High-Performance Complementary Electrochromic Device Based on Iridium Oxide as a Counter Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Transparent and Electrochromic Electrodes and Electrolyte Materials
2.2. Measurements and Characterizations
3. Results and Discussion
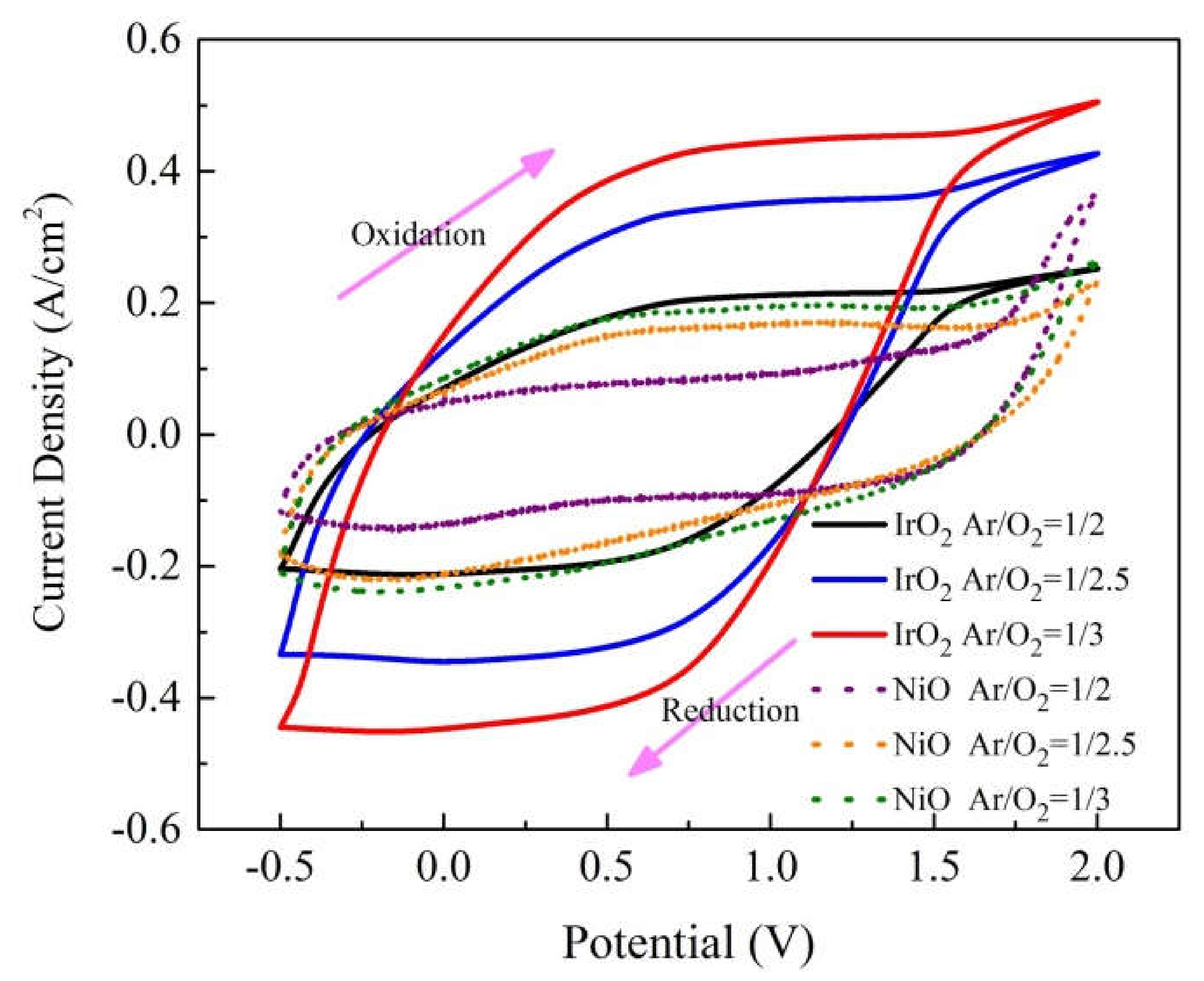
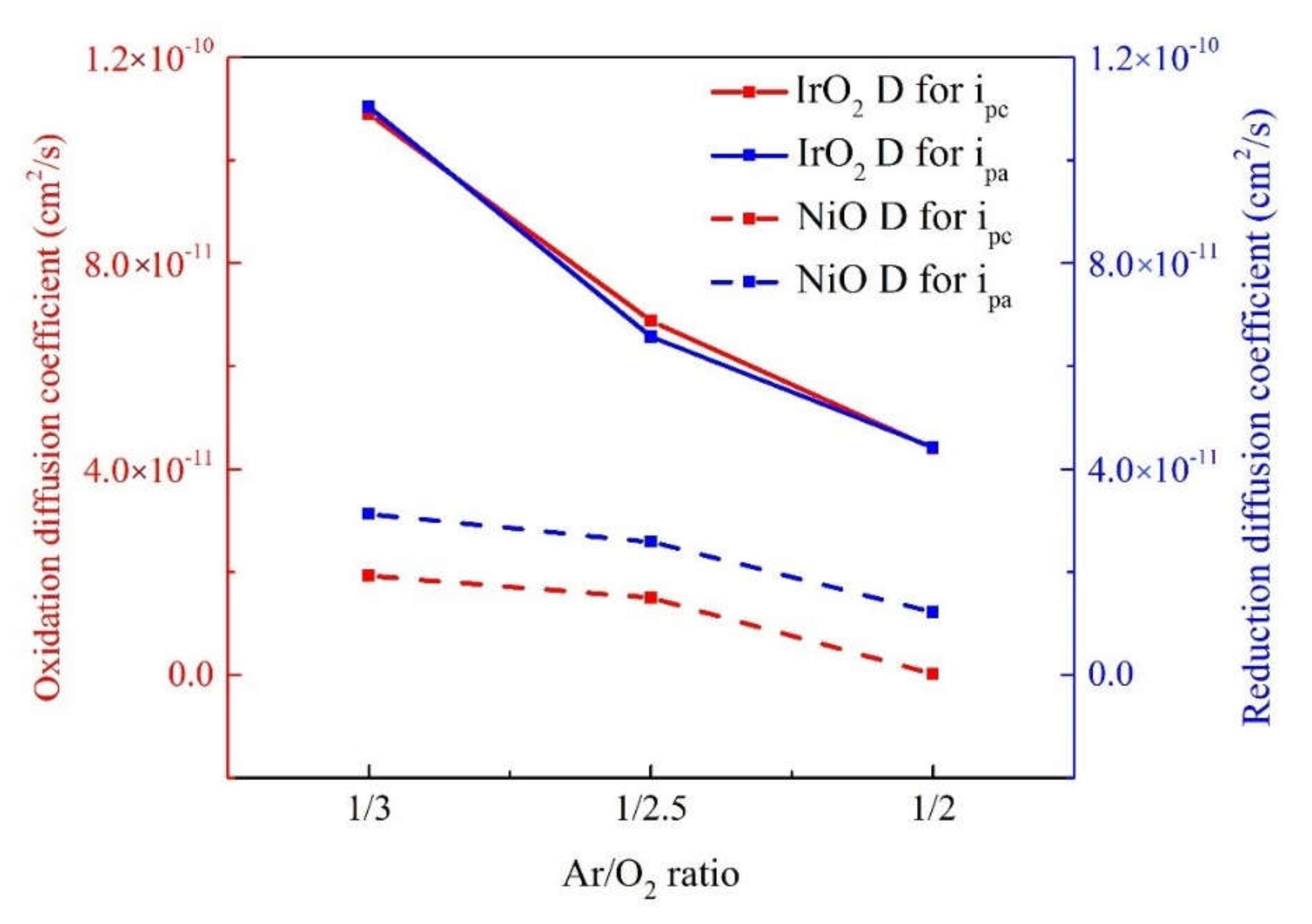
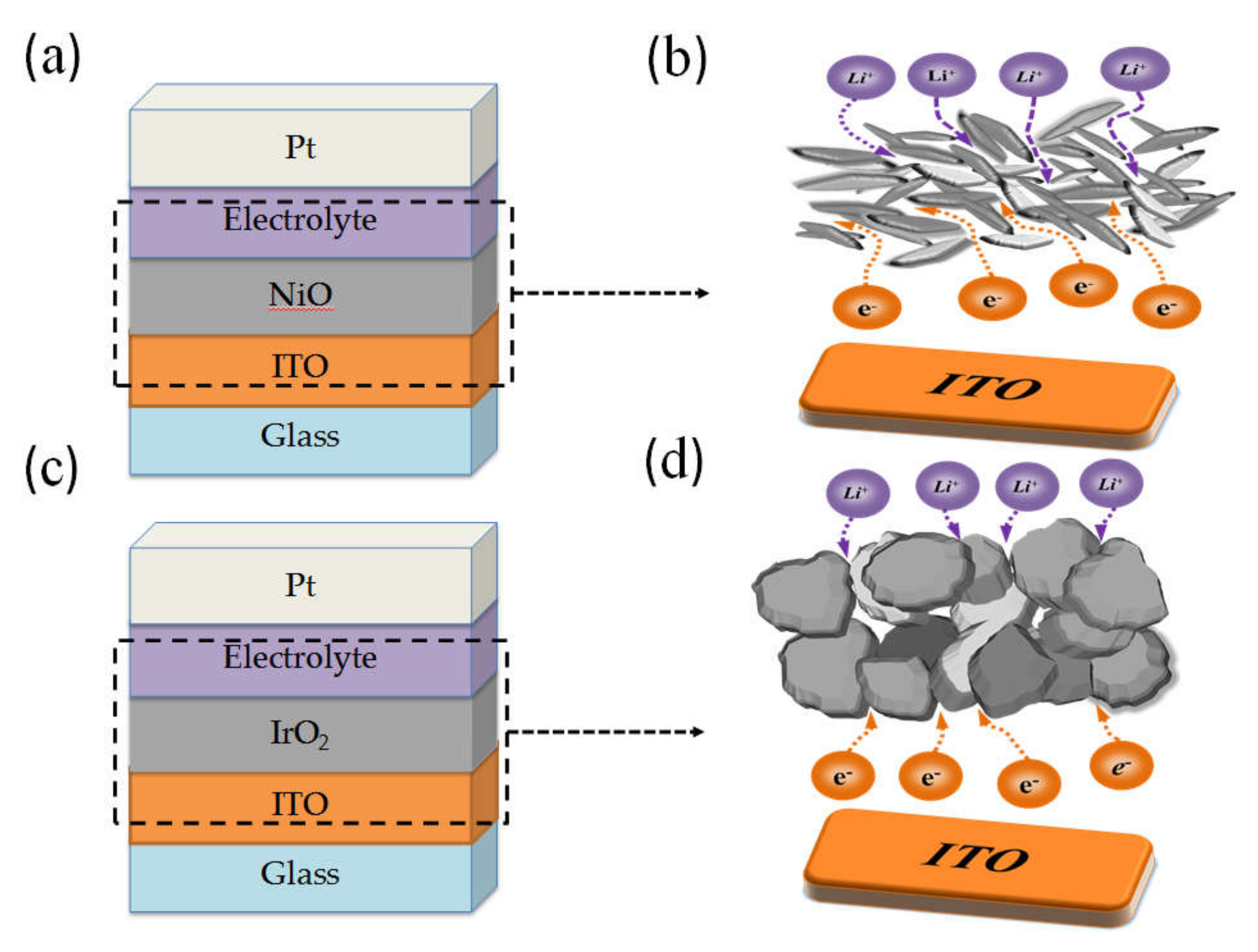
3.1. IrO2/ITO and NiO/ITO Films: Ionic Diffusion
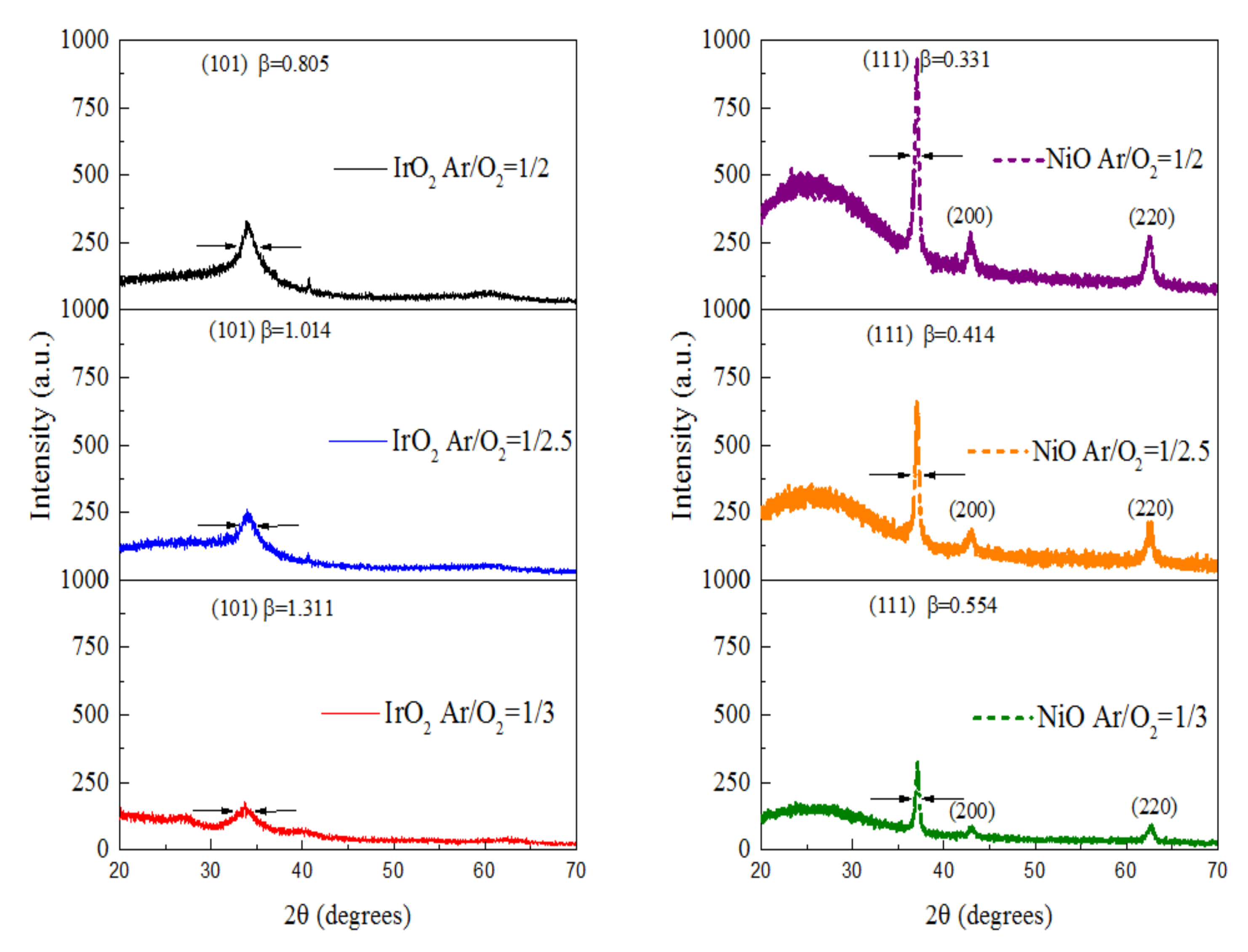
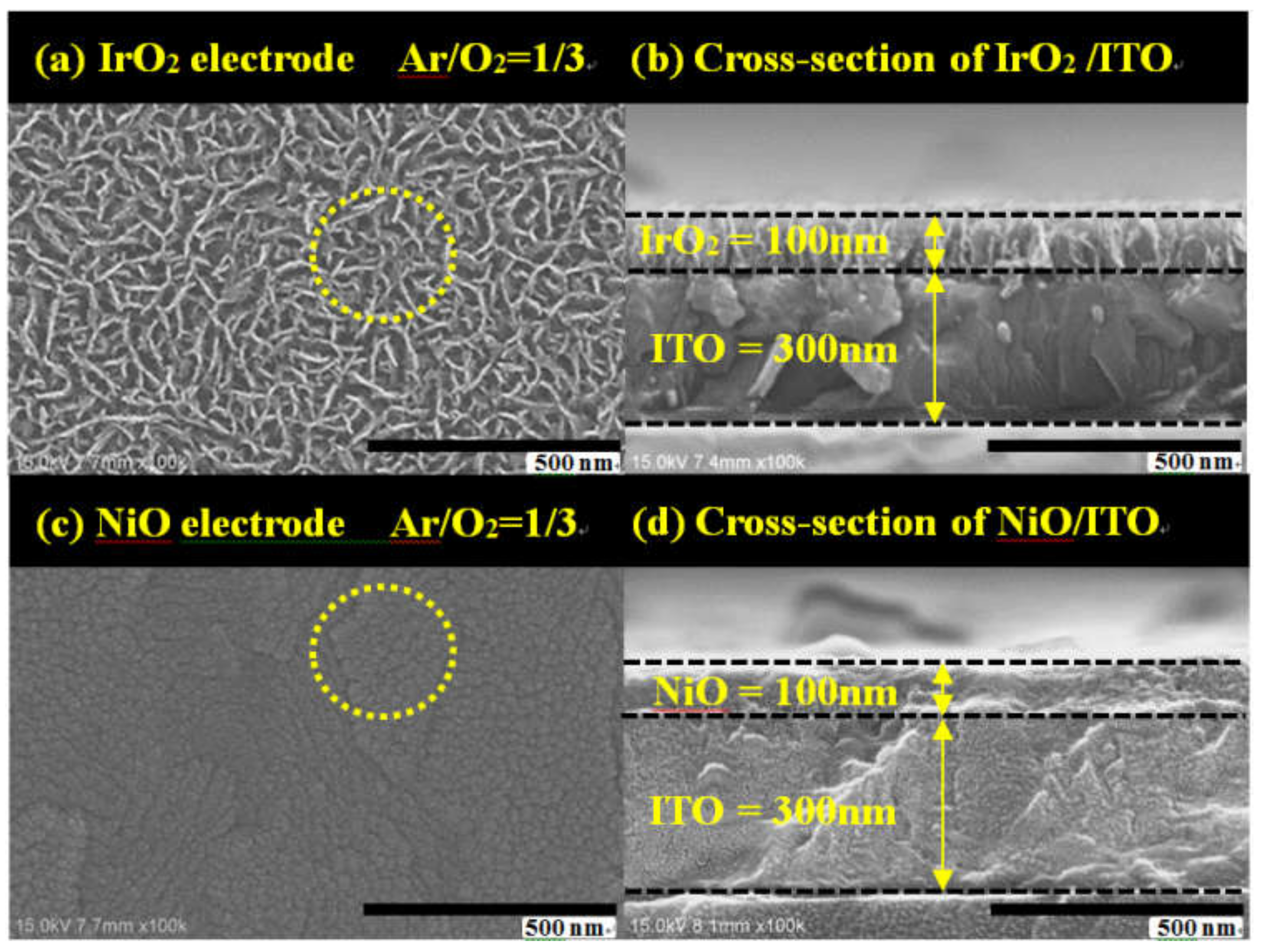
3.2. Material Structure and Surface Morphology Analysis
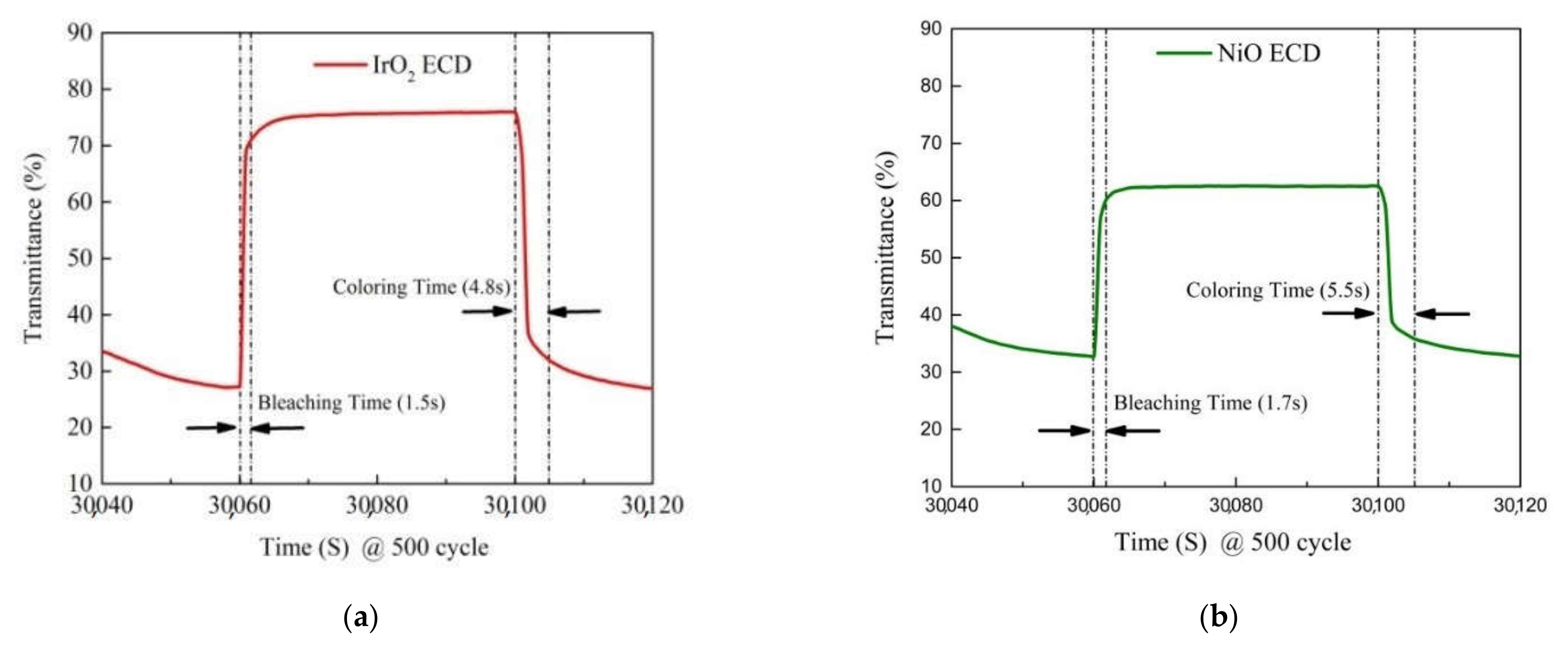
3.3. Optical Transmittance and Cycle Durability Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granqvist, C.G.; Arvizu, M.A.; Bayrak Pehlivan, I.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings:A critical review. Electrochim. Acta 2018, 259, 1170–1182. [Google Scholar] [CrossRef]
- Tie, S.F.; Tan, C.W. A review of energy sources and energy management system in electric vehicles. Renew. Sustain. Energy Rev. 2013, 20, 82–102. [Google Scholar] [CrossRef]
- Sami, O.; Cedric, G.G.; Christophe, D.; Cedric, D.; Raphael, S. All inorganic thin film electrochromic device using LiPON as the ion conductor. Sol. Energy Mater. Sol. Cells 2016, 145, 2–7. [Google Scholar]
- Cai, G.; Peter, D.; Cui, M.; Chen, J.; Wang, X.; Eh, A.L.S.; Magdassi, S.; Lee, P.S. Inkjetprinted all solid-state electrochromic devices based on NiO/WO3 nanoparticle complementary electrodes. Nanoscale 2016, 8, 348–357. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, H.; Li, N.; Tong, Z.; Wang, J.; Zhao, J.; Li, Y. A rapid-response electrochromic device with significantly enhanced electrochromic performance. RSC Adv. 2015, 5, 803–806. [Google Scholar] [CrossRef]
- Cai, G.F.; Tu, J.P.; Zhou, D.; Wang, X.L.; Gu, C.D. Growth of vertically aligned hierarchical WO3 nano-architecture arrays on transparent conducting substrates with outstandingelectrochromic performance. Sol. Energy Mater. Sol. Cells 2014, 124, 103–110. [Google Scholar] [CrossRef]
- Li, H.; McRae, L.; Elezzabi, A.Y. Solution-processed interfacial PEDOT: PSS assemblyinto porous tungsten molybdenum oxide nanocomposite films for electrochromic applications. ACS Appl. Mater. Interfaces 2018, 10, 10520–10527. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ko, T.F.; Hsu, S.C.; Li, K.D.; Wu, J.Y. Fast response ofcomplementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020, 10, 8430–8442. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chi, P.W.; Chandan, P.; Lin, C.K. Electrochemistry and Rapid ElectrochromismControl of MoO3/V2O5 Hybrid Nano bilayers. Nat. Mater. 2019, 12, 2475. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wang, X.L.; Xia, X.H.; Yao, Z.J.; Zhong, Y.; Tu, J.P. Enhanced electrochromic and energy storage performance in mesoporous WO3 film and its application in abifunctional smart window. Nanoscale 2018, 10, 8162–8169. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Yu, G.; Lu, Y.; Wang, L.; Wujcik, E.; Wei, S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C 2017, 5, 1569. [Google Scholar] [CrossRef]
- Heydari Gharahcheshmeh, M.; Gleason, K. Device Fabrication Based on oxidative Chemical Vapor Deposition (OCVD) Synthesis of Conducting Polymers and Related Conjugated Organic Materials. Adv. Mater. Interfaces 2019, 6, 1801564. [Google Scholar] [CrossRef]
- Kim, H.; Choi, D.; Kim, K.; Chu, W.; Chun, D.M.; Lee, C.S. Effect of particle size andamorphous phase on the electrochromic properties of kinetically deposited WO3 films. Sol. Energy Mater. Sol. Cells 2018, 177, 44–50. [Google Scholar] [CrossRef]
- Shien, L.; Zheng, J.; Xu, C. Enhanced electrochromic switches and tunable green fluorescencebased on terbium ion doped WO3 films. Nanoscale 2019, 11, 23049–23057. [Google Scholar] [CrossRef]
- Yue, Y.; Li, H.; Li, K.; Wang, J.; Wang, H.; Zhang, Q.; Li, Y.; Chen, P. High-performancecomplementary electrochromic device based on WO3·0.33H2O/PEDOT and prussian blueelectrodes. J. Phys. Chem. Solids 2017, 110, 284–289. [Google Scholar] [CrossRef]
- Deniz, D.; Frankel, D.J.; Lab, R.J. Nanostructured tungsten and tungsten trioxide filmsprepared by glancing angle deposition. Thin Solid Films 2010, 518, 4095–4099. [Google Scholar] [CrossRef]
- Usha, K.S.; Sivakumar, R.; Sanjeeviraja, C.; Sathe, V.; Ganesan, V.; Wang, T.Y. Improvedelectrochromic performance of a radio frequency magnetron sputtered NiO thin film with high optical switching speed. Thin Solid Films 2016, 6, 79668–79680. [Google Scholar]
- Wnag, L.; Yuan, L.; Wu, X.; Hou, C.; Feng, S. Electrochromic response of pulsed laser deposition prepared WO3–TiO2 composite film. RSC Adv. 2014, 4, 47670–47676. [Google Scholar] [CrossRef]
- Liao, C.C. Lithium-driven electrochromic properties of electrodeposited nickel hydroxide electrodes. Sol. Energy Mater. Sol. Cells 2012, 99, 26–30. [Google Scholar] [CrossRef]
- Lin, Y.S.; Wu, S.S.; Tsai, T.H. Electrochromic properties of novel atmospheric pressureplasma jet-synthesized-organotungsten oxide films for flexible electrochromic devices. Sol. Energy Mater. Sol. Cells 2010, 94, 2283–2291. [Google Scholar] [CrossRef]
- White, C.M.; Gillaspie, D.T.; Whitney, E.; Lee, S.H.; Dillon, A.C. Flexible electrochromicdevices based on crystalline WO3 nanostructures produced with hot-wire chemical vapor deposition. Thin Solid Films 2009, 517, 3596–3599. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, X.; Zhao, Y.; Wang, L.; Liu, D.; Li, X.; Chen, M.; Zhao, J.; Li, Y. Preparation and performance of fast-response ITO/Li-NiO/Li-WO3/ITO all-solid-stateelectrochromic devices by evaporation method. Mater. Mater. Lett. 2020, 265, 127464–127466. [Google Scholar] [CrossRef]
- Li, S.; Yao, Z.; Zhou, J.; Zhang, R.; Shen, H. Fabrication and characterization of WO3 thinfilms on silicon surface by thermal evaporation. Mater. Mater. 2017, 195, 213–216. [Google Scholar]
- Kim, S.K.; Seok, H.J.; Kim, D.H.; Choi, D.H.; Nam, S.J.; Kim, S.C.; Kim, H.K. Comparisonof NiOx thin film deposited by spin-coating or thermal evaporation for application as a holetransport layer of perovskite solar cells. RSC Adv. 2014, 4, 47670–47676. [Google Scholar]
- Zhi, M.; Huang, W.; Shi, Q.; Wang, M.; Wnag, Q. Sol–gel fabrication of WO3/RGO nanocomposite film with enhanced electrochromic performance. RSC Adv. 2016, 6, 67488–67495. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Guo, K.; Guo, D.; Shi, M. Effect of the Ammonium Tungsten Precursor Solutionwith the Modification of Glycerol on Wide Band Gap WO3 Thin Film and Its Electrochromic Properties. Adv. Micromach. 2020, 11, 311. [Google Scholar] [CrossRef]
- Xie, Z.; Zhu, Y.G.; Xu, J.; Huang, H.; Chen, D.; Shen, G. Porous WO3 with enhancedphotocatalytic and selective gas sensing properties. Cryst. Eng. Commun. 2011, 13, 6393–6398. [Google Scholar] [CrossRef]
- Boelens, S.; Veltrop, H. Hard coatings of TiN, (TiHf) N and (TiNb) N deposited by randomand steered arc evaporation. Surf. Coat. Technol. 1987, 33, 63–71. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Z.; Li, W.; Song, X.; Jia, S.; Wang, L. IEEE Transactions on Plasma Science. IEEE 2015, 43, 2267–2274. [Google Scholar]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666. [Google Scholar] [CrossRef]
- Anders, A.; Jüttner, B. Cathode mode transition in high-pressure discharge lamps at start-up Light. Res. Technol. 1990, 22, 111–115. [Google Scholar]
- Chen, P.W.; Chang, C.T.; Ali, M.M.; Wu, J.Y.; Li, Y.C.; Chen, M.H.; Jan, D.-J.; Yuan, C.T. Tantalum oxide flm deposited by vacuum cathodic arc plasma with improved electrochromic performance. Sol. Energy Mater. Sol. Cells 2018, 182, 188–195. [Google Scholar] [CrossRef]
- Lee, K.D. Indium-Zinc-Tin-Oxide flm prepared by DC reactive magnetron sputtering for electrochromic application. Materials 2018, 11, 2221. [Google Scholar]
- Liu, Q.; Chen, Q.; Zhang, Q.; Xiao, Y.; Zhong, X.; Dong, G.; Terryn, H.; Baert, K.; Reiners, F.; Diao, X. In situ electrochromic efficiency of a nickel oxide thin film: Origin of electrochemical process and electrochromic degradation. J. Mater. Chem. C 2018, 6, 646. [Google Scholar] [CrossRef]
- Pereira, S.; Goncalves, A.; Correia, N.; Pinto, J.; Pereira, L.; Martins, R.; Fortunato, E. Electrochromic behavior of NiO thin films deposited by e-beam evaporation at room temperature. Sol. Energy Mater. Sol. Cells 2014, 120, 109–115. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Zhang, J.; Tu, J.; Xia, X.; Qiao, Y.; Lu, Y. An all-solid-state electrochromic device based on NiO/WO3 complementary structure and solid hybrid polyelectrolyte. Sol. Energy Mater. Sol. Cells 2009, 93, 1840–1845. [Google Scholar] [CrossRef]
- Yang, L.; Ge, D.; Zhao, J.; Ding, Y.; Kong, X.; Li, Y. Improved electrochromic performance of ordered macroporous tungstenoxide films for IR electrochromic device. Sol. Energy Mater. Sol. Cells 2012, 100, 251–257. [Google Scholar] [CrossRef]
- Ma, D.; Li, T.; Xu, Z.; Wang, L.; Wang, J. Electrochromic devices based on tungsten oxide films with honeycomblike nanostructures and nanoribbons array. Sol. Energy Mater. Sol. Cells 2018, 177, 51–56. [Google Scholar] [CrossRef]
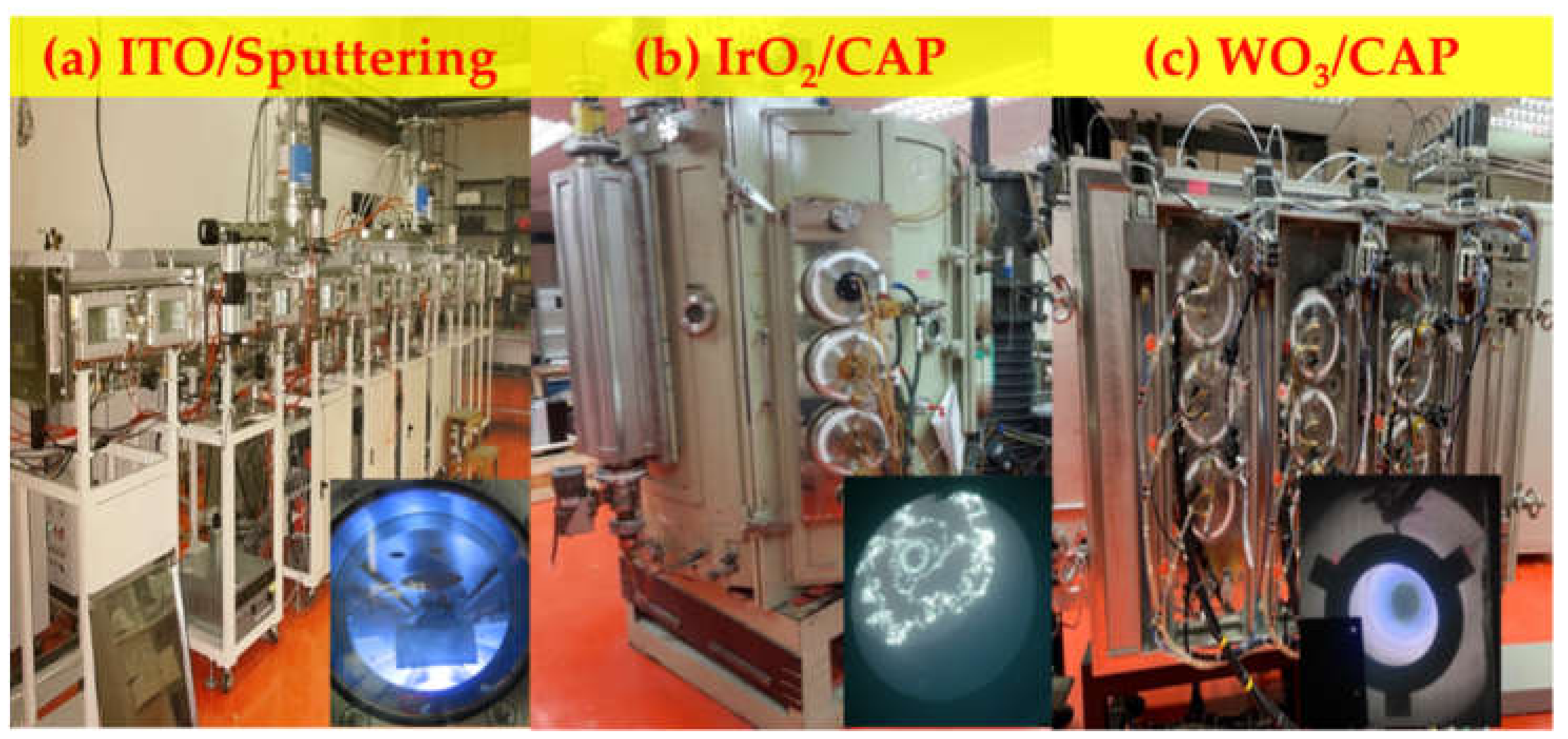


| No. | Electrode | Ar/O2 (Ar = 20 sccm) | W.P. (Torr) | DC Power (W) | Deposition Temp (°C) | Deposition Time (s) | Thickness (nm) |
|---|---|---|---|---|---|---|---|
| Sample 1 | IrO2 | 1/2 | 1.2 × 10−3 | 1250 | 100 | 40 | 100 |
| Sample 2 | IrO2 | 1/2.5 | 1.7 × 10−3 | 1250 | 100 | 40 | 100 |
| Sample 3 | IrO2 | 1/3 | 1.9 × 10−3 | 1250 | 100 | 40 | 100 |
| Sample 4 | NiO | 1/2 | 1.2 × 10−3 | 1250 | 100 | 100 | 100 |
| Sample 5 | NiO | 1/2.5 | 1.7 × 10−3 | 1250 | 100 | 100 | 100 |
| Sample 6 | NiO | 1/3 | 1.9 × 10−3 | 1250 | 100 | 100 | 100 |
| Target | W.P. (Torr) | Ar/O2 (sccm) | DC power (W) | Deposition Time (min) | Deposition Rate (nm/min) | Deposition Temp °C | Thickness (nm) |
|---|---|---|---|---|---|---|---|
| ITO | 3 × 10−3 | 1/3 (Ar = 100) | 500 | 60 | 5 | 200 | 300 |
| W Metal | 8 × 10−3 | 1/3 (Ar = 100) | 1500 | 15 | 13.3 | 50 | 200 |
| No. | Electrode | Ar/O2 (Ar = 20 sccm) | Anodic Current (jpa) | Cathodic Current (jpc) | D for jpa Oxidation | D for jpc Reduction |
|---|---|---|---|---|---|---|
| Sample 1 | IrO2 | 1/2 | 2.82 × 10−4 | 2.83 × 10−4 | 4.40 × 10−11 | 4.42 × 10−11 |
| Sample 2 | IrO2 | 1/2.5 | 3.53 × 10−4 | 3.45 × 10−4 | 6.88 × 10−11 | 6.57 × 10−11 |
| Sample 3 | IrO2 | 1/3 | 4.44 × 10−4 | 4.47 × 10−4 | 1.09 × 10−10 | 1.10 × 10−10 |
| Sample 4 | NiO | 1/2 | 8.70 × 10−5 | 1.49 × 10−4 | 4.18 × 10−12 | 1.22 × 10−11 |
| Sample 5 | NiO | 1/2.5 | 1.65 × 10−4 | 2.16 × 10−4 | 1.50 × 10−11 | 2.59 × 10−11 |
| Sample 6 | NiO | 1/3 | 1.87 × 10−4 | 2.38 × 10−4 | 1.93 × 10−11 | 3.12 × 10−11 |
| No. | Electrode | Ar/O2 (sccm) | 2θ (deg) | FWHM (β) | Ave Grain Size (nm) |
|---|---|---|---|---|---|
| Sample 1 | IrO2 | 1/2 | 34.832° | 0.805° | 10.03 |
| Sample 2 | IrO2 | 1/2.5 | 34.644° | 1.014° | 8.20 |
| Sample 3 | IrO2 | 1/3 | 34.312° | 1.311° | 6.35 |
| Sample 4 | NiO | 1/2 | 37.815° | 0.331° | 25.30 |
| Sample 5 | NiO | 1/2.5 | 37.613° | 0.414° | 20.27 |
| Sample 6 | NiO | 1/3 | 37.282° | 0.554° | 15.15 |
| Materials/Device | Method | ∆T (%) | CE (cm2/C) | Switching Time (tc/tb) | Ref. |
|---|---|---|---|---|---|
| WO3/IrO2 | CAP | 50 | - | 4.8/1.5 s | This work |
| WO3/NiO | CAP | 46 | 90 | 3.1/4.6 s | [8] |
| WO3/NiO | DC Sputtering | 55 | 87 | 10/20 s | [37] |
| WO3/PANI | Electro polymerization | 37.4 | 98.4 | 9.9/13.6 s | [38] |
| WO3/PANI | Dip-coating | 54.3 | 79.7 | 1.4/1.1 s | [10] |
| WO3 | Spray | - | - | - | [13] |
| (NH4)0.33 WO3 | Hydrothermal | 60.9 | 60.9 | 5.7/4.2 s | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, T.-F.; Chen, P.-W.; Li, K.-M.; Young, H.-T.; Chang, C.-T.; Hsu, S.-C. High-Performance Complementary Electrochromic Device Based on Iridium Oxide as a Counter Electrode. Materials 2021, 14, 1591. https://doi.org/10.3390/ma14071591
Ko T-F, Chen P-W, Li K-M, Young H-T, Chang C-T, Hsu S-C. High-Performance Complementary Electrochromic Device Based on Iridium Oxide as a Counter Electrode. Materials. 2021; 14(7):1591. https://doi.org/10.3390/ma14071591
Chicago/Turabian StyleKo, Tien-Fu, Po-Wen Chen, Kuan-Ming Li, Hong-Tsu Young, Chen-Te Chang, and Sheng-Chuan Hsu. 2021. "High-Performance Complementary Electrochromic Device Based on Iridium Oxide as a Counter Electrode" Materials 14, no. 7: 1591. https://doi.org/10.3390/ma14071591
APA StyleKo, T.-F., Chen, P.-W., Li, K.-M., Young, H.-T., Chang, C.-T., & Hsu, S.-C. (2021). High-Performance Complementary Electrochromic Device Based on Iridium Oxide as a Counter Electrode. Materials, 14(7), 1591. https://doi.org/10.3390/ma14071591







