The Influence of Nanometals, Dispersed in the Electrophoretic Nanohydroxyapatite Coatings on the Ti13Zr13Nb Alloy, on Their Morphology and Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Electrophoretic Deposition
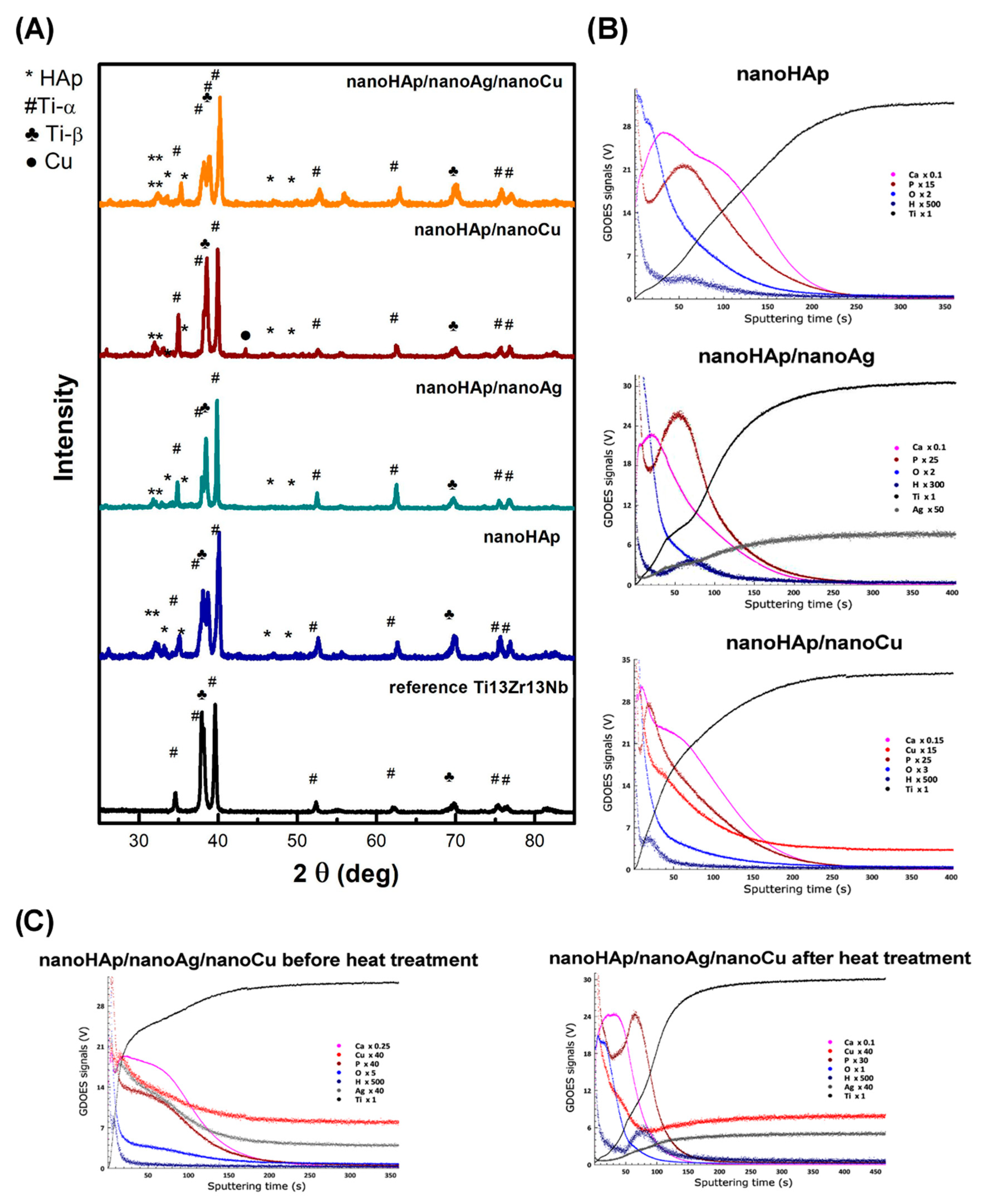
2.3. Microstructure, Phase, and Chemical Examinations
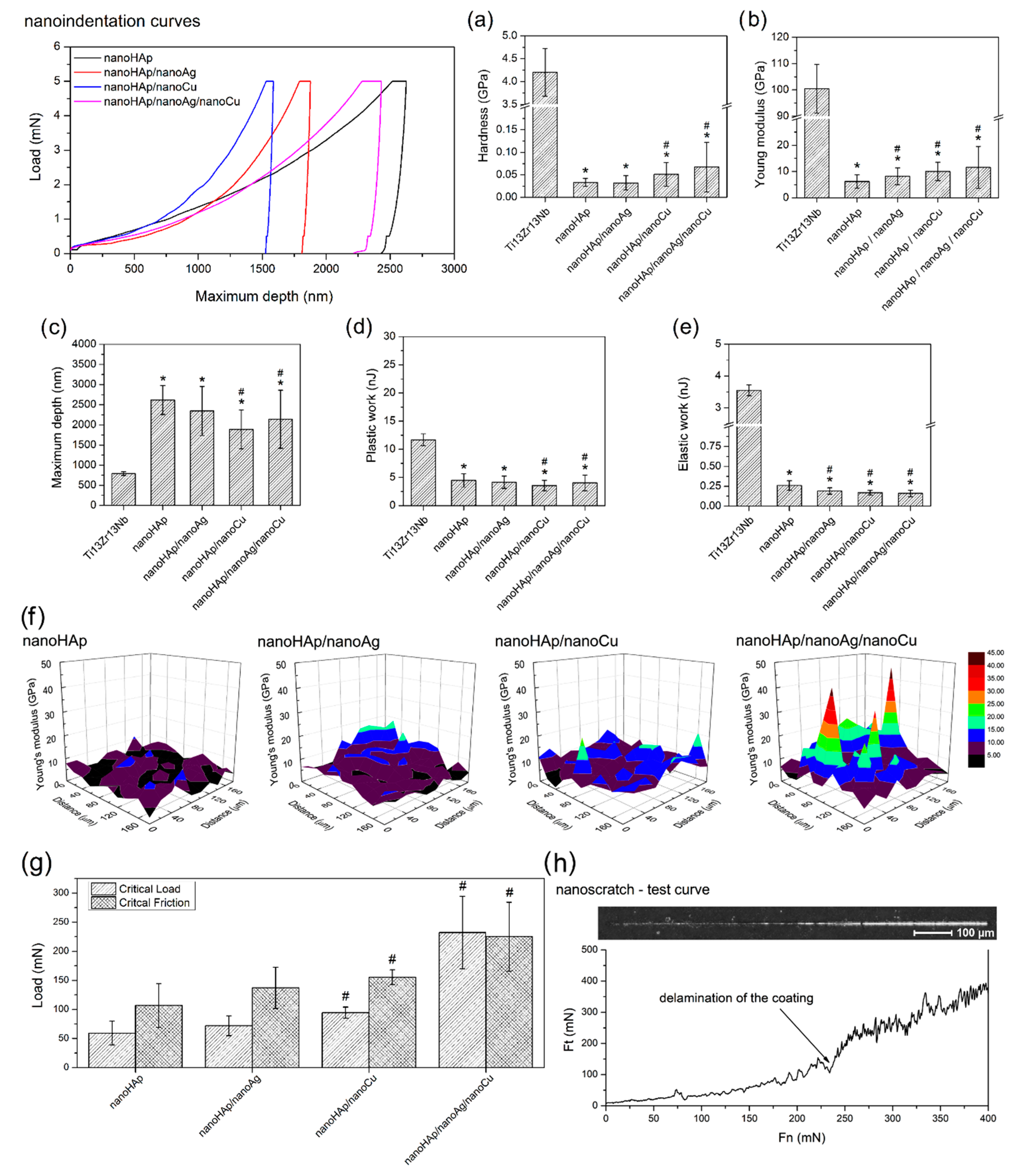
2.4. Nanoindentation and Nanoscratch Tests
2.5. Statistical Analysis
3. Results
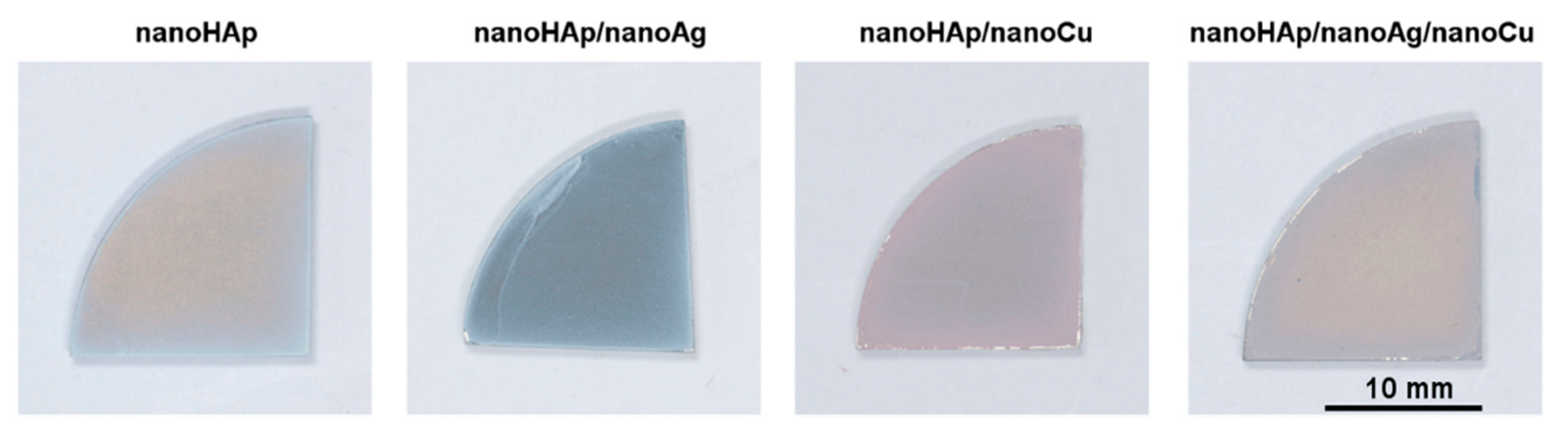
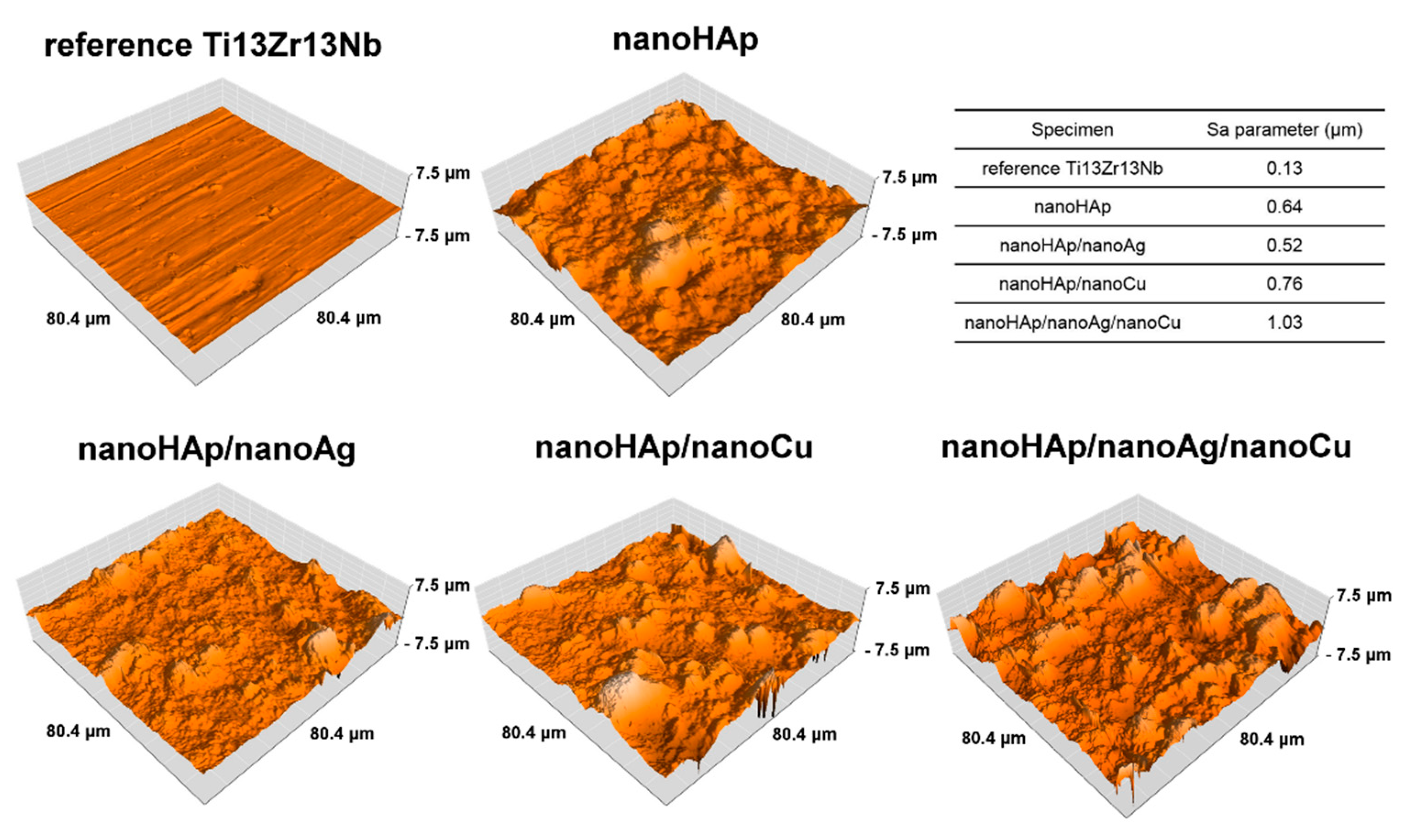
3.1. Morphology and Topography of Coatings
3.2. Nanoindentation and Nanoscratch Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, J.C.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Harun, W.; Asri, R.; Alias, J.; Zulkifli, F.; Kadirgama, K.; Ghani, S.; Shariffuddin, J. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Bral, A.; Mommaerts, M.Y. In vivo biofunctionalization of titanium patient-specific implants with nano hydroxyapatite and other nano calcium phosphate coatings: A systematic review. J. Cranio-Maxillofacial Surg. 2016, 44, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Bartmanski, M.; Zielinski, A.; Majkowska-Marzec, B.; Strugala, G. Effects of solution composition and electrophoretic deposition voltage on various properties of nanohydroxyapatite coatings on the Ti13Zr13Nb alloy. Ceram. Int. 2018, 44, 19236–19246. [Google Scholar] [CrossRef]
- Riau, A.K.; Lwin, N.C.; Gelfand, L.; Hu, H.; Liedberg, B.; Chodosh, J.; Venkatraman, S.S.; Mehta, J.S. Surface modification of corneal prosthesis with nano-hydroxyapatite to enhance in vivo biointegration. Acta Biomater. 2020, 107, 299–312. [Google Scholar] [CrossRef]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, X.; Li, C.; Huang, Y.; Ding, Q. Preparation and characterization of chitosan-silver/hydroxyapatite composite coatings on TiO2 nanotube for biomedical applications. Appl. Surf. Sci. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Grubova, I.Y.; Surmeneva, M.A.; Ivanova, A.A.; Kravchuk, K.; Prymak, O.; Epple, M.; Buck, V.; Surmenev, R.A. The effect of patterned titanium substrates on the properties of silver-doped hydroxyapatite coatings. Surf. Coat. Technol. 2015, 276, 595–601. [Google Scholar] [CrossRef]
- Geng, Z.; Cui, Z.; Li, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Li, X.; He, X.; Yu, X.; Wang, R.; et al. Strontium incorporation to optimize the antibacterial and biologicaal characteristics of silver-substituted hydroxyapatite coating. Mater. Sci. Eng. C 2016, 58, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sikder, P.; Koju, N.; Ren, Y.; Goel, V.K.; Phares, T.; Lin, B.; Bhaduri, S.B. Development of single-phase silver-doped antibacterial CDHA coatings on Ti6Al4V with sustained release. Surf. Coat. Technol. 2018, 342, 105–116. [Google Scholar] [CrossRef]
- Zhang, X.; Chaimayo, W.; Yang, C.; Yao, J.; Miller, B.L.; Yates, M.Z. Silver-hydroxyapatite composite coatings with enhanced antimicrobial activities through heat treatment. Surf. Coat. Technol. 2017, 325, 39–45. [Google Scholar] [CrossRef]
- Karthika, A. Aliovalent ions substituted hydroxyapatite coating on titanium for improved medical applications. Mater. Today Proc. 2018, 5, 8768–8774. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Strugała, G.; Mielewczyk-Gryń, A.; Zieliński, A. Properties of Nanohydroxyapatite Coatings Doped with Nanocopper, Obtained by Electrophoretic Deposition on Ti13Zr13Nb Alloy. Materials 2019, 12, 3741. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted Hydroxyapatites with Antibacterial Properties. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Godymchuk, A.Y.; Gusev, A.A.; Gulchenko, S.I.; Vasyukova, I.A.; Kuznetsov, D.V. Considerable Variation of Antibacterial Activity of Cu Nanoparticles Suspensions Depending on the Storage Time, Dispersive Medium, and Particle Sizes. BioMed Res. Int. 2015, 2015, 412530. [Google Scholar] [CrossRef]
- Zia, R.; Riaz, M.; Farooq, N.; Qamar, A.; Anjum, S. Antibacterial activity of Ag and Cu nanoparticles synthesized by chemical reduction method: A comparative analysis. Mater. Res. Express 2018, 5, 075012. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Sidane, D.; Chicot, D.; Yala, S.; Ziani, S.; Khireddine, H.; Iost, A.; Decoopman, X. Study of the mechanical behavior and corrosion resistance of hydroxyapatite sol–gel thin coatings on 316 L stainless steel pre-coated with titania film. Thin Solid Films 2015, 593, 71–80. [Google Scholar] [CrossRef]
- Singh, G.; Singh, S.; Prakash, S. Surface characterization of plasma sprayed pure and reinforced hydroxyapatite coating on Ti6Al4V alloy. Surf. Coat. Technol. 2011, 205, 4814–4820. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Qiao, H.; Nian, X.; Zhang, X.; Wang, W.; Zhang, X.; Chang, X.; Han, S.; Pang, X. Anticorrosive effects and In Vitro cytocompatibility of calcium silicate/zinc-doped hydroxyapatite composite coatings on titanium. Appl. Surf. Sci. 2015, 357, 1776–1784. [Google Scholar] [CrossRef]
- Zhong, Z.; Qin, J.; Ma, J. Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium. Ceram. Int. 2015, 41, 8878–8884. [Google Scholar] [CrossRef]
- Galindo, T.G.P.; Kataoka, T.; Fujii, S.; Okuda, M.; Tagaya, M. Preparation of nanocrystalline zinc-substituted hydroxyapatite films and their biological properties. Colloid Interface Sci. Commun. 2016, 10–11, 15–19. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Ramya, S.; Kavitha, L.; Gopi, D. Development of zinc-halloysite nanotube/minerals substituted hydroxyapatite bilayer coatings on titanium alloy for orthopedic applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 357–365. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chen, C.-C.; Wang, J.-B.; Wang, Y.-C.; Lin, F.-H. Flame sprayed zinc doped hydroxyapatite coating with antibacterial and biocompatible properties. Ceram. Int. 2017, 43, S829–S835. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, K.; Gao, J.; Yang, Y.; Qin, Y.-X.; Zheng, Y.; Zhu, D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Bose, S.; Vu, A.A.; Emshadi, K.; Bandyopadhyay, A. Effects of polycaprolactone on alendronate drug release from Mg-doped hydroxyapatite coating on titanium. Mater. Sci. Eng. C 2018, 88, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Cacciotti, I.; De Bonis, A.; Fosca, M.; Komlev, V.; Latini, A.; Santagata, A.; Teghil, R. Fe-doped hydroxyapatite coatings for orthopedic and dental implant applications. Appl. Surf. Sci. 2014, 307, 301–305. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Sima, F.; Axente, E.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium and zoledronate hydroxyapatites graded composite coatings for bone prostheses. J. Colloid Interface Sci. 2015, 448, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiao, H.; Nian, X.; Zhang, X.; Zhang, X.; Song, G.; Xu, Z.; Zhang, H.; Han, S. Improving the bioactivity and corrosion resistance properties of electrodeposited hydroxyapatite coating by dual doping of bivalent strontium and manganese ion. Surf. Coat. Technol. 2016, 291, 205–215. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Murugan, N.; Karthikeyan, P.; Sathishkumar, S.; Anbarasu, G.; Rajavel, R. Development of antibacterial activity and corrosion resistance properties of electrodeposition of mineralized hydroxyapatite coated on titanium alloy for biomedical applications. Mater. Today: Proc. 2017, 4, 12393–12400. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.J.; Fernández-Villa, D.; Serrano, M.C.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Feito, M.J.; Serrano, M.C.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; et al. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater. 2020, 101, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef]
- Ke, D.; Vu, A.A.; Bandyopadhyay, A.; Bose, S. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2019, 84, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Durdu, S.; Usta, M.; Berkem, A.S. Bioactive coatings on Ti6Al4V alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 301, 85–93. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Liang, C.; Wang, H.; Qiao, Z. Formation mechanism and adhesive strength of a hydroxyapatite/TiO2 composite coating on a titanium surface prepared by micro-arc oxidation. Appl. Surf. Sci. 2016, 362, 109–114. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Mariappan, A.; Neyvasagam, K.; Ayeshamariam, A.; Pandi, P.; Palanichamy, R.R.; Gopinathan, C.; Mola, G.T.; Maaza, M. Photocatalytic performance and antimicrobial activities of HAp-TiO2 nanocomposite thin films by sol-gel method. Surf. Interfaces 2017, 6, 247–255. [Google Scholar] [CrossRef]
- Długoń, E.; Niemiec, W.; Frączek-Szczypta, A.; Jeleń, P.; Sitarz, M.; Błażewicz, M. Spectroscopic studies of electrophoretically deposited hybrid HAp/CNT coatings on titanium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 872–875. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Kavitha, L. Influence of ionic substitution in improving the biological property of carbon nanotubes reinforced hydroxyapatite composite coating on titanium for orthopedic applications. Ceram. Int. 2015, 41, 5454–5463. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Rubini, K.; Fini, M.; Bigi, A. Antioxidant and bone repair properties of quercetin-functionalized hydroxyapatite: An in vitro osteoblast–osteoclast–endothelial cell co-culture study. Acta Biomater. 2016, 32, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Kim, J.; Lee, K.; Hwang, J.; Choi, Y.; Seo, Y.; Jeon, H.; Kang, H.C.; Woo, H.-M.; Kang, B.-J.; et al. DNA aptamer immobilized hydroxyapatite for enhancing angiogenesis and bone regeneration. Acta Biomater. 2019, 99, 469–478. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, B.; Wang, Y.; Zhou, X.; Weng, J.; Qu, S.; Feng, B.; Watari, F.; Ding, Y.; Leng, Y. Nano-Ag-loaded hydroxyapatite coatings on titanium surfaces by electrochemical deposition. J. R. Soc. Interface 2010, 8, 529–539. [Google Scholar] [CrossRef]
- Guo, C.; Xue, J.; Dong, Y. Fabrication and characterization of hydroxyapatite nanomaterial dual deposited with nano silver and zinc oxide. Mater. Lett. 2018, 219, 182–185. [Google Scholar] [CrossRef]
- Jelinek, M.; Kocourek, T.; Remsa, J.; Weiserova, M.; Jurek, K.; Mikšovský, J.; Strnad, J.; Galandáková, A.; Ulrichová, J. Antibacterial, cytotoxicity and physical properties of laser—Silver doped hydroxyapatite layers. Mater. Sci. Eng. C 2013, 33, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- He, L.-H.; Standard, O.C.; Huang, T.T.; Latella, B.A.; Swain, M.V. Mechanical behaviour of porous hydroxyapatite. Acta Biomater. 2008, 4, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Ruys, A.J.; Milthorpe, B.K.; Sorrell, C.C. Precipitation of hydroxyapatite nanoparticles: Effects of precipitation method on electrophoretic deposition. J. Mater. Sci. Mater. Med. 2005, 16, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Bigham, A.; Saebnoori, E.; Hassanzadeh-Tabrizi, S.; Rahmati, S.; Alizadeh, Z.M.; Nasirian, V.; Rafienia, M. Electrophoretic-deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications. Surf. Coat. Technol. 2017, 321, 171–179. [Google Scholar] [CrossRef]
- Lyasnikova, A.V.; Markelova, O.A.; Dudareva, O.A.; Lyasnikov, V.N.; Barabash, A.P.; Shpinyak, S.P. Comprehensive Characterization of Plasma-Sprayed Coatings Based Silver- and Copper-Substituted Hydroxyapatite. Powder Met. Met. Ceram. 2016, 55, 328–333. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M. Effect of morphology on the electrophoretic deposition of hydroxyapatite nanoparticles. J. Alloys Compd. 2018, 741, 211–222. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Jazdzewska, M.; Głodowska, J.; Kalka, P. Effects of electrophoretic deposition times and nanotubular oxide surfaces on properties of the nanohydroxyapatite/nanocopper coating on the Ti13Zr13Nb alloy. Ceram. Int. 2019, 45, 20002–20010. [Google Scholar] [CrossRef]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P.; Pawlowski, L.; Pieper, M.; Zielinski, A. Electrophoretic deposition (EPD) of nanohydroxyapatite—Nanosilver coatings on Ti13Zr13Nb alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Drevet, R.; Ben Jaber, N.; Faure, J.P.; Tara, A.; Larbi, A.B.C.; Benhayoune, H. Electrophoretic deposition (EPD) of nano-hydroxyapatite coatings with improved mechanical properties on prosthetic Ti6Al4V substrates. Surf. Coat. Technol. 2016, 301, 94–99. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, H.-S.; Hahn, H.T. Reactive Sintering of Copper Nanoparticles Using Intense Pulsed Light for Printed Electronics. J. Electron. Mater. 2010, 40, 42–50. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Hai, J.; Li, T. Synthesis of silver–hydroxyapatite composite with improved antibacterial properties. Vacuum 2018, 152, 132–137. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Huang, Y.; Ding, Q.; Pang, X. Antibacterial and bioactivity of silver substituted hydroxyapatite/TiO 2 nanotube composite coatings on titanium. Appl. Surf. Sci. 2014, 314, 348–357. [Google Scholar] [CrossRef]
- Gross, K.A.; Babovic, M. Influence of abrasion on the surface characteristics of thermally sprayed hydroxyapatite coatings. Biomater. 2002, 23, 4731–4737. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.; Qu, S.; Zhang, X. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials 2003, 24, 4663–4670. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.-H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Wu, Y.; Zitelli, J.P.; TenHuisen, K.S.; Yu, X.; Libera, M.R. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials 2011, 32, 951–960. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, J.-Y.; Ihn, K.J. Preparation of Silver Nanoparticles Having Low Melting Temperature Through a New Synthetic Process without Solvent. J. Nanosci. Nanotechnol. 2007, 7, 3805–3809. [Google Scholar] [CrossRef]
- Asoro, M.; Damiano, J.; Ferreira, P. Size Effects on the Melting Temperature of Silver Nanoparticles: In-Situ TEM Observations. Microsc. Microanal. 2009, 15, 706–707. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Dmitruk, I.M.; Alexeenko, A.A.; Dmytruk, A.M. Size-dependent melting of spherical copper nanoparticles embedded in a silica matrix. Phys. Rev. B 2007, 75, 1–6. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-containing calcium phosphates: Effectiveness of Ag-incorporated β-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, Q.; Huang, Y.; Han, S.; Pang, X. Magnesium substituted hydroxyapatite coating on titanium with nanotublar TiO2 intermediate layer via electrochemical deposition. Appl. Surf. Sci. 2014, 305, 77–85. [Google Scholar] [CrossRef]
- Araghi, A.; Hadianfard, M. Fabrication and characterization of functionally graded hydroxyapatite/TiO2 multilayer coating on Ti–6Al–4V titanium alloy for biomedical applications. Ceram. Int. 2015, 41, 12668–12679. [Google Scholar] [CrossRef]
- Bartmanski, M. The Properties of Nanosilver—Doped Nanohydroxyapatite Coating on the Ti13zr13Nb Alloy. Adv. Mater. Sci. 2017, 17, 18–28. [Google Scholar] [CrossRef]
- Clèries, L.; Fernández-Pradas, J.; Morenza, J. Behavior in simulated body fluid of calcium phosphate coatings obtained by laser ablation. Biomaterials 2000, 21, 1861–1865. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M. Electrophoretic deposition of fiber hydroxyapatite/titania nanocomposite coatings. Ceram. Int. 2018, 44, 622–630. [Google Scholar] [CrossRef]
- Kumar, R.M.; Kuntal, K.K.; Singh, S.K.; Gupta, P.; Bhushan, B.; Gopinath, P.; Lahiri, D. Electrophoretic deposition of hydroxyapatite coating on Mg–3Zn alloy for orthopaedic application. Surf. Coat. Technol. 2016, 287, 82–92. [Google Scholar] [CrossRef]

| Element | Zr | Nb | Fe | C | N | O | Ti |
|---|---|---|---|---|---|---|---|
| wt.% | 13.0 | 13.0 | 0.05 | 0.04 | 0.019 | 0.11 | rem. |
| Specimen | Amount of nanoHAp (g/L) | Amount of nanoAg (g/L) | Amount of nanoCu (g/L) |
|---|---|---|---|
| nanoHAp | 0.1 | - | - |
| nanoHAp/nanoAg | 0.1 | 0.01 | - |
| nanoHAp/nanoCu | 0.1 | - | 0.01 |
| nanoHAp/nanoAg/nanoCu | 0.1 | 0.005 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartmański, M.; Pawłowski, Ł.; Mielewczyk-Gryń, A.; Strugała, G.; Rokosz, K.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Zieliński, A. The Influence of Nanometals, Dispersed in the Electrophoretic Nanohydroxyapatite Coatings on the Ti13Zr13Nb Alloy, on Their Morphology and Mechanical Properties. Materials 2021, 14, 1638. https://doi.org/10.3390/ma14071638
Bartmański M, Pawłowski Ł, Mielewczyk-Gryń A, Strugała G, Rokosz K, Gaiaschi S, Chapon P, Raaen S, Zieliński A. The Influence of Nanometals, Dispersed in the Electrophoretic Nanohydroxyapatite Coatings on the Ti13Zr13Nb Alloy, on Their Morphology and Mechanical Properties. Materials. 2021; 14(7):1638. https://doi.org/10.3390/ma14071638
Chicago/Turabian StyleBartmański, Michał, Łukasz Pawłowski, Aleksandra Mielewczyk-Gryń, Gabriel Strugała, Krzysztof Rokosz, Sofia Gaiaschi, Patrick Chapon, Steinar Raaen, and Andrzej Zieliński. 2021. "The Influence of Nanometals, Dispersed in the Electrophoretic Nanohydroxyapatite Coatings on the Ti13Zr13Nb Alloy, on Their Morphology and Mechanical Properties" Materials 14, no. 7: 1638. https://doi.org/10.3390/ma14071638
APA StyleBartmański, M., Pawłowski, Ł., Mielewczyk-Gryń, A., Strugała, G., Rokosz, K., Gaiaschi, S., Chapon, P., Raaen, S., & Zieliński, A. (2021). The Influence of Nanometals, Dispersed in the Electrophoretic Nanohydroxyapatite Coatings on the Ti13Zr13Nb Alloy, on Their Morphology and Mechanical Properties. Materials, 14(7), 1638. https://doi.org/10.3390/ma14071638












