Lyophilized Emulsions in the Form of 3D Porous Matrices as a Novel Material for Topical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polylactide Microparticles
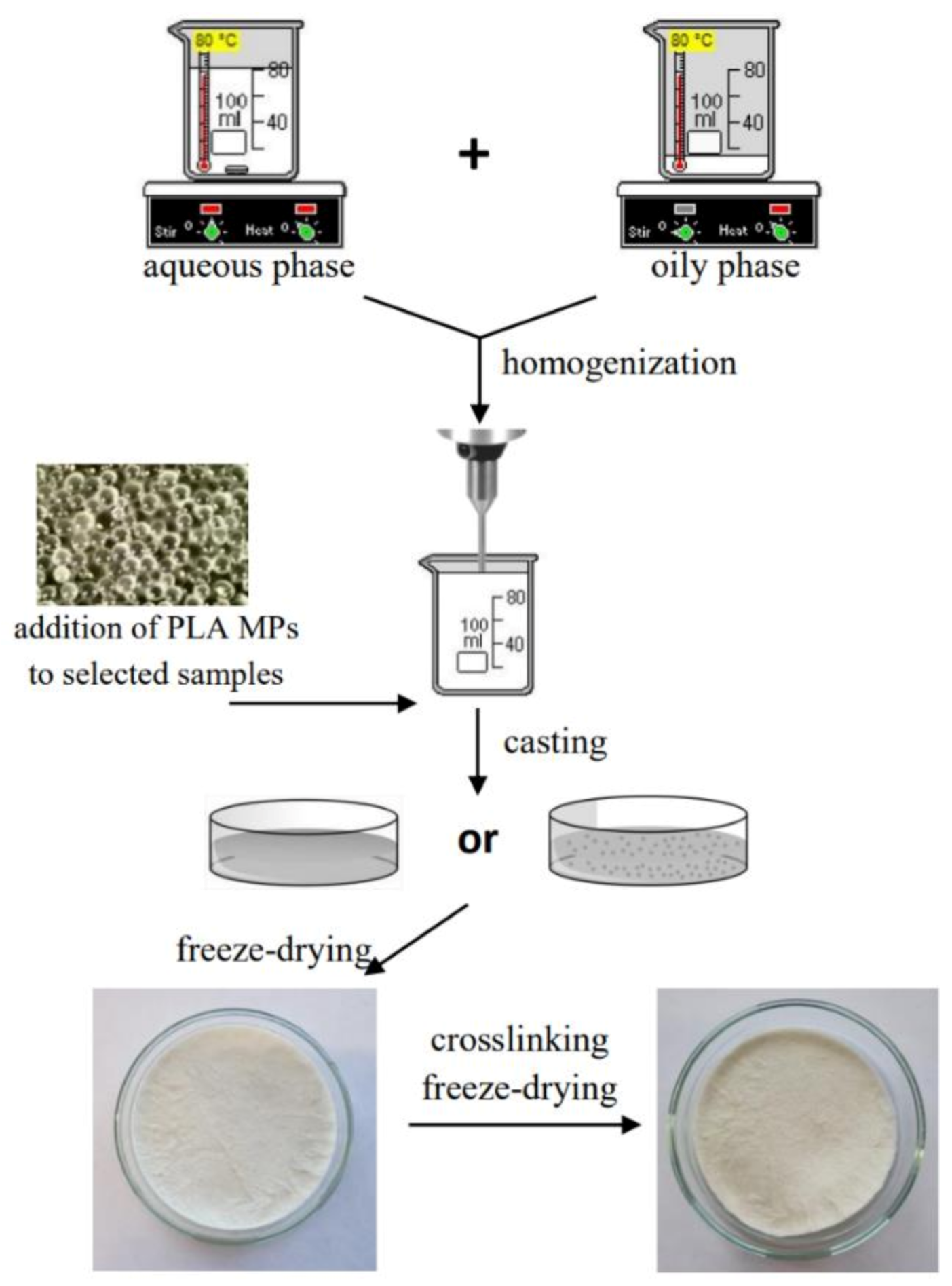
2.2.2. Preparation of Freeze-Dried Emulsion Matrices
2.2.3. Characterization of Materials
Scanning Electron Microscopy (SEM) Analysis
Attenuated Total Reflection Infrared Spectroscopy (ATR-FTIR)
Moisture Content Measurements
Porosity and Density Measurements
Swelling Properties
Degradation Measurements
Mechanical Properties
Loading Capacity of Materials with PLA Microparticles
In Vitro Release of Calendula Officinalis Flower Extract
3. Results and Discussion
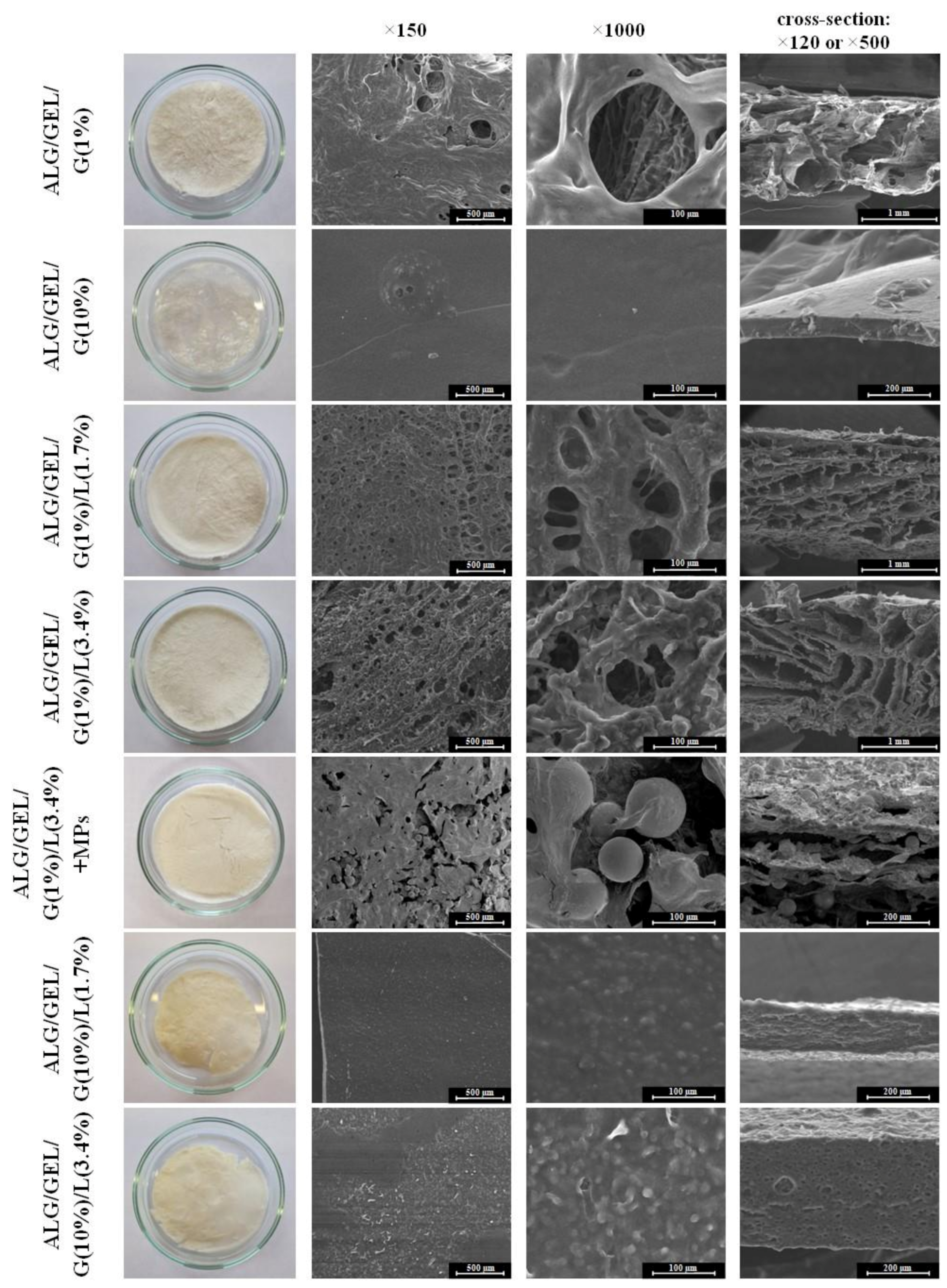
3.1. Structure of Materials
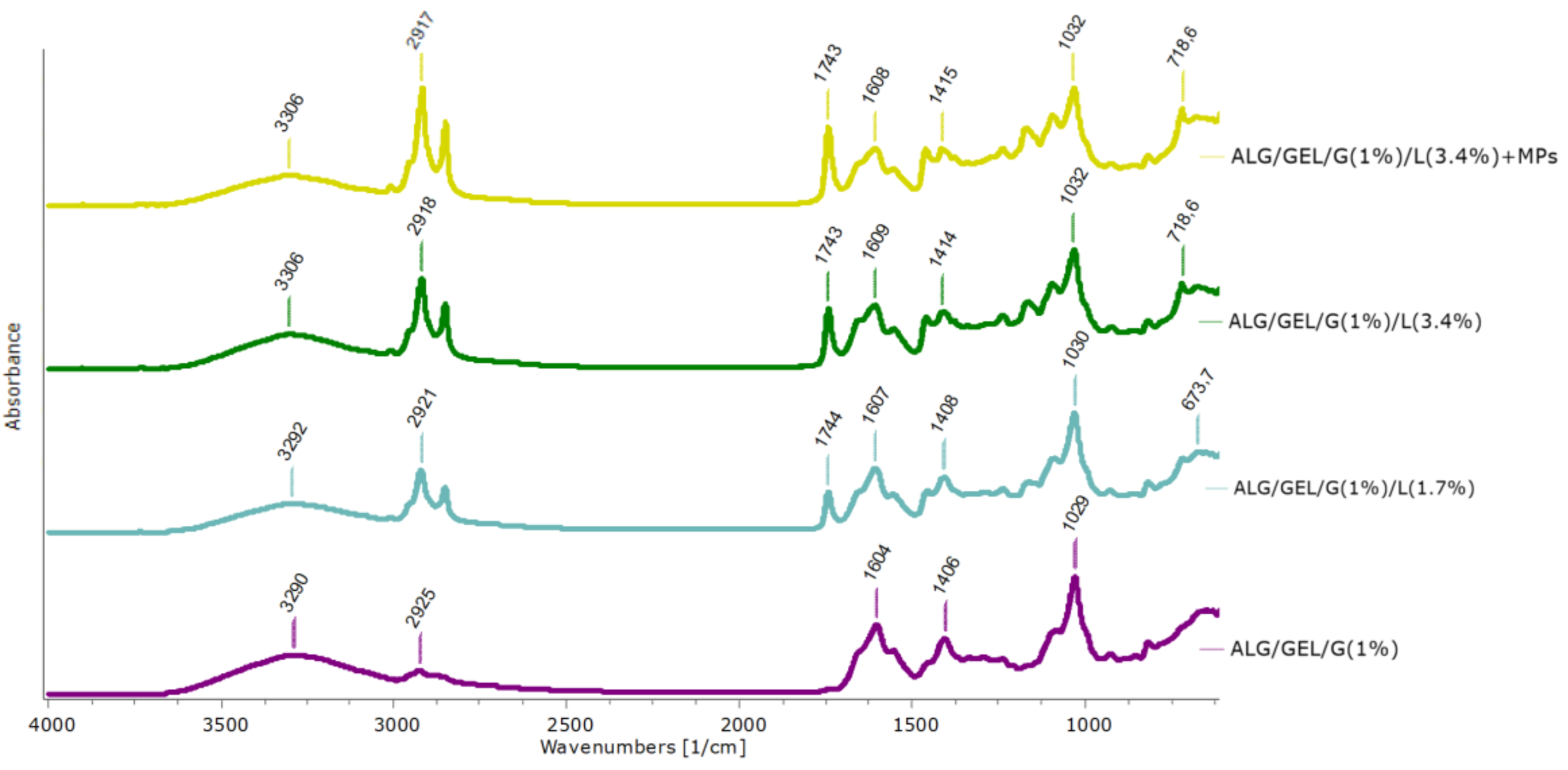
3.2. ATR-FTIR Spectroscopy
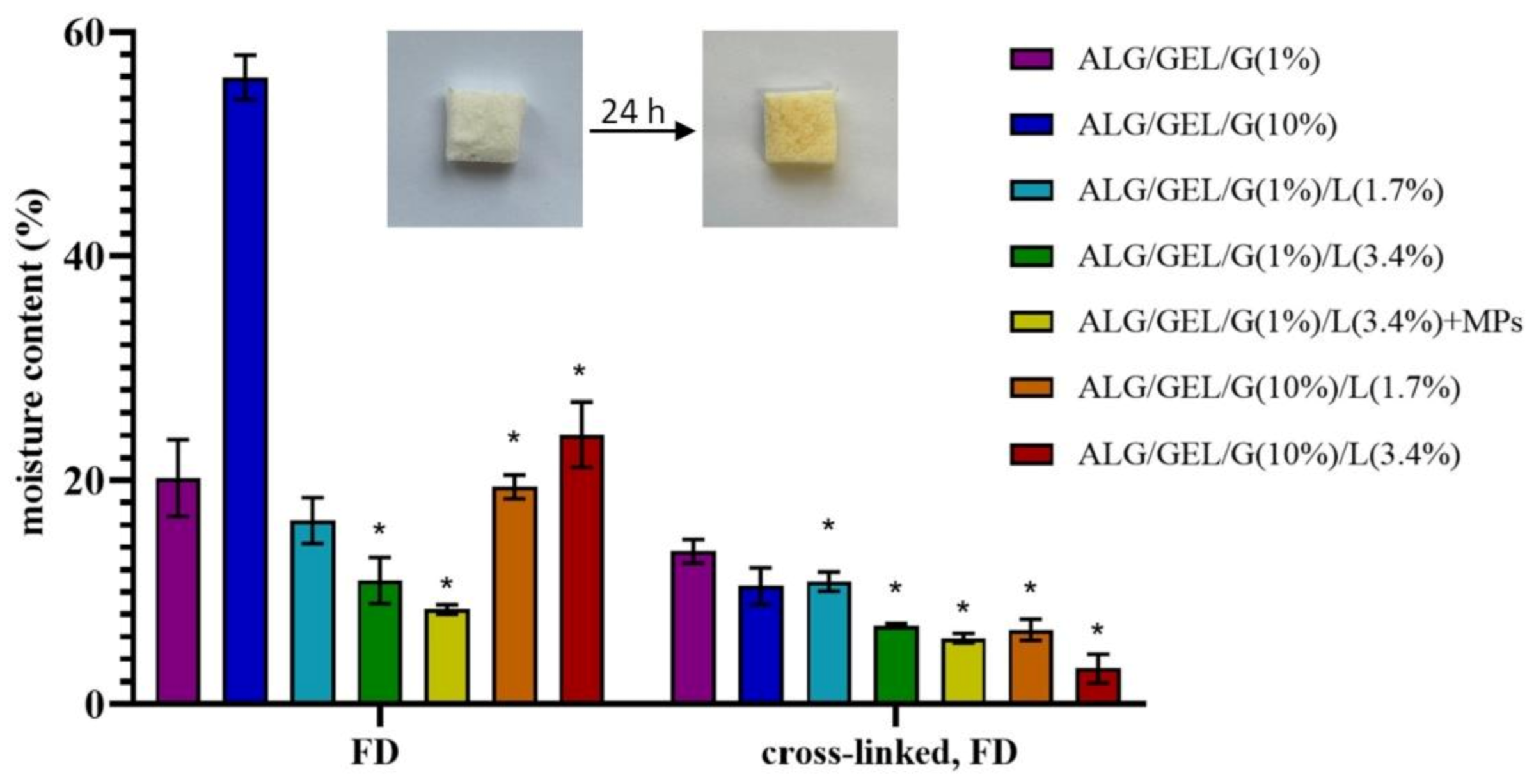
3.3. Moisture Content
3.4. Porosity and Density Measurements
3.5. Swelling Properties
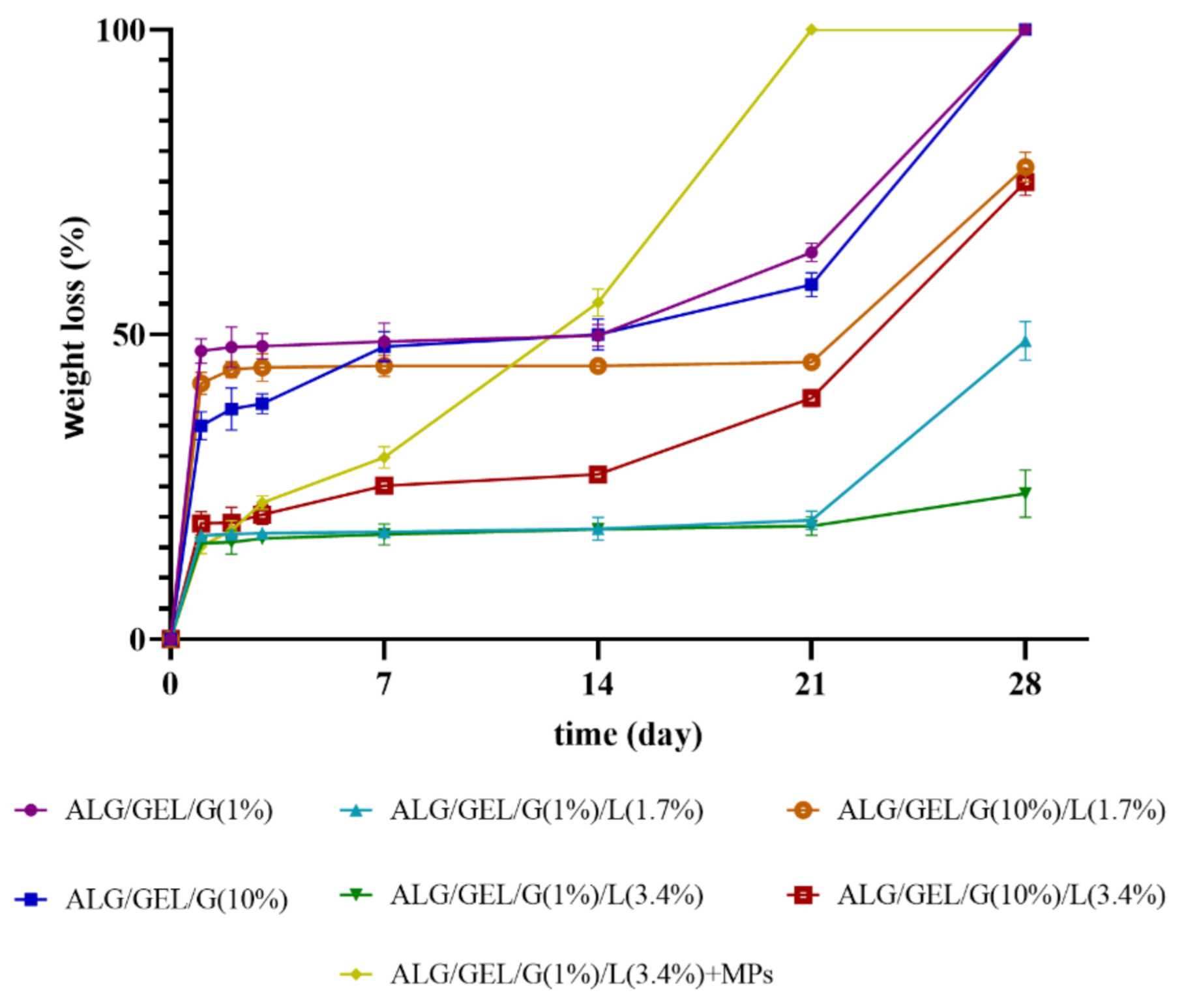
3.6. Degradation Measurements
3.7. Mechanical Properties
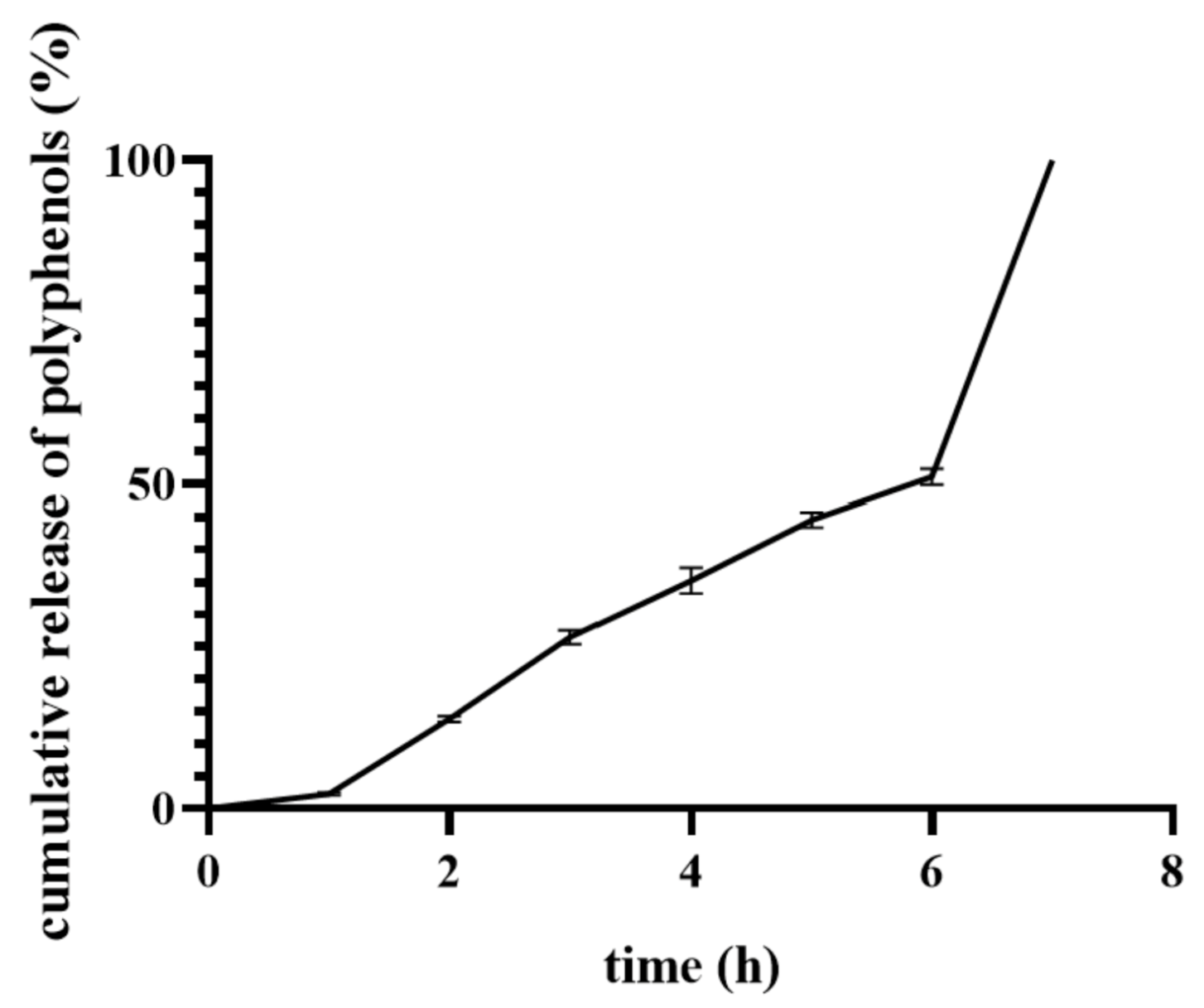
3.8. Loading Capacity and In Vitro Release of Calendula Officinalis Flower Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2010, 86, 172–184. [Google Scholar] [CrossRef]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Preparation of interconnected highly porous polymeric structures by a replication and freeze-drying process. J. Biomed. Mater. Res. 2003, 67, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Heal. Mater. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Karahaliloglu, Z.; Kilicay, E.; Denkbas, E.B. Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1172–1185. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Liu, J.; Yang, J.; Jia, N.; Zhu, C.; Zhang, J. Optimizing microenvironment by integrating negative pressure and exogenous electric fields via a flexible porous conductive dressing to accelerate wound healing. Biomater. Sci. 2021, 9, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Kuroyanagi, Y. Development of a Wound Dressing Composed of Hyaluronic Acid and Collagen Sponge with Epidermal Growth Factor. J. Biomater. Sci. Polym. Ed. 2012, 23, 629–643. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Huang, S.; Yu, F.; Bei, Y.; Zhang, Y.; Tang, J.; Huang, Y.; Xiang, Q. Hybrid Freeze-Dried Dressings Composed of Epidermal Growth Factor and Recombinant Human-Like Collagen Enhance Cutaneous Wound Healing in Rats. Front. Bioeng. Biotechnol. 2020, 8, 742. [Google Scholar] [CrossRef]
- O’Brien, F.J. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Geanaliu-Nicolae, R.-E.; Andronescu, E. Blended Natural Support Materials—Collagen Based Hydrogels Used in Biomedicine. Materials 2020, 13, 5641. [Google Scholar] [CrossRef]
- Forero, J.C.; Roa, E.; Reyes, J.G.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef]
- Han, X.; Honda, Y.; Tanaka, T.; Imura, K.; Hashimoto, Y.; Yoshikawa, K.; Yamamoto, K. Gas Permeability of Mold during Freezing Process Alters the Pore Distribution of Gelatin Sponge and Its Bone-Forming Ability. Materials 2020, 13, 4705. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, I.; Francolini, I.; Di Lisio, V.; Martinelli, A.; Pietrelli, L.; D’Abusco, A.S.; Scoppio, A.; Piozzi, A. Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering. Materials 2020, 13, 3577. [Google Scholar] [CrossRef]
- Lv, Q.; Feng, Q. Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique. J. Mater. Sci. Mater. Electron. 2006, 17, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Chen, X.; Xiong, G.; Guo, R.; Luo, H. Synthesis and characterization of three-dimensional porous graphene oxide/sodium alginate scaffolds with enhanced mechanical properties. Mater. Express 2014, 4, 429–434. [Google Scholar] [CrossRef]
- Adachi, T.; Boschetto, F.; Miyamoto, N.; Yamamoto, T.; Marin, E.; Zhu, W.; Kanamura, N.; Tahara, Y.; Akiyoshi, K.; Mazda, O.; et al. In Vivo Regeneration of Large Bone Defects by Cross-Linked Porous Hydrogel: A Pilot Study in Mice Combining Micro Tomography, Histological Analyses, Raman Spectroscopy and Synchrotron Infrared Imaging. Materials 2020, 13, 4275. [Google Scholar] [CrossRef]
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152. [Google Scholar] [CrossRef]
- Morais, A.R.D.V.; Alencar, É.d.N.; Júnior, F.H.X.; De Oliveira, C.M.; Marcelino, H.R.; Barratt, G.; Fessi, H.; Egito, E.S.T.D.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef]
- Hou, Y.; Fang, G.; Jiang, Y.; Song, H.; Zhang, Y.; Zhao, Q. Emulsion Lyophilization as a Facile Pathway to Fabricate Stretchable Polymer Foams Enabling Multishape Memory Effect and Clip Application. ACS Appl. Mater. Interfaces 2019, 11, 32423–32430. [Google Scholar] [CrossRef]
- Sultana, N.; Wang, M. Fabrication of HA/PHBV composite scaffolds through the emulsion freezing/freeze-drying process and characterisation of the scaffolds. J. Mater. Sci. Mater. Med. 2007, 19, 2555–2561. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. One-step synthesis of protein-encapsulated microspheres in a porous scaffold by freeze-drying double emulsions and tuneable protein release. Chem. Commun. 2013, 49, 8833–8835. [Google Scholar] [CrossRef]
- Lee, J.; Chang, J.Y. Pickering Emulsion Stabilized by Microporous Organic Polymer Particles for the Fabrication of a Hierarchically Porous Monolith. Langmuir 2018, 34, 11843–11849. [Google Scholar] [CrossRef]
- Wandrey, C.; Bartkowiak, A.; Harding, S.E. Materials for Encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing, 1st ed.; Zuidam, N.J., Nedovic, V., Eds.; Springer: New York, NY, USA, 2010; ISBN 9781441910073. [Google Scholar]
- Armendáriz-Barragán, B.; Zafar, N.; Badri, W.; Galindo-Rodríguez, S.A.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant extracts: From encapsulation to application. Expert Opin. Drug Deliv. 2016, 13, 1165–1175. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Donsì, F.; Barba, F.J.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Mudrić, J.; Ibrić, S.; Đuriš, J. Microencapsulation methods for plants biologically active compounds: A review. Lek. Sirovine 2018, 38, 62–67. [Google Scholar] [CrossRef]
- Re, T.; Mooney, D.; Antignac, E.; Dufour, E.; Bark, I.; Srinivasan, V.; Nohynek, G. Application of the threshold of toxicological concern approach for the safety evaluation of calendula flower (Calendula officinalis) petals and extracts used in cosmetic and personal care products. Food Chem. Toxicol. 2009, 47, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Zaman, S.U.; Khan, B.A.; Amir, M.N.; Ebrahimzadeh, M.A. Calendula extract: Effects on mechanical parameters of human skin. Acta Pol. Pharm. Drug Res. 2011, 68, 693–701. [Google Scholar]
- Kozlowska, J.; Stachowiak, N.; Sionkowska, A. Collagen/Gelatin/Hydroxyethyl Cellulose Composites Containing Microspheres Based on Collagen and Gelatin: Design and Evaluation. Polymers 2018, 10, 456. [Google Scholar] [CrossRef]
- Kozlowska, J.; Stachowiak, N.; Prus, W. Stability studies of collagen-based microspheres with Calendula officinalis flower extract. Polym. Degrad. Stab. 2019, 163, 214–219. [Google Scholar] [CrossRef]
- Kozlowska, J.; Prus, W.; Stachowiak, N. Microparticles based on natural and synthetic polymers for cosmetic applications. Int. J. Biol. Macromol. 2019, 129, 952–956. [Google Scholar] [CrossRef]
- Kozlowska, J.; Kaczmarkiewicz, A. Collagen matrices containing poly(vinyl alcohol) microcapsules with retinyl palmitate—Structure, stability, mechanical and swelling properties. Polym. Degrad. Stab. 2019, 161, 108–113. [Google Scholar] [CrossRef]
- Prus-Walendziak, W.; Kozlowska, J. Design of Sodium Alginate/Gelatin-Based Emulsion Film Fused with Polylactide Microparticles Charged with Plant Extract. Materials 2021, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, R.; Jin, H.-J.; Kaplan, D.L. Porous 3-D Scaffolds from Regenerated Silk Fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar]
- Pradini, D.; Juwono, H.; Madurani, K.A.; Kurniawan, F. A preliminary study of identification halal gelatin using Quartz Crystal Microbalance (QCM) sensor. Malays. J. Fundam. Appl. Sci. 2018, 14, 325–330. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’Ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Man, Y.B.C. A new method for determining gossypol in cottonseed oil by FTIR spectroscopy. J. Am. Oil Chem. Soc. 2003, 80, 625–628. [Google Scholar] [CrossRef]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; PRĐUN, S.; Bubalo, D.; Gajger, I.T. An Approach for Routine Analytical Detection of Beeswax Adulteration Using FTIR-ATR Spectroscopy. J. Apic. Sci. 2015, 59, 37–49. [Google Scholar] [CrossRef]
- Li, F.; Wang, T.; He, H.B.; Tang, X. The properties of bufadienolides-loaded nano-emulsion and submicro-emulsion during lyophilization. Int. J. Pharm. 2008, 349, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Nandagiri, V.K.; Gentile, P.; Chiono, V.; Tonda-Turo, C.; Matsiko, A.; Ramtoola, Z.; Montevecchi, F.M.; Ciardelli, G. Incorporation of PLGA nanoparticles into porous chitosan–gelatin scaffolds: Influence on the physical properties and cell behavior. J. Mech. Behav. Biomed. Mater. 2011, 4, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ghazanlou, S.I.; Jalaly, M.; Sadeghzadeh, S.; Korayem, A.H. A comparative study on the mechanical, physical and morphological properties of cement-micro/nanoFe3O4 composite. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing Porous Bone Tissue Engineering Scaffolds with Enhanced Mechanical Properties from Composite Hydrogels Composed of Modified Alginate, Gelatin, and Bioactive Glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, T.R.; Erices, A.A.; Aguilera, J.M. Porous matrix of calcium alginate/gelatin with enhanced properties as scaffold for cell culture. J. Mech. Behav. Biomed. Mater. 2015, 46, 331–342. [Google Scholar] [CrossRef]
- Ahumada, M.; Jacques, E.; Calderon, C.; Martínez-Gómez, F. Porosity in Biomaterials: A Key Factor in the Development of Applied Materials in Biomedicine. Handb. Ecomater. 2019, 5, 3503–3522. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Stancu, I.-C.; Dragusin, D.M.; Vasile, E.; Trusca, R.; Antoniac, I.; Vasilescu, D.S. Porous calcium alginate–gelatin interpenetrated matrix and its biomineralization potential. J. Mater. Sci. Mater. Electron. 2011, 22, 451–460. [Google Scholar] [CrossRef]
- Wang, L.; Shansky, J.; Borselli, C.; Mooney, D.; VanDenburgh, H. Design and Fabrication of a Biodegradable, Covalently Crosslinked Shape-Memory Alginate Scaffold for Cell and Growth Factor Delivery. Tissue Eng. Part A 2012, 18, 2000–2007. [Google Scholar] [CrossRef]
- Varde, N.K.; Pack, D.W. Influence of particle size and antacid on release and stability of plasmid DNA from uniform PLGA microspheres. J. Control. Release 2007, 124, 172–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, W.; Palazzo, A.; Hennink, W.E.; Kok, R.J. Effect of Particle Size on Drug Loading and Release Kinetics of Gefitinib-Loaded PLGA Microspheres. Mol. Pharm. 2017, 14, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Stojanović, R.; Manojlović, V.; Komes, D.; Cindrić, I.J.; Nedović, V.; Bugarski, B. Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate–chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res. Int. 2011, 44, 1094–1101. [Google Scholar] [CrossRef]


| Sample | Composition of Materials (% w/w) | Addition (% w/w) | |||||
|---|---|---|---|---|---|---|---|
| Aqueous Phase | Oily Phase | PLA MPs | |||||
| ALG | GEL | G | Cottonseed Oil | Beeswax | Span-80 | ||
| ALG/GEL/G(1%) | 2 | 1 | 1 | – | – | – | – |
| ALG/GEL/G(10%) | 2 | 1 | 10 | – | – | – | – |
| ALG/GEL/G(1%)/L(1.7%) | 2 | 1 | 1 | 1.2 | 0.5 | 0.35 | – |
| ALG/GEL/G(1%)/L(3.4%) | 2 | 1 | 1 | 2.4 | 1 | 0.7 | – |
| ALG/GEL/G(1%)/L(3.4%) + MPs | 2 | 1 | 1 | 2.4 | 1 | 0.7 | 6 |
| ALG/GEL/G(10%)/L(1.7%) | 2 | 1 | 10 | 1.2 | 0.5 | 0.35 | – |
| ALG/GEL/G(10%)/L(3.4%) | 2 | 1 | 10 | 2.4 | 1 | 0.7 | – |
| Sample | Porosity (Є) (%) | Density (d) (mg/mL) | ||
|---|---|---|---|---|
| FD | Cross-Linked, FD | FD | Cross-Linked, FD | |
| ALG/GEL/G(1%) | 75.0 ± 0.0 | 66.7 ± 0.0 | 57.8 ± 6.5 | 116.3 ± 9.2 |
| ALG/GEL/G(10%) | 44.3 ± 5.2 | 72.2 ± 4.8 | 351.5 ± 1.7 | 151.9 ± 19.0 |
| ALG/GEL/G(1%)/L(1.7%) | 80.0 ± 0.0 | 41.0 ± 1.6 | 125.6 ± 5.0 | 173.6 ± 3.4 |
| ALG/GEL/G(1%)/L(3.4%) | 43.3 ± 2.9 | 63.3 ± 4.7 | 98.2 ± 6.8 | 137.4 ± 5.2 |
| ALG/GEL/G(1%)/L(3.4%) + MPs | 63.9 ± 2.4 | 64.4 ± 3.8 | 166.4 ± 7.9 | 220.0 ± 15.5 |
| ALG/GEL/G(10%)/L(1.7%) | 41.9 ± 1.6 | 40.0 ± 0.0 | 393.6 ± 11.9 | 318.0 ± 14.1 |
| ALG/GEL/G(10%)/L(3.4%) | 40.0 ± 0.0 | 59.0 ± 1.6 | 460.9 ± 2.2 | 259.4 ± 22.3 |
| Sample | Ec (kPa) | ||
|---|---|---|---|
| FD | Cross-Linked, FD | ||
| Dry | Dry | Soaked in PBS | |
| ALG/GEL/G(1%) | 102.9 ± 16.4 | 576.3 ± 51.4 | 9.1 ± 0.7 |
| ALG/GEL/G(10%) | 2.0 ± 0.1 | 555.3 ± 27.8 | 7.1 ± 0.4 |
| ALG/GEL/G(1%)/L(1.7%) | 92.3 ± 12.0 | 317.8 ± 83.2 | 10.5 ± 0.9 |
| ALG/GEL/G(1%)/L(3.4%) | 107.0 ± 20.9 | 592.0 ± 47.8 | 14.3 ± 1.6 |
| ALG/GEL/G(1%)/L(3.4%) + MPs | 76.2 ± 4.7 | 338.0 ± 53.4 | 7.3 ± 0.3 |
| ALG/GEL/G(10%)/L(1.7%) | 1.8 ± 0.2 | 222.3 ± 32.6 | 14.5 ± 0.9 |
| ALG/GEL/G(10%)/L(3.4%) | 4.9 ± 0.8 | 465.0 ± 14.9 | 33.6 ± 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prus-Walendziak, W.; Kozlowska, J. Lyophilized Emulsions in the Form of 3D Porous Matrices as a Novel Material for Topical Application. Materials 2021, 14, 950. https://doi.org/10.3390/ma14040950
Prus-Walendziak W, Kozlowska J. Lyophilized Emulsions in the Form of 3D Porous Matrices as a Novel Material for Topical Application. Materials. 2021; 14(4):950. https://doi.org/10.3390/ma14040950
Chicago/Turabian StylePrus-Walendziak, Weronika, and Justyna Kozlowska. 2021. "Lyophilized Emulsions in the Form of 3D Porous Matrices as a Novel Material for Topical Application" Materials 14, no. 4: 950. https://doi.org/10.3390/ma14040950
APA StylePrus-Walendziak, W., & Kozlowska, J. (2021). Lyophilized Emulsions in the Form of 3D Porous Matrices as a Novel Material for Topical Application. Materials, 14(4), 950. https://doi.org/10.3390/ma14040950







