Magnetoelectric Multiferroicity and Magnetic Anisotropy in Guanidinium Copper(II) Formate Crystal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
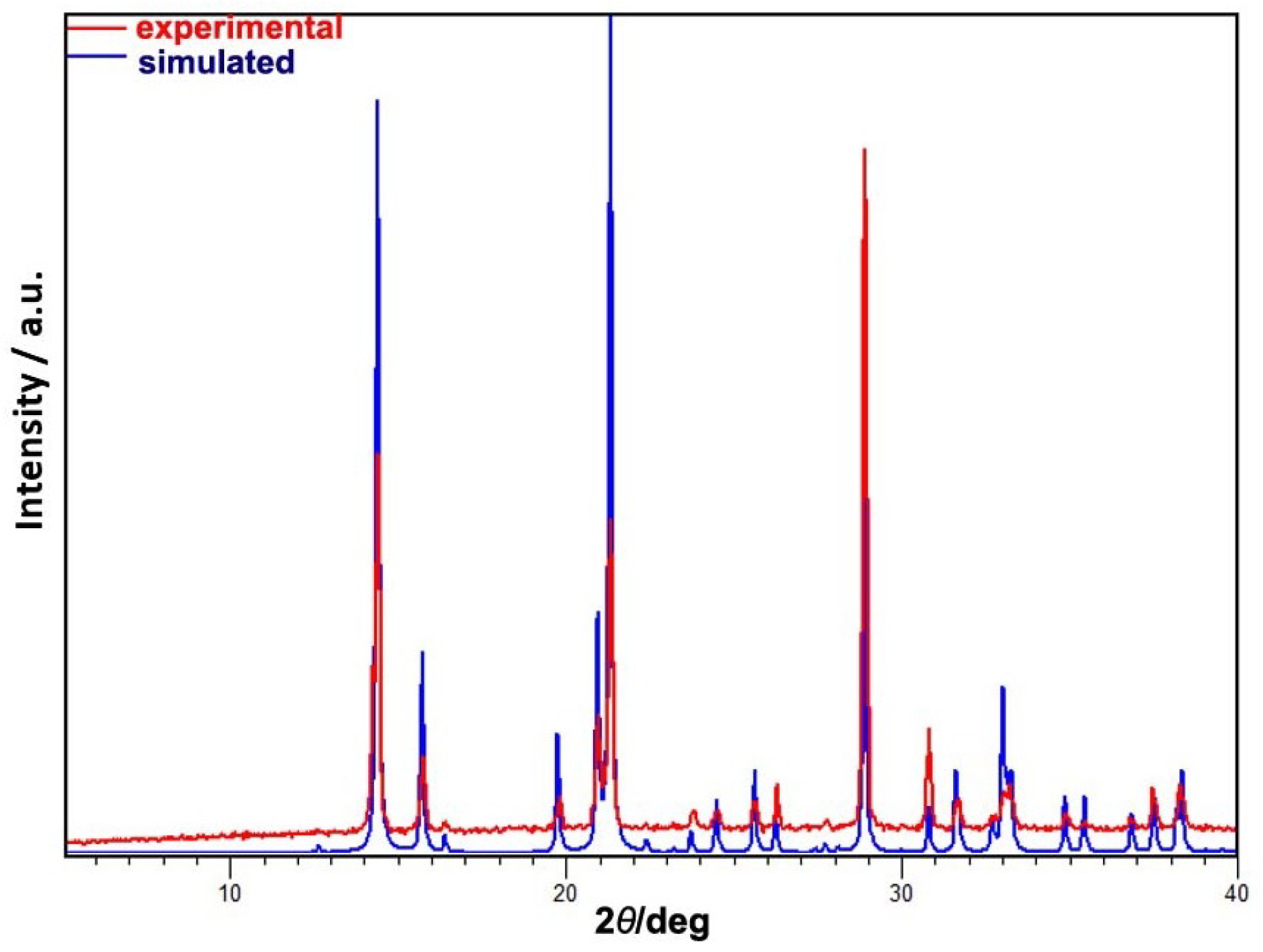
2.2. Single-Crystal X-ray Diffraction
2.3. Density Functional Theory (DFT) Calculations
2.4. Magnetic Measurements
2.5. Magneto-Electric Measurements
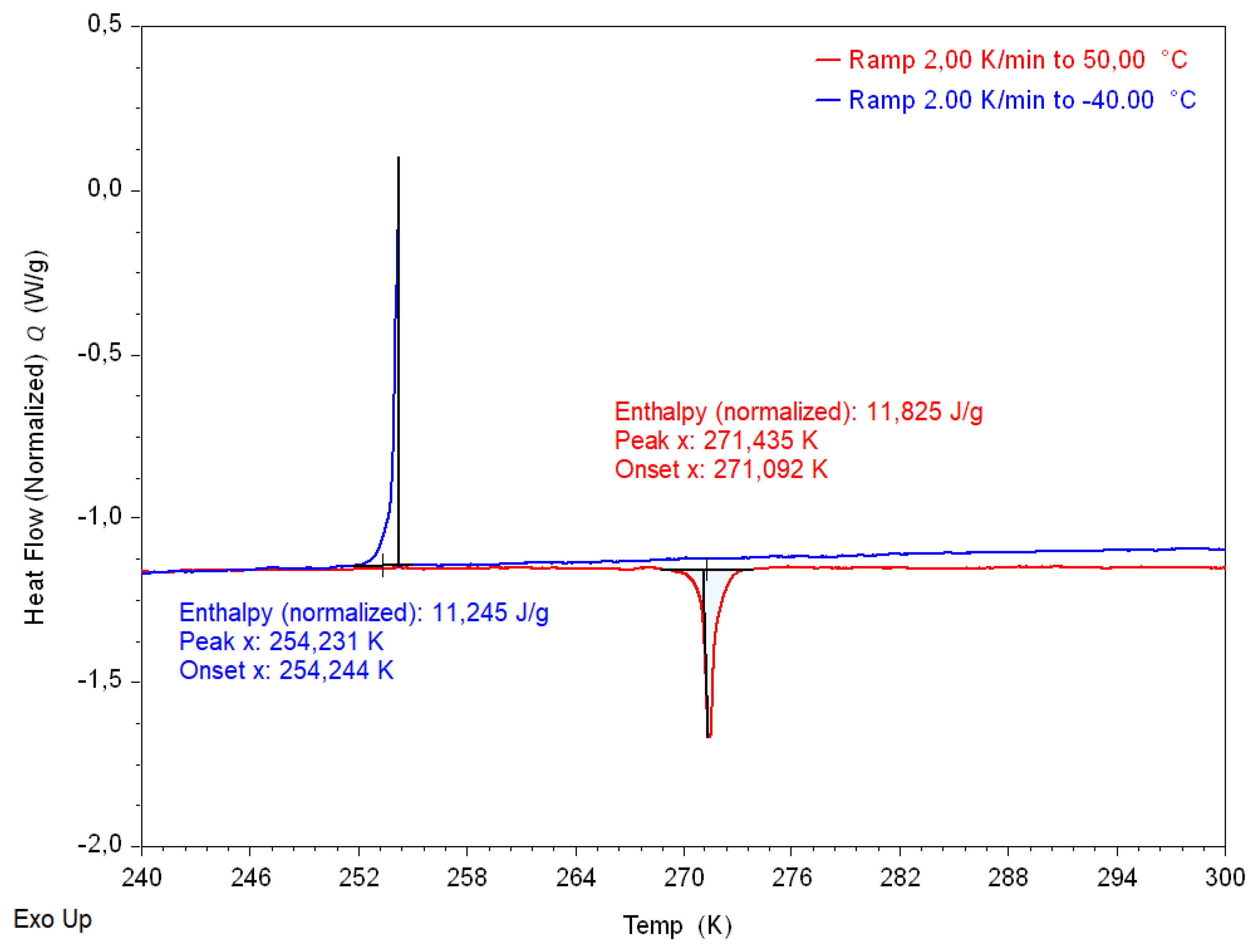
2.6. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
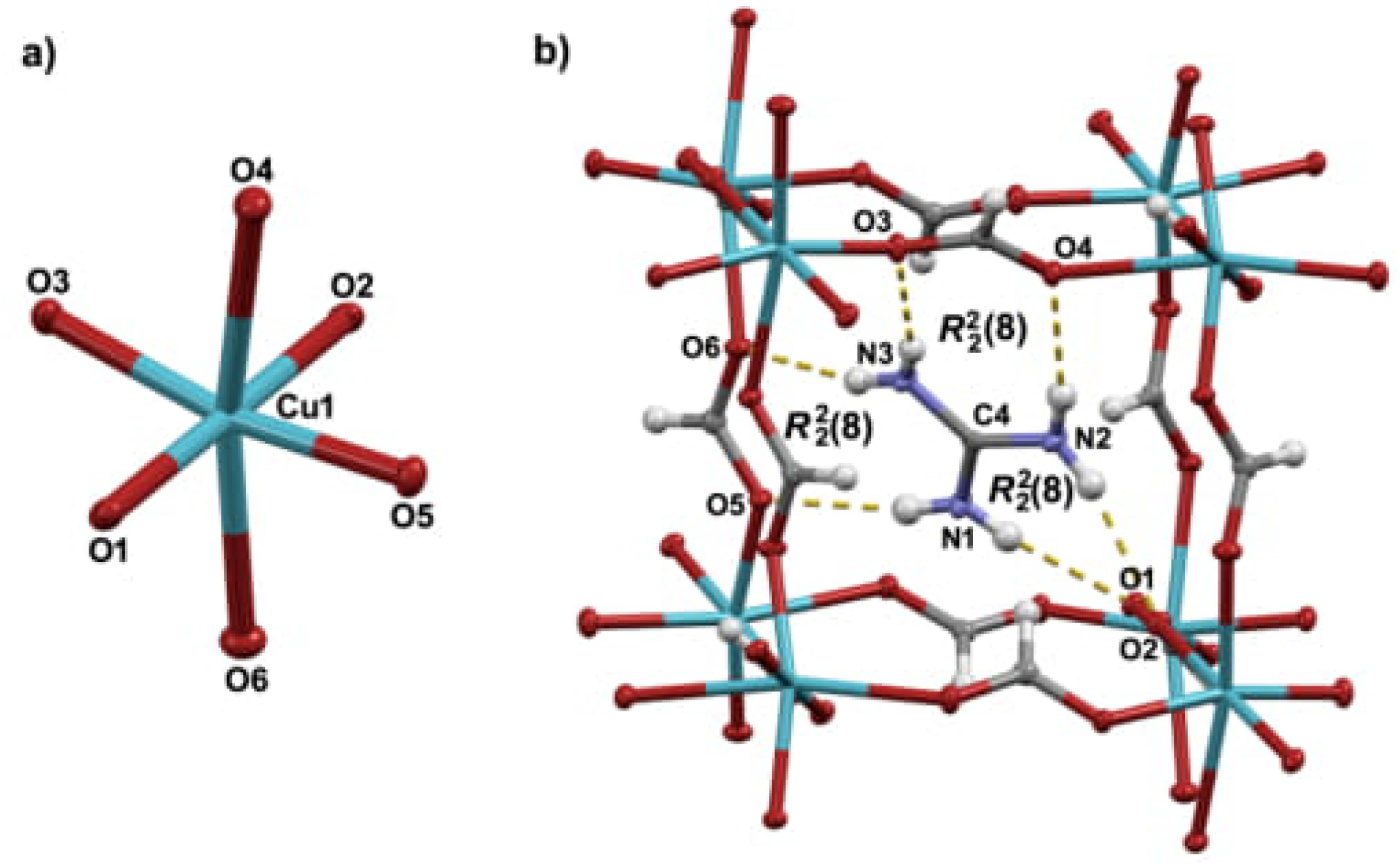
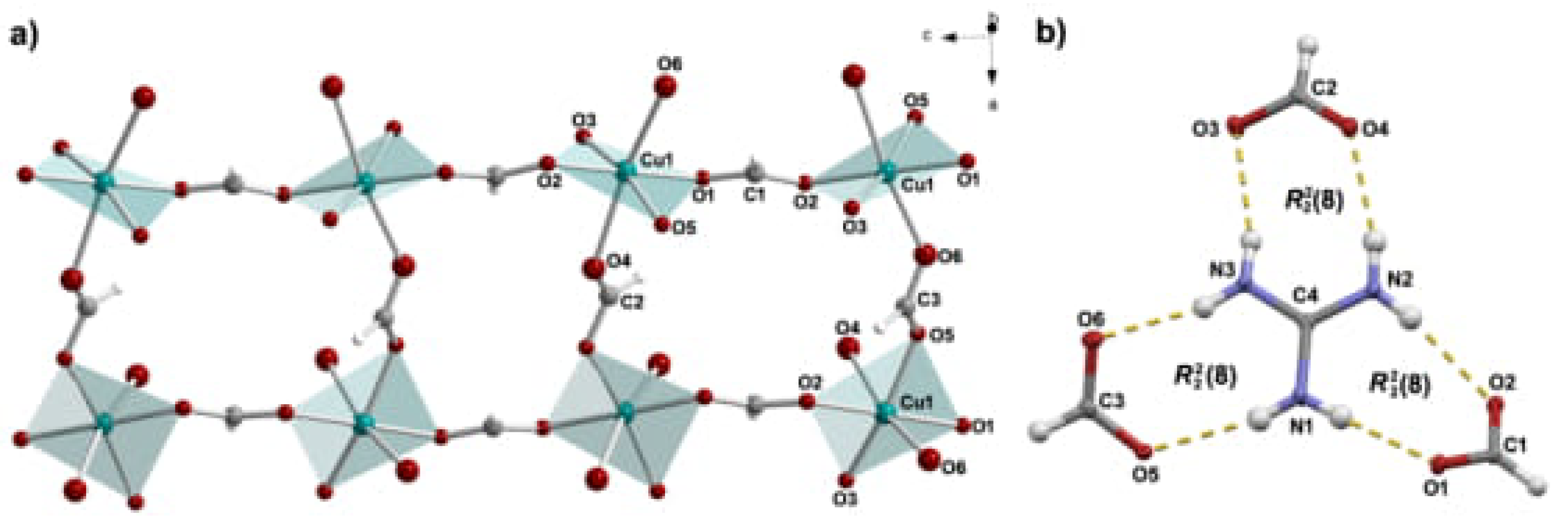
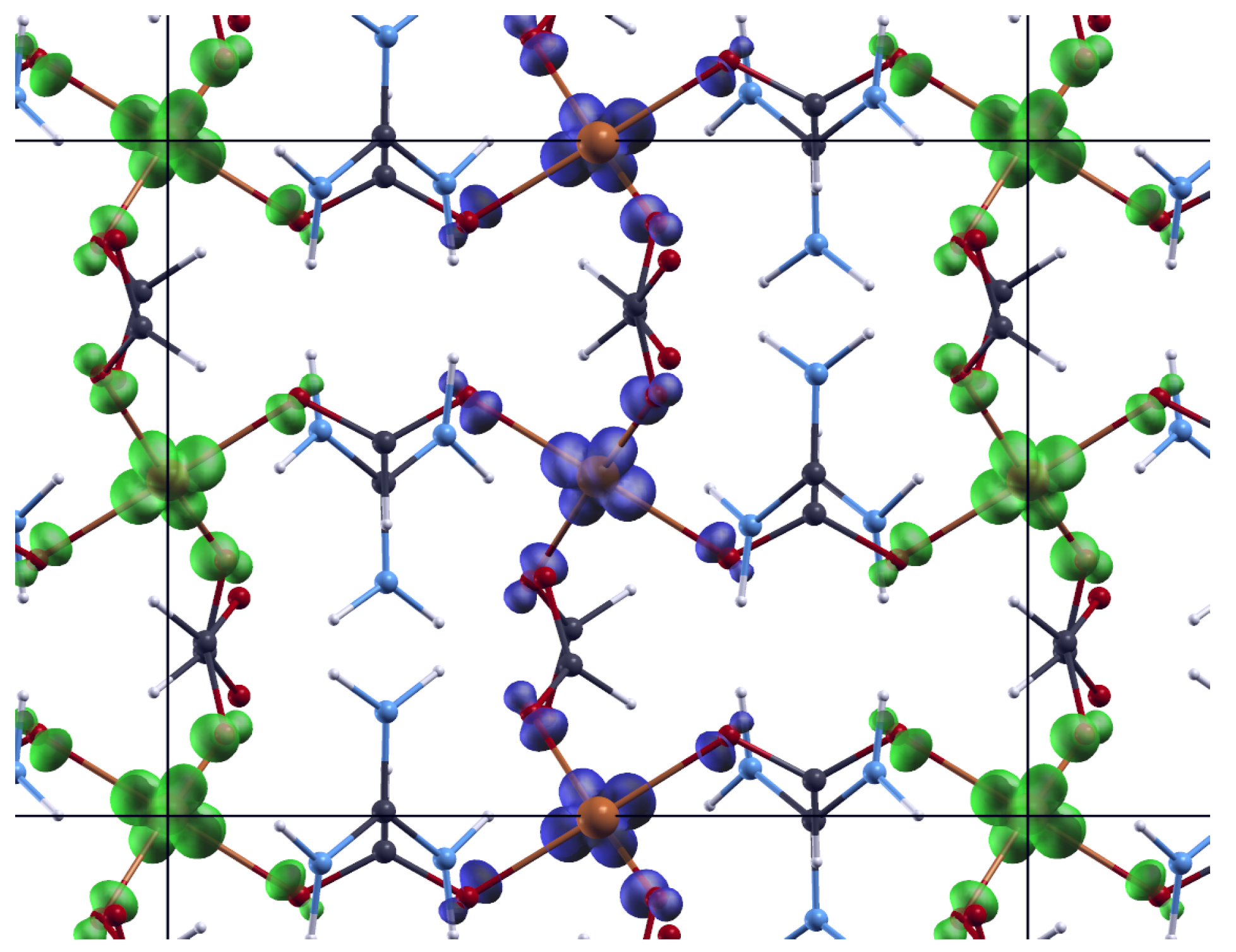
3.1. Molecular and Crystal Structure
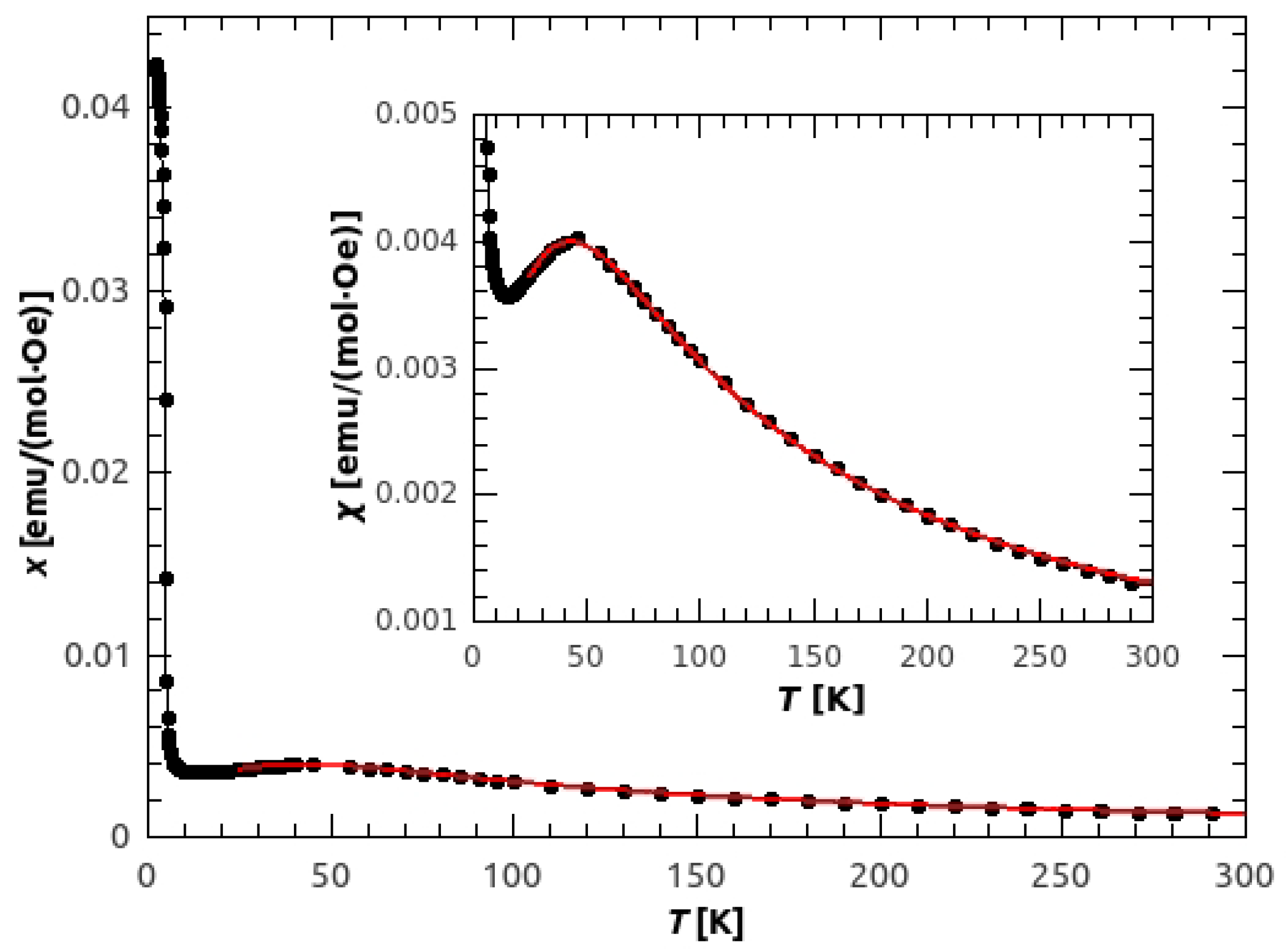
3.2. Magnetic Susceptibility
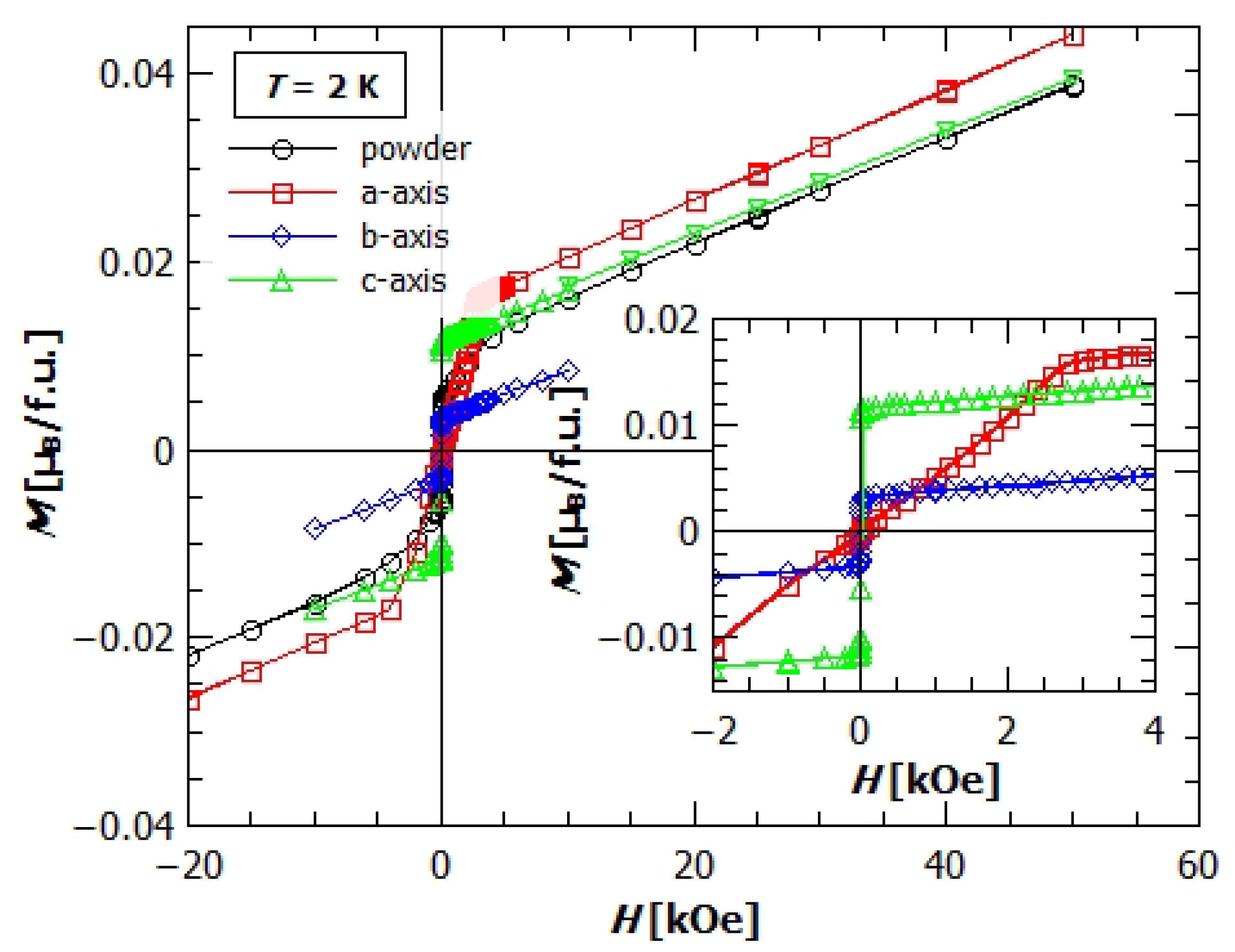
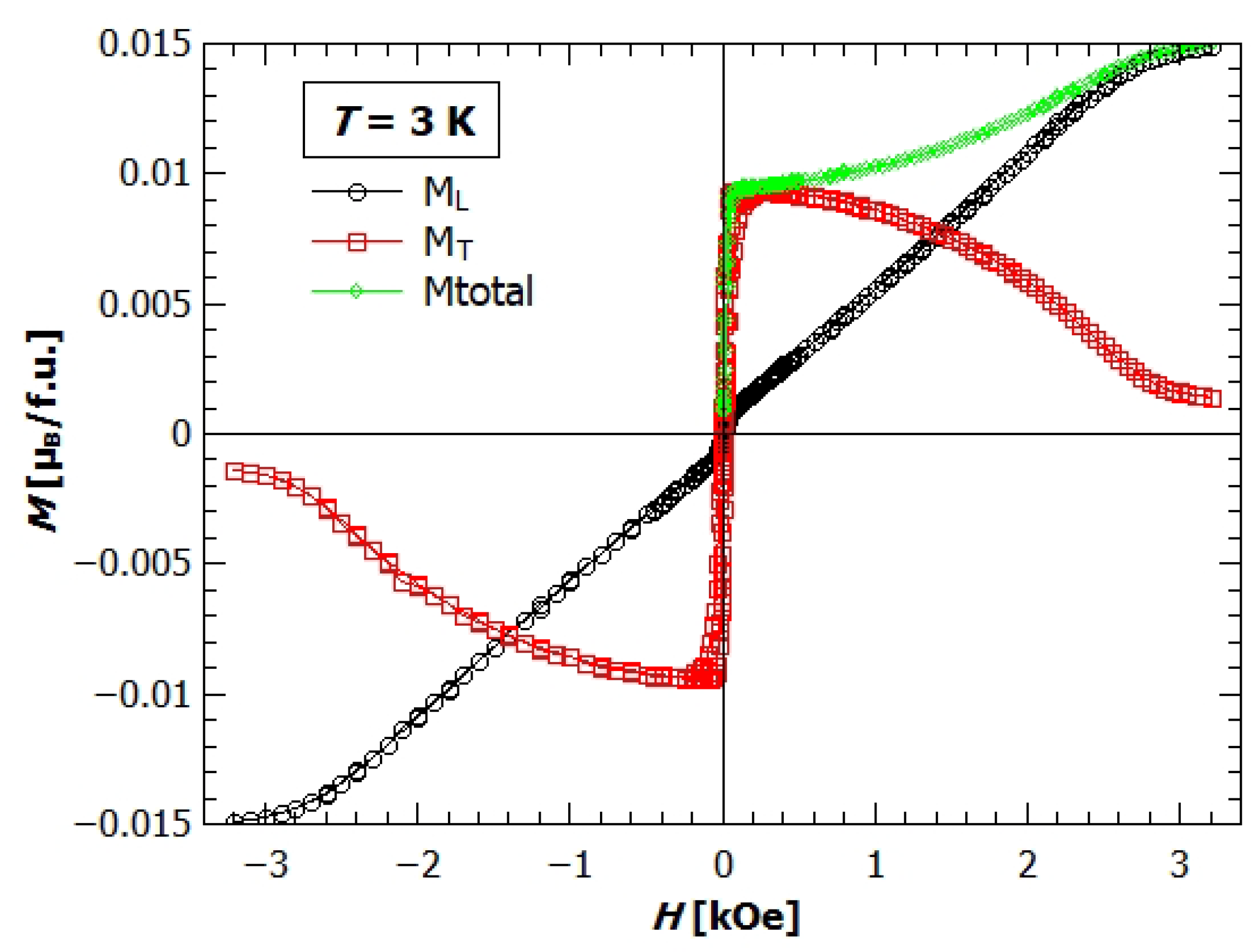
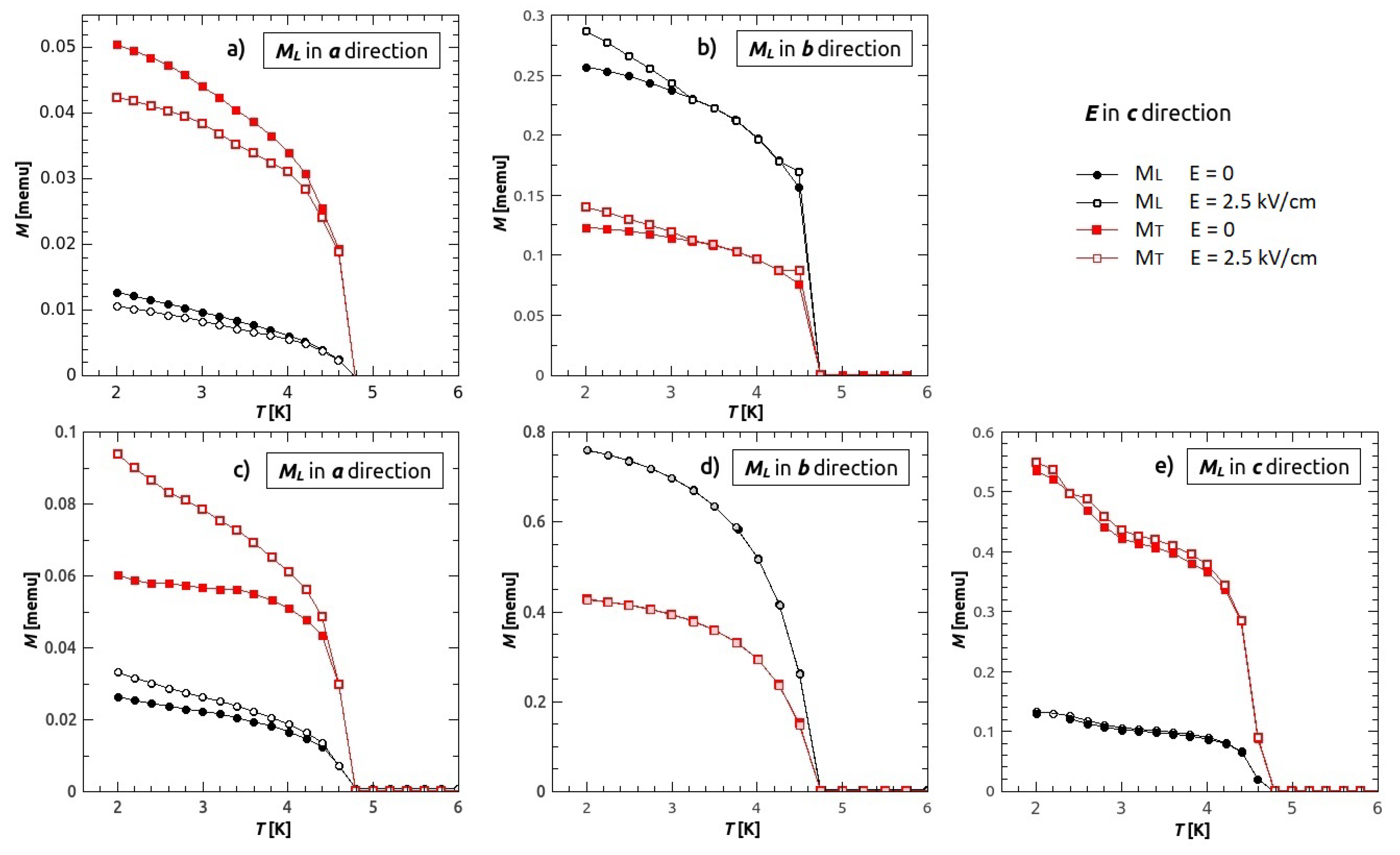
3.3. Magnetic Anisotropy
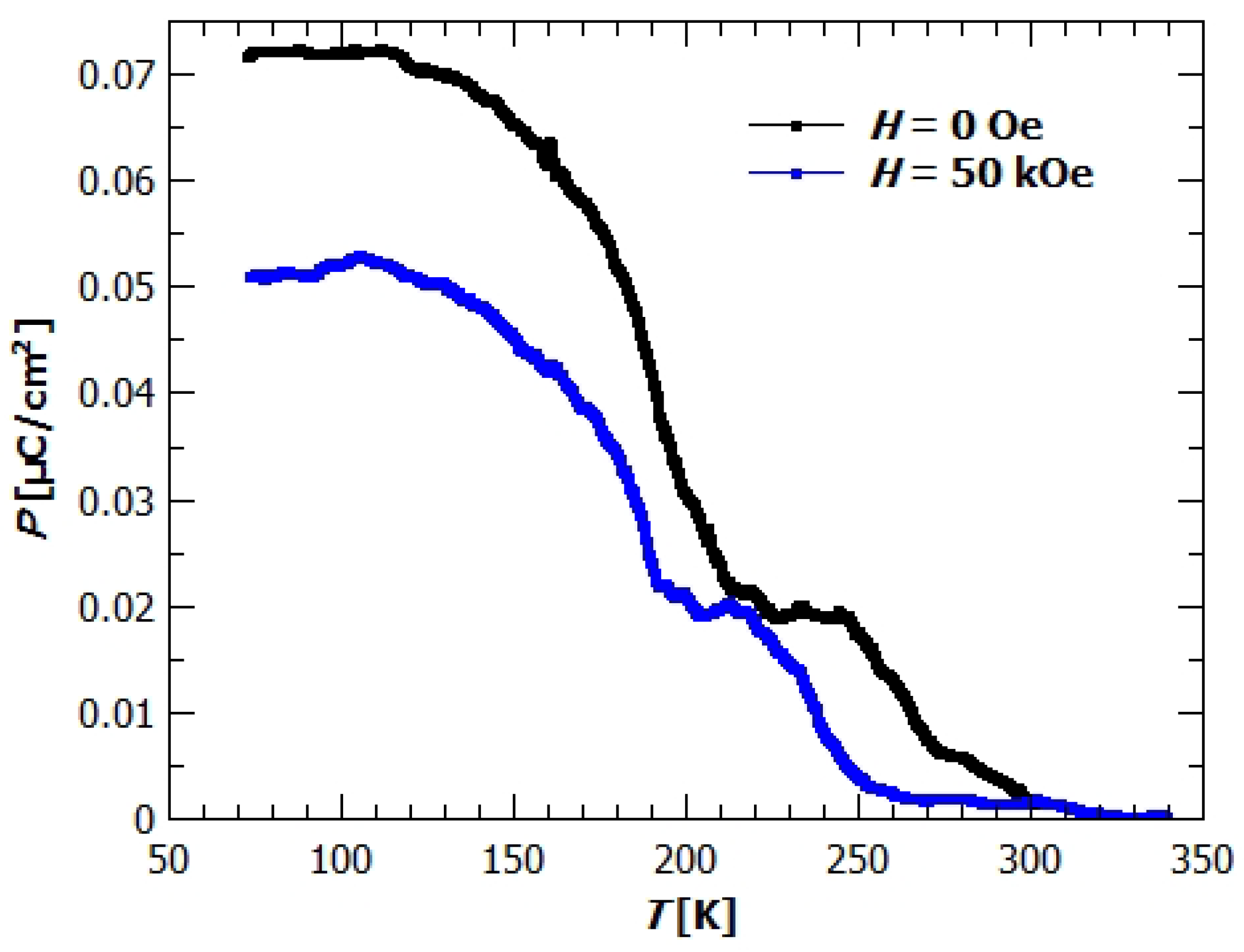
3.4. Magneto-Electric Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Structural Features
| Complex | 1 |
|---|---|
| Chemical formula | C4H9CuN3O6 |
| Mr | 258.69 |
| Crystal system, colour and habit | orthorhombic, blue, prism |
| Crystal dimensions/mm3 | 0.06 × 0.08 × 0.21 |
| Space group | Pna21(No. 33) |
| Z | 4 |
| Unit cell parameters: | |
| a /Å | 8.4350(2) |
| b /Å | 9.0145(2) |
| c /Å | 11.2820(4) |
| /° | 90 |
| /° | 90 |
| /° | 90 |
| V/Å3 | 857.85(4) |
| Dcalc/g cm–3 | 2.003 |
| μ/mm–1 | 2.558 |
| F(000) | 524 |
| No. refined parameters, Np/restraints | 146/6 |
| Reflections collected, unique (Rint), observed [I ≥ 2(I)] | 8136, 2072 (0.016), 1996 |
| R1a [I ≥ 2(I)] | 0.0202 |
| g1, g2 in w b | 0.0326, 0.2077 |
| wR2c (all data) | 0.0554 |
| Goodness of fit on F2, S d | 1.08 |
| Bond lengths | |||
| A−B | d(A−B)/Å | A−B | d(A−B)/Å |
| Cu1–O1 | 1.992(3) | O5–C3 1 | 1.272(3) |
| Cu1–O2 | 1.991(3) | C2–O3 2 | 1.269(3) |
| Cu1–O3 | 1.953(2) | O1–C1 3 | 1.254(6) |
| Cu1–O4 | 2.3597(19) | O4–C2 | 1.238(3) |
| Cu1–O5 | 1.9674(19) | O2–C1 | 1.251(6) |
| Cu1–O6 | 2.331(2) | O6–C3 | 1.235(3) |
| N3–C4 | 1.321(2) | ||
| N2–C4 | 1.306(12) | ||
| C4–N1 | 1.352(13) | ||
| Bond angles | |||
| A–B–C | ∠(A–B–C)/° | ||
| O1– Cu1–O2 | 179.36(8) | C3–O6–Cu1 | 134.59(19) |
| O1– Cu1–O3 | 89.54(9) | C1–O2–Cu1 | 121.3(2) |
| O1– Cu1–O4 | 88.91(8) | C2–O4–Cu1 | 130.41(18) |
| O1– Cu1–O5 | 89.57(12) | C3 1–O5–Cu1 | 128.22(18) |
| O1– Cu1–O6 | 90.70(12) | O4–C2–O3 2 | 124.1(3) |
| O2– Cu1–O3 | 89.95(13) | C1 3–O1–Cu1 | 121.6(2) |
| O2–Cu1–O4 | 91.44(11) | N2–C4–N3 | 121.7(10) |
| O2–Cu1–O5 | 91.02(9) | N2–C4–N1 | 119.87(19) |
| O2–Cu1–O6 | 89.07(8) | N3–C4–N1 | 118.3(11) |
| O3–Cu1–O4 | 84.94(8) | C2 4–O3–Cu1 | 129.3(2) |
| O3–Cu1–O5 | 166.90(8) | O2–C1–O1 5 | 124.0(2) |
| O3–Cu1–O6 | 106.03(6) | O6–C3–O5 6 | 124.0(2) |
| O4–Cu1–O5 | 81.97(6) | ||
| O4–Cu1–O6 | 169.02(8) | ||
| O5–Cu1–O6 | 87.05(9) | ||
| D–HA | D–H | HA | DA | ∠D–HA | Symmetry Code |
|---|---|---|---|---|---|
| N1–H1AO5 | 0.84(3) | 2.15(3) | 2.969(3) | 167(3) | −x,1−y,1/2 + z |
| N1–H1BO1 | 0.820(19) | 2.12(2) | 2.925(3) | 170(3) | 1/2−x,−1/2 + y,1/2 + z |
| N2–H2AO4 | 0.87(3) | 2.07(3) | 2.920(3) | 165(3) | - |
| N2– H2BO2 | 0.818(18) | 2.18(2) | 2.981(3) | 167(3) | −1/2 + x,1/2−y,z |
| N3–H3AO6 | 0.84(3) | 2.10(3) | 2.911(4) | 165(3) | 1/2−x,1/2 + y,1/2 + z |
| N3–H3BO3 | 0.83(3) | 2.17(3) | 2.970(4) | 164(3) | −1/2 + x,3/2−y,z |
Appendix B. Electric Properties

Appendix C. Thermodynamic Properties
References
- Fiebig, M. Revival of the magnetoelectric effect. J. Phys. D Appl. Phys. 2005, 38, R123. [Google Scholar] [CrossRef]
- Khomskii, D. Classifying multiferroics: Mechanisms and effects. Physics 2009, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Liu, J.M.; Cheong, S.W.; Ren, Z. Multiferroic materials and magnetoelectric physics: Symmetry, entanglement, excitation, and topology. Adv. Phys. 2015, 64, 519–626. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liu, J.-M.; Ren, Z. Multiferroicity: The coupling between magnetic and polarization orders. Adv. Phys. 2009, 58, 321–448. [Google Scholar] [CrossRef]
- Gajek, M.; Bibes, M.; Fusil, S.; Bouzehouane, K.; Fontcuberta, J.; Barthélémy, A.; Fert, A. Tunnel junctions with multiferroic barriers. Nat. Mater. 2007, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Spaldin, N.A. Multiferroics: Progress and prospects in thin films. Nat. Mater. 2007, 6, 21. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, C.; Chen, Z.; Xi, S.; Shao, W.; Zhang, P.; Chen, G.; Li, J. Four-state memory based on a giant and non-volatile converse magnetoelectric effect in FeAl/PIN-PMN-PT structure. Sci. Rep. 2016, 6, 30002. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.A. Why are there so few magnetic ferroelectrics? J. Phys. Chem. B 2000, 104, 6694–6709. [Google Scholar] [CrossRef]
- Hill, N.A.; Filippetti, A. Why are there any magnetic ferroelectrics? J. Magn. Magn. Mater. 2002, 242, 976–979. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and Applications of Bismuth Ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Rogez, G.; Viart, N.; Drillon, M. Multiferroic Materials: The Attractive Approach of Metal–Organic Frameworks (MOFs). Angew. Chem. Int. Ed. 2010, 49, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2017, 2, 16099. [Google Scholar] [CrossRef]
- Cheetham, A.K.; Rao, C.N.R. There’s room in the middle. Science 2007, 318, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Stropa, A.; Wang, Z.M.; Gao, S. Hybrid Organic-Inorganic Perovskites; Wiley-VCH: Weinheim, Germany, 2020. [Google Scholar]
- Wang, Z.; Zhang, B.; Otsuka, T.; Inoue, K.; Kobayashi, H.; Kurmoo, M. Anionic NaCl-type frameworks of MnII(HCOO)3−, templated by alkylammonium, exhibit weak ferromagnetism. Dalton Trans. 2004, 15, 2209–2216. [Google Scholar] [CrossRef]
- Jain, P.; Dalal, N.S.; Toby, B.H.; Kroto, H.W.; Cheetham, A.K. Order-Disorder Antiferroelectric Phase Transition in a Hybrid Inorganic-Organic Framework with the Perovskite Architecture. J. Am. Chem. Soc. 2008, 130, 10450–10451. [Google Scholar] [CrossRef]
- Sánchez-Andújar, M.; Presedo, S.; Yáñez-Vilar, S.; Castro-García, S.; Shamir, J.; Señarís-Rodríguez, M.A. Characterization of the order-disorder dielectric transition in the hybrid organic-inorganic perovskite-like formate Mn(HCOO)3[(CH3)2NH2]. Inorg. Chem. 2010, 49, 1510–1516. [Google Scholar] [CrossRef]
- Hu, K.-L.; Kurmoo, M.; Wang, Z.; Gao, S. Metal–Organic Perovskites: Synthesis, Structures, and Magnetic Properties of [C(NH2)3][MII(HCOO)3] (M=Mn, Fe, Co, Ni, Cu, and Zn; C(NH2)3=Guanidinium). Chem. Eur. J. 2009, 15, 12050–12064. [Google Scholar] [CrossRef]
- Stroppa, A.; Jain, P.; Barone, P.; Marsman, M.; Perez-Mato, J.M.; Cheetham, A.K.; Kroto, H.W.; Picozzi, S. Electric Control of Magnetization and Interplay between Orbital Ordering and Ferroelectricity in a Multiferroic Metal–Organic Framework. Angew. Chem. Int. Ed. 2011, 50, 5847–5850. [Google Scholar] [CrossRef]
- Tian, Y.; Stroppa, A.; Chai, Y.-S.; Barone, P.; Perez-Mato, M.; Picozzi, S.; Sun, Y. High-temperature ferroelectricity and strong magnetoelectric effects in a hybrid organic–inorganic perovskite framework. Phys. Status Solidi RRL 2015, 9, 62–67. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.40.67a (Rigaku OD, 2019), CrysAlis PRO, Agilent Technologies, Version 1.171.37.35 (release 13-08-2014 CrysAlis171 .NET) (compiled Aug 13 2014, 18:06:01). Available online: https://www.rigakuxrayforum.com/ (accessed on 29 March 2021).
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Cryst. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849. [Google Scholar] [CrossRef]
- Gui, D.; Ji, L.; Muhammad, A.; Li, W.; Cai, W.; Li, Y.; Li, X.; Wu, X.; Lu, P. Jahn–Teller Effect on Framework Flexibility of Hybrid Organic–Inorganic Perovskites. J. Phys. Chem. Lett. 2018, 9, 751–755. [Google Scholar] [CrossRef]
- Viswanathan, M. Insights on the Jahn–Teller distortion, hydrogen bonding and local environment correlations in a promised multiferroic hybrid perovskite. J. Phys. Condens. Matter 2019, 31, 45LT01. [Google Scholar] [CrossRef]
- Viswanathan, M. Enhancement of the guest orderliness in a low-symmetric perovskite-type metal–organic framework influenced by Jahn–Teller distortion. Phys. Chem. Chem. Phys. 2018, 20, 21809–21813. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M. Disorder in the hydrogen-atoms uninvolved in hydrogen bonds in a metal–organic framework. Phys. Chem. Chem. Phys. 2018, 20, 24527–24534. [Google Scholar] [CrossRef]
- Viswanathan, M. Neutron diffraction studies on the thermal expansion and anomalous mechanics in the perovskite-type [C(ND2)3]Me2+(DCOO)3[Me = Cu, Mn, Co]. Phys. Chem. Chem. Phys. 2018, 20, 17059–17070. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M. Stability of Hydrogen Bonds in the Metal Guanidinium Formate Hybrid Perovskites: A Single-Crystal Neutron Diffraction Study. Cryst. Growth Des. 2019, 19, 4287–4292. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, 65, 148. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Towler, M.; Van der Streek, J.; et al. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466. [Google Scholar] [CrossRef]
- Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Australia, 2021; Available online: http://www.povray.org/ (accessed on 28 March 2021).
- Diamond—Crystal and Molecular Structure Visualization, Crystal Impact—Dr. H. Putz and Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: http://www.crystalimpact.com/diamond (accessed on 28 March 2021).
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrity, K.F.; Bennett, J.W.; Rabe, K.M.; Vanderbilt, D. Pseudopotentials for high-throughput DFT calculations. Comput. Mater. Sci. 2014, 81, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- King-Smith, R.D.; Vanderbilt, D. Theory of polarization of crystalline solids. Phys. Rev. B 1993, 47, 1651. [Google Scholar] [CrossRef]
- Khan, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993. [Google Scholar]
- Kanižaj, L.; Molčanov, K.; Torić, F.; Pajić, D.; Lončarić, I.; Šantić, A.; Jurić, M. Ladder-like [CrCu] coordination polymers containing unique bridging modes of [Cr(C2O4)3] and [Cr2O7]2−. Dalton Trans. 2019, 48, 7891–7898. [Google Scholar] [CrossRef]
- Jurić, M.; Androš Dubraja, L.; Pajić, D.; Torić, F.; Zorko, A.; Ozarowski, A.; Despoja, V.; Lafargue-Dit-Hauret, W.; Rocquefelte, X. Experimental and Theoretical Investigation of the Anti-Ferromagnetic Coupling of Cr(III) Ions through Diamagnetic -O-Nb(V)-O- Bridges. Inorg. Chem. 2017, 56, 6879–6889. [Google Scholar] [CrossRef] [PubMed]
- Herak, M.; Zorko, A.; Pregelj, M.; Zaharko, O.; Posnjak, G.; Jagličić, Z.; Potočnik, A.; Luetkens, H.; van Tol, J.; Ozarowski, A.; et al. Magnetic order and low-energy excitations in the quasi-one-dimensional antiferromagnet CuSe2O5 with staggered fields. Phys. Rev. B 2013, 87, 104413. [Google Scholar] [CrossRef] [Green Version]
- Živković, I.; Djokić, D.M.; Herak, M.; Pajić, D.; Prša, K.; Pattison, P.; Dominko, D.; Micković, Z.; Cinčić, D.; Forró, L.; et al. Site-selective quantum correlations revealed by magnetic anisotropy in the tetramer system SeCuO3. Phys. Rev. B 2012, 86, 054405. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šenjug, P.; Dragović, J.; Torić, F.; Lončarić, I.; Despoja, V.; Smokrović, K.; Topić, E.; Đilović, I.; Rubčić, M.; Pajić, D. Magnetoelectric Multiferroicity and Magnetic Anisotropy in Guanidinium Copper(II) Formate Crystal. Materials 2021, 14, 1730. https://doi.org/10.3390/ma14071730
Šenjug P, Dragović J, Torić F, Lončarić I, Despoja V, Smokrović K, Topić E, Đilović I, Rubčić M, Pajić D. Magnetoelectric Multiferroicity and Magnetic Anisotropy in Guanidinium Copper(II) Formate Crystal. Materials. 2021; 14(7):1730. https://doi.org/10.3390/ma14071730
Chicago/Turabian StyleŠenjug, Pavla, Jure Dragović, Filip Torić, Ivor Lončarić, Vito Despoja, Kristina Smokrović, Edi Topić, Ivica Đilović, Mirta Rubčić, and Damir Pajić. 2021. "Magnetoelectric Multiferroicity and Magnetic Anisotropy in Guanidinium Copper(II) Formate Crystal" Materials 14, no. 7: 1730. https://doi.org/10.3390/ma14071730
APA StyleŠenjug, P., Dragović, J., Torić, F., Lončarić, I., Despoja, V., Smokrović, K., Topić, E., Đilović, I., Rubčić, M., & Pajić, D. (2021). Magnetoelectric Multiferroicity and Magnetic Anisotropy in Guanidinium Copper(II) Formate Crystal. Materials, 14(7), 1730. https://doi.org/10.3390/ma14071730







