Humidity-Sensing Properties of an 1D Antiferromagnetic Oxalate-Bridged Coordination Polymer of Iron(III) and Its Temperature-Induced Structural Flexibility
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Measurements
2.2. Synthetic Procedures
2.3. Single-Crystal X-ray Structural Study
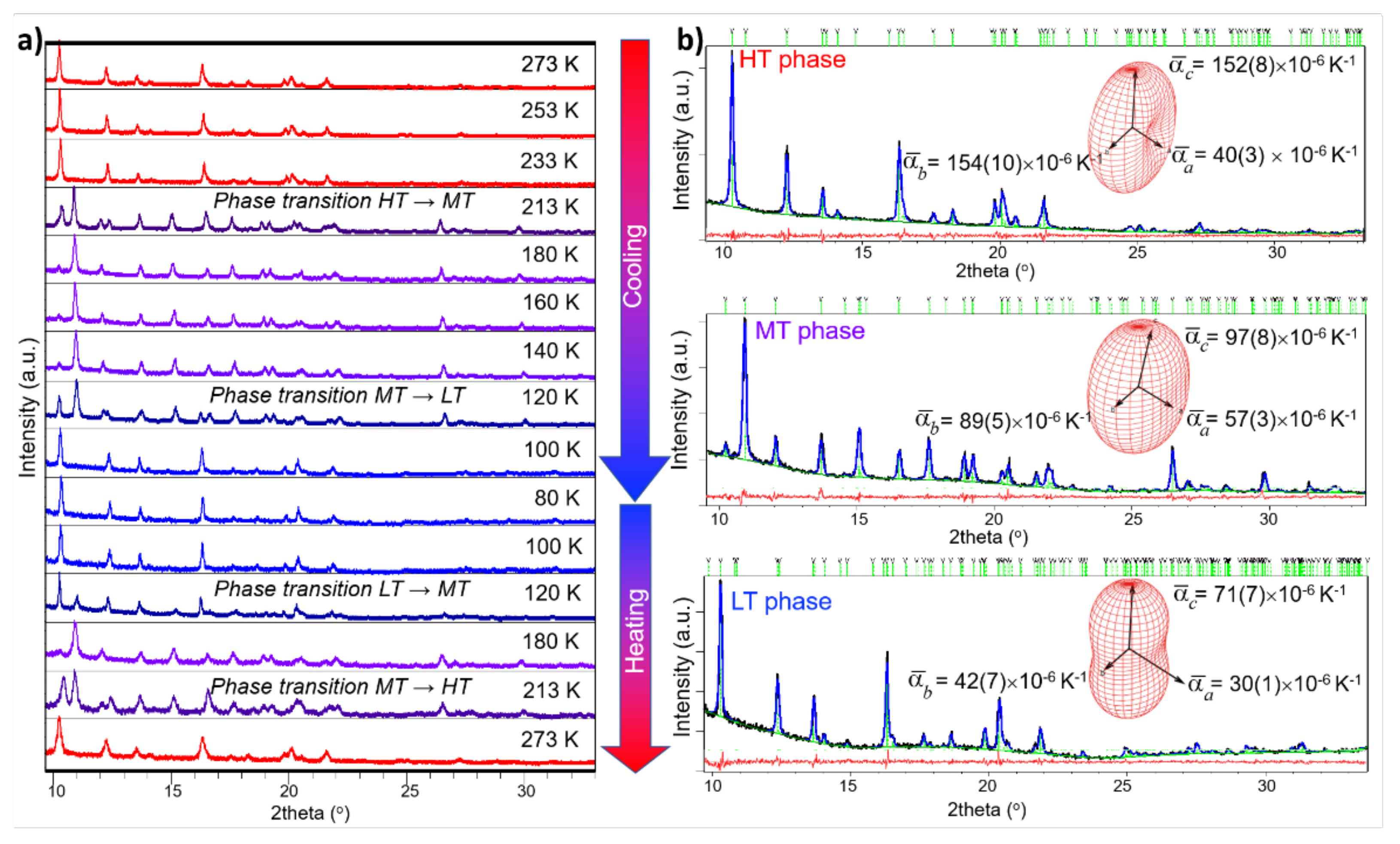
2.4. Powder X-ray Structural Study
2.5. Magnetization Study
2.6. Proton Conductivity Study
3. Results and Discussion
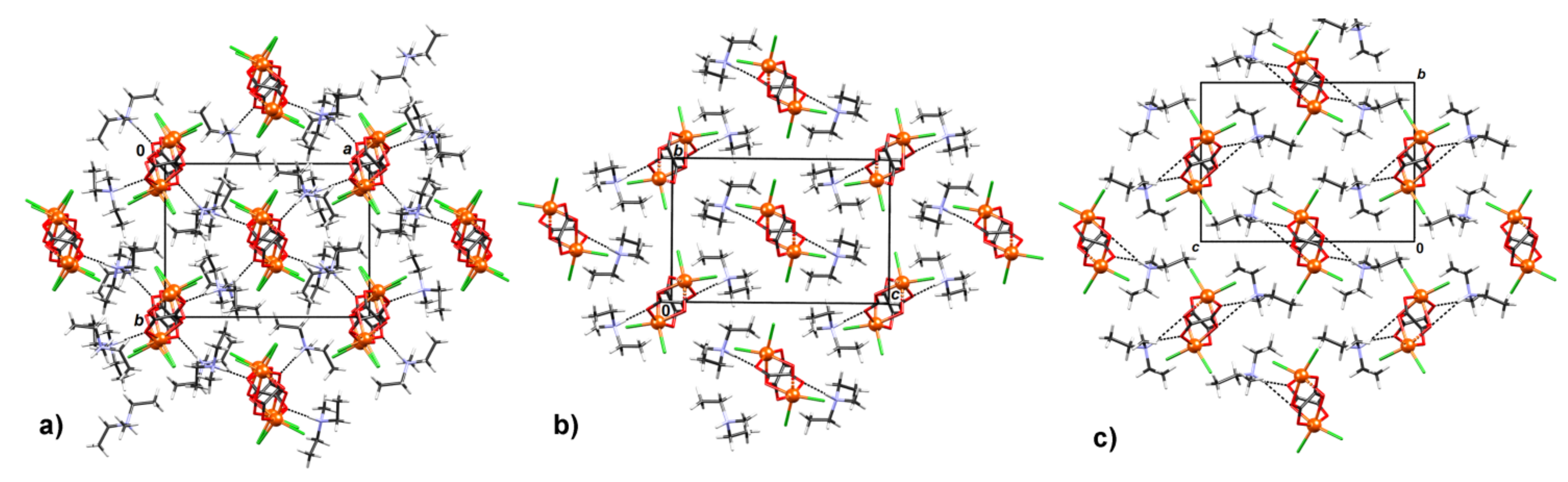
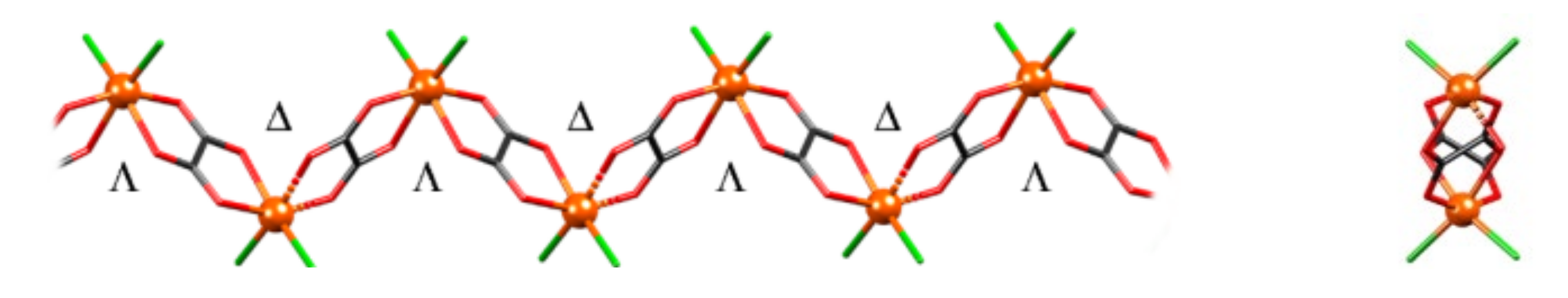
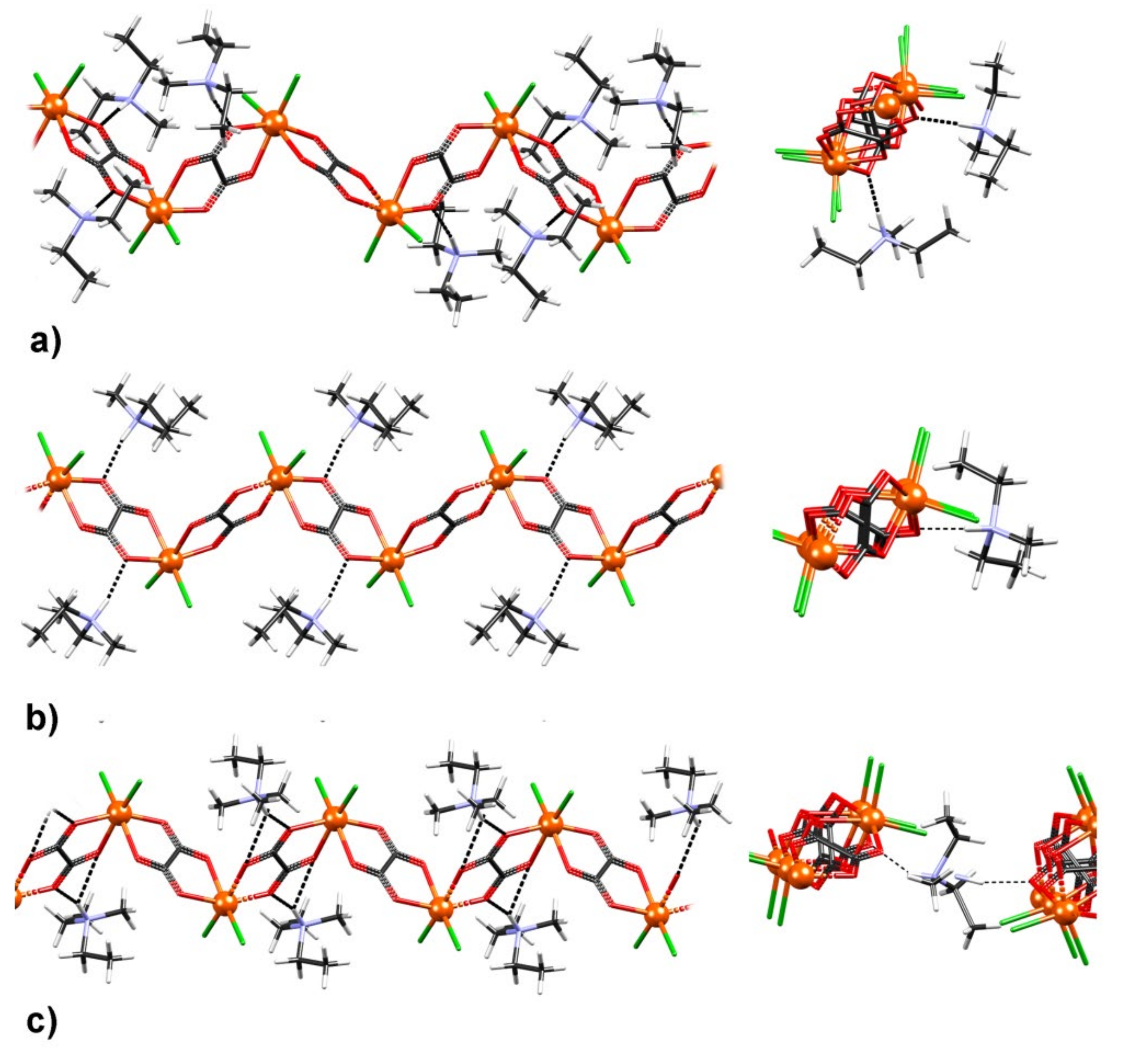
3.1. Synthesis and Crystal Structures of Compound 1
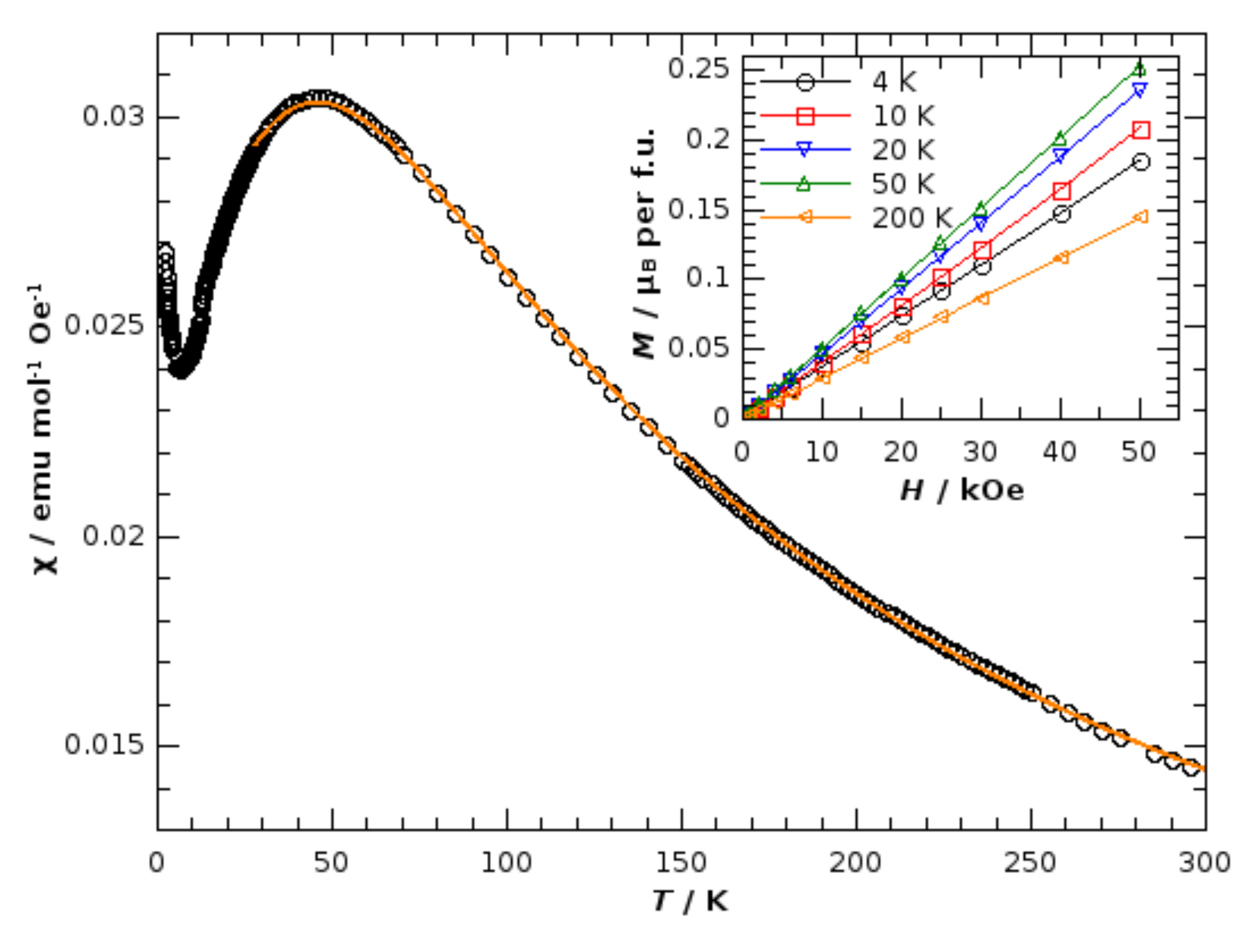
3.2. Magnetic and Charge Transport Properties of Compound 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronado, E.; Espallargas, G.M. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-León, M.; Coronado, E.; Martí-Gastaldoz, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kanižaj, L.; Barišić, D.; Torić, F.; Pajić, D.; Molčanov, K.; Šantić, A.; Lončarić, I.; Jurić, M. Structural, Electrical, and Magnetic Versatility of the Oxalate-Based [CuFe] Compounds Containing 2,2′:6′,2″-Terpyridine: Anion-Directed Synthesis. Inorg. Chem. 2020, 59, 18078–18089. [Google Scholar] [CrossRef] [PubMed]
- Kreuer, K.-D. Proton conductivity: Materials and Applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Lim, D.-W.; Kitagawa, H. Proton Transport in Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-X.; Yang, Y.-C.; Dou, B.-H.; Li, Z.-F.; Li, G. Proton conductive carboxylate-based metal-organic frameworks. Coord. Chem. Rev. 2020, 404, 213100. [Google Scholar] [CrossRef]
- Lim, D.-W.; Sadakiyo, M.; Kitagawa, H. Proton transfer in hydrogen-bonded degenerate systems of water and ammonia in metal–organic frameworks. Chem. Sci. 2019, 10, 16–33. [Google Scholar] [CrossRef] [Green Version]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Hydrated Proton-Conductive Metal-Organic Frameworks. ChemPlusChem 2016, 81, 691–701. [Google Scholar] [CrossRef]
- Yamada, T.; Otsubo, K.; Makiura, R.; Kitagawa, H. Designer coordination polymers: Dimensional crossover architectures and proton conduction. Chem. Soc. Rev. 2013, 42, 6655–6669. [Google Scholar] [CrossRef]
- Mon, M.; Vallejo, J.; Pasán, J.; Fabelo, O.; Train, C.; Verdaguer, M.; Ohkoshi, S.-I.; Tokoro, H.; Nakagawa, K.; Pardo, E. A novel oxalate-based three-dimensional coordination polymer showing magnetic ordering and high proton conductivity. Dalton Trans. 2017, 46, 15130–15137. [Google Scholar] [CrossRef] [PubMed]
- Maxim, C.; Ferlay, S.; Tokoro, H.; Ohkoshid, S.-I.; Train, C. Atypical stoichiometry for a 3D bimetallic oxalate-based long-range ordered magnet exhibiting high proton conductivity. Chem. Commun. 2014, 50, 5629–5632. [Google Scholar] [CrossRef]
- Pardo, E.; Train, C.; Gontard, G.; Boubekeur, K.; Fabelo, O.; Liu, H.; Dkhil, B.; Lloret, F.; Nakagawa, K.; Tokoro, H.; et al. High Proton Conduction in a Chiral Ferromagnetic Metal-Organic Quartz-like Framework. J. Am. Chem. Soc. 2011, 133, 15328–15331. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Ōkawa, H.; Shigematsu, A.; Ohba, M.; Yamada, T.; Kitagawa, H. Promotion of Low-Humidity Proton Conduction by Controlling Hydrophilicity in Layered Metal–Organic Frameworks. J. Am. Chem. Soc. 2012, 134, 5472–5475. [Google Scholar] [CrossRef]
- Ōkawa, H.; Shigematsu, A.; Sadakiyo, M.; Miyagawa, T.; Yoneda, K.; Ohba, M.; Kitagawa, H. Oxalate-Bridged Bimetallic Complexes {NH(prol)3}[MCr(ox)3] (M = MnII, FeII, CoII; NH(prol)3+ = Tri(3-hydroxypropyl)ammonium) Exhibiting Coexistent Ferromagnetism and Proton Conduction. J. Am. Chem. Soc. 2009, 131, 13516–13522. [Google Scholar] [CrossRef] [PubMed]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Rational Designs for Highly Proton-Conductive Metal-Organic Frameworks. J. Am. Chem. Soc. 2009, 131, 9906–9907. [Google Scholar] [CrossRef] [PubMed]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Proton Conductivity Control by Ion Substitution in a Highly Proton-Conductive Metal-Organic Framework. J. Am. Chem. Soc. 2014, 136, 13166–13169. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Yamada, T.; Honda, K.; Matsui, H.; Kitagawa, H. Control of Crystalline Proton-Conducting Pathways by Water-Induced Transformations of Hydrogen-Bonding Networks in a Metal-Organic Framework. J. Am. Chem. Soc. 2014, 136, 7701–7707. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Unni, S.M.; Sharma, A.; Kurungot, S.; Ghosh, S.K. Two-in-One: Inherent Anhydrous and Water-Assisted High Proton Conduction in a 3D Metal-Organic Framework. Angew. Chem. Int. Ed. 2014, 53, 2638–2642. [Google Scholar] [CrossRef]
- Wang, X.; Qin, T.; Bao, S.S.; Zhang, Y.C.; Shen, X.; Zheng, L.M.; Zhu, D.R. Facile synthesis of a water stable 3D Eu-MOF showing high proton conductivity and its application as a sensitive luminescent sensor for Cu2+ ions. J. Mater. Chem. A 2016, 4, 16484–16489. [Google Scholar] [CrossRef]
- Tominaka, S.; Coudert, F.; Dao, T.D.; Nagao, T.; Cheetham, A.K. Insulator-to-Proton-Conductor Transition in a Dense Metal-Organic Framework. J. Am. Chem. Soc. 2015, 137, 6428–6431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Sadakiyo, M.; Kitagawa, H. High Proton Conductivity of One-Dimensional Ferrous Oxalate Dihydrate. J. Am. Chem. Soc. 2009, 131, 3144–3145. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-B.; Wang, Z.-M.; Liu, T.; Gao, S. Synthesis, Structure, and Magnetic Properties of (A)[FeIII(oxalate)Cl2] (A = Alkyl Ammonium Cations) with Anionic 1D [FeIII(oxalate)Cl2]- Chains. Inorg. Chem. 2007, 46, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis PRO; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2018.
- SAINT V8.34A; Bruker AXS Inc.: Madison, WI, USA, 2013.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Cliffe, M.J.; Goodwin, A.L. PASCal: A principal axis strain calculator for thermal expansion and compressibility determination. J. Appl. Crystallogr. 2012, 45, 1321–1329. [Google Scholar] [CrossRef] [Green Version]
- Khan, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993; p. 258. [Google Scholar]
- Kanižaj, L.; Šenjug, P.; Pajić, D.; Pavić, L.; Molčanov, K.; Jurić, M. Magnetic and Electrical Behaviors of the Homo- and Heterometallic 1D and 3D Coordination Polymers Based on the Partial Decomposition of the [Cr(C2O4)3]3− Building Block. Materials 2020, 13, 5341. [Google Scholar] [CrossRef] [PubMed]
- Panda, M.K.; Runčevski, T.; Sahoo, S.C.; Belik, A.A.; Nath, N.K.; Dinnebier, R.E.; Naumov, P. Colossal positive and negative thermal expansion and thermosalient effect in a pentamorphic organometallic martensite. Nat. Commun. 2014, 5, 4811. [Google Scholar]
- Lončarić, I.; Popović, J.; Despoja, V.; Burazer, S.; Grgičević, I.; Popović, D.; Skoko, Ž. Reversible Thermosalient Effect of N′-2-Propylidene-4-hydroxybenzohydrazide Accompanied by an Immense Negative Compressibility: Structural and Theoretical Arguments Aiming toward the Elucidation of Jumping Phenomenon. Cryst. Growth Des. 2017, 17, 4445–4453. [Google Scholar] [CrossRef]
- Yao, Z.-S.; Guan, H.; Shiota, Y.; He, C.-T.; Wang, X.-L.; Wu, S.-Q.; Zheng, X.; Su, S.-Q.; Yoshizawa, K.; Kong, X.; et al. Giant anisotropic thermal expansion actuated by thermodynamically assisted reorientation of imidazoliums in a single crystal. Nat. Commun. 2019, 10, 4805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.; Fujiwara, H.; Kobayashi, H.; Kurmoo, M.; Inoue, K.; Mori, T.; Gao, S.; Zhang, Y.; Zhu, D. Tetrathiafulvalene [FeIII(C2O4)Cl2]: An Organic–Inorganic Hybrid Exhibiting Canted Antiferromagnetism. Adv. Mater. 2005, 17, 1988–1991. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Zhang, Y.; Takahashi, K.; Okano, Y.; Cui, H.; Kobayashi, H.; Inoue, K.; Kurmoo, M.; Pratt, F.L.; et al. Hybrid Organic-Inorganic Conductor with a Magnetic Chain Anion: κ-BETS2[FeIII(C2O4)Cl2] [BETS = Bis(ethylenedithio)tetraselenafulvalene]. Inorg. Chem. 2006, 45, 3275–3280. [Google Scholar] [CrossRef]
- Armentano, D.; Mastropietro, T.F.; De Munno, G.; Rossi, P.; Lloret, F.; Julve, M. New Extended Magnetic Systems Based on Oxalate and Iron(III) Ions. Inorg. Chem. 2008, 47, 3772–3786. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Marino, N.; De Munno, G.; Lloret, F.; Julve, M.; Pardo, E.; Armentano, D. Selective Guest Inclusion in Oxalate-Based Iron(III) Magnetic Coordination Polymers. Inorg. Chem. 2016, 55, 11160–11169. [Google Scholar] [CrossRef]
- Sanda, S.; Biswas, S.; Konar, S. Study of Proton Conductivity of a 2D Flexible MOF and a 1D Coordination Polymer at Higher Temperature. Inorg. Chem. 2015, 54, 1218–1222. [Google Scholar] [CrossRef]
- Morikawa, S.; Yamada, T.; Kitagawa, H. Crystal structure and proton conductivity of a one-dimensional coordination polymer, {Mn(DHBQ)(H2O)2}. Chem. Lett. 2009, 38, 654–655. [Google Scholar] [CrossRef]
- Guo, Z.-C.; Shi, Z.-Q.; Wang, X.-Y.; Li, Z.-F.; Li, G. Proton conductive covalent organic frameworks. Coord. Chem. Rev. 2020, 422, 213465. [Google Scholar] [CrossRef]

| Compound | LT-1 (100 K) | MT-1 (180 K) | HT-1 (243 K) |
|---|---|---|---|
| Empirical formula | C7H14Cl2FeNO4 | C7H14Cl2FeNO4 | C7H14Cl2FeNO4 |
| Formula wt. (g·mol−1) | 302.94 | 302.94 | 302.94 |
| Color | yellow | yellow | yellow |
| Crystal dimensions/mm | 0.18 × 0.09 × 0.07 | 0.20 × 0.18 × 0.09 | 0.20 × 0.18 × 0.08 |
| Space group | P21/n | P21/c | P21/c |
| a (Å) | 14.962(3) | 8.720(2) | 8.5125(8) |
| b (Å) | 10.791(2) | 9.762(2) | 10.7755(14) |
| c (Å) | 16.944(3) | 14.830(3) | 14.9703(16) |
| α (°) | 90 | 90 | 90 |
| β (°) | 105.96(3) | 95.43(3) | 104.798(9) |
| γ (°) | 90 | 90 | 90 |
| Z | 8 | 4 | 4 |
| V (Å3) | 2630.1(9) | 1256.8(5) | 1327.6(3) |
| Dcalc (g·cm−3) | 1.530 | 1.601 | 1.516 |
| λ (Å) | 0.71073 (MoKα) | 0.71073 (MoKα) | 1.54179 (CuKα) |
| μ (mm−1) | 1.547 | 1.618 | 12.807 |
| Θ range (°) | 2.26–27.57 | 2.35–26.53 | 5.12–74.32 |
| T (K) | 100(2) | 180(2) | 243(2) |
| Diffractometer type | D8 Venture | D8 Venture | Xcalibur Nova |
| Range of h, k, l | 19 < h < 18; 13 < k < 13; −21 < l < 22 | −10 < h < 10; −12 < k < 10; −18 < l < 17 | −10 < h < 6; −13 < k < 13; −18 < l < 17 |
| Reflections collected | 58295 | 10852 | 5948 |
| Independent reflections | 6028 | 2595 | 2423 |
| Observed reflections (I≥2σ) | 4917 | 1937 | 1440 |
| Absorption correction | None | None | Multi-scan |
| Tmin, Tmax | − | − | 0.21266; 1.0000 |
| Rint | 0.0597 | 0.0590 | 0.0542 |
| R (F) | 0.0349 | 0.0416 | 0.0873 |
| Rw (F2) | 0.0904 | 0.1155 | 0.2990 |
| Goodness of fit | 1.043 | 1.066 | 1.010 |
| H atom treatment | Mixed | Constrained | Constrained |
| No. of parameters | 315 | 139 | 147 |
| No. of restraints | 91 | 0 | 77 |
| Δρmax, Δρmin (eÅ–3) | 0.890; −0.520 | 0.570; −0.467 | 0.444; −0.381 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burazer, S.; Molčanov, K.; Šantić, A.; Klaser, T.; Wenger, E.; Pajić, D.; Jagličić, Z.; Popović, J.; Jurić, M. Humidity-Sensing Properties of an 1D Antiferromagnetic Oxalate-Bridged Coordination Polymer of Iron(III) and Its Temperature-Induced Structural Flexibility. Materials 2021, 14, 5543. https://doi.org/10.3390/ma14195543
Burazer S, Molčanov K, Šantić A, Klaser T, Wenger E, Pajić D, Jagličić Z, Popović J, Jurić M. Humidity-Sensing Properties of an 1D Antiferromagnetic Oxalate-Bridged Coordination Polymer of Iron(III) and Its Temperature-Induced Structural Flexibility. Materials. 2021; 14(19):5543. https://doi.org/10.3390/ma14195543
Chicago/Turabian StyleBurazer, Sanja, Krešimir Molčanov, Ana Šantić, Teodoro Klaser, Emmanuel Wenger, Damir Pajić, Zvonko Jagličić, Jasminka Popović, and Marijana Jurić. 2021. "Humidity-Sensing Properties of an 1D Antiferromagnetic Oxalate-Bridged Coordination Polymer of Iron(III) and Its Temperature-Induced Structural Flexibility" Materials 14, no. 19: 5543. https://doi.org/10.3390/ma14195543
APA StyleBurazer, S., Molčanov, K., Šantić, A., Klaser, T., Wenger, E., Pajić, D., Jagličić, Z., Popović, J., & Jurić, M. (2021). Humidity-Sensing Properties of an 1D Antiferromagnetic Oxalate-Bridged Coordination Polymer of Iron(III) and Its Temperature-Induced Structural Flexibility. Materials, 14(19), 5543. https://doi.org/10.3390/ma14195543










