. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and Nitrogen-Containing Polyols: Synergistic Effect on the Thermal Property and Flame Retardancy of Rigid Polyurethane Foam Composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Gao, M. Thermal Degradation and Flame Retardant Mechanism of the Rigid Polyurethane Foam Including Functionalized Graphene Oxide. Polymers 2019, 11, 78. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, T.; Zou, B.; Hu, W.; Wang, B.; Zhan, J.; Ma, C.; Hu, Y. Synthesis of a Novel Liquid Phosphorus-Containing Flame Retardant for Flexible Polyurethane Foam: Combustion Behaviors and Thermal Properties. Polym. Degrad. Stab. 2020, 171, 109029. [Google Scholar] [CrossRef]
- Tang, G.; Zhou, L.; Zhang, P.; Han, Z.; Chen, D.; Liu, X.; Zhou, Z. Effect of Aluminum Diethylphosphinate on Flame Retardant and Thermal Properties of Rigid Polyurethane Foam Composites. J. Anal. Calorim. 2020, 140, 625–636. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Gao, J.; Zhou, Z.; Tang, G.; Wang, C. Research on Two Sides Horizontal Flame Spread over Rigid Polyurethane with Different Flame Retardants. J. Anal. Calorim. 2021. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Preparation of Flame-Retardant Rigid Polyurethane Foams by Combining Modified Melamine–Formaldehyde Resin and Phosphorus Flame Retardants. ACS Omega 2020, 5, 9658–9667. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Levchik, S.V.; Schartel, B. Bubbles and Collapses: Fire Phenomena of Flame-retarded Flexible Polyurethane Foams. Polym. Adv. Technol. 2020, pat.4939. [Google Scholar] [CrossRef]
- Xu, Z.; Duan, L.; Hou, Y.; Chu, F.; Jiang, S.; Hu, W.; Song, L. The Influence of Carbon-Encapsulated Transition Metal Oxide Microparticles on Reducing Toxic Gases Release and Smoke Suppression of Rigid Polyurethane Foam Composites. Compos. Part A Appl. Sci. Manuf. 2020, 131, 105815. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Liu, M.; Zhu, Q.; Liu, X.; Zhang, B.; Chen, D.; Liu, X.; Zhang, K.; Tang, G. Flame Retarded Rigid Polyurethane Foam Composites Based on Gel-Silica Microencapsulated Ammonium Polyphosphate. J. Sol-Gel. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Liu, D.; Hu, A. The Influence of Environmentally Friendly Flame Retardants on the Thermal Stability of Phase Change Polyurethane Foams. Materials 2020, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Qian, L.; Xu, B.; Xi, W. Addition Flame-Retardant Effect of Nonreactive Phosphonate and Expandable Graphite in Rigid Polyurethane Foams. J. Appl. Polym. Sci. 2018, 135, 45960. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, S.; Zhu, J.; Li, G.; Peng, X. Establishment of a Highly Efficient Flame-Retardant System for Rigid Polyurethane Foams Based on Bi-Phase Flame-Retardant Actions. RSC Adv. 2018, 8, 9985–9995. [Google Scholar] [CrossRef]
- Ma, S.; Xiao, Y.; Zhou, F.; Schartel, B.; Chan, Y.Y.; Korobeinichev, O.P.; Trubachev, S.A.; Hu, W.; Ma, C.; Hu, Y. Effects of Novel Phosphorus-Nitrogen-Containing DOPO Derivative Salts on Mechanical Properties. Thermal Stability and Flame Retardancy of Flexible Polyurethane Foam. Polym. Degrad. Stab. 2020, 177, 109160. [Google Scholar] [CrossRef]
- Chao, C.; Gao, M.; Chen, S. Expanded Graphite: Borax Synergism in the Flame-Retardant Flexible Polyurethane Foams. J. Anal. Calorim. 2018, 131, 71–79. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Lv, H.-N.; Sun, J.; Cai, Z.-S. Flame Retardant and Thermal Decomposition Properties of Flexible Polyurethane Foams Filled with Several Halogen-Free Flame Retardants. Polym. Eng. Sci. 2014, 54, 2497–2507. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid Polyurethane Foams with Halogen-free Flame Retardants: Thermal Insulation, Mechanical, and Flame Retardant Properties. J. Appl. Polym. Sci. 2020, 137, 47611. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Li, L. Flame Retardant Behavior of Ternary Synergistic Systems in Rigid Polyurethane Foams. Polymers 2019, 11, 207. [Google Scholar] [CrossRef]
- Yue, X.; Li, C.; Ni, Y.; Xu, Y.; Wang, J. Flame Retardant Nanocomposites Based on 2D Layered Nanomaterials: A Review. J. Mater. Sci. 2019, 54, 13070–13105. [Google Scholar] [CrossRef]
- Brannum, D.J.; Price, E.J.; Villamil, D.; Kozawa, S.; Brannum, M.; Berry, C.; Semco, R.; Wnek, G.E. Flame-Retardant Polyurethane Foams: One-Pot, Bioinspired Silica Nanoparticle Coating. ACS Appl. Polym. Mater. 2019, 1, 2015–2022. [Google Scholar] [CrossRef]
- Han, S.; Zhu, X.; Chen, F.; Chen, S.; Liu, H. Flame-Retardant System for Rigid Polyurethane Foams Based on Diethyl Bis(2-Hydroxyethyl)Aminomethylphosphonate and in-Situ Exfoliated Clay. Polym. Degrad. Stab. 2020, 177, 109178. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Kremensas, A. Melamine, Silica, and Ionic Liquid as a Novel Flame Retardant for Rigid Polyurethane Foams with Enhanced Flame Retardancy and Mechanical Properties. Polym. Test. 2020, 87, 106511. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, K.; Jin, X.; Sun, S.; Tian, Y.; Wang, F.; Liu, P.; Yang, M. Investigation of Flame Retardant Flexible Polyurethane Foams Containing DOPO Immobilized Titanium Dioxide Nanoparticles. Polymers 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z. High Performance Nano-Zinc Amino-Tris-(Methylenephosphonate) in Rigid Polyurethane Foam with Improved Mechanical Strength, Thermal Stability and Flame Retardancy. Polym. Degrad. Stab. 2018, 154, 62–72. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, Q.; Zhao, Y.; Gao, S.; Zhang, Z.; He, Y. Novel MoS2–DOPO Hybrid for Effective Enhancements on Flame Retardancy and Smoke Suppression of Flexible Polyurethane Foams. ACS Omega 2020, 5, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Gosz, K.; Haponiuk, J.; Mielewczyk-Gryń, A.; Piszczyk, Ł. Physico-mechanical Properties and Flammability of PUR/PIR Foams Containing Expandable Graphite Core-shell Composite Particles. Polym. Compos. 2019, 40, 3805–3813. [Google Scholar] [CrossRef]
- Li, Y.; Zou, J.; Zhou, S.; Chen, Y.; Zou, H.; Liang, M.; Luo, W. Effect of Expandable Graphite Particle Size on the Flame Retardant, Mechanical, and Thermal Properties of Water-Blown Semi-Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- de Souza, F.M.; Choi, J.; Bhoyate, S.; Kahol, P.K.; Gupta, R.K. Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams. C J. Carbon Res. 2020, 6, 27. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Dittrich, B.; Schartel, B.; Roso, M.; Modesti, M. Expandable Graphite in Polyurethane Foams: The Effect of Expansion Volume and Intercalants on Flame Retardancy. J. Appl. Polym. Sci. 2017, 134, 45173. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, H.; Tian, H.; Wu, J.; Xiang, A.; Wang, C.; Ma, S.; Wu, Q. Mechanical and Flame-resistance Properties of Polyurethane-imide Foams with Different-sized Expandable Graphite. Polym. Eng. Sci. 2020, 60, 2324–2332. [Google Scholar] [CrossRef]
- Li, J.; Mo, X.; Li, Y.; Zou, H.; Liang, M.; Chen, Y. Influence of Expandable Graphite Particle Size on the Synergy Flame Retardant Property between Expandable Graphite and Ammonium Polyphosphate in Semi-Rigid Polyurethane Foam. Polym. Bull. 2018, 75, 5287–5304. [Google Scholar] [CrossRef]
- Pang, X.-Y.; Xin, Y.-P.; Shi, X.-Z.; Xu, J.-X. Effect of different size-modified expandable graphite and ammonium polyphosphate on the flame retardancy, thermal stability, physical, and mechanical properties of rigid polyurethane foam. Polym. Eng. Sci. 2019, 59, 1381–1394. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Cabulis, U.; Kirpluks, M.; Ryszkowska, J.; Auguścik, M. Innovative Porous Polyurethane-Polyisocyanurate Foams Based on Rapeseed Oil and Modified with Expandable Graphite. Ind. Crops Prod. 2017, 95, 316–323. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, G.; Zhou, Y.; Hu, L.; Feng, G.; Zhang, M. Synergistic Effect of Expandable Graphite, Diethyl Ethylphosphonate and Organically-Modified Layered Double Hydroxide on Flame Retardancy and Fire Behavior of Polyisocyanurate-Polyurethane Foam Nanocomposite. Polym. Degrad. Stab. 2014, 101, 92–101. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Synergistic Effects of Expandable Graphite and Ammonium Pentaborate Octahydrate on the Flame-retardant, Thermal Insulation, and Mechanical Properties of Rigid Polyurethane Foam. Polym. Compos. 2020, 41, 1749–1762. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Shi, Y.; Rao, X.; Cai, S.; Fu, L.; Feng, Y.; Wang, L.; Zheng, X.; Yang, W. Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite. Molecules 2020, 25, 4741. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, Y.; Li, X.; Shao, H.; Xu, W.; Li, D. A Facile, Environmentally and Friendly Flame-Retardant: Synergistic Flame Retardant Property of Polyurethane Rigid Foam. Mater. Lett. 2020, 267, 127542. [Google Scholar] [CrossRef]
- Thi, N.H.; Pham, D.L.; Hanh, N.T.; Oanh, H.T.; Yen Duong, T.H.; Nguyen, T.N.; Tuyen, N.D.; Phan, D.L.; Trinh, H.T.; Nguyen, H.T.; et al. Influence of Organoclay on the Flame Retardancy and Thermal Insulation Property of Expandable Graphite/Polyurethane Foam. J. Chem. 2019, 1–8. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z. Synergistic effect of nano magnesium amino-tris-(methylenephosphonate) and expandable graphite on improving flame retardant, mechanical and thermal insulating properties of rigid polyurethane foam. Mater. Chem. Phys. 2018, 219, 318–327. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Zhu, M. Flame Retardant, Mechanical and Thermal Insulating Properties of Rigid Polyurethane Foam Modified by Nano Zirconium Amino-Tris-(Methylenephosphonate) and Expandable Graphite. Polym. Degrad. Stab. 2019, 170, 108997. [Google Scholar] [CrossRef]
- Asimakopoulou, E.; Zhang, J.; Mckee, M.; Wieczorek, K.; Krawczyk, A.; Andolfo, M.; Kozlecki, T.; Scatto, M.; Sisani, M.; Bastianini, M.; et al. Effect of Layered Double Hydroxide, Expanded Graphite and Ammonium Polyphosphate Additives on Thermal Stability and Fire Performance of Polyisocyanurate Insulation Foam. Thermochim. Acta 2020, 693, 178724. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Wu, X. Construction of an Efficient Ternary Flame Retardant System for Rigid Polyurethane Foam Based on Bi-phase Flame Retardant Effect. Polym. Adv. Technol. 2020, 31, 3202–3210. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Y.; Guo, X.; Chen, L.; Jia, D. The Synergistic Effect of Ionic Liquid-Modified Expandable Graphite and Intumescent Flame-Retardant on Flame-Retardant Rigid Polyurethane Foams. Materials 2020, 13, 3095. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-Based Polyurethane Prepared from Kraft Lignin and Modified Castor Oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Acuña, P.; Zhang, J.; Yin, G.-Z.; Liu, X.-Q.; Wang, D.-Y. Bio-Based Rigid Polyurethane Foam from Castor Oil with Excellent Flame Retardancy and High Insulation Capacity via Cooperation with Carbon-Based Materials. J. Mater. Sci. 2021, 56, 2684–2701. [Google Scholar] [CrossRef]
- Ribeiro da Silva, V.; Mosiewicki, M.A.; Yoshida, M.I.; Coelho da Silva, M.; Stefani, P.M.; Marcovich, N.E. Polyurethane Foams Based on Modified Tung Oil and Reinforced with Rice Husk Ash I: Synthesis and Physical Chemical Characterization. Polym. Test. 2013, 32, 438–445. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Jia, P.; Bo, C.; Zhang, M.; Hu, L.; Zhou, Y. Phosphorus-Containing Tung Oil-Based Siloxane Toughened Phenolic Foam with Good Mechanical Properties, Fire Performance and Low Thermal Conductivity. Mater. Des. 2020, 192, 108668. [Google Scholar] [CrossRef]
- Kairyt, A.; Vejelis, S. Evaluation of forming mixture composition impact on properties of water blown rigid polyurethane (PUR) foam from rapeseed oil polyol. Ind. Crops Prod. 2015, 66, 210–215. [Google Scholar] [CrossRef]
- Kuranska, M.; Prociak, A.; Michalowski, S.; Cabulis, U.; Kirpluks, M. Microcellulose as a natural filler in polyurethane foams based on the biopolyol from rapeseed oil. Polimery 2016, 61, 625–632. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. A Solvent-Free and Scalable Method to Prepare Soybean-Oil-Based Polyols by Thiol–Ene Photo-Click Reaction and Biobased Polyurethanes Therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly Flame-Retardant Polyurethane Foam Based on Reactive Phosphorus Polyol and Limonene-Based Polyol: Research Article. J. Appl. Polym. Sci. 2018, 135, 46224. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Highly Flame Retardant and Bio-Based Rigid Polyurethane Foams Derived from Orange Peel Oil. Polym. Eng. Sci. 2018, 58, 2078–2087. [Google Scholar] [CrossRef]
- Otto, G.P.; Moisés, M.P.; Carvalho, G.; Rinaldi, A.W.; Garcia, J.C.; Radovanovic, E.; Fávaro, S.L. Mechanical Properties of a Polyurethane Hybrid Composite with Natural Lignocellulosic Fibers. Compos. Part B Eng. 2017, 110, 459–465. [Google Scholar] [CrossRef]
- Rodrigues Pereira de Paula, C.; Trianoski, R.; Azevedo, E. Development and Characterization of Sawdust and Sisal Fiber Reinforced Vegetable Based Polyurethane Foam Hybrid Composites. J. Nat. Fibers 2020, 1–10. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and Applicational Evaluation of the Rigid Polyurethane Foam Composites with Egg Shell Waste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Oushabi, A.; Sair, S.; Abboud, Y.; Tanane, O.; Bouari, A.E. An Experimental Investigation on Morphological, Mechanical and Thermal Properties of Date Palm Particles Reinforced Polyurethane Composites as New Ecological Insulating Materials in Building. Case Stud. Constr. Mater. 2017, 7, 128–137. [Google Scholar] [CrossRef]
- Bryśkiewicz, A.; Zieleniewska, M.; Przyjemska, K.; Chojnacki, P.; Ryszkowska, J. Modification of Flexible Polyurethane Foams by the Addition of Natural Origin Fillers. Polym. Degrad. Stab. 2016, 132, 32–40. [Google Scholar] [CrossRef]
- Antunes, M. Esparto Wool as Reinforcement in Hybrid Polyurethane Composite Foams. Ind. Crops Prod. 2011, 34, 1641–1648. [Google Scholar] [CrossRef]
- Olcay, H.; Kocak, E.D. Rice Plant Waste Reinforced Polyurethane Composites for Use as the Acoustic Absorption Material. Appl. Acoust. 2021, 173, 107733. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, Q.; Liu, H.; Guo, W.; Jiang, Y.; Chen, L.; He, L.; Qi, J.; Xiao, H.; Chen, Y.; et al. Renewable Natural Resources Reinforced Polyurethane Foam for Use of Lightweight Thermal Insulation. Mater. Res. Express 2020, 7, 055302. [Google Scholar] [CrossRef]
- Choe, H.; Sung, G.; Kim, J.H. Chemical Treatment of Wood Fibers to Enhance the Sound Absorption Coefficient of Flexible Polyurethane Composite Foams. Compos. Sci. Technol. 2018, 156, 19–27. [Google Scholar] [CrossRef]
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Polyurethane Hybrid Composites Reinforced with Lavender Residue Functionalized with Kaolinite and Hydroxyapatite. Materials 2021, 14, 415. [Google Scholar] [CrossRef]
- Olcay, H.; Kocak, E.D. The Mechanical, Thermal and Sound Absorption Properties of Flexible Polyurethane Foam Composites Reinforced with Artichoke Stem Waste Fibers. J. Ind. Text. 2020, 152808372093419. [Google Scholar] [CrossRef]
- Soundhar, A.; Rajesh, M.; Jayakrishna, K.; Sultan, M.T.H.; Shah, A.U.M. Investigation on Mechanical Properties of Polyurethane Hybrid Nanocomposite Foams Reinforced with Roselle Fibers and Silica Nanoparticles. Nanocomposites 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Zanini, N.C.; Souza, A.G.; Barbosa, R.F.S.; Rosa, D.S.; Mulinari, D.R. A Novel Hybrid Polyurethane Composites with ZNO Particles and Sheath Palm Residues: Synergistic Effect. Polym. Compos. 2021, 42, 532–542. [Google Scholar] [CrossRef]
- Yun, G.W.; Lee, J.H.; Kim, S.H. Flame Retardant and Mechanical Properties of Expandable Graphite/Polyurethane Foam Composites Containing Iron Phosphonate Dopamine-Coated Cellulose. Polym. Compos. 2020, 41, 2816–2828. [Google Scholar] [CrossRef]
- Kuranska, M.; Prociak, A.; Cabulis, U.; Kirpiuks, M. Water-Blown Polyurethane-Polyisocyanurate Foams Based on Bio-Polyols with Wood Fibers. Polimery 2015, 61, 705–712. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Kinyanjui, J.M.; Sapochak, L. FTIR Analysis of Chemical Gradients in Thermally Processed Molded Polyurethane Foam. J. Cell. Plast. 2007, 43, 183–196. [Google Scholar] [CrossRef]
- Harikrishnan, G.; Singh, S.N.; Kiesel, E.; Macosko, C.W. Nanodispersions of carbon nanofiber for polyurethane foaming. Polymer 2010, 51, 3349–3353. [Google Scholar] [CrossRef]
- Heintz, A.M.; Duffy, D.J.; Nelson, C.M.; Hua, Y.; Hsu, S.L.; Suen, W.; Paul, C.W. A Spectroscopic Analysis of the Phase Evolution in Polyurethane Foams. Macromolecules 2005, 38, 9192–9199. [Google Scholar] [CrossRef]
- Schuur, M.; Noordover, B.; Gajmans, R. Polyurethane elastomers with amide chain extenders of uniform length. Polymer 2006, 47, 1091–1100. [Google Scholar] [CrossRef]
- Gama, N. Insights into the Physical Properties of Biobased Polyurethane/Expanded Graphite Composite Foams. Compos. Sci. Technol. 2017, 138, 24–31. [Google Scholar] [CrossRef]
- Mazurek, M.M.; Tomczyk, K.; Auguścik, M.; Ryszkowska, J.; Rokicki, G. Influence of the Soft Segment Length on the Properties of Water-Cured Poly(Carbonate-Urethane-Urea)s. Polym. Adv. Technol. 2015, 26, 57–67. [Google Scholar] [CrossRef]
- Bourbigot, S.; Bras, M.L.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal Stability and Flame Retardancy of Polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Saiani, A.; Rochas, C.; Eeckhaut, G.; Daunch, W.A.; Higgins, J.S. Origin of Multiple Melting Endotherms in a High Hard Block Content Polyurethane. 2. Structural Investigation. Macromolecules 2004, 37, 1411–1421. [Google Scholar] [CrossRef]
- Rightor, E.G.; Urquhart, S.G.; Hitchcock, A.P.; Ade, H.; Smith, A.P.; Mitchell, G.E.; Priester, R.D.; Aneja, A.; Appel, G.; Wilkes, G.; et al. Identification and Quantitation of Urea Precipitates in Flexible Polyurethane Foam Formulations by X-ray Spectromicroscopy. Macromolecules 2002, 35, 5873–5882. [Google Scholar] [CrossRef]
- Barksby, N.; Allen, G.L. Polyurethanes World Congress; Technomic: Lancaster, PA, USA, 1993; pp. 445–450. [Google Scholar]
- Wang, X.; Kalali, E.N.; Wang, D.-Y. Renewable Cardanol-Based Surfactant Modified Layered Double Hydroxide as a Flame Retardant for Epoxy Resin. ACS Sustain. Chem. Eng. 2015, 3, 3281–3290. [Google Scholar] [CrossRef]
- Wang, W.; Pan, H.; Yu, B.; Pan, Y.; Song, L.; Liew, K.M.; Hu, Y. Fabrication of Carbon Black Coated Flexible Polyurethane Foam for Significantly Improved Fire Safety. RSC Adv. 2015, 5, 55870–55878. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Pospiech, P.; Strzelec, K. Effects of Chemically Treated Eucalyptus Fibers on Mechanical, Thermal and Insulating Properties of Polyurethane Composite Foams. Materials 2020, 13, 1781. [Google Scholar] [CrossRef] [PubMed]
- Członka, S.; Strąkowska, A.; Kairytė, A.; Kremensas, A. Nutmeg Filler as a Natural Compound for the Production of Polyurethane Composite Foams with Antibacterial and Anti-Aging Properties. Polym. Test. 2020, 86, 106479. [Google Scholar] [CrossRef]
- Ribeiro da Silva, V.; Mosiewicki, M.A.; Yoshida, M.I.; Coelho da Silva, M.; Stefani, P.M.; Marcovich, N.E. Polyurethane Foams Based on Modified Tung Oil and Reinforced with Rice Husk Ash II: Mechanical Characterization. Polym. Test. 2013, 32, 665–672. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, Z.; Chen, Y.; Li, G.; Wang, L.; Tang, Y.; Pang, R.; Khan, Z.U.H.; Wan, P. Preparation and Characterization of Flame Retardant Polyurethane Foams Containing Phosphorus–Nitrogen-Functionalized Lignin. RSC Adv. 2014, 4, 55271–55279. [Google Scholar] [CrossRef]
- Malińska, K. Laboratoryjne wyznaczanie porowatości powietrznej materiałów przeznaczonych do kompostowania. Inżynieria Ochr. Sr. 2012, 15, 155–167. (In Polish) [Google Scholar]
- Zhang, Z.; Li, D.; Xu, M.; Li, B. Synthesis of a Novel Phosphorus and Nitrogen-Containing Flame Retardant and Its Application in Rigid Polyurethane Foam with Expandable Graphite. Polym. Degrad. Stab. 2020, 173, 109077. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials–interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Schartel, B.; Wilkie, C.A.; Camino, G. Recommendations on the scientific approach to polymer flame retardancy: Part 2—Concepts. J. Fire Sci. 2017, 35, 3–20. [Google Scholar] [CrossRef]


| Trade Name | Component | Manufacturer |
|---|---|---|
| Daltocel F442 | polyetherol with functionality 3, hydroxyl number 42 mg KOH/g, ethoxy (EO) group content approximately 76% | Huntsman, TX, USA |
| Daltocel F526 | polyetherol with functionality 3, hydroxyl number 126 mg KOH/g, molecular weight 1300 g/mol, EO group content over 70% | Huntsman, TX, USA |
| Rokopol M1170 | polyetherol with functionality 3, hydroxyl number 35 mg KOH/g, EO group content over 50% | PCC Rokita SA, Brzeg Dolny, Poland |
| Rokopol F3600 | polyetherol with functionality 3, hydroxyl number 48 mg KOH/g, molecular weight 3600 g/mol | PCC Rokita SA, Brzeg Dolny, Poland |
| Ongronat 4040 | Isocyanate: a commercial mixture of mixture 4,4′-diphenylmethane diisocyanate and o-(p-isocyanarobenzyl) phenylisocyanate and polyisocyanate polyphenylmethane, functionality 2, NCO groups content 32.4% | BorsodChem, Kazincbarcika, Hungary |
| Jeffcat DPA | gelation catalyst: (N-(3-dimethylaminopropyl)—N,N-diisopropanolamine) | Huntsman, TX, USA |
| Jeffcat ZF10 | foaming catalyst: (N,N,N′-trimethylethyl-N′-hydroxyethyl-bisaminoethyl ether) | Huntsman, TX, USA |
| Tegostab B4900 | surfactant: polyether modified polysiloxane, | Evonik Industries AG, Essen, Germany |
| Symbol | Type | Minimum Carbon Content, [%] | Particle Size, [mm] | Expansion, [mL/g] | Maximum Volatile Matter Content, [%] | Bulk Density, [g/cm3] |
|---|---|---|---|---|---|---|
| EG S | EG 096 | 96 | 0–0.15 | 40–80 | <10 | ~0.62 |
| EG L | EG 290 | 90 | 0.2–0.6 | 200–300 | <15 | ~0.64 |
| EG X | EG 399 | 99 | 0.4–1.0 | 250–400 | <15 | ~0.70 |
| Sample Symbol | Content in Polyol, php of Polyol | Content in Foam, wt. % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EG | BCP | EG | BCP | Matrix | |||||
| S | L | X | S | L | X | ||||
| VEF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100.00 |
| VEFS3 | 3 | 0 | 0 | 0 | 1.9 | 0 | 0 | 0 | 98.1 |
| VEFS4 | 4 | 0 | 0 | 0 | 2.5 | 0 | 0 | 0 | 97.5 |
| VEFS5 | 5 | 0 | 0 | 0 | 3.1 | 0 | 0 | 0 | 96.9 |
| VEFS6 | 6 | 0 | 0 | 0 | 3.7 | 0 | 0 | 0 | 96.3 |
| VEFL5 | 0 | 5 | 0 | 0 | 0 | 3.1 | 0 | 0 | 96.9 |
| VEFL10 | 0 | 10 | 0 | 0 | 0 | 6.0 | 0 | 0 | 94.0 |
| VEFL15 | 0 | 15 | 0 | 0 | 0 | 8.7 | 0 | 0 | 91.3 |
| VEFX5 | 0 | 0 | 5 | 0 | 0 | 0 | 3.1 | 0 | 96.9 |
| VEFX10 | 0 | 0 | 10 | 0 | 0 | 0 | 6.0 | 0 | 94.0 |
| VEFX15 | 0 | 0 | 15 | 0 | 0 | 0 | 8.7 | 0 | 91.3 |
| VEFBCP30 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 16.0 | 84.0 |
| VEFBCP30S6 | 6 | 0 | 0 | 30 | 3.1 | 0 | 0 | 15.5 | 81.4 |
| VEFBCP30L15 | 0 | 15 | 0 | 30 | 0 | 7.3 | 0 | 14.8 | 77.9 |
| VEFBCP30X15 | 0 | 0 | 15 | 30 | 0 | 0 | 7.3 | 14.8 | 77.9 |
| Sample Symbol | R | DPS | UA, [%] | UR, [%] |
|---|---|---|---|---|
| VEF | 1.02 | 0.50 | 38.2 | 61.8 |
| VEFS6 | 1.44 | 0.59 | 42.8 | 57.2 |
| VEFL15 | 1.24 | 0.55 | 45.2 | 54.8 |
| VEFX15 | 1.03 | 0.51 | 45.6 | 54.4 |
| VEFBCP30 | 1.22 | 0.54 | 44.6 | 55.4 |
| VEFBCP30S6 | 1.58 | 0.61 | 48.4 | 51.6 |
| VEFBCP30L15 | 1.81 | 0.64 | 48.9 | 51.1 |
| VEFBCP30X15 | 1.99 | 0.67 | 49.1 | 50.9 |
| Sample Symbol | m150, [%] | T5%, [°C] | Δm1, [%] | Tmax1, [°C] | Vmax1, [%/°C] | Δm2, [%] | Tmax2, [°C] | Vmax2, [%/°C] | Δm3, [%] | Tmax3, [°C] | Vmax3, [%/°C] | m600, [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VEF | 0.3 | 278 | 14.1 | 304 | 0.30 | 18.6 | 338 | 0.43 | 57.0 | 413 | 1.23 | 10.0 |
| VEFS3 | 0.3 | 284 | 15.5 | 311 | 0.29 | 17.4 | 345 | 0.37 | 55.6 | 418 | 1.26 | 11.2 |
| VEFS4 | 0.3 | 280 | 14.7 | 309 | 0.30 | 18.2 | 343 | 0.38 | 55.1 | 419 | 1.27 | 11.5 |
| VEFS5 | 0.3 | 280 | 13.2 | 305 | 0.26 | 19.8 | 344 | 0.39 | 54.9 | 419 | 1.24 | 11.7 |
| VEFS6 | 0.3 | 284 | 12.9 | 307 | 0.27 | 20.8 | 345 | 0.42 | 54.1 | 420 | 1.24 | 11.9 |
| VEFL5 | 0.3 | 282 | 11.2 | 310 | 0.25 | 21.8 | 348 | 0.37 | 55.3 | 420 | 1.25 | 11.7 |
| VEFL10 | 0.3 | 286 | 11.5 | 311 | 0.26 | 21.3 | 342 | 0.40 | 54.3 | 420 | 1.25 | 12.2 |
| VEFL15 | 0.3 | 284 | 11.9 | * | * | 19.8 | 339 | 0.48 | 55.1 | 418 | 1.17 | 12.8 |
| VEFX5 | 0.3 | 287 | 12.9 | 310 | 0.30 | 20.7 | 343 | 0.41 | 54.7 | 416 | 1.26 | 11.4 |
| VEFX10 | 0.3 | 284 | 13.7 | * | * | 20.7 | 339 | 0.48 | 51.8 | 416 | 1.18 | 13.5 |
| VEFX15 | 0.3 | 282 | 14.1 | * | * | 20.5 | 338 | 0.48 | 49.0 | 415 | 1.11 | 15.8 |
| BCP | 5.8 | 127 | 21.7 | 289 | 0.34 | 16.1 | 334 | 0.38 | 31.8 | 407 | 0.36 | 24.5 |
| VEFBCP30 | 1.6 | 252 | 14.3 | 302 | 0.28 | 20.3 | 340 | 0.40 | 45.2 | 418 | 1.01 | 12.7 |
| VEFBCP30S6 | 1.7 | 259 | 13.8 | * | * | 20.1 | 342 | 0.38 | 46.3 | 420 | 0.97 | 14.2 |
| VEFBCP30L15 | 1.7 | 249 | 13.5 | * | * | 20.6 | 331 | 0.41 | 45.5 | 416 | 0.96 | 15.6 |
| VEFBCP30X15 | 1.4 | 253 | 13.3 | * | * | 21.2 | 335 | 0.42 | 43.2 | 417 | 0.91 | 17.9 |
| Sample Symbol | Tg1 [°C] | Tg2 [°C] | Td1 [°C] | ΔHd1 [J/g] | Tg1′ [°C] | Tg2′ [°C] | Td1′ [°C] | ΔHd1′ [J/g] |
|---|---|---|---|---|---|---|---|---|
| VEF | −63.8 | - | 68.5 | 56.9 | −62.9 | - | 57.9 | 33.7 |
| VEFS3 | −62.9 | - | 54.6 | 52.7 | −63.2 | - | 61.2 | 40.7 |
| VEFS4 | −63.1 | - | 50.4 | 46.8 | −62.5 | - | 44.5 | 35.1 |
| VEFS5 | −62.5 | - | 60.1 | 46.7 | −62.6 | - | 43.4 | 33.2 |
| VEFS6 | −64.2 | - | 65.6 | 46.2 | −62.7 | - | 42.4 | 31.9 |
| VEFL5 | −63.1 | −6.0 | 46.3 | 39.6 | −63.1 | −5.9 | 43.8 | 30.2 |
| VEFL10 | −62.8 | −5.7 | 45.7 | 48.5 | −63.4 | −6.0 | 45.2 | 36.4 |
| VEFL15 | −62.8 | −5.6 | 53.8 | 55.4 | −63.3 | −6.0 | 52.3 | 43.9 |
| VEFX5 | −63.7 | −3.6 | 52.1 | 42.1 | −63.5 | −3.8 | 46.2 | 30.3 |
| VEFX10 | −62.3 | −3.5 | 52.0 | 41.1 | −62.4 | −4.7 | 45.9 | 30.2 |
| VEF X15 | −62.6 | −3.1 | 45.6 | 46.8 | −63.4 | −3.2 | 45.2 | 35.0 |
| VEFBCP30 | −65.5 | - | 100.1 | 60.4 | −64.6 | - | 63.2 | 28.6 |
| VEFBCP30S6 | −59.9 | - | 60.2 | 34.5 | −59.7 | - | 54.6 | 25.0 |
| VEFBCP30L15 | −59.6 | - | 75.3 | 75.4 | −59.0 | - | 70.4 | 70.5 |
| VEFBCP30X15 | −60.3 | - | 85.6 | 86.0 | −59.5 | - | 78.5 | 80.3 |
| Sample Symbol | D, [kg/m3] | H, [kPa] | Cf | ε50, [%] | ε75, [%] | ε90, [%] |
|---|---|---|---|---|---|---|
| VEF | 39.7 ± 0.5 | 2.28 ± 0.11 | 2.09 ± 0.06 | 2.1 ± 0.2 | 10.0 ± 3.5 | 85.2 ± 12.8 |
| VEFS3 | 42.7 ± 0.6 | 2.34 ± 0.18 | 2.24 ± 0.08 | 2.3 ± 0.4 | 12.5 ± 3.9 | 17.9 ± 3.6 |
| VEFS4 | 43.3 ± 0.5 | 2.33 ± 0.11 | 1.98 ± 0.06 | 1.8 ± 0.5 | 2.5 ± 0.9 | 12.4 ± 0.3 |
| VEFS5 | 43.7 ± 0.6 | 2.24 ± 0.10 | 2.23 ± 0.09 | 1.5 ± 0.3 | 3.1 ± 0.8 | 10.3 ± 0.6 |
| VEFS6 | 43.9 ± 0.4 | 2.42 ± 0.13 | 2.11 ± 0.08 | 0.8 ± 0.1 | 1.3 ± 0.3 | 3.7 ± 0.4 |
| VEFL5 | 44.4 ± 0.7 | 2.18 ± 0.08 | 2.26 ± 0.12 | 2.2 ± 0.1 | 2.4 ± 0.4 | 74.2 ± 5.6 |
| VEFL10 | 47.1 ± 0.9 | 2.32 ± 0.13 | 2.23 ± 0.12 | 2.8 ± 0.1 | 10.3 ± 1.1 | 26.2 ± 4.5 |
| VEFL15 | 48.9 ± 1.5 | 2.43 ± 0.12 | 2.24 ± 0.09 | 2.9 ± 0.2 | 10.5 ± 1.5 | 8.0 ± 0.9 |
| VEFX5 | 42.4 ± 0.9 | 2.19 ± 0.14 | 2.27 ± 0.08 | 5.9 ± 0.6 | 5.9 ± 0.9 | 8.9 ± 0.5 |
| VEFX10 | 43.9 ± 0.8 | 2.29 ± 0.13 | 2.29 ± 0.09 | 4.9 ± 0.5 | 9.9 ± 1.5 | 86.7 ± 9.8 |
| VEFX15 | 47.9 ± 0.6 | 2.46 ± 0.14 | 2.33 ± 0.07 | 4.2 ± 0.7 | 5.4 ± 0.6 | 68.6 ± 7.5 |
| VEFBCP30 | 52.2 ± 1.5 | 2.30 ± 0.18 | 2.60 ± 0.08 | 3.9 ± 0.5 | 6.2 ± 0.9 | 62.0 ± 7.2 |
| VEFBCP30S6 | 54.8 ± 1.4 | 2.10 ± 0.19 | 2.66 ± 0.09 | 1.8 ± 0.5 | 2.5 ± 0.9 | 82.4 ± 0.3 |
| VEFBCP30L15 | 57.5 ± 1.7 | 2.51 ± 0.17 | 2.59 ± 0.12 | 1.5 ± 0.3 | 3.1 ± 0.8 | 10.3 ± 0.6 |
| VEFBCP30X15 | 57.1 ± 2.0 | 2.65 ± 0.19 | 2.65 ± 0.10 | 0.8 ± 0.1 | 1.3 ± 0.3 | 3.7 ± 0.4 |
| Sample | LOI, [%] | UL 94 | |||
|---|---|---|---|---|---|
| Flammability Class | Loss of Weight [%] | Burning Time 1 [s] | Burning Speed [mm/min] 2 | ||
| VEF | 18.0 | HB75 | Burned | 103 | 43.5 |
| VEFS4 | 19.0 | HB75 | Burned | 64 | 70.3 |
| VEFL15 | 22.0 | HB40b | 18.3 | 10 | - |
| VEFX15 | 23.6 | HB40b | 21.0 | 10 | - |
| VEFBCP30 | 17.4 | HB75 | Burned | 70 | 64.3 |
| VEFBCP30L15 | 23.7 | HB40b | 23.7 | 15 | - |
| VEFBCP30X15 | 26.0 | HB40a | 21.3 | - | - |
| Sample Symbol | TTI [s] | THR [MJ/m2] | PML [%] | MLR [g/s] | HRR [kW/m2] | HRRm [kW/m2] | THRRm [s] | EHC [MJ/kg] | FIGRA [kW/m2s] |
|---|---|---|---|---|---|---|---|---|---|
| VEF | 36 | 6.4 | 92.0 | 0.079 | 79.6 | 122.8 | 90 | 8.7 | 1.36 |
| VEFS3 | 19 | 5.9 | 92.1 | 0.088 | 75.6 | 110.9 | 62 | 7.7 | 1.79 |
| VEFS4 | 14 | 6.8 | 91.8 | 0.058 | 54.7 | 95.6 | 62 | 9.0 | 1.54 |
| VEFS5 | 9 | 6.5 | 92.0 | 0.061 | 56.9 | 93.5 | 60 | 8.6 | 1.56 |
| VEFS6 | 14 | 4.8 | 91.5 | 0.054 | 59.0 | 91.1 | 70 | 6.9 | 1.30 |
| VEFL5 | 18 | 4.4 | 90.3 | 0.067 | 57.3 | 88.9 | 65 | 7.2 | 1.37 |
| VEFL10 | 16 | 5.0 | 84.4 | 0.041 | 40.8 | 63.5 | 97 | 8.2 | 0.65 |
| VEFL15 | 6 | 4.3 | 77.8 | 0.025 | 21.1 | 27.3 | 115 | 7.7 | 0.24 |
| VEFX5 | 13 | 6.0 | 89.9 | 0.048 | 45.3 | 78.7 | 73 | 8.1 | 1.08 |
| VEFX10 | 11 | 6.2 | 82.5 | 0.034 | 30.1 | 47.7 | 109 | 8.3 | 0.44 |
| VEFX15 | 16 | 3.4 | 72.2 | 0.022 | 14.0 | 18.5 | 125 | 5.7 | 0.15 |
| VEFBCP30 | 21 | 9.7 | 92.7 | 0.065 | 84.2 | 124.0 | 65 | 11.0 | 1.91 |
| VEFBCP30S6 | 8 | 9.0 | 87.8 | 0.051 | 52.3 | 86.0 | 85 | 9.1 | 1.01 |
| VEFBCP30L15 | 9 | 5.0 | 76.5 | 0.025 | 15.5 | 21.2 | 137 | 5.8 | 0.15 |
| VEFBCP30X15 | 7 | 2.9 | 63.4 | 0.020 | 11.4 | 15.5 | 54 | 4.6 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliwa, R.; Ryszkowska, J.; Oleksy, M.; Auguścik-Królikowska, M.; Gzik, M.; Bartoń, J.; Budzik, G. Effects of Various Types of Expandable Graphite and Blackcurrant Pomace on the Properties of Viscoelastic Polyurethane Foams. Materials 2021, 14, 1801. https://doi.org/10.3390/ma14071801
Oliwa R, Ryszkowska J, Oleksy M, Auguścik-Królikowska M, Gzik M, Bartoń J, Budzik G. Effects of Various Types of Expandable Graphite and Blackcurrant Pomace on the Properties of Viscoelastic Polyurethane Foams. Materials. 2021; 14(7):1801. https://doi.org/10.3390/ma14071801
Chicago/Turabian StyleOliwa, Rafał, Joanna Ryszkowska, Mariusz Oleksy, Monika Auguścik-Królikowska, Małgorzata Gzik, Joanna Bartoń, and Grzegorz Budzik. 2021. "Effects of Various Types of Expandable Graphite and Blackcurrant Pomace on the Properties of Viscoelastic Polyurethane Foams" Materials 14, no. 7: 1801. https://doi.org/10.3390/ma14071801
APA StyleOliwa, R., Ryszkowska, J., Oleksy, M., Auguścik-Królikowska, M., Gzik, M., Bartoń, J., & Budzik, G. (2021). Effects of Various Types of Expandable Graphite and Blackcurrant Pomace on the Properties of Viscoelastic Polyurethane Foams. Materials, 14(7), 1801. https://doi.org/10.3390/ma14071801










