Comparison of the Tribological Properties of the Thermal Diffusion Zinc Coating to the Classic and Heat Treated Hot-Dip Zinc Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
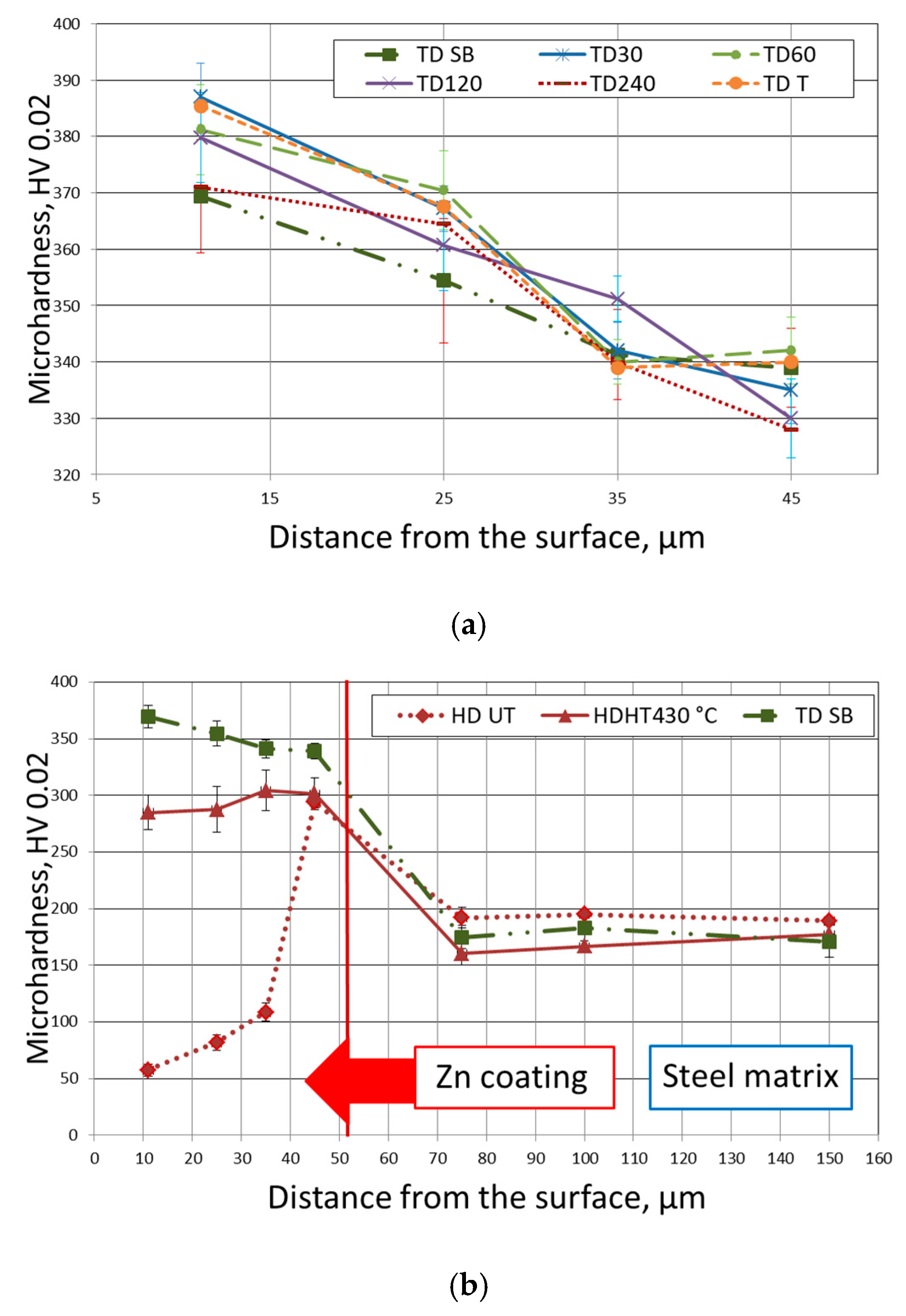
3.1. Metallographic Observations and Microhardness Distribution
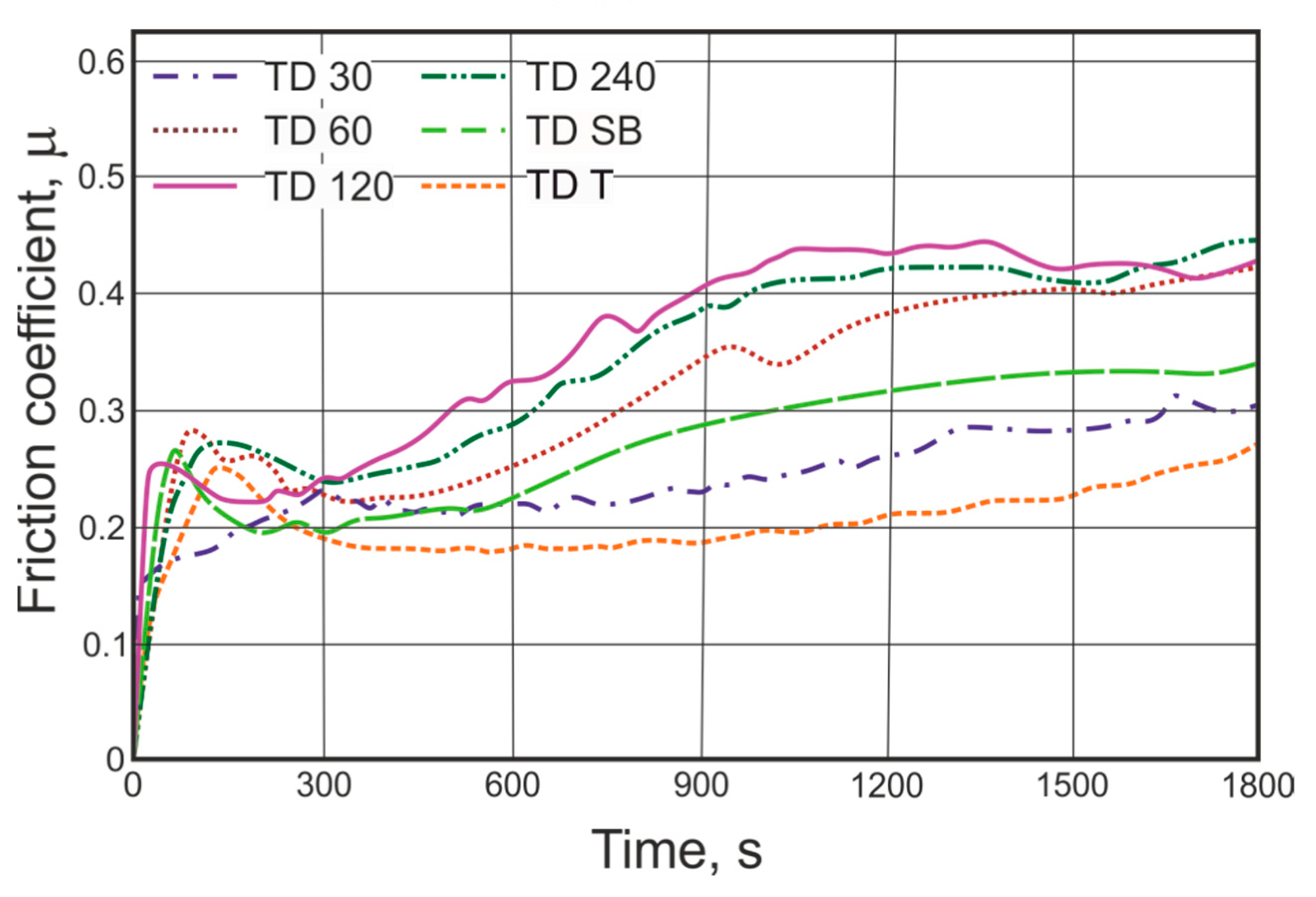
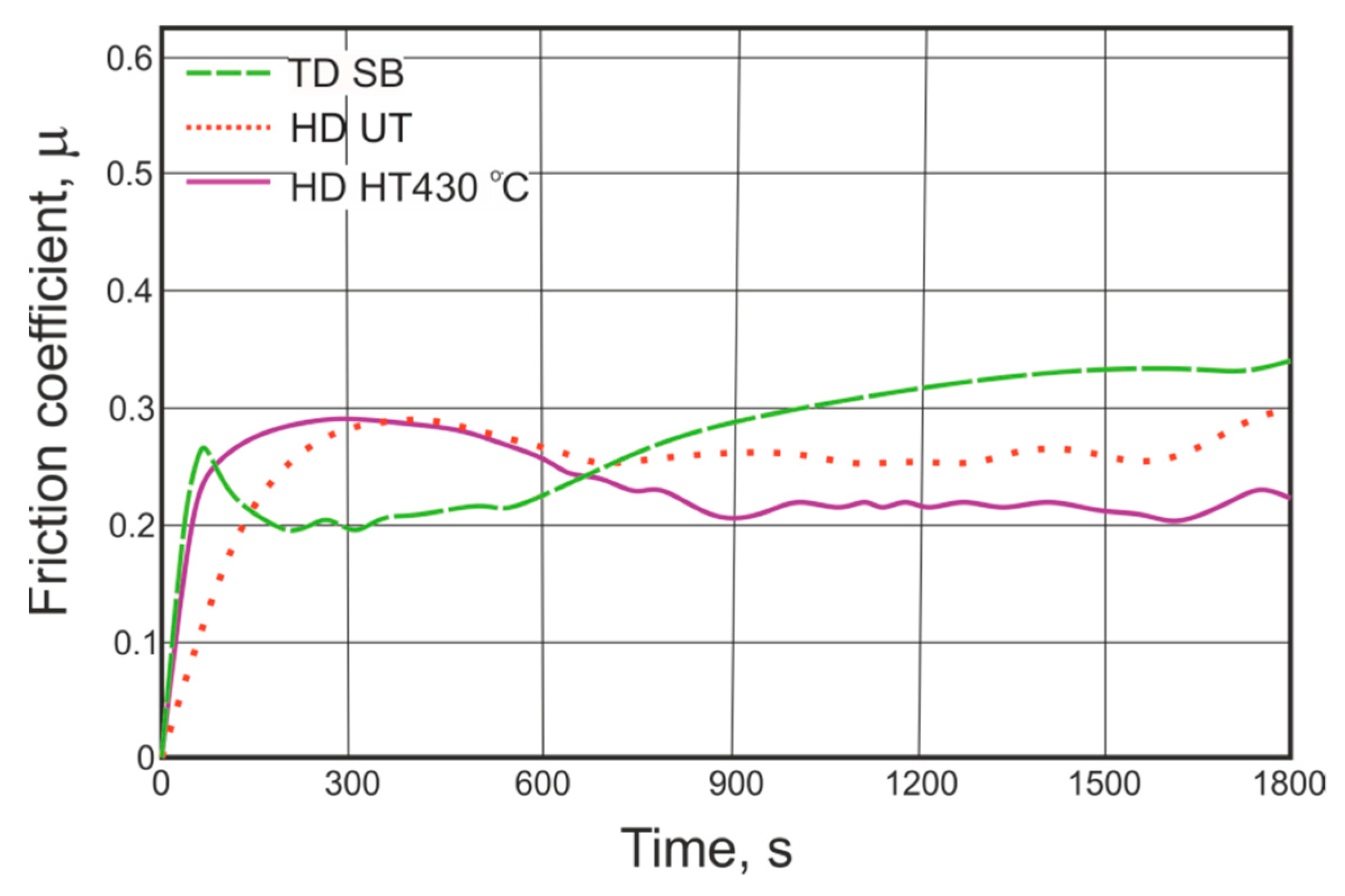
3.2. Friction Coefficient Measurements
4. Conclusions
- (1)
- The method of the base steel surface preparation affects the friction coefficient value, thickness and wear resistance of the TD zinc coating.
- (2)
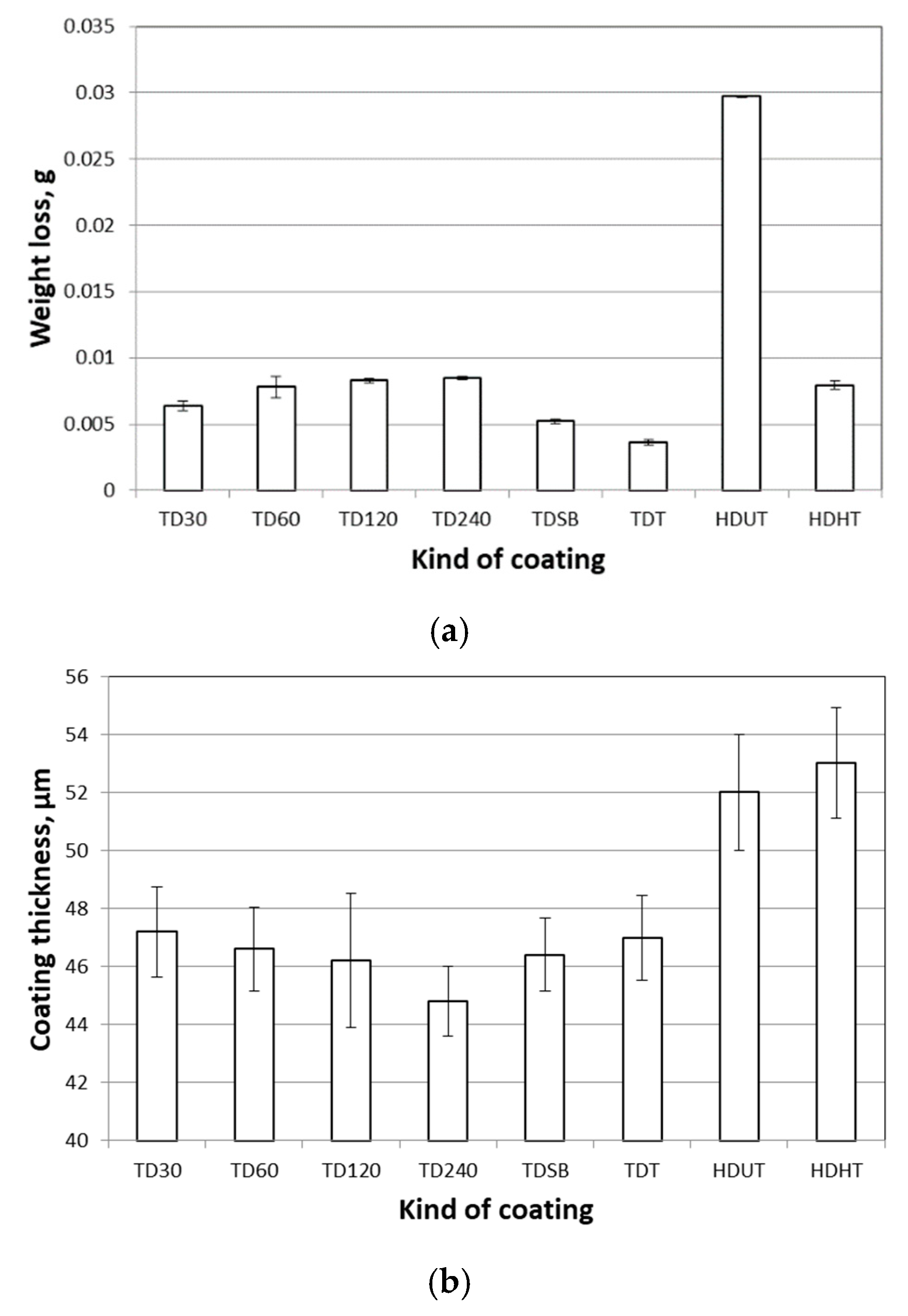
- In the applied test conditions, the value of the friction coefficient of the TD coating varied within the range 0.20–0.39, with a coating thickness of 44.5 to 47.5 µm, respectively.
- (3)
- The measured friction coefficient values correspond well with the microhardness profile determined on the cross section of the TD coating and the weight loss trend of changes obtained during the “pin-on-disc” test. With increasing of the coating’s hardness, both the TD coating’s coefficient of friction and the weight loss are reduced.
- (4)
- The lower values of the friction coefficient were measured for samples with higher roughness of the base steel surface. The observed changes may be caused by the increase of the steel reactivity and in consequence extending the range of occurrence of the harder δ phase.
- (5)
- The changes in properties of compared coatings are due to the differentiation in the microstructure (verified by results of EDS analysis), caused by specific growth or diffusion conditions during individual coating formation. The TD coating was composed of δ (outer) and Г (inner) phases. The microstructure of a tested HD zinc coating was formed by phases η, ζ, δ and Г1, whereas in a HD zinc coating after heat treatment, only three phases occurred: ζ, δ and Г.
- (6)
- The TD coating (δ+Г) showed higher abrasion resistance (in comparison to HD UT coating—η+ζ+δ), which was expressed in a reduction in weight loss measured during the tribological test. In the conducted test the HD zinc coating weight loss was four times greater.
- (7)
- The abrasion resistance of the TD zinc coating (δ+Г) is similar to the HD HT coating (ζ+δ+Г)—the measured difference in the weight loss was a maximum of 0.004 g.
- (8)
- The hardness of the TD zinc coating reached the values of 325–385 HV 0.02 and was greater by 40–90 HV 0.02 than the values obtained for the HD HT zinc coating.
- (9)
- With the use of the proper method of the base steel surface preparation, it is possible to improve the tribological properties of the thermal diffusion zinc coating, decrease its wear and to adjust/change the coefficient of friction according to the requirements within the range of 0.20–0.39.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.H.; Ma, A.B.; Fan, X.D.; Gong, M.Z. Sherardizing and Characteristic of Zinc Protective Coating on High-Strength Steel Bridge Cable Wires. Adv. Mater. Res. 2010, 97, 1368–1372. [Google Scholar] [CrossRef]
- Natrup, F.; Graf, W. 20-Sherardizing: Corrosion protection of steel by zinc diffusion coating. In Thermochemical Surface Engineering of Steels; Mittemeijer, E.J., Somers, M.A., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 737–750. [Google Scholar] [CrossRef]
- Kania, H.; Sipa, J. Thermal diffusion zinc coating technology with reactive atmosphere recirculation. Part 1, General description of technology and structure of coatings. Ochr. Przed. Korozją. 2018, 11, 338–345. [Google Scholar] [CrossRef]
- Kania, H.; Sipa, J.; Skupińska, A. Thermal diffusion zinc coating technology with reactive atmosphere recirculation. Part 2: Corrosion resistance of coatings. Ochr. Przed. Korozją. 2018, 12, 375–382. [Google Scholar] [CrossRef]
- Biryukov, A.I.; Galin, R.G.; Zakharyevich, D.A.; Wassilkovska, A.V.; Batmanova, T.V. The effect of the chemical composition of intermetallic phases on the corrosion of thermal diffusion zinc coatings. Surf. Coat. Technol. 2019, 372, 166–172. [Google Scholar] [CrossRef]
- Melhem, G.N. Aerospace Fasteners: Use in Structural Applications. In Encyclopedia of Aluminum and Its Alloys, 30–45th ed.; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Pang, X.; Gao, K.; Yang, H.; Qiao, L.; Wang, Y.; Volinsky, A.A. Interfacial Microstructure of Chromium Oxide Coatings. Adv. Eng. Mater. 2007, 9, 594–599. [Google Scholar] [CrossRef]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef] [PubMed]
- Buchtík, M.; Krystýnová, M.; Másilko, J.; Wasserbauer, J. The Effect of Heat Treatment on Properties of Ni–P Coatings Deposited on a AZ91 Magnesium Alloy. Coatings 2019, 9, 461. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Tian, X.; Zou, L.; Zhao, X.; Wang, S.; Wang, S. The Hardness and Corrosion Properties of Trivalent Chromium Hard Chromium. Mater. Sci. Appl. 2017, 8, 1014–1026. [Google Scholar] [CrossRef][Green Version]
- Panda, A.; Dyadyura, K.; Hovorun, T.; Pylypenko, O.; Dunaeva, M.; Pandova, I. Nanostructured Wear-Resistant Coatings based on Refractory Metals Nitrides: Physical-Mechanical Properties and Structural-Phase State. Manag. Prod. Eng. Rev. 2019, 10, 133–139. [Google Scholar] [CrossRef]
- Metals Price List—London Metal Exchange. Available online: www.lme.com (accessed on 10 February 2021).
- Maaß, P.; Peißker, P. Handbook of Hot-Dip Galvanization; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Kania, H.; Sipa, J. Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained on High-Strength Grade 10.9 Bolts Using a New Thermal Diffusion Process. Materials 2019, 12, 1400. [Google Scholar] [CrossRef]
- Votava, J. Anticorrosion protection of strength bolts. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 181–188. [Google Scholar] [CrossRef]
- Chung, P.P.; Wang, J.; Durandet, Y. Deposition processes and properties of coatings on steel fasteners-A review. Friction 2019, 7, 389–416. [Google Scholar] [CrossRef]
- Bondareva, O. Study of the Temperature Effect on the Structure and Thickness of Hot-Dip Zinc Coatings on Fixing Products. Appl. Mech. Mater. 2014, 698, 355–359. [Google Scholar] [CrossRef]
- Chen, K.J.; Hung, F.Y.; Lui, T.S.; Tseng, C.H. Decrease in Hydrogen Embrittlement Susceptibility of 10B21 Screws by Bake Aging. Metals 2016, 6, 211. [Google Scholar] [CrossRef]
- Rebak, R.B.; Muchjin, L.; Szklarska-Smialowska, Z. Hydrogen diffusion and accumulation in automotive fasteners. Corrosion 1997, 53, 481–488. [Google Scholar] [CrossRef]
- Ferraz, M.T.; Oliveira, M. Steel fasteners failure by hydrogen embrittlement. Ciência E Tecnol. Dos Mater. 2008, 20, 128–133. [Google Scholar]
- Szłapa, I.; Jędrzejczyk, D.; Hajduga, M.; Węgrzynkiewicz, S.; Sołek, D. Evaluation of Corrosion Resistance of Zinc Coatings on Component of the Ambulance. In Proceedings of the Metal 2013 Conference, Brno, Czech Republic, 15–17 May 2013; pp. 1–6. [Google Scholar]
- Wołczyński, W.; Kucharska, B.; Garzeł, G.; Sypien, A.; Pogoda, Z.; Okane, T. Part III. Kinetics of the (Zn)—Coating Deposition during Stable and Meta-Stable Solidifications. Arch. Met. Mater. 2015, 60, 199–207. [Google Scholar] [CrossRef]
- Evans, D.W. Next generation technology for corrosion protection in ground support. In Proceedings of the 2014 Coal Operators’ Conference, Northfields Ave, Australia, 12–14 February 2014; pp. 177–185. [Google Scholar]
- Marder, A. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Pokorný, P.; Cinert, J.; Pala, Z. Fe-Zn intermetallic phases prepared by diffusion annealing and spark-plasma sintering. Mater. Technol. 2016, 50, 253–256. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Wegrzynkiewicz, S.; Jedrzejczyk, D.; Szłapa, I.; Hajduga, M.; Boczkal, S. Influence of a Substrate Surface on the (Zn)—Coating Formation/Wpływ Powierzchni Podłoża Na Kształtowanie Się Powłoki (Zn). Arch. Met. Mater. 2014, 59, 1373–1378. [Google Scholar] [CrossRef]
- PKN. Standard—Hot-Dip Galvanized Coatings on Fabricated Iron and Steel Articles—Specifications and Test Methods; PN EN ISO 1461:2011; Polish Committee for Standardization: Warsaw, Poland, 2011. [Google Scholar]
- PKN. Standard, Zinc Coatings—Guidelines and Recommendations for the Protection against Corrosion of Iron and Steel in Structures—Part 2: Hot Dip Galvanizing; PN EN ISO 14713-2:2020; Polish Committee for Standardization: Warsaw, Poland, 2020. [Google Scholar]
- Jedrzejczyk, D.; Hajduga, M. Effect of the Surface Oxidation on the Hot-Dip Zinc Galvanizing of Cast Iron. Arch. Met. Mater. 2011, 56, 839–849. [Google Scholar] [CrossRef]
- Jedrzejczyk, D. Effect of High Temperature Oxidation on Structure and Corrosion Resistance of the Zinc Coating Deposited on Cast Iron. Arch. Met. Mater. 2012, 57, 145–154. [Google Scholar] [CrossRef]
- Che, C.; Lu, J.; Kong, G.; Xu, Q. Role of silicon in steels on galvanized coatings. Acta Met. Sin. Engl. Lett. 2009, 22, 138–145. [Google Scholar] [CrossRef]
- Che, C.; Lu, J.; Kong, G. Interpretation of Sebisty effect of hot dip galvanized steels. Trans. Nonferrous Met. Soc. China 2005, 15, 1275–1279. [Google Scholar]
- Kania, H.; Liberski, P.P. Synergistic influence of Al, Ni, Bi and Sn addition to a zinc bath upon growth kinetics and the structure of coatings. IOP Conf. Series: Mater. Sci. Eng. 2012, 35, 012004. [Google Scholar] [CrossRef]
- Kania, H.; Liberski, P. Synergistic Influence of the Addition of Al, Ni and Pb to a Zinc Bath upon Growth Kinetics and Structure of Coatings. Solid State Phenom. 2013, 212, 115–120. [Google Scholar] [CrossRef]
- Pistofidis, N.; Vourlias, G.; Konidaris, S.; Pavlidou, E.; Stergiou, A.; Stergioudis, G. The effect of bismuth on the structure of zinc hot-dip galvanized coatings. Mater. Lett. 2007, 61, 994–997. [Google Scholar] [CrossRef]
- Katiforis, N.; Papadimitriou, G. Influence of copper, cadmium and tin additions in the galvanizing bath on the structure, thickness and cracking behaviour of the galvanized coatings. Surf. Coat. Technol. 1996, 78, 185–195. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Stergioudis, G.; Tsipas, D. The effect of alloying elements on the crystallization behaviour and on the properties of galvanized coatings. Cryst. Res. Technol. 2004, 39, 23–29. [Google Scholar] [CrossRef]
- Lekbir, C.; Dahoun, N.; Guetitech, A.; Hacid, A.; Ziouche, A.; Ouaad, K.; Djadoun, A. Effect of Immersion Time and Cooling Mode on the Electrochemical Behavior of Hot-Dip Galvanized Steel in Sulfuric Acid Medium. J. Mater. Eng. Perform. 2017, 26, 2502–2511. [Google Scholar] [CrossRef]
- Bondareva, O.S.; Melnikov, A.A.; Amosov, A. Influence of hot-dip galvanizing temperature on formation of zinc coating on a steel with a high silicon content. Adv. Environ. Biol. 2014, 8, 943–948. [Google Scholar]
- Gapsari, F.; Setyarini, P.H.; Anam, K.; Azizah, S.; Yuliati, R. The Effect of Hot Dip Galvanizing Temperature to Corrosion Rate of Steel as the Material for Chopper Machine. Solid State Phenom. 2019, 291, 148–154. [Google Scholar] [CrossRef]
- Bicao, P.; Jianhua, W.; Su, X.P.; Zhi, L.; Fucheng, Y. Effects of zinc bath temperature on the coatings of hot-dip galvanizing. Surf. Coat. Technol. 2008, 202, 1785–1788. [Google Scholar] [CrossRef]
- Chatterjee, B. Sherardizing. Met. Finish. 2004, 102, 40–46. [Google Scholar] [CrossRef]
- Wortelen, D.; Frieling, R.; Bracht, H.; Graf, W.; Natrup, F. Impact of zinc halide addition on the growth of zinc-rich layers generated by sherardizing. Surf. Coat. Tech. 2015, 263, 66–77. [Google Scholar] [CrossRef]
- Konstantinov, V.M.; Buloichyk, I.A. Some aspects of sherardizing implementation during anticorrosion protection of heat-treated metal parts. IOP Conf. Ser.: Mater. Sci. Eng. 2015, 71, 012063. [Google Scholar] [CrossRef]
- Pokorny, P.; Kolisko, J.; Balik, L.; Novak, P. Reaction kinetics of the formation of intermetallic Fe-Zn during hot-dip galvanizing of steel. Metalurgija 2016, 55, 111–114. [Google Scholar]
- Wienströer, S.; Fransen, M.; Mittelstädt, H.; Nazikkol, C.; Völker, M. Zinc/Iron Phase Transformation Studies on Galvannealed Steel Coatings by X-Ray Diffraction. Adv. X-ray Anal. 2003, 46, 291–296. [Google Scholar]
- Sonntag, B.; Thom, K.; Dambrowsky, N.; Dingwerth, B. Zinc-Niciel electroplating—Best Suited. Electrolytes for a diversity of apliccations. Galvanotechnik 2009, 7, 1499–1513. [Google Scholar]
- Fayomi, O.; Popoola, A. An Investigation of the Properties of Zn Coated Mild Steel. Int. J. Electrochem. Sci. 2012, 7, 6555–6570. [Google Scholar]
- Jędrzejczyk, D.; Szatkowska, E. The Impact of Heat Treatment on the Behavior of a Hot-Dip Zinc Coating Applied to Steel During Dry Friction. Materials 2021, 14, 660. [Google Scholar] [CrossRef]
- Borawski, A. Common Methods in Analysing the Tribological Properties of Brake Pads and Discs—A Review. Acta Mech. Et Autom. 2019, 13, 189–199. [Google Scholar] [CrossRef]
- Posmyk, A.; Myalski, J. The influence of using conditions on tribological properties of brake systems with composite disc. Tribologia 2019, 2, 117–124. [Google Scholar] [CrossRef]
- PKN. Standard–Zinc Diffusion Coatings on Steel Products–Sherardizing–Specification; PN-EN ISO 17668:2016-04; Polish Committee for Standardization: Warsaw, Poland, 2016. [Google Scholar]
- PKN. Standard—Fasteners, Zinc Coatings Applied by Hot-Dip Method; PN EN ISO 10684:2006/Ap1:2013-08; Polish Committee for Standardization: Warsaw, Poland, 2011. [Google Scholar]
- Azadeh, M.; Toroghinejad, M.R. Effect of Heat Treatment on Formability of Hot-dip Galvanized Low Carbon Steel Sheet. ISIJ Int. 2009, 49, 1945–1951. [Google Scholar] [CrossRef]
- Porter, F.C. Zinc Handbook: Properties, Processing, and Use in Design; CRC Press: New York, NY, USA, 1991. [Google Scholar]
- Fang, F.; Du, X.F.; Chen, Y.M. Galvanealed coating evolution for hot forming steel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012101. [Google Scholar] [CrossRef]
- Liberski, P.; Tatarek, A.; Kania, H.; Podolski, P. Coating growth on silicon-containing iron alloys in hot dip galvanizing process. In Proceedings of the 22nd International Galvanizing Conference Intergalva 2009, EGGA, Madrid, Spain, 8–12 June 2009; pp. 181–187. [Google Scholar]
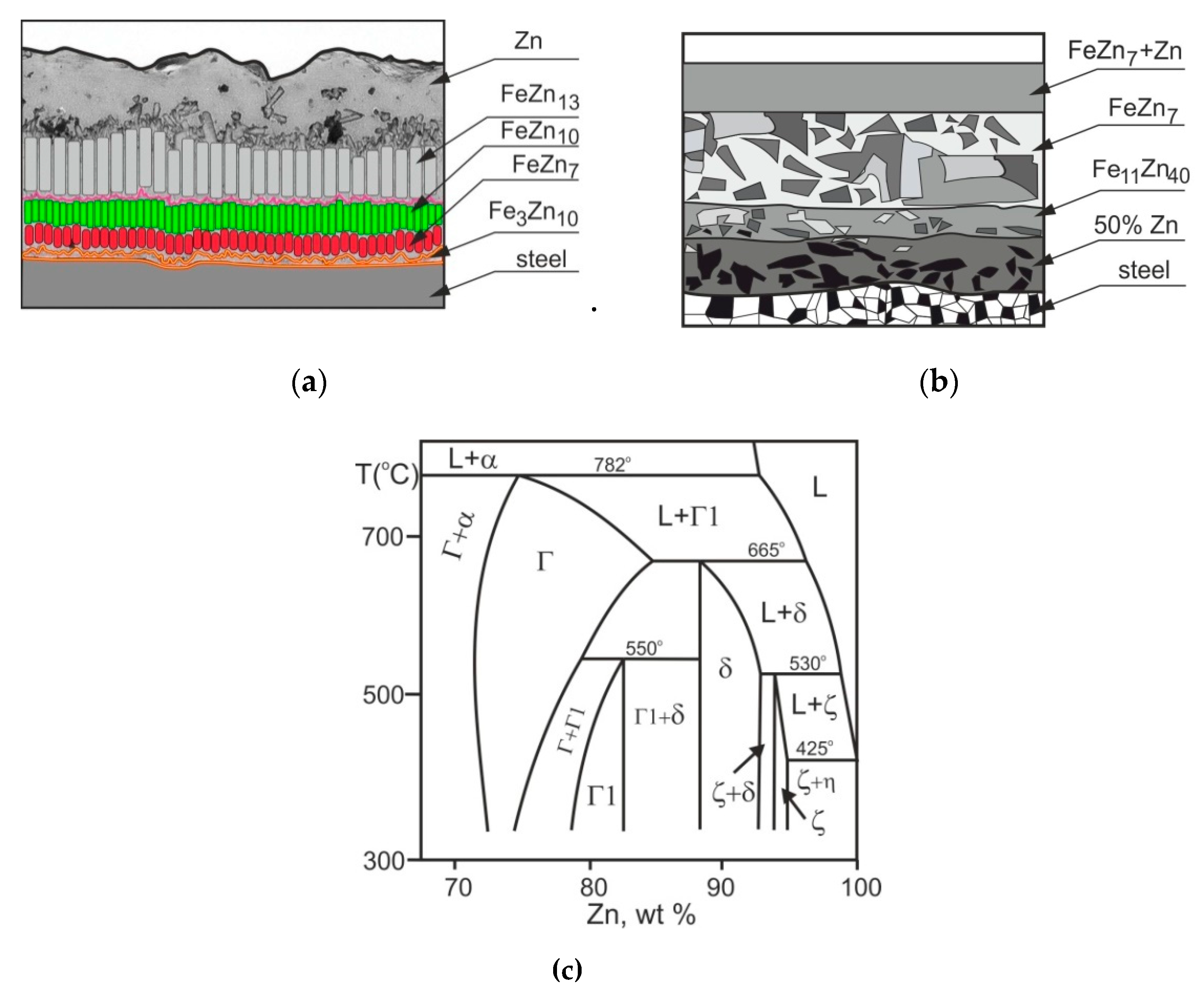

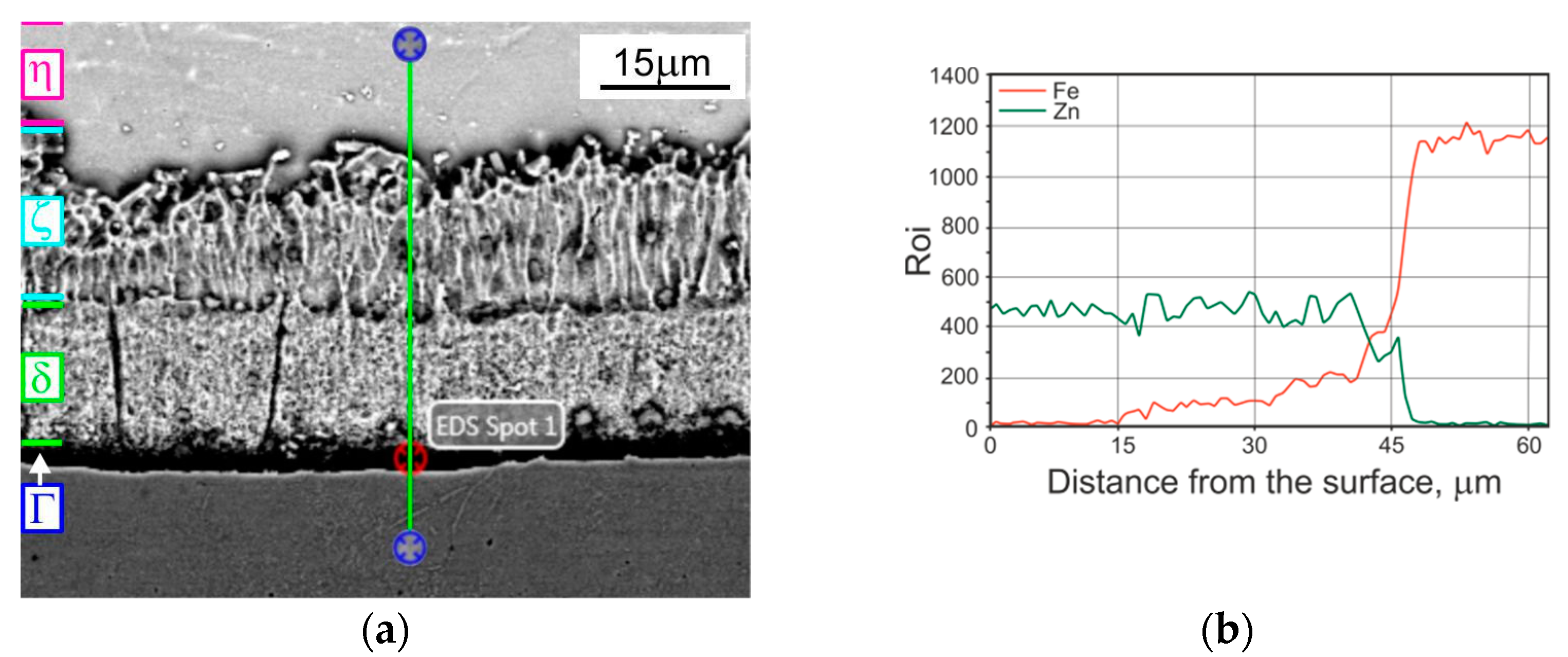
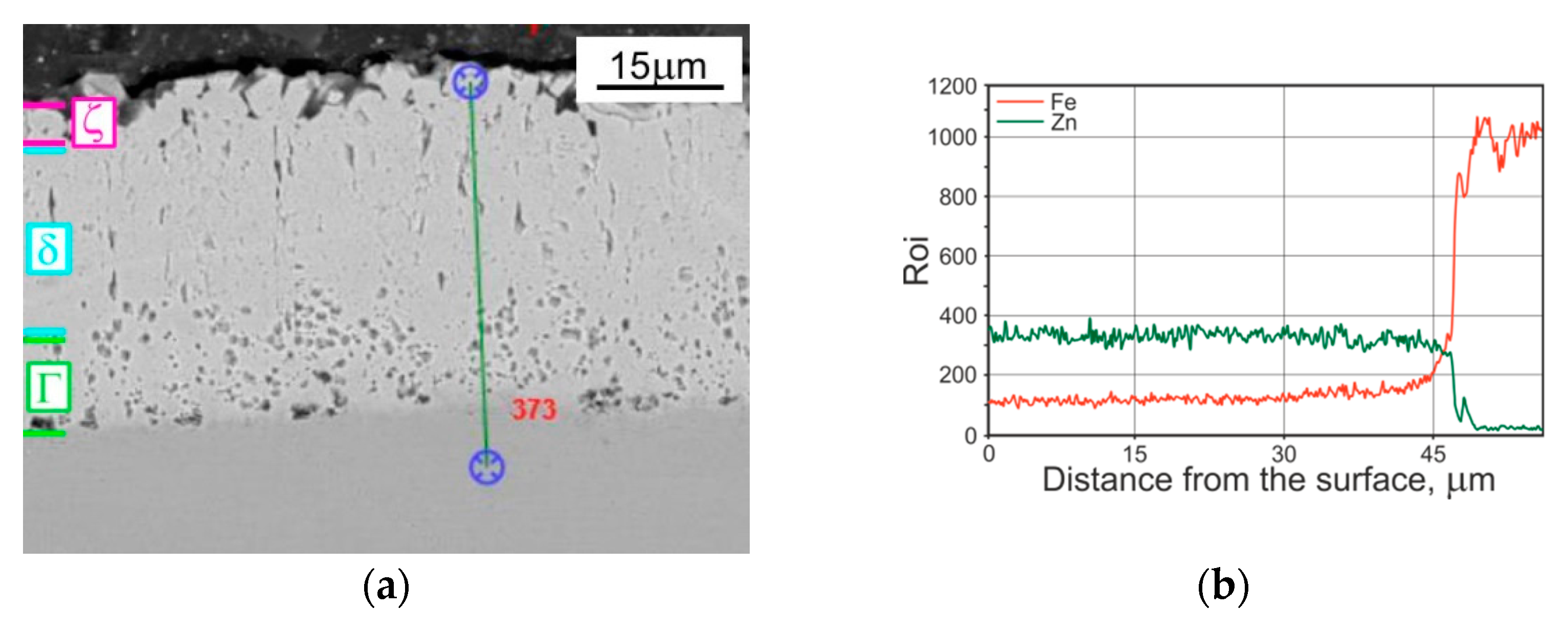
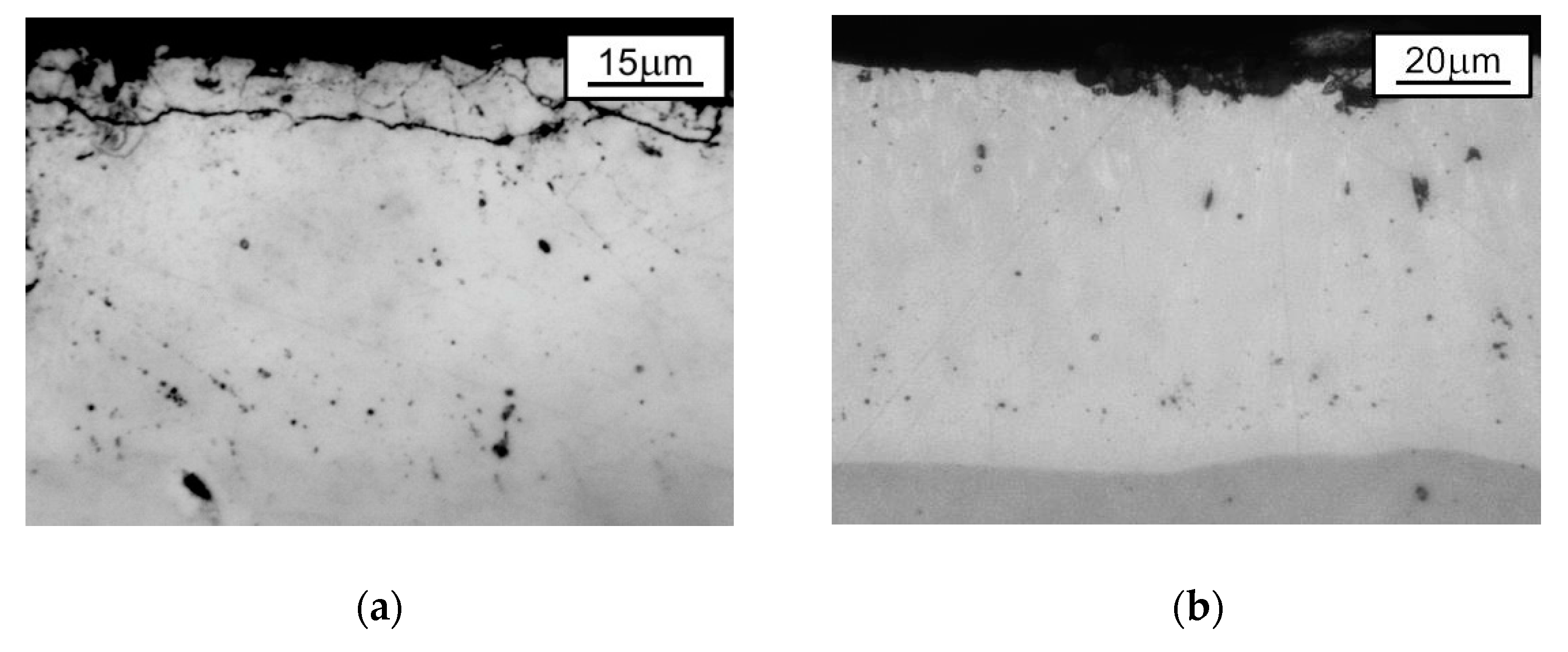
| Phase | Iron Content, wt. % | Hardness | |
|---|---|---|---|
| HD Coating | TD Coating | ||
| η–Zn | 0.03 [46] | 52 HV [16] 70 HB [23] | n.d. n.d. |
| ζ–FeZn13 | 6 [43]; 6.17 [46]; 5–6 [16]; 5.9–7.1[47] | 208 HV [16] 220 HB [23] | n.d. n.d. |
| δ–FeZn10 | 7–11.5 [16]; 7.87 [46] | 358HV [16] | n.d. |
| δ–(FeZn11–FeZn6.67) | 8.1–13.2 [47] | n.d. | n.d. |
| δ–FeZn7 | 7–10 [43]; 10.87 [46] | 270 HB [23] | 300 HB [23] |
| Г1–Fe5Zn21 | 17–19.6 [16]; 16.90 [46] | 505 HV [16] | n.d. |
| Г1–Fe11Zn40 | 19.02 [46] | n.d. | 350 HB [23] |
| Г–Fe3Zn10 | 23.5–28 [16]; 20.40 [46] | 326 HV [23] | n.d. |
| Г–(Fe5Zn21–Fe4Zn9) | 18–31 [47] | n.d. | 600 HB [23] |
| Element | Distance from the Coating Surface, µm | |||||||
| 5 | 10 | 15 | 20 | 25 | 30 | 35 | 45 | |
| Content of the Element, Wt% | ||||||||
| Fe | 11.07 | 11.10 | 10.96 | 13.20 | 18.03 | 20.21 | 22.45 | 91.95 |
| Zn | 88.93 | 88.90 | 89.04 | 86.80 | 81.97 | 79.26 | 77.55 | 1.13 |
| Roughness (Sa), µm | |||||||
| TD30 | TD60 | TD120 | TD240 | SB | T | HD | HDHT |
| Before Galvanizing | |||||||
| 6.22 | 5.66 | 4.59 | 4.19 | 6.80 | 8.38 | 3.63 | 2.29 |
| After Galvanizing | |||||||
| 2.50 | 2.81 | 2.60 | 2.44 | 2.84 | 3.35 | 2.94 | 3.43 |
| Average Friction Coefficient Value of Zinc Coating, µ | |||||||
| 0.25 | 0.36 | 0.39 | 0.38 | 0.28 | 0.20 | 0.27 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejczyk, D.; Skotnicki, W. Comparison of the Tribological Properties of the Thermal Diffusion Zinc Coating to the Classic and Heat Treated Hot-Dip Zinc Coatings. Materials 2021, 14, 1655. https://doi.org/10.3390/ma14071655
Jędrzejczyk D, Skotnicki W. Comparison of the Tribological Properties of the Thermal Diffusion Zinc Coating to the Classic and Heat Treated Hot-Dip Zinc Coatings. Materials. 2021; 14(7):1655. https://doi.org/10.3390/ma14071655
Chicago/Turabian StyleJędrzejczyk, Dariusz, and Wojciech Skotnicki. 2021. "Comparison of the Tribological Properties of the Thermal Diffusion Zinc Coating to the Classic and Heat Treated Hot-Dip Zinc Coatings" Materials 14, no. 7: 1655. https://doi.org/10.3390/ma14071655
APA StyleJędrzejczyk, D., & Skotnicki, W. (2021). Comparison of the Tribological Properties of the Thermal Diffusion Zinc Coating to the Classic and Heat Treated Hot-Dip Zinc Coatings. Materials, 14(7), 1655. https://doi.org/10.3390/ma14071655







