Quantification of Carbonic Contamination of Fused Silica Surfaces at Different Stages of Classical Optics Manufacturing
Abstract
:1. Introduction
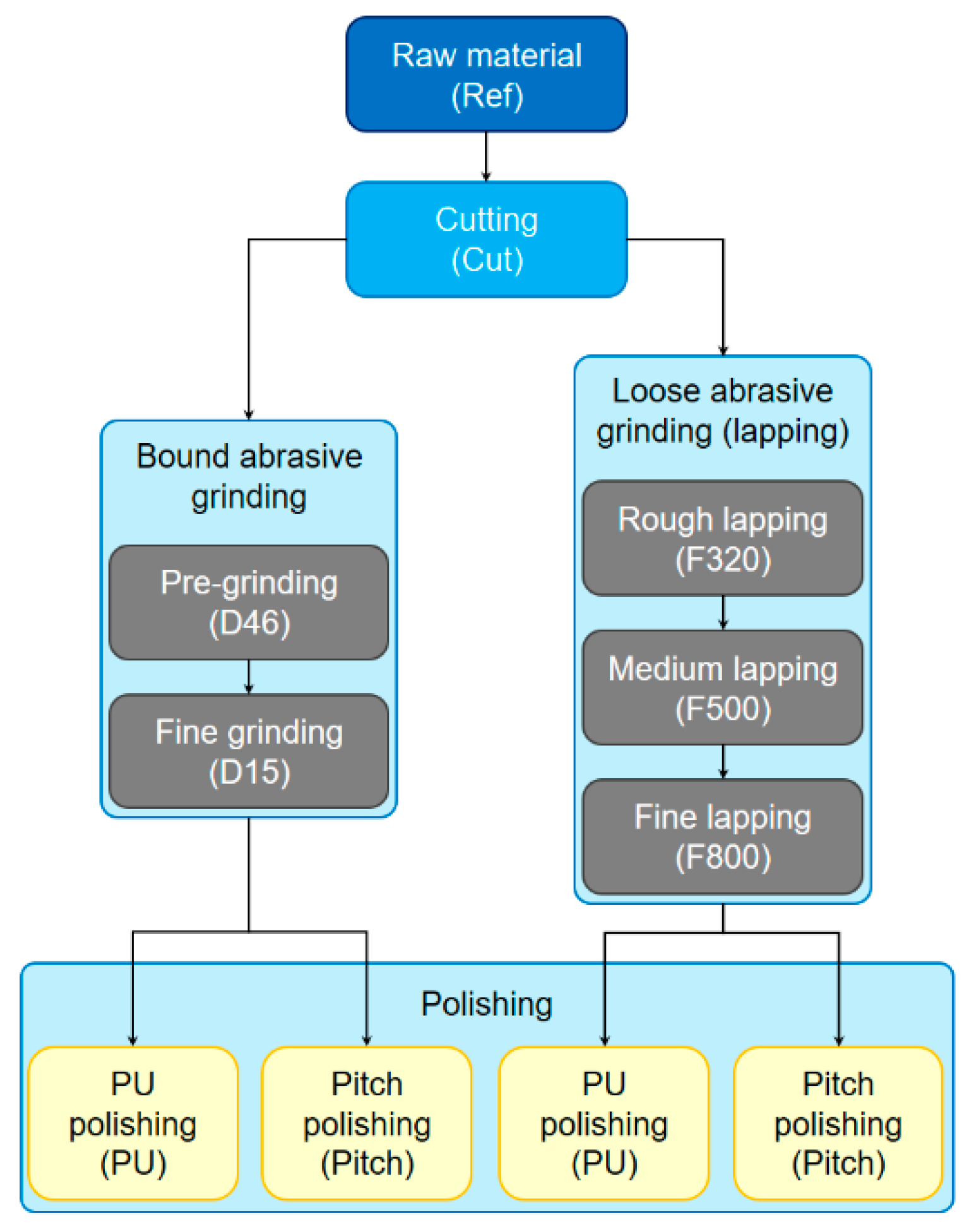
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterisation
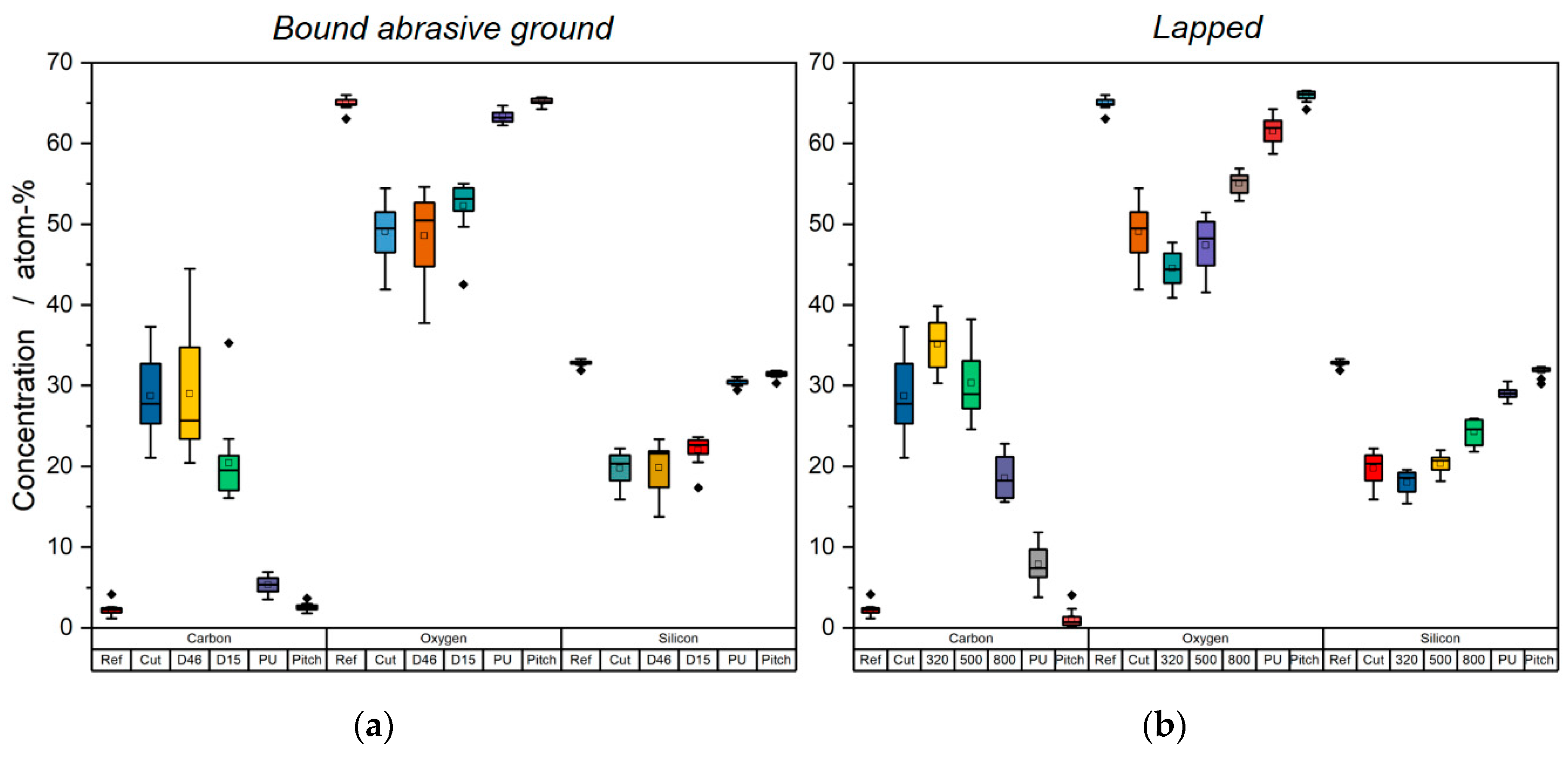
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerhard, C. Optics Manufacturing: Components and Systems; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-4987-6459-9. [Google Scholar]
- van der Kooij, D.; Visser, A.; Hijnen, W.A.M. Determining the concentration of easily assimilable organic carbon in drinking water. J. Am. Water Work. Assoc. 1982, 74, 540–545. [Google Scholar] [CrossRef]
- Servais, P.; Anzil, A.; Ventresque, C. Simple method for determination of biodegradable dissolved organic carbon in water. Appl. Environ. Microbiol. 1989, 55, 2732–2734. [Google Scholar] [CrossRef] [Green Version]
- Neauport, J.; Lamaignere, L.; Bercegol, H.; Pilon, F.; Birolleau, J.-C. Polishing-induced contamination of fused silica optics and laser induced damage density at 351 nm. Opt. Express 2005, 13, 10163–10171. [Google Scholar] [CrossRef] [Green Version]
- Suratwala, T.; Steele, W.; Wong, L.; Feit, M.D.; Miller, P.E.; Dylla-Spears, R.; Shen, N.; Desjardin, R. Chemistry and Formation of the Beilby Layer During Polishing of Fused Silica Glass. J. Am. Ceram. Soc. 2015, 98, 2395–2402. [Google Scholar] [CrossRef]
- Pfiffer, M.; Longuet, J.-L.; Labrugère, C.; Fargin, E.; Bousquet, B.; Dussauze, M.; Lambert, S.; Cormont, P.; Néauport, J. Characterization of the polishing induced contamination of fused silica optics. In Laser-Induced Damage in Optical Materials 2016, proceedings of the SPIE Laser Damage, Boulder, CO, USA, 25 September 2016; Exarhos, G.J., Gruzdev, V.E., Menapace, J.A., Ristau, D., Soileau, M.J., Eds.; SPIE: Bellingham, WA, USA, 2016; p. 100141F. [Google Scholar]
- Gerhard, C.; Tasche, D.; Uteza, O.; Hermann, J. Investigation of nonuniform surface properties of classically manufactured fused silica windows. Appl. Opt. 2017, 56, 7427–7434. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, C.; Tasche, D.; Munser, N.; Dyck, H. Increase in nanosecond laser-induced damage threshold of sapphire windows by means of direct dielectric barrier discharge plasma treatment. Opt. Lett. 2017, 42, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Ross, D.; Tastepe, E.; Lamb, M.; Funamoto, Y.; Shima, D.; Kamimura, T.; Yamaguchi, H. Fused silica contamination layer removal using magnetic field-assisted finishing. J. Am. Ceram. Soc. 2020, 103, 3008–3019. [Google Scholar] [CrossRef]
- Gerhard, C.; Taleb, A.; Pelascini, F.; Hermann, J. Quantification of surface contamination on optical glass via sensitivity-improved calibration-free laser-induced breakdown spectroscopy. Appl. Surf. Sci. 2021, 537, 147984. [Google Scholar] [CrossRef]
- Walton, J.; Alexander, M.R.; Fairley, N.; Roach, P.; Shard, A.G. Film thickness measurement and contamination layer correction for quantitative XPS. Surf. Interface Anal. 2016, 48, 164–172. [Google Scholar] [CrossRef]
- Seah, M.P.; Spencer, S.J. Ultrathin SiO2 on Si. I. Quantifying and removing carbonaceous contamination. J. Vac. Sci. Technol. A Vac. Surf. Film. 2003, 21, 345–352. [Google Scholar] [CrossRef]
- Smith, G.C. Evaluation of a simple correction for the hydrocarbon contamination layer in quantitative surface analysis by XPS. J. Electron Spectrosc. Relat. Phenom. 2005, 148, 21–28. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, C.; Stappenbeck, M. Impact of the Polishing Suspension Concentration on Laser Damage of Classically Manufactured and Plasma Post-Processed Zinc Crown Glass Surfaces. Appl. Sci. 2018, 8, 1556. [Google Scholar] [CrossRef] [Green Version]
- Haisma, J.; Spierings, G.A.C.M. Contact bonding, including direct-bonding in a historical and recent context of materials science and technology, physics and chemistry. Mater. Sci. Eng. R Rep. 2002, 37, 1–60. [Google Scholar] [CrossRef]
- Greco, V.; Marchesini, F.; Molesini, G. Optical contact and van der Waals interactions: The role of the surface topography in determining the bonding strength of thick glass plates. J. Opt. A Pure Appl. Opt. 2001, 3, 85–88. [Google Scholar] [CrossRef]
- Smith, H.I. Optical-Contact Bonding. J. Acoust. Soc. Am. 1965, 37, 928–929. [Google Scholar] [CrossRef]
- Jin, J.; Hanefeld, U. The selective addition of water to C=C bonds; enzymes are the best chemists. Chem. Commun. 2011, 47, 2502–2510. [Google Scholar] [CrossRef]
- Frankel, G.S.; Vienna, J.D.; Lian, J.; Scully, J.R.; Gin, S.; Ryan, J.V.; Wang, J.; Kim, S.H.; Windl, W.; Du, J. A comparative review of the aqueous corrosion of glasses, crystalline ceramics, and metals. Npj Mater. Degrad. 2018, 2, 5. [Google Scholar] [CrossRef]
- Lampman, S.R. Fatigue and Fracture, 10th ed.; 3. Print; ASM International: Materials Park, OH, USA, 2002; ISBN 978-0-87170-385-9. [Google Scholar]
- Bliedtner, J.; Gräfe, G. Optiktechnologie: Grundlagen-Verfahren-Anwendungen-Beispiele; Hanser: München, Germany, 2010; ISBN 978-3-446-40896-8. [Google Scholar]
- Del Hoyo, J.; Kim, D.W.; Burge, J.H. Super-smooth optical fabrication controlling high-spatial frequency surface irregularity. In Proceedings of the SPIE Optical Engineering + Applications, San Diego, CA, USA, 25 August 2013; Fähnle, O.W., Williamson, R., Kim, D.W., Eds.; SPIE: Bellingham, WA, USA, 2013; p. 88380T. [Google Scholar]
- Leistner, A.J.; Thwaite, E.G.; Lesha, F.; Bennett, J.M. Polishing study using Teflon and pitch laps to produce flat and supersmooth surfaces. Appl. Opt. 1992, 31, 1472–1482. [Google Scholar] [CrossRef]
- Lindquist, A. Pitch, Polytron, and polyurethane: A comparison. Appl. Opt. 1986, 25, 3796–3797. [Google Scholar] [CrossRef]
- Cheng, H.; Dong, Z.; Ye, X.; Tam, H.-Y. Subsurface damages of fused silica developed during deterministic small tool polishing. Opt. Express 2014, 22, 18588–18603. [Google Scholar] [CrossRef] [PubMed]
- Rupp, W.J. Conventional Optical Polishing Techniques. Opt. Acta Int. J. Opt. 1971, 18, 1–16. [Google Scholar] [CrossRef]
- Liebl, J.; Biskup, H.; Draxinger, S.; Rascher, R.; Wünsche, C. Process development for the reproducible roughness measurement of optical surfaces using white light interferometry. Int. J. Metrol. Qual. Eng. 2014, 5, 104. [Google Scholar] [CrossRef] [Green Version]
- Douglas, J.F. How does surface roughness affect polymer-surface interactions? Macromolecules 1989, 22, 3707–3716. [Google Scholar] [CrossRef]
- Neauport, J.; Cormont, P.; Legros, P.; Ambard, C.; Destribats, J. Imaging subsurface damage of grinded fused silica optics by confocal fluorescence microscopy. Opt. Express 2009, 17, 3543–3554. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhler, R.; Hellrung, D.; Tasche, D.; Gerhard, C. Quantification of Carbonic Contamination of Fused Silica Surfaces at Different Stages of Classical Optics Manufacturing. Materials 2021, 14, 1620. https://doi.org/10.3390/ma14071620
Köhler R, Hellrung D, Tasche D, Gerhard C. Quantification of Carbonic Contamination of Fused Silica Surfaces at Different Stages of Classical Optics Manufacturing. Materials. 2021; 14(7):1620. https://doi.org/10.3390/ma14071620
Chicago/Turabian StyleKöhler, Robert, Domenico Hellrung, Daniel Tasche, and Christoph Gerhard. 2021. "Quantification of Carbonic Contamination of Fused Silica Surfaces at Different Stages of Classical Optics Manufacturing" Materials 14, no. 7: 1620. https://doi.org/10.3390/ma14071620
APA StyleKöhler, R., Hellrung, D., Tasche, D., & Gerhard, C. (2021). Quantification of Carbonic Contamination of Fused Silica Surfaces at Different Stages of Classical Optics Manufacturing. Materials, 14(7), 1620. https://doi.org/10.3390/ma14071620






