The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates for Biochar Production
2.2. Parameters of the Pyrolysis Process
2.3. Physicochemical and Physical Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. Pyrolysis Process Yield
3.2. Chemical Characteristics of the Biochar Produced
3.3. BET Specific Surface Area and Microstructure of Biochars
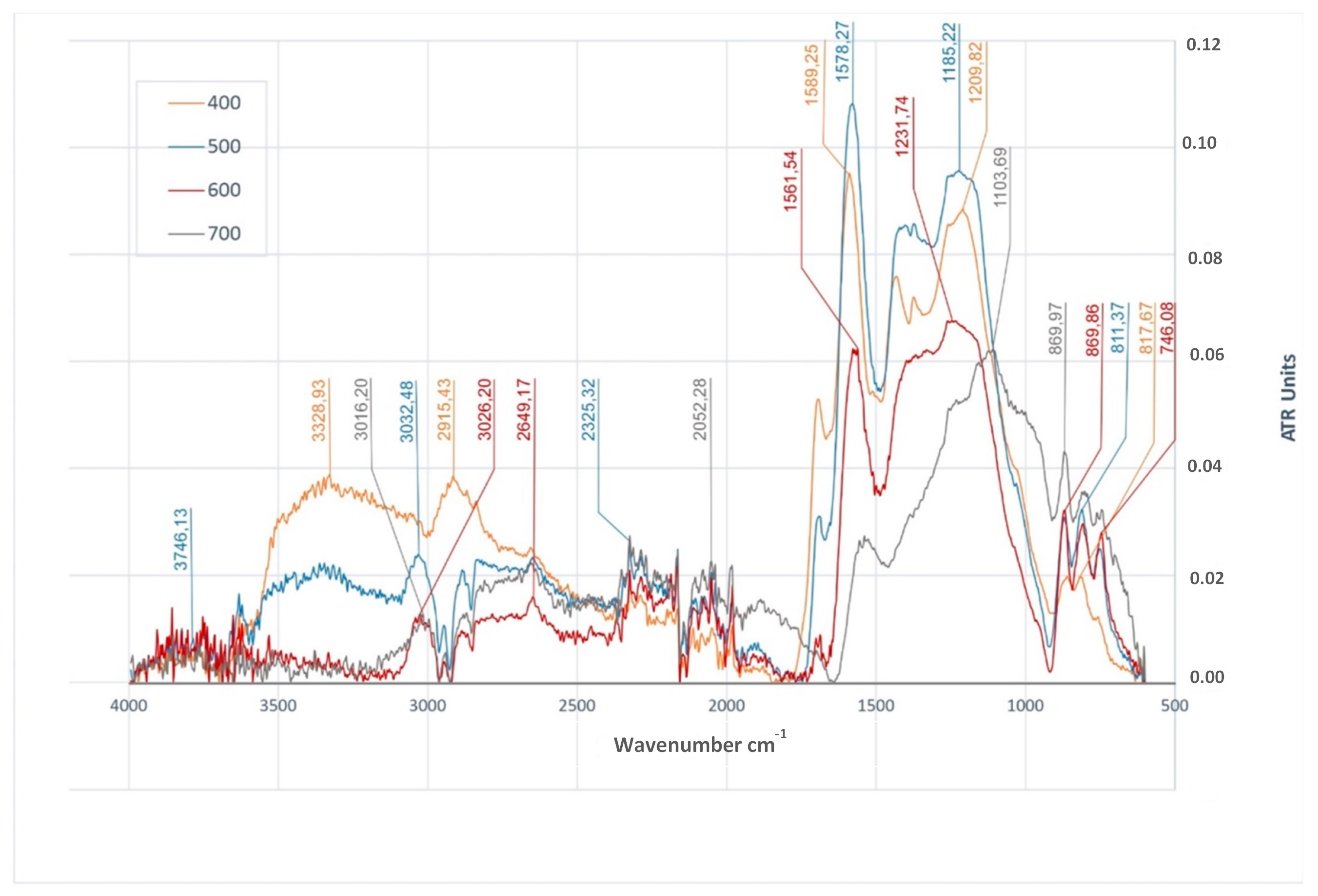
3.4. Functional Groups in Biochars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunnigan, L.; Morton, B.J.; Ashman, P.J.; Zhang, X.; Kwong, C.W. Emission characteristics of a pyrolysis-combustion system for the co-production of biochar and bioenergy from agricultural wastes. Waste Manag. 2018, 77, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.W.; Wang, M.Y.; Cao, Y.C.; Wu, S.C.; Liang, P.; Li, Y.N.; Zhang, J.Y.; Zhang, J.; Wong, M.H.; Shan, S.D.; et al. Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: Biochar properties and environmental risk from metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-Y.; Tsai, W.-T.; Chen, J.-W.; Lin, Y.-Q.; Chang, Y.-M. Characterization of biochar prepared from biogas digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef]
- Taherymoosavi, S.; Verheyen, V.; Munroe, P.; Joseph, S.; Reynolds, A. Characterization of organic compounds in biochar derived from municipal solid waste. Waste Manag. 2017, 67, 131–142. [Google Scholar] [CrossRef]
- Mohammadi, A.; Cowie, A.L.; Mai, T.L.A.; Brandão, M.; de la Rosa, R.A.; Kristiansen, P.; Joseph, S. Climate-change and health effects of using rice husk for biochar-compost: Comparing three pyrolysis systems. J. Clean. Prod. 2017, 162, 260–272. [Google Scholar] [CrossRef]
- Barber, S.T.; Yin, J.; Draper, K.; Trabold, T.A. Closing nutrient cycles with biochar- from filtration to fertilizer. J. Clean. Prod. 2018, 197, 1597–1606. [Google Scholar] [CrossRef]
- Efika, C.E.; Onwudili, J.A.; Williams, P.T. Infuence of heating rates on the products of high-temperature pyrolysis of waste wood pellets and biomass model compounds. Waste Manag. 2018, 76, 497–506. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, H.; Zhang, T.; Nan, X.; Ma, F. Effect of pyrolysis temperature on sulfur content, extractable fraction and release of sulfate in corn straw biochar. RSC Adv. 2018, 8, 35611–35617. [Google Scholar] [CrossRef]
- Bavariani, M.Z.; Ronaghi, A.; Ghasemi, R. Influence of pyrolysis tempeartures on FTIR analysis, nutrient bioavailability, and agricultural use of poultry manure biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Adsorption behavior of salicylic acid on biochar as derived from the thermal pyrolysis of barley straws. J. Clean. Prod. 2018, 195, 1162–1169. [Google Scholar] [CrossRef]
- Bai, X.; Li, Z.; Zhang, Y.; Ni, J.; Wang, X.; Zhou, X. Recovery of ammonium in urine by biochar derived from faecal sludge and its application as soil conditioner. Waste Biomass Valorization 2018, 9, 1619–1628. [Google Scholar] [CrossRef]
- Batool, S.; Idrees, M.; Hussain, Q.; Kong, J. Adsorption of copper (II) by using derived-farmyard and poultry manure biochars: Efficiency and mechanism. Chem. Phys. Lett. 2017, 689, 160–198. [Google Scholar] [CrossRef]
- Kleemann, R.; Chenoweth, J.; Clift, R.; Morse, S.; Pearce, P. Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolysed sewage sludge char (PSSC). Waste Manag. 2017, 60, 201–210. [Google Scholar] [CrossRef]
- Malińska, K.; Dach, J. Biochar as a supplementary material for biogas production. Ecol. Eng. 2015, 41, 117–124. (In Polish) [Google Scholar] [CrossRef]
- Malińska, K. Biochar is a response to current environmental problems. Eng. Prot. Environ. 2012, 15, 387–403. (In Polish) [Google Scholar]
- Roberst, D.A.; Cole, A.J.; Whelan, A.; de Nys, R.; Paul, N.A. Slow pyrolysis enhances the recovery and reuse of phosphorus and reduces metal leaching from biosolids. Waste Manag. 2017, 64, 133–139. [Google Scholar]
- Li, F.; Liang, X.; Niyungeko, C.; Sun, T.; Liu, F.; Arai, Y. Effects of biochar amendments on soil phosphorus transformation in agricultural soils. Adv. Agron. 2019, 158, 131–172. [Google Scholar] [CrossRef]
- Ibarrola, R.; Shackley, S.; Hammond, J. Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manag. 2012, 32, 859–868. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, B.; Sun, M.; Chen, X.; Zhang, M.; Li, H.; Shaucong, X. Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell by TG-DTG-MS and residue analysis. Waste Manag. 2017, 62, 91–100. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Charcterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Mia, S.; Uddin, M.E.; Kader, M.A.; Ahsan, A.; Mannan, M.A.; Hossain, M.M.; Solaiman, Z.M. Pyrolysis and co-composting of municipal organic waste in Bangladesh: A quantitative estimate of recyclable nutrients, greenhouse gas emissions, and economic benefits. Waste Manag. 2018, 75, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Wang, M.; Chen, H.; Zhang, Z. Combing biochar, zeolite and wood vinegar for composting of pig manure: The effect on greenhouse gas emission and nitrogen conservation. Waste Manag. 2018, 74, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent development in biochor utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, H.; Zhao, X.; Cui, H.; Wei, Z. Reducing nitrogen loss and phytotoxicity during beer vinasse composting with biochar addition. Waste Manag. 2017, 61, 150–156. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Kalus, K.; Andersen, D.S.; Opalinski, S. Pilot-scale testing of non-activated biochar for swine manure treatment and mitigation of ammonia, hydrogen sulfide, odorous volatile organic compounds (VOCs), and greenhouse gas emissions. Sustainability 2017, 9, 929. [Google Scholar] [CrossRef]
- Janczak, D.; Malińska, K.; Czekała, W.; Caceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Malińska, K.; Zabochnicka-Świątek, M.; Dach, J. Effects of biochar amendment on ammonia emission during composting of sewage sludge. Ecol. Eng. 2014, 71, 474–478. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Siles, J.A.; Diz, J.; Chica, A.F.; Martin, M.A. Modeling of composting process of different organic waste at pilot scale: Biodegradability and odor emissions. Waste Manag. 2017, 59, 48–58. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Manera, C.; Silvestre, W.P.; Pauletti, G.F.; Altafini, C.R.; Godinho, M. Use of biochar produced from elephant grass by pyrolysis in a screw reactor as a soil amendment. Waste Biomass Valorization 2019, 10, 3089–3100. [Google Scholar] [CrossRef]
- Czekała, W.; Jeżowska, A.; Chełkowski, D. The use of biochar for the production of organic fertilizers. J. Ecol. Eng. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of feedstock and pyrolysis temperature on properties of biochor governing end use efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Rycaj, M.; Lehmann, J.; Cornelissen, G. Influence of activated carbon and biochar on phytotoxicity of air- dried sewage sludge to Lepidium sativum. Ecotoxicol. Environ. Saf. 2012, 80, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef]
- Zama, E.F.; Zhu, Y.-G.; Reid, B.J.; Sun, G.-X. The role of biochar properties in influencing the sorption and desorption of Pb (II), Cd (II) and as (III) in aqueous solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200, 673–680. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, R.; Ma, F.; Li, Y.; Wang, L. Effects of biochar derived from chicken manure and rape straw on speciation and phytoavailability of Cd to maize in artificially contaminated loess soil. J. Environ. Manag. 2016, 184, 569–574. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E. Effects of pyrolysis temperature on soybean stover–and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 activetd biochar from solid waste of a beer industry and its application for methylene blue adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pitman, C.U., Jr. Modeling and evaluation of chromium remediation from using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Dinelli, F.D.; Kenar, J.A.; Jackson, M.A.; Thomas, A.J.; Peterson, S.C. Physical and chemical properties of pyrolyzed biosolids for utilization in sand-based turfgrass rootzones. Waste Manag. 2018, 76, 98–105. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. pyrolysis 2012, 9, 138–145. [Google Scholar] [CrossRef]
- Wystalska, K.; Malińska, K.; Włodarczyk, R.; Chajczyk, O. Effects of pyrolysis parameters on the yield and properties of biochar from pelletized sunflower husk. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 44, p. 00197. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Production and characterization of a value added biochar mix using seaweed, rice husk and pine sawdust: A parametric study. J. Clean. Prod. 2018, 200, 641–656. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Yuan, Y.; Wang, R.; Wen, Y.; Man, J. Comparative study for microcystin-LR sorption onto biochars produced from various plant- and animal- wastes at different pyrolysis temperatures: Influencing mechanisms of biochar properties. Bioresour. Technol. 2018, 24, 794–803. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Steibel, M.; Spliethoff, H.; Glarborg, P. Influence of fast pyrolysis conditions on yield and structural transformation of biomass chars. Fuel Process. Technol. 2015, 140, 205–214. [Google Scholar] [CrossRef]
- Konczak, M.; Oleszczuk, P. Co-pyrolysis of sewage sludge and biomass in carbon dioxide as a carrier gas affects the total and leachable metals in biochars. J. Hazard. Mater. 2020, 400, 123144. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Martoglio-Smith, P.A.; Hajaligol, M.R. Characterization of char from the pyrolysis of tobacco. J. Agirc. Food Chem. 2002, 50, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanism of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Hasnan, F.I.; Iamail, K.N.; Mus, M.; Jaapar, J.; Alwi, H.; Hamid, K.K. Characterization of bio char derived from tapioca skin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012016. [Google Scholar] [CrossRef]
- Giudicianni, P.; Pindozzi, S.; Grottola, C.M.; Stanzione, F.; Faugno, S.; Fagnano, M.; Fiorentino, N.; Ragucci, R. Pyrolysis for exploitation of biomasses selected for soil phytoremediation: Characterization of gaseous and solid products. Waste Manag. 2017, 61, 288–299. [Google Scholar] [CrossRef]
- Shoi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar climate and soil, A review to guide future research. CSIRO Land Water Sci. Rep. 2009, 5, 1–59. [Google Scholar]
- Lehman, J. Bio-energy in the black. Front. Ecol Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Chuanming, X.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering application of biochar for removal organic pollutants. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Mosa, A.; Gao, B.; Yin, X.; Ahmad, Z.; Wang, H. Sorption of lead ions onto oxidized bagasse-biochar mitigates Pb-induced oxidative stress on hydroponically grown chicory: Experimental observations and mechanisms. Chemosphere 2018, 208, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Regkouzas, P.; Diamadopoulos, E. Adsorption of selected organic micro-pollutants on sewage sludge biochar. Chemosphere 2019, 224, 840–851. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Zheng, Y.; Hu, X.; Creamer, A.E.; Annable, M.D.; Li, Y. Biochar for volatile organic compound (VOC) removal: Sorption performance and governing mechanisms. Bioresour. Technol. 2017, 245, 606–614. [Google Scholar] [CrossRef]
- Yao, S.; Li, X.; Cheng, H.; Zhang, C.; Bian, Y.; Jiang, X.; Song, Y. Resource utylization of a typical vegetable waste as biochars in removing phthalate acid esters from water: A sorption case study. Bioresour. Technol. 2019, 293, 122081. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.A.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.J.; Liu, Y. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Li, A.-Y.; Deng, H.; Ye, C.-H.; Wu, Y.-Q.; Linmu, Y.-D.; Hang, H.-L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Xu, D.; Cao, J.; Li, Y.; Howard, A.; Kewei, Y. Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: A case study on ammonium adsorption capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolisis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solution: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Qi, F.; Yan, Y.; Lamb, D.; Naidu, R.; Bolan, N.S.; Liu, Y.; Ok, Y.S.; Donne, S.W.; Semple, K.T. Thermal stability of biochar and its effects on cadmium sorption capacity. Bioresour. Technol. 2017, 246, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Sarkar, B.; Igalavithana, A.D.; Ok, Y.S.; Yang, X.; Lombi, E.; Bolan, N. Mechanistic insights of 2,4-D sorption onto biochar: Influence of feedstock material and biochar properties. Bioresour. Technol. 2017, 246, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Viglasova, E.; Galambos, M.; Dankova, Z.; Krivosudsky, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matik, M.; Briancin, J. Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef]
- Rittl, T.F.; Butterbach-Bahl, K.; Basile, C.M.; Pereira, L.A.; Alms, V.; Dannenmann, M.; Couto, E.G.; Ceri, C.E.P. Greenhouse gas emissions from soil amended with agricultural residua biochars: Effects of feedstock type, production temperature and soil moisture. Biomass Bioenergy 2017, 117, 1–9. [Google Scholar] [CrossRef]
- PN-EN ISO 18122:2016-01-Polish Version, Solid Biofuels—Determination of Ash Content.
- PN-ISO 10694:2002—Soil Quality—Determination of Organic Carbon Content and Total Carbon Content after Dry Combustion (Elemental Analysis).
- PN-EN 16169:2012—Polish Standard. Sewage Sludge, Treated Bio-Waste and Soil. Determination of Nitrogen by the Kjeldahl Method.
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, D.M.; Vithanage, M.; Lee, S.S.; Ok, S.Y. Biochar as a sorbent for contaminant management In soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, R. Biochar production technology. In Biochar for Environmental Management Science and Technology: Earth Scans; Lehmann, J., Joseph, S., Eds.; Earthscan Publ.: London, UK, 2009; pp. 127–146. [Google Scholar]
- De Wildt, P.; Reith, H.; Heeres, H.J. Biomass pyrolysis for chemicals. Biofuels 2011, 2, 185–208. [Google Scholar] [CrossRef]
- Yang, S.I.; Wu, M.S.; Wu, C.Y. Application of biomass fast pyrolysis part I: Pyrolysis characteristics and products. Energy 2014, 66, 162–171. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Lopez-Capel, E.; Pena, J.; Rieradevall, J.; Montero, J.I.; Puy, N. Technical feasibility and carbon footprint of biochar co-production with tomato plant residue. Waste Manag. 2017, 67, 121–130. [Google Scholar] [CrossRef]
- Ruiz-Gomez, N.; Quispe, V.; Abrego, J.; Atienza-Martinez, M.; Murillo, M.B.; Gea, G. Co-pyrolysis of sewage sludge and manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef]
- EBC. European Biochar Certificate—Guidelines for Sustainable Production of Biochar. European Biochar Foundation; EBC: Arbaz, Switzerland, 2012. Available online: http://www.european-biochar.org/en/download (accessed on 30 August 2018). [CrossRef]
- Srinivasan, P.; Sarmah, A.K.; Smernik, R.; Das, O.; Farid, M.; Gao, W. A feasibility study of agricultural and sewage biomass as biochar, bioenergy and biocomposite feedstock: Production, characterization and potential application. Sci. Total Environ. 2015, 512, 495–505. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, F.; Zhang, H.; Shao, L.; Chen, D.; He, P. Multiscale visualization of the structural and characteristic changes of sewage sludge biochar oriented towards potential agronomic and environmental implication. Sci. Rep. 2015, 5, 9406. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, K.K. A short note on investigation and remediation of contaminated groundwater and soil in Korea. J. Eng. Geol. 2004, 14, 123–130. [Google Scholar]
- Molenda, J.; Swat, M.; Osuch-Słomka, E. Effects of thermal conditions of pyrolysis process on the quality of biochar obtained from vegetable waste. Eng. Prot. Environ. 2018, 21, 289–302. [Google Scholar] [CrossRef]
- Schulzki, G.; Nublein, B.; Sievers, H. Transition rates of selected metals determined in various types of teas (Camellia sinensis L. Kuntze) and herbal/fruit infusions. Food Chem. 2017, 215, 22–30. [Google Scholar] [CrossRef]
- Hu, X.; Guo, H.; Gholizadeh, M.; Sattari, B.; Liu, Q. Pyrolysis of different wood species: Impacts of C/H ratio in feedstock on distribution of pyrolysis products. Biomass Bioenergy 2019, 120, 28–39. [Google Scholar] [CrossRef]

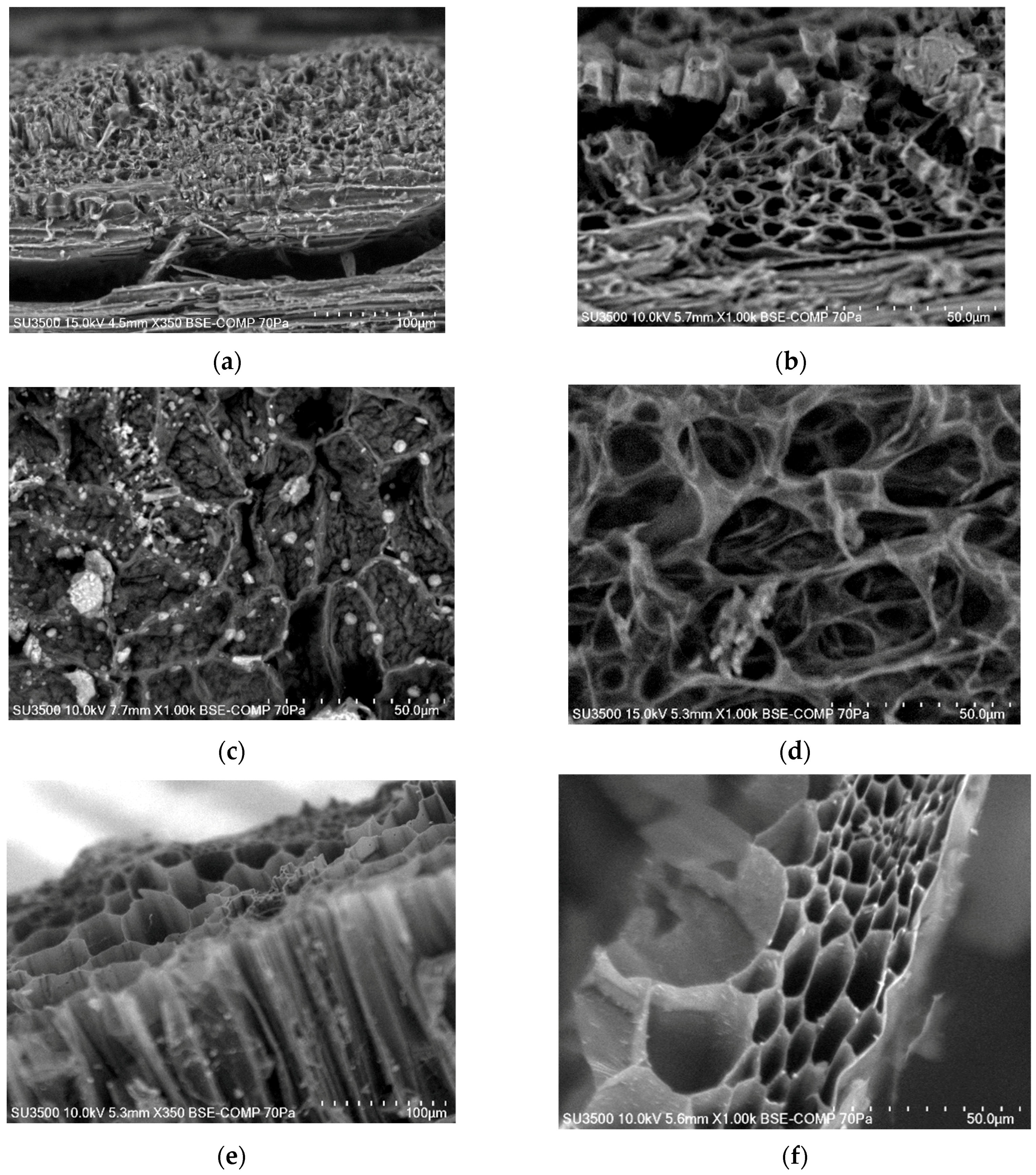
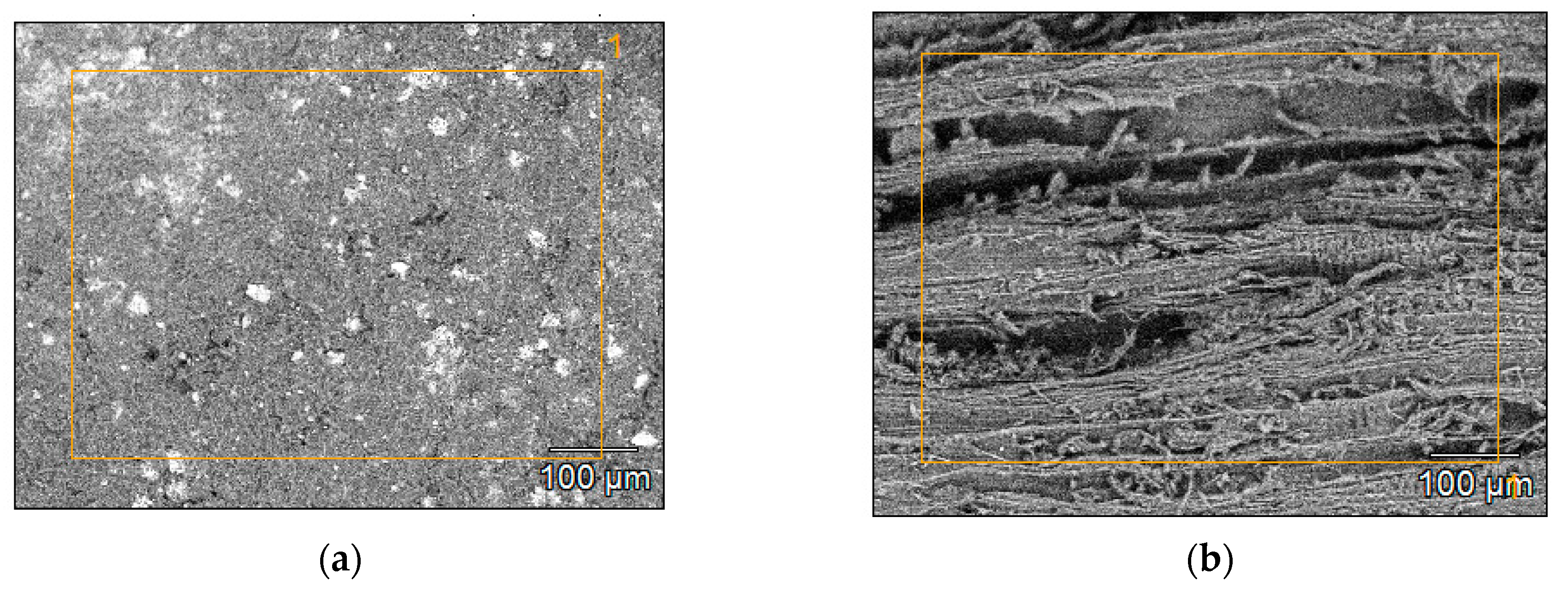
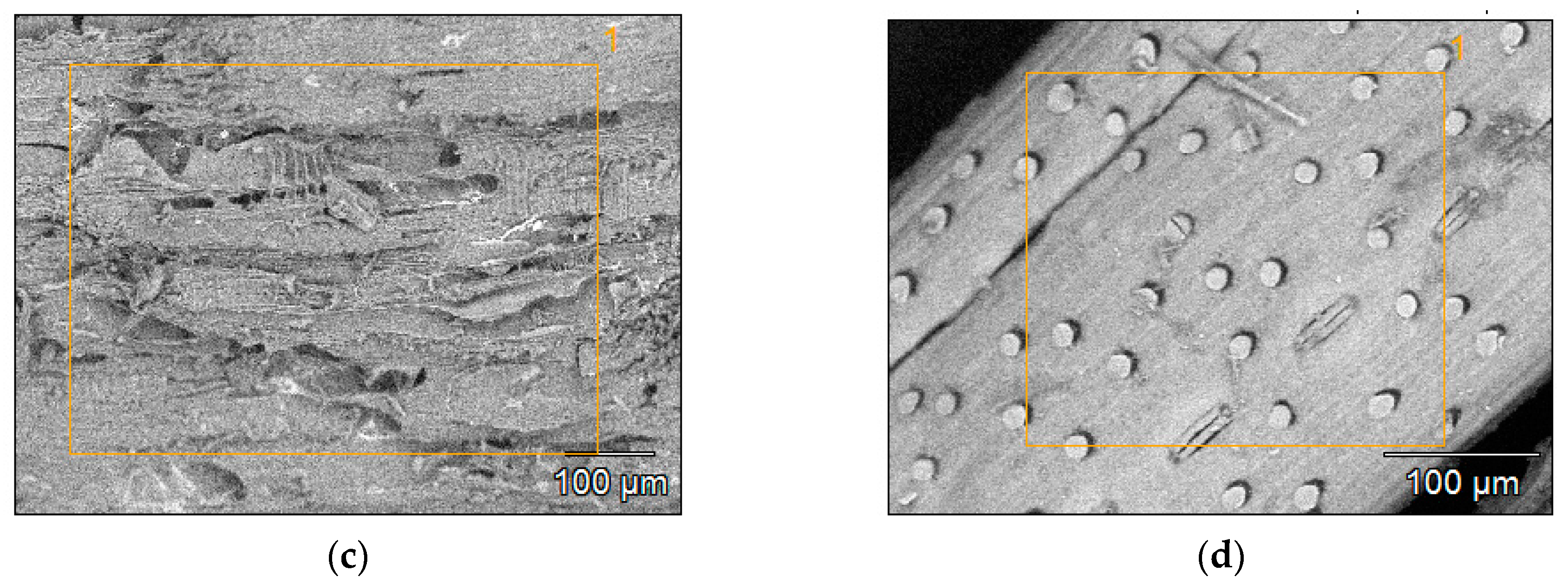
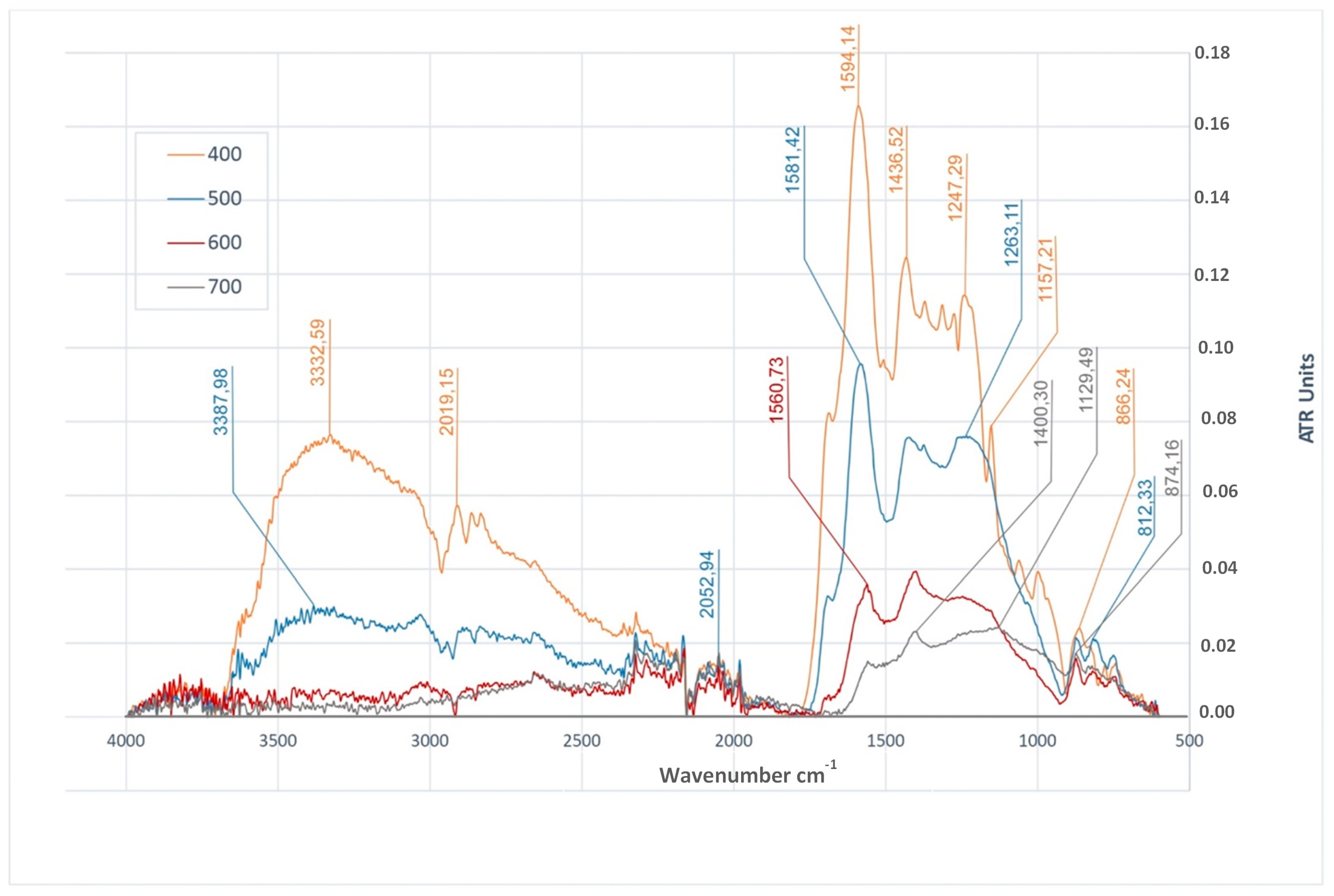
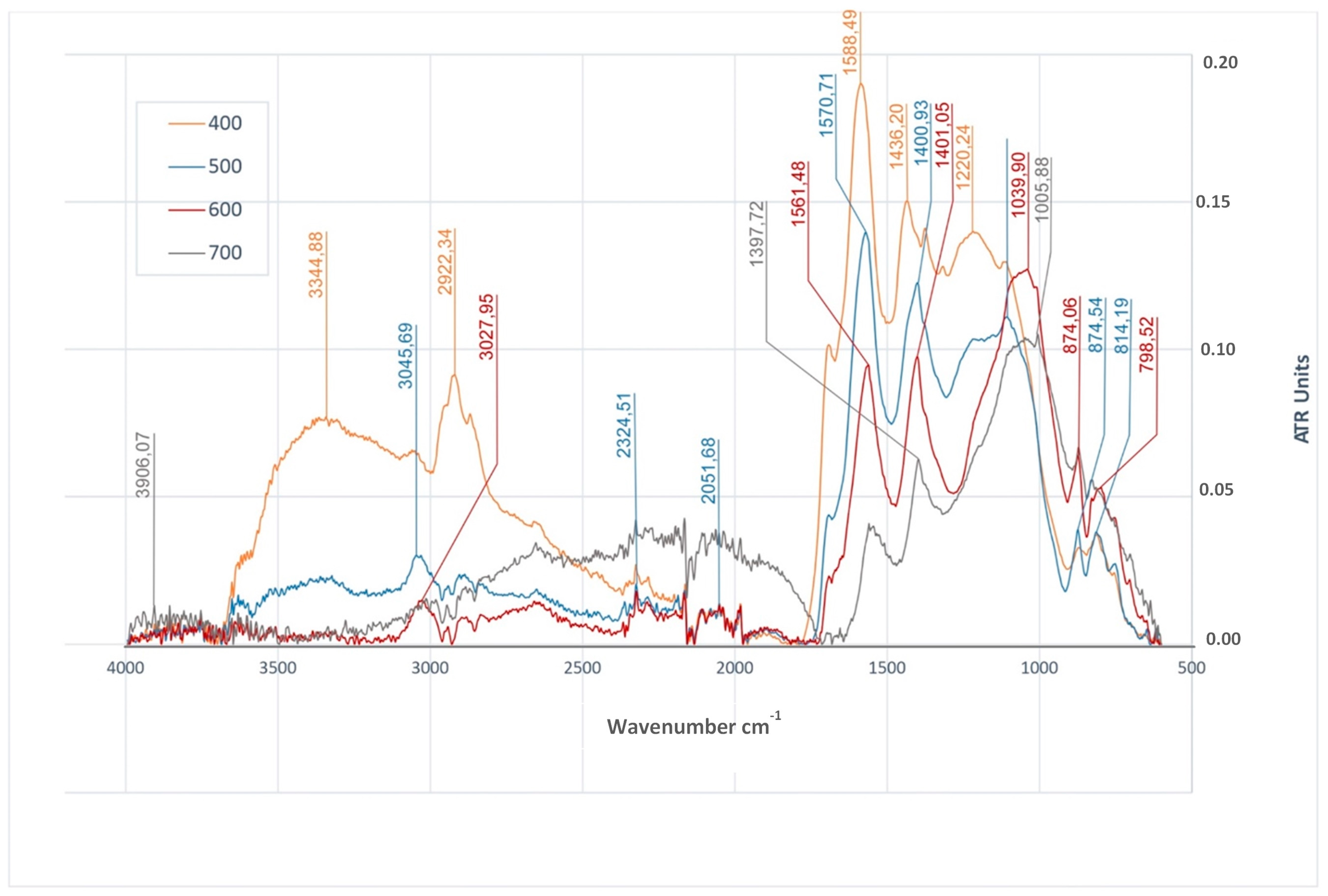
| MC% | OM%dm | Ash%dm | pHH2O | NK% | TC%dm | |
|---|---|---|---|---|---|---|
| WRS | 11.96 ± 1.52 | 96.08 ± 1.75 | 3.91 ± 1.75 | 7.05 | 0.507 | 44.260 |
| WS | 13.38 ± 0.17 | 98.94 ± 0.10 | 1.06 ± 0.10 | 4.98 | 0.299 | 49.923 |
| WC | 7.19 ± 0.04 | 99.34 ± 0.41 | 0.66 ± 0.41 | 5.11 | 0.213 | 50.101 |
| Type of Biochar | Yield | pHH2O | OM | Ash | N | C | H | S | TOC | H/C |
|---|---|---|---|---|---|---|---|---|---|---|
| % | % d.m. | |||||||||
| BWRS400 | 34.4 | 8.35 ± 0.08 | 89.45 ± 0.45 | 10.55 ± 0.45 | 0.28 ± 0.07 | 69.11 ± 0.69 | 3.87 ± 0.04 | - | 69.0 | 0.67 |
| BWRS500 | 29.5 | 9.52 ± 0.04 | 88.36 ± 0.50 | 11,64 ± 0.50 | 0.32 ± 0.05 | 74.40 ± 0.13 | 2.97 ± 0.01 | - | 69.9 | 0.48 |
| BWRS600 | 29.1 | 9.89 ± 0.01 | 86.12 ± 0.22 | 13.88 ± 0.22 | 0.65 ± 0.01 | 77.32 ± 0.12 | 2.33 ±0.13 | - | 76.5 | 0.36 |
| BWRS700 | 25.5 | 10.10 ± 0.11 | 85.56 ± 0.61 | 14.44 ± 0.61 | 0.98 ± 0.12 | 78.48 ± 0.19 | 1.45 ± 0.12 | - | 77.9 | 0.22 |
| BWS400 | 40.5 | 5.98 ± 0.06 | 98.94 ± 0.28 | 1.06 ± 0.28 | - | 79.40 ± 1.16 | 3.96 ± 0.06 | - | 79.2 | 0.60 |
| BWS500 | 34.2 | 6.90 ± 0.05 | 98.05 ± 0.09 | 1.95 ± 0.09 | - | 78.03 ± 5.93 | 3.22 ± 0.03 | - | 77.3 | 0.49 |
| BWS600 | 31.4 | 7.37 ± 0.01 | 97.38 ± 0.17 | 2.62 ± 0.17 | - | 63.24 ± 0.95 | 2.20 ± 0.02 | - | 62.5 | 0.42 |
| BWS700 | 30.2 | 9.85 ± 0.01 | 97.00 ± 0.31 | 3.00 ± 0.31 | - | 77.38 ± 0.67 | 1.38 ± 0.05 | - | 76.4 | 0.21 |
| BWC400 | 31.7 | 4.45 ± 0.04 | 98.65 ± 0.08 | 1.35 ± 0.08 | - | 76.65 ± 0.35 | 4.20 ± 0.11 | - | 75.6 | 0.66 |
| BWC500 | 25.1 | 5.18 ± 0.03 | 98.81 ± 0.06 | 1.18 ± 0.06 | - | 82.39 ± 0.81 | 3.02 ± 0.01 | - | 81.9 | 0.44 |
| BWC600 | 23.2 | 6.57 ± 0.04 | 98.51 ± 0.05 | 1.49 ± 0.05 | - | 88.03 ± 6.61 | 2.53 ± 0.32 | - | 87.0 | 0.34 |
| BWC700 | 22.0 | 7.82 ± 0.03 | 98.37 ± 0.08 | 1.61 ± 0.08 | - | 84.61 ± 10.03 | 1.47 ± 0.14 | - | 84.0 | 0.21 |
| pH | Ash | N | C | H | BET | |
|---|---|---|---|---|---|---|
| Main effect—type of substrate | 13,749.03 *** | 5134.79 *** | 681.22 *** | 0.08 | 5.08 * | 12,447.77 *** |
| Main effect—temperature | 5486.10 *** | 86.53 *** | 58.53 *** | 1.85 | 737.42 *** | 8096.43 *** |
| Interactive effect—type of substrate * temperature | 446.88 *** | 24.27 *** | 58.53 *** | 2.16 | 3.56 * | 2807.52 *** |
| pH | Ash | N | C | H | BET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| BWRS | 9.47 | 0.71 | 12.63 | 1.71 | 0.56 | 0.30 | 74.83 | 3.79 | 2.66 | 0.93 | 7.41 | 3.94 | |
| BWS | 7.53 | 1.50 | 2.16 | 0.79 | 0.00 | 0.00 | 74.51 | 7.33 | 2.69 | 1.03 | 81.79 | 80.23 | |
| BWC | 6.01 | 1.35 | 1.41 | 0.18 | 0.00 | 0.00 | 76.53 | 24.94 | 2.81 | 1.04 | 191.51 | 156.48 | |
| 400 | 6.26 | 1.70 | 4.32 | 4.68 | 0.09 | 0.14 | 66.54 | 25.38 | 4.01 | 0.16 | 2.70 | 1.45 | |
| 500 | 7.20 | 1.89 | 4.92 | 5.05 | 0.11 | 0.16 | 78.27 | 4.58 | 3.07 | 0.12 | 37.28 | 39.40 | |
| 600 | 7.94 | 1.50 | 6.00 | 5.93 | 0.22 | 0.33 | 76.20 | 11.27 | 2.35 | 0.23 | 161.65 | 131.26 | |
| 700 | 9.26 | 1.08 | 6.35 | 6.11 | 0.33 | 0.49 | 80.16 | 6.05 | 1.43 | 0.10 | 172.66 | 156.15 | |
| BWRS | 400 | 8.35 | 0.08 | 10.55 | 0.45 | 0.28 | 0.07 | 69.11 | 0.69 | 3.87 | 0.04 | 4.52 | 0.06 |
| 500 | 9.52 | 0.04 | 11.64 | 0.50 | 0.32 | 0.05 | 74.40 | 0.13 | 2.97 | 0.01 | 12.99 | 0.07 | |
| 600 | 9.89 | 0.01 | 13.88 | 0.22 | 0.65 | 0.01 | 77.32 | 0.12 | 2.33 | 0.13 | 8.71 | 0.05 | |
| 700 | 10.10 | 0.11 | 14.44 | 0.61 | 0.98 | 0.12 | 78.48 | 0.19 | 1.45 | 0.12 | 3.43 | 0.06 | |
| BWS | 400 | 5.98 | 0.06 | 1.06 | 0.28 | 0.00 | 0.00 | 79.40 | 1.16 | 3.96 | 0.06 | 1.23 | 0.02 |
| 500 | 6.90 | 0.05 | 1.95 | 0.09 | 0.00 | 0.00 | 78.03 | 5.93 | 3.22 | 0.03 | 9.11 | 0.13 | |
| 600 | 7.37 | 0.01 | 2.62 | 0.17 | 0.00 | 0.00 | 63.24 | 0.95 | 2.20 | 0.02 | 164.48 | 1.83 | |
| 700 | 9.85 | 0.01 | 3.00 | 0.31 | 0.00 | 0.00 | 77.38 | 0.67 | 1.38 | 0.05 | 152.35 | 4.00 | |
| BWC | 400 | 4.45 | 0.04 | 1.35 | 0.08 | 0.00 | 0.00 | 51.10 | 44.26 | 4.20 | 0.11 | 2.36 | 0.05 |
| 500 | 5.18 | 0.03 | 1.18 | 0.06 | 0.00 | 0.00 | 82.39 | 0.81 | 3.02 | 0.01 | 89.73 | 2.86 | |
| 600 | 6.57 | 0.04 | 1.49 | 0.05 | 0.00 | 0.00 | 88.03 | 6.61 | 2.53 | 0.32 | 311.77 | 4.04 | |
| 700 | 7.82 | 0.03 | 1.61 | 0.08 | 0.00 | 0.00 | 84.61 | 10.03 | 1.47 | 0.14 | 362.19 | 7.44 | |
| Type of Biochar | 400 °C | 500 °C | 600 °C | 700 °C |
|---|---|---|---|---|
| m2·g−1 | ||||
| BWRS | 4 | 12 | 8 | 3 |
| BWS | 1 | 9 | 164 | 152 |
| BWC | 2 | 89 | 311 | 363 |
| FTIR Peak cm−1 | Characteristic Vibrations | BWC | BWS | BWRS | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | 700 °C | 400 °C | 500 °C | 600 °C | 700 °C | 400 °C | 500 °C | 600 °C | 700 °C | |||
| 3328–3387 | O–H stretching | + | − | − | − | + | + | − | − | + | + | − | − | [8,33,36,48,71,88] |
| 2915–3050 | C–H stretching | + | + | + | + | + | + | + | − | + | + | + | − | [8,33,48,49,88,93] |
| 1700 | C=O stretching | + | + | + | − | + | + | − | − | + | + | + | − | [8,36,52,71,88] |
| 1540–1594 | C=C stretching | + | + | + | + | + | + | + | + | + | + | + | + | [8,33,36,48,71,88,91,93] |
| 1397–1470 | C–H deformation | + | + | + | − | + | + | + | + | + | + | + | + | [10,36,71] |
| 1200–1290 | O–H deformation | + | − | − | − | + | + | − | − | + | + | − | − | [8,93] |
| 1005–1231 | C–O stretching | − | + | + | + | + | − | + | + | + | + | + | + | [8,33,52,88,91] |
| 746–874 | =C–H bending | + | + | + | + | + | + | + | + | + | + | + | − | [8,36,48,52,88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wystalska, K.; Kwarciak-Kozłowska, A. The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties. Materials 2021, 14, 1644. https://doi.org/10.3390/ma14071644
Wystalska K, Kwarciak-Kozłowska A. The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties. Materials. 2021; 14(7):1644. https://doi.org/10.3390/ma14071644
Chicago/Turabian StyleWystalska, Katarzyna, and Anna Kwarciak-Kozłowska. 2021. "The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties" Materials 14, no. 7: 1644. https://doi.org/10.3390/ma14071644
APA StyleWystalska, K., & Kwarciak-Kozłowska, A. (2021). The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties. Materials, 14(7), 1644. https://doi.org/10.3390/ma14071644






