Evaluation of Biodegradable PVA-Based 3D Printed Carriers during Dissolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. FDM Printing
2.3. Preparation of Riboflavin Containing Liquid Fill
2.4. Physical Characterisation of 3D Printed Carriers
2.5. PVA-Based Carrier and Drug Release Study
2.5.1. Study of PVA-Based Carrier Erosion
2.5.2. Riboflavin Release
3. Results and Discussion
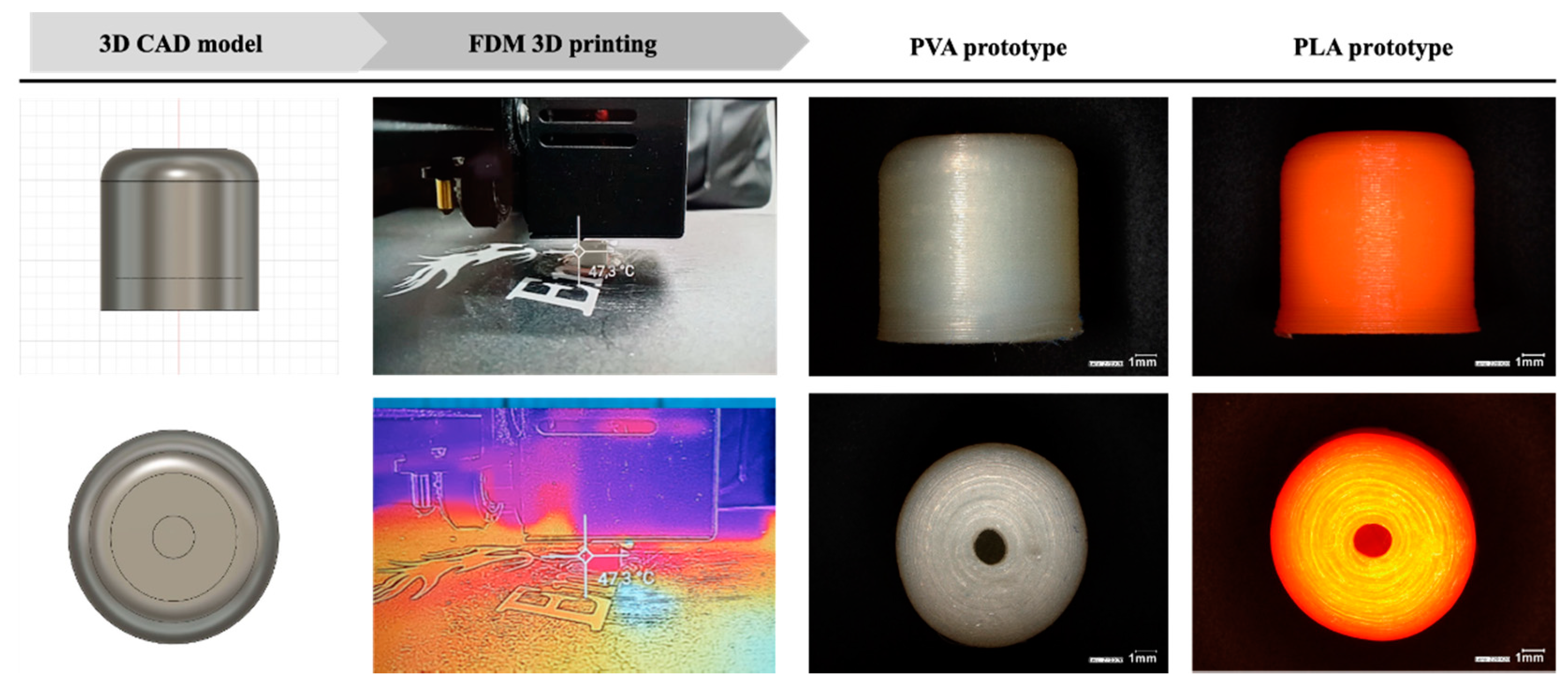
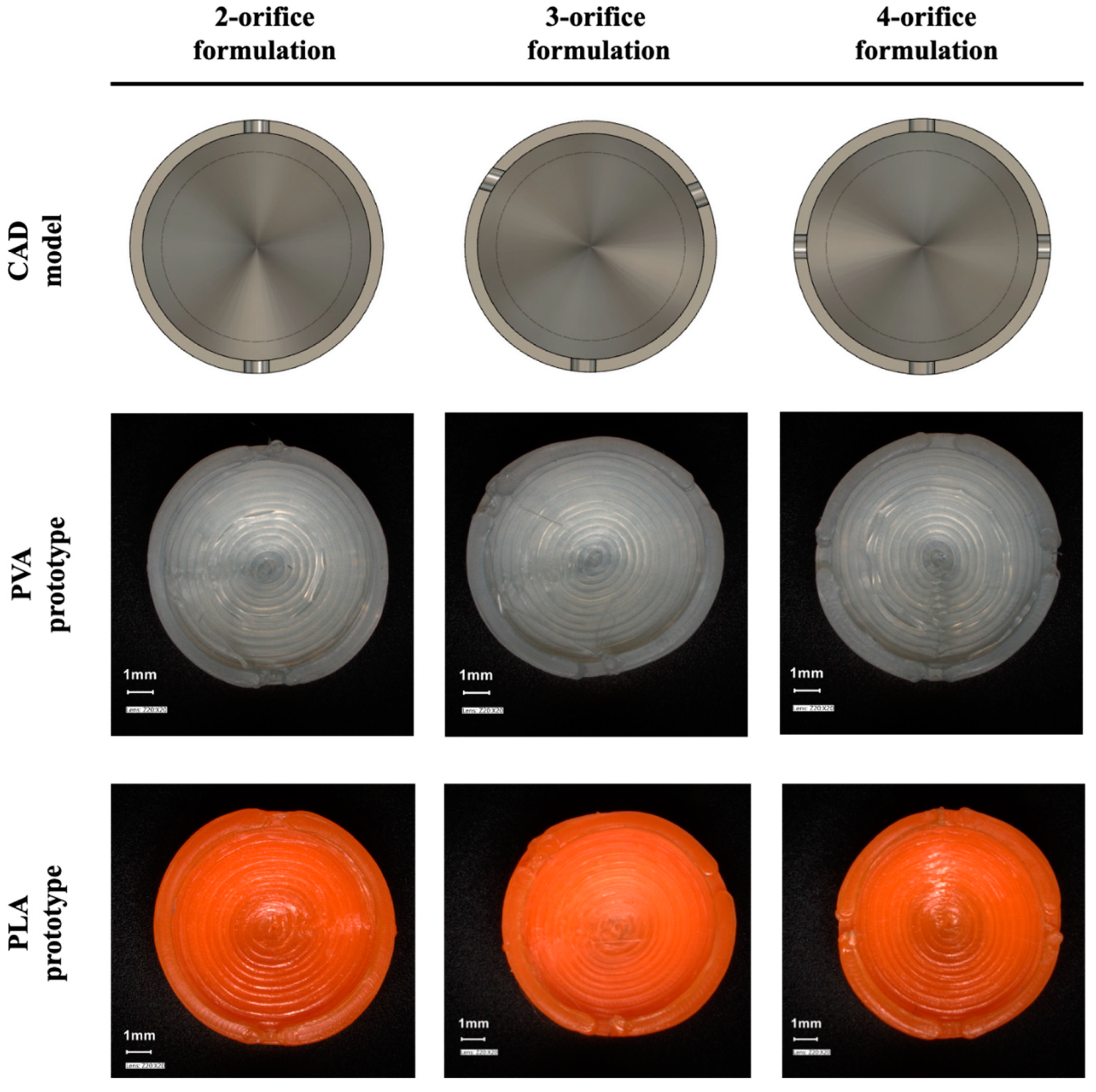
3.1. The CAD Design and the Tracking of the Printlet
3.2. Physical Characterisation of 3D Printed Carrier Systems
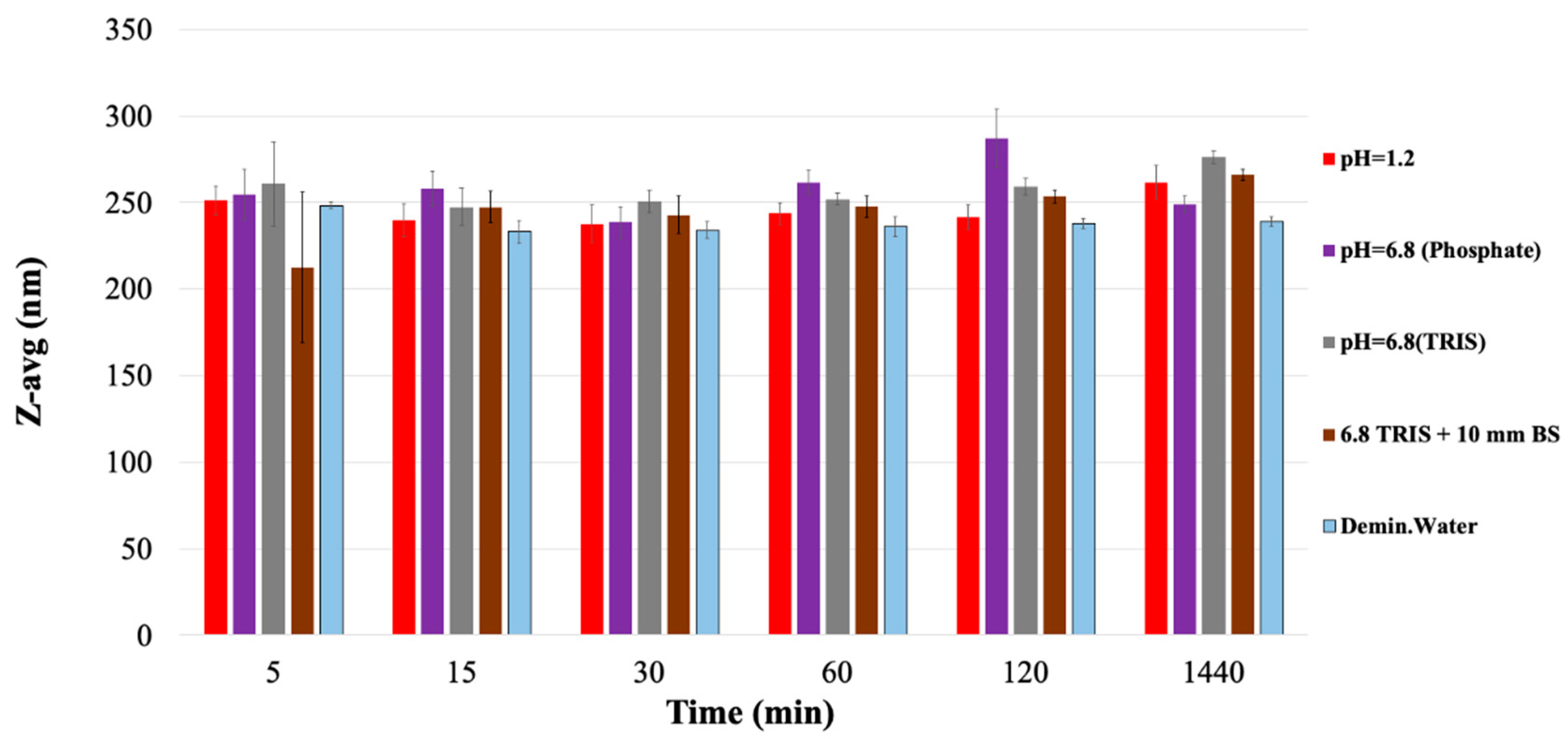
3.3. Erosion of the PVA Carrier
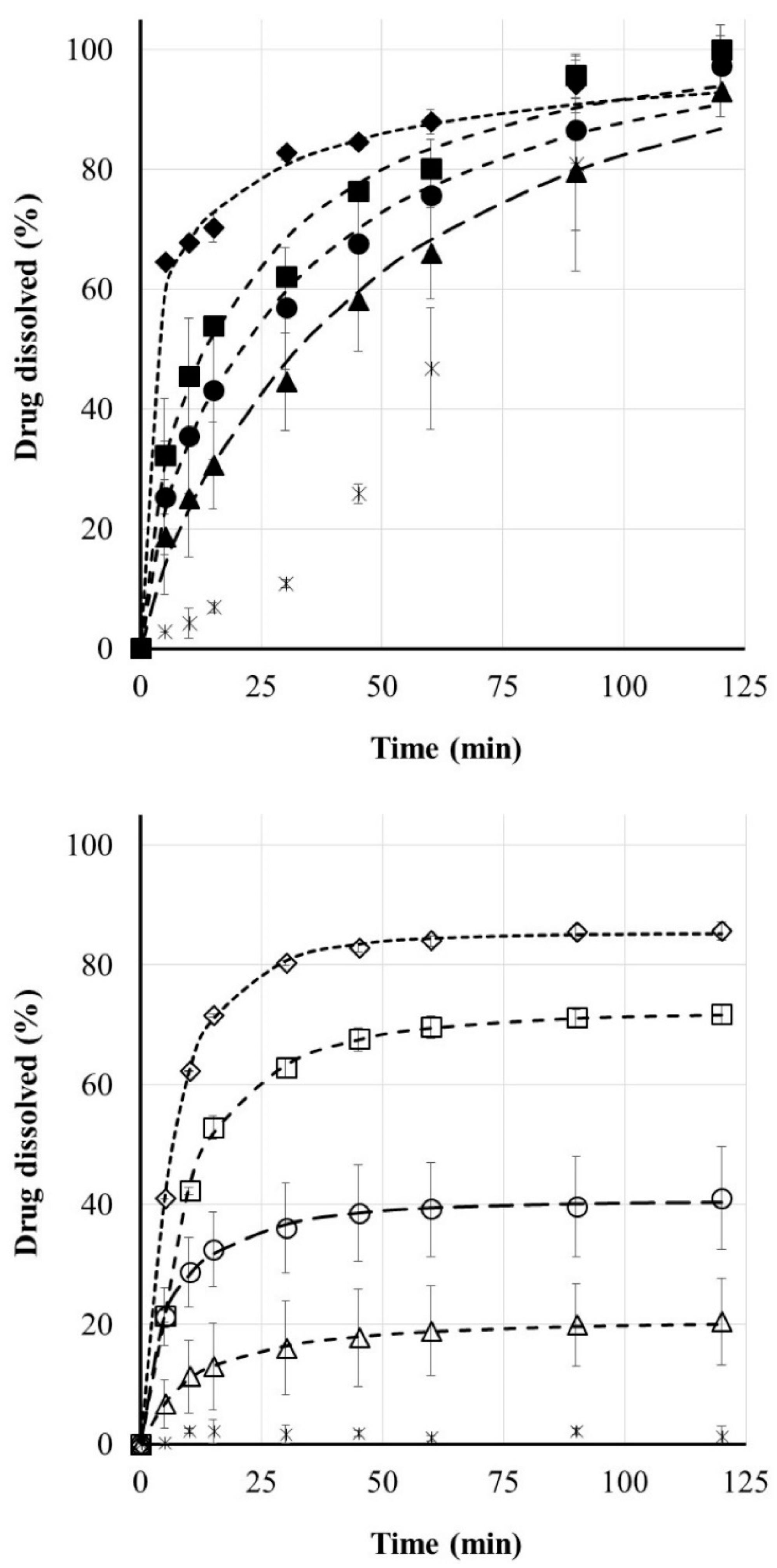
3.4. Riboflavin Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Center for Drug Evaluation and Research (CDER). Approval Package for SPRITAM; CDER: Rockville, MD, USA, 2015.
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.-W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Öblom, H. 3D-Printed Drugs for Children—Are We Ready Yet? AAPS PharmSciTech 2017, 18, 303–308. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. 3D-Printed Network Structures as Controlled-Release Drug Delivery Systems: Dose Adjustment, API Release Analysis and Prediction. AAPS PharmSciTech 2018, 19, 3333–3342. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 1–22. [Google Scholar] [CrossRef]
- Mechanics, A.; Devices, O.; di Prima, M. Technical Considerations for Additive Manufactured Medical Devices. Guidance for Industry and Food and Drug Administration Staff; FDA: Rockville, MD, USA, 2017.
- FDA; CDER. Advancement of Emerging Technology Applications for Pharmaceutical Innovation and Modernization Guidance for Industry; CDER: Rockville, MD, USA, 2017.
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D printed medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- AlGahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef] [PubMed]
- Rattanakit, P.; Moulton, S.E.; Santiago, K.S.; Liawruangrath, S.; Wallace, G.G. Extrusion printed polymer structures: A facile and versatile approach to tailored drug delivery platforms. Int. J. Pharm. 2012, 422, 254–263. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- McMains, S. Layered Manufacturing Technologies; ACM: New York, NY, USA, 2005; Volume 48. [Google Scholar]
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, M.L.; Ariffin, M.K.A.M. The Effects of Combined Infill Patterns on Mechanical Properties in FDM Process. Polymers 2020, 12, 2792. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D Printing of Tunable Zero-Order Release Printlets. Polymers 2020, 12, 1769. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Nasereddin, J.; Belton, P.; Qi, S. Impact of Processing Parameters on the Quality of Pharmaceutical Solid Dosage Forms Produced by Fused Deposition Modeling (FDM). Pharmaceutics 2019, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-Y.; Chou, Y.-C.; Lai, Y.-H.; Lin, Y.-T.; Lu, C.-J.; Liu, S.-J. Fabrication of Drug-Eluting Nano-Hydroxylapatite Filled Polycaprolactone Nanocomposites Using Solution-Extrusion 3D Printing Technique. Polymers 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef]
- Arafat, B.; Qinna, N.; Cieszynska, M.; Forbes, R.T.; Alhnan, M.A. Tailored on demand anti-coagulant dosing: An in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. Eur. J. Pharm. Biopharm. 2018, 128, 282–289. [Google Scholar] [CrossRef]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maccagnan, S.; Ortenzi, M.A.; Antenucci, S.; Briatico-Vangosa, F.; Maroni, A.; Gazzaniga, A.; Zema, L. Industrial Development of a 3D-Printed Nutraceutical Delivery Platform in the Form of a Multicompartment HPC Capsule. AAPS PharmSciTech 2018, 19, 3343–3354. [Google Scholar] [CrossRef]
- Smith, D.; Kapoor, Y.; Hermans, A.; Nofsinger, R.; Kesisoglou, F.; Gustafson, T.P.; Procopio, A. 3D printed capsules for quantitative regional absorption studies in the GI tract. Int. J. Pharm. 2018, 550, 418–428. [Google Scholar] [CrossRef]
- Khezri, M.; Rasmussen, K. An energy-based approach to buckling modal decomposition of thin-walled members with arbitrary cross sections, Part 1: Derivation. Thin Walled Struct. 2019, 138, 496–517. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.-W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Shah, M.K.; Vora, N. Formulation strategies for solid oral dosage form using 3D printing technology: A minireview. J. Drug Deliv. Sci. Technol. 2018, 46, 148–155. [Google Scholar] [CrossRef]
- Araújo, M.R.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. The Digital Pharmacies Era: How 3D Printing Technology Using Fused Deposition Modeling Can Become a Reality. Pharmaceutics 2019, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Soares, C.; Gollwitzer, V.; Habashy, R.; Timmins, P.; Alhnan, M.A. On demand manufacturing of patient-specific liquid capsules via co-ordinated 3D printing and liquid dispensing. Eur. J. Pharm. Sci. 2018, 118, 134–143. [Google Scholar] [CrossRef]
- Solaro, R.; Corti, A.; Chiellini, E. Biodegradation of poly(vinyl alcohol) with different molecular weights and degree of hydrolysis. Polym. Adv. Technol. 2000, 11, 873–878. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. On the environmental impacts of 3D printing technology. Appl. Mater. Today 2020, 20, 100689. [Google Scholar] [CrossRef]
- Krishna, N.; Brow, F. Polyvinyl alcohol as an ophthalmic vehicle. Effect on regeneration of corneal epithelium. Am. J. Ophthalmol. 1964, 57, 99–106. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Trapani, A.; Di Franco, C.; Mandracchia, D.; Trapani, G.; Franchini, C.; Corbo, F.; Tripodo, G.; Kolev, I.N.; Stoyanov, G.S.; et al. In vitro and ex vivo studies on diltiazem hydrochloride-loaded microsponges in rectal gels for chronic anal fissures treatment. Int. J. Pharm. 2019, 557, 53–65. [Google Scholar] [CrossRef]
- Debevec, V.; Ljubin, T.S.; Jeraj, Ž; Peterka, T.R.; Bratuž, B.; Gašperlin, D.; Srčič, S.; Horvat, M. Step-wise approach to developing a scale-independent design space for functional tablet coating process. Drug Dev. Ind. Pharm. 2020, 46, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Z.; Zhang, X.; Li, J.; Huang, Y.; Chen, W.; Pan, X.; Wu, C. Development of Paroxetine Hydrochloride Single Layer Controlled-Release Tablets Based on 32 Factorial Design. Pharmaceutics 2018, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Samaha, D.; Shehayeb, R.; Kyriacos, S. Modeling and Comparison of Dissolution Profiles of Diltiazem Modified-Release Formulations. Dissolution Technol. 2009, 16, 41–46. [Google Scholar] [CrossRef]
- Kállai, N.; Luhn, O.; Dredán, J.; Kovács, K.; Lengyel, M.; Antal, I. Evaluation of drug release from coated pellets based on isomalt, sugar, and microcrystalline cellulose inert cores. AAPS PharmSciTech 2010, 11, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.; Róka, E.; Mollet, L.; Coleman, A.W.; Perret, F.; Kim, B.; Kovács, R.; Kazsoki, A.; Zelkó, R.; Gesztelyi, R.; et al. Fused deposition modeling 3D printing: Test platforms for evaluating post-fabrication chemical modifications and in-vitro biological properties. Pharmaceutics 2019, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, N.; Yamamoto, T.; Arakawa, K.; Teshima, A. Preparation of PVA/Polymer Colloid nanocomposite Hydrogel Using PS-PNVA Particles. Chem. Lett. 2019, 48, 378–381. [Google Scholar] [CrossRef]
- Bastiat, G.; Pritz, C.O.; Roider, C.; Fouchet, F.; Lignières, E.; Jesacher, A.; Glueckert, R.; Ritsch-Marte, M.; Schrott-Fischer, A.; Saulnier, P.; et al. A new tool to ensure the fluorescent dye labeling stability of nanocarriers: A real challenge for fluorescence imaging. J. Control. Release 2013, 170, 334–342. [Google Scholar] [CrossRef]
- Sheskey, P.J.; Cook, W.G.; Cable, C.G. (Eds.) Handbook of Pharmaceutical Excipients, 8th ed.; Pharmaceutical Press: London, UK, 2017. [Google Scholar]


| PVA Carrier | PLA Carrier | |
|---|---|---|
| Weight (g) (n = 20; mean ± SD) | 0.42 0.007 | 0.49 0.004 |
| Height (mm) (n = 20; mean ± SD) | 9.75 0.053 | 9.75 0.036 |
| Diameter (mm) (n = 20; mean ± SD) | 9.66 0.298 | 9.66 0.239 |
| Hardness (N) (n = 10; mean ± SD) | 212.63 75.87 | 300.0 1.00 |
| Friability (%) | 0.016 | 0.024 |
| Medium | 5 min | 15 min | 30 min | 60 min | 120 min | 1440 min |
|---|---|---|---|---|---|---|
| pH = 1.2 | 1.83 ± 0.92 | 15.94 ± 1.26 | 36.3 ± 1.4 | 75.15 ± 0.92 | 94.79 ± 0.66 | 100 ± 1.18 |
| pH = 6.8 (Phosphate) | 4.49 ± 0.46 | 19.77 ± 0.38 | 57.83 ± 1.28 | 83.21 ± 0.42 | 85.76 ± 1.01 | 100 ± 1.49 |
| pH = 6.8 (TRIS) | 0.55 ± 0.15 | 2.81 ± 0.4 | 10.95 ± 2.61 | 19.33 ± 0.11 | 36.0 ± 0.43 | 100 ± 1.01 |
| pH = 6.8 (TRIS) + 10 mm BS | 1.0 ± 0.1 | 3.57 ± 1.56 | 7.4 ± 1.02 | 13.8 ± 1.12 | 25.7 ± 1.54 | 100 ± 6.9 |
| Demineralized water | 2.37 ± 1.0 | 10.97 ± 0.76 | 30.01 ± 0.61 | 50.76 ± 0.8 | 73.89 ± 0.52 | 100 ± 0.54 |
| Filament Base | PLA | PVA | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of orifices | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| M∞ (%) | 20.46 | 40.52 | 72.15 | 85.28 | 100.00 | 100.00 | 99.92 | 100.00 |
| t0 (min) | 2.42 | 0.11 | 3.71 | 2.43 | 0.00 | 0.00 | 0.00 | 0.00 |
| τd (min) | 12.17 | 7.54 | 7.53 | 5.00 | 50.77 | 34.48 | 23.96 | 6.90 |
| β | 0.59 | 0.62 | 0.60 | 0.63 | 0.82 | 0.70 | 0.64 | 0.34 |
| r | 0.9991 | 0.9992 | 0.9999 | 0.9999 | 0.9947 | 0.9960 | 0.9925 | 0.9950 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basa, B.; Jakab, G.; Kállai-Szabó, N.; Borbás, B.; Fülöp, V.; Balogh, E.; Antal, I. Evaluation of Biodegradable PVA-Based 3D Printed Carriers during Dissolution. Materials 2021, 14, 1350. https://doi.org/10.3390/ma14061350
Basa B, Jakab G, Kállai-Szabó N, Borbás B, Fülöp V, Balogh E, Antal I. Evaluation of Biodegradable PVA-Based 3D Printed Carriers during Dissolution. Materials. 2021; 14(6):1350. https://doi.org/10.3390/ma14061350
Chicago/Turabian StyleBasa, Bálint, Géza Jakab, Nikolett Kállai-Szabó, Bence Borbás, Viktor Fülöp, Emese Balogh, and István Antal. 2021. "Evaluation of Biodegradable PVA-Based 3D Printed Carriers during Dissolution" Materials 14, no. 6: 1350. https://doi.org/10.3390/ma14061350
APA StyleBasa, B., Jakab, G., Kállai-Szabó, N., Borbás, B., Fülöp, V., Balogh, E., & Antal, I. (2021). Evaluation of Biodegradable PVA-Based 3D Printed Carriers during Dissolution. Materials, 14(6), 1350. https://doi.org/10.3390/ma14061350






