A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective
Abstract
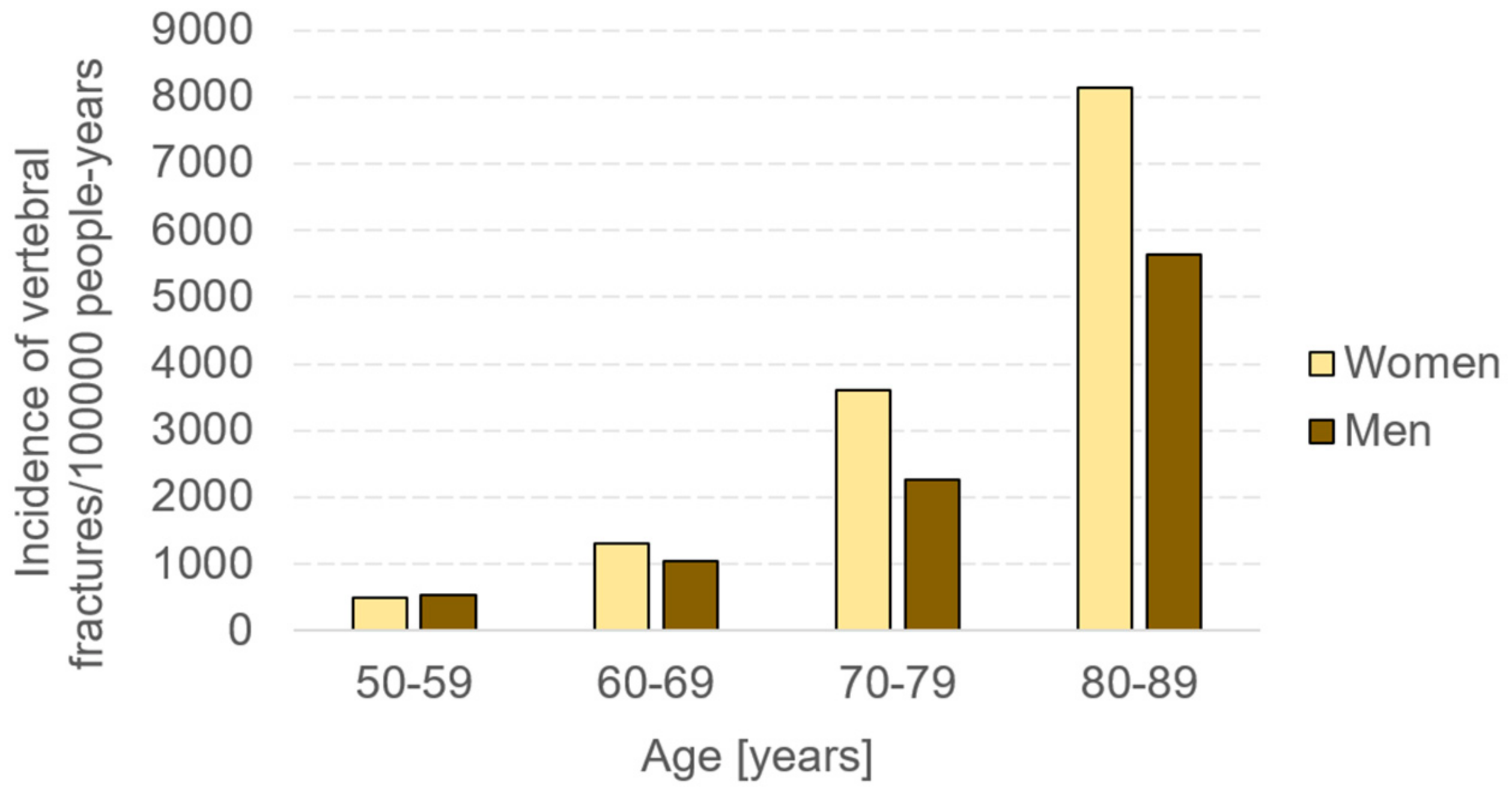
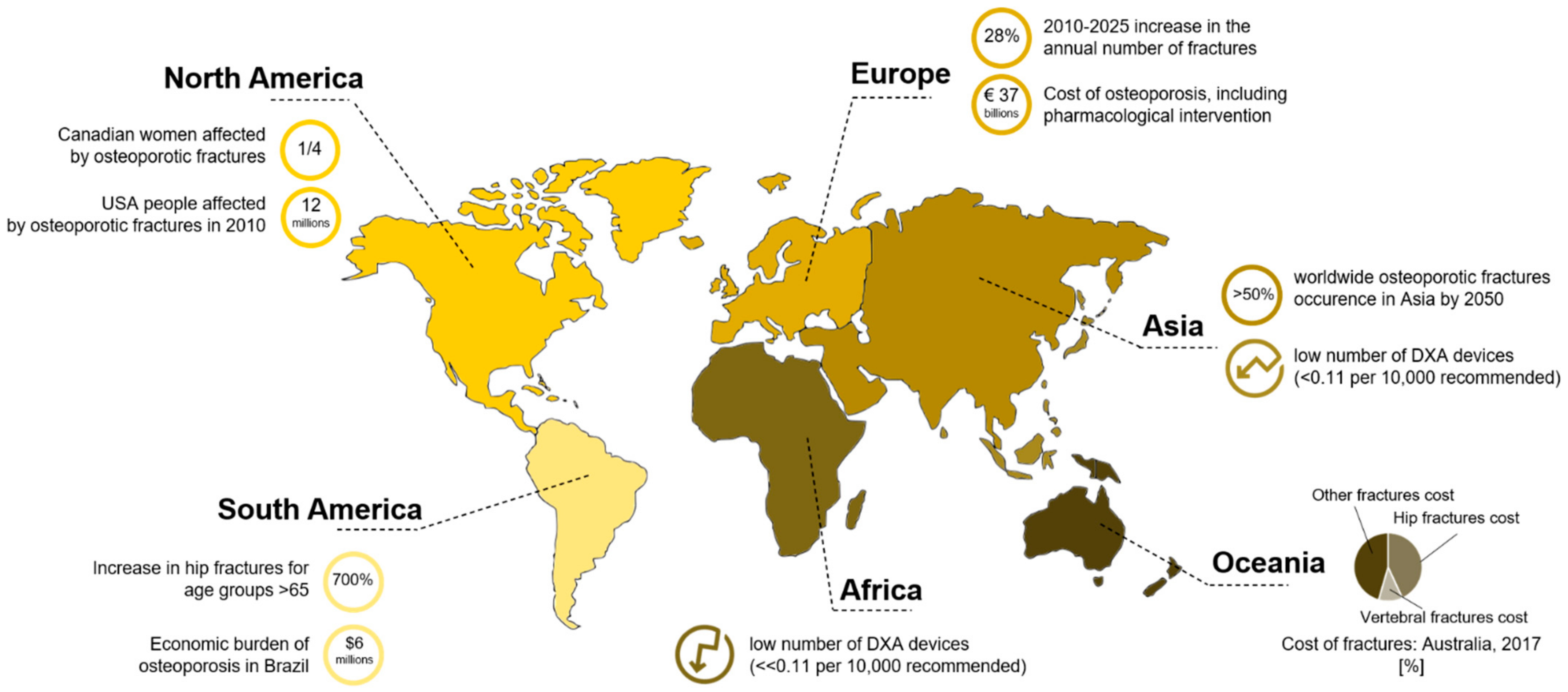
1. Introduction
2. Imaging Techniques for Multiscale Damage Assessment and Prediction
2.1. Macro- and Meso-Scale Imaging
2.2. Micro- and Nano-Scale Imaging
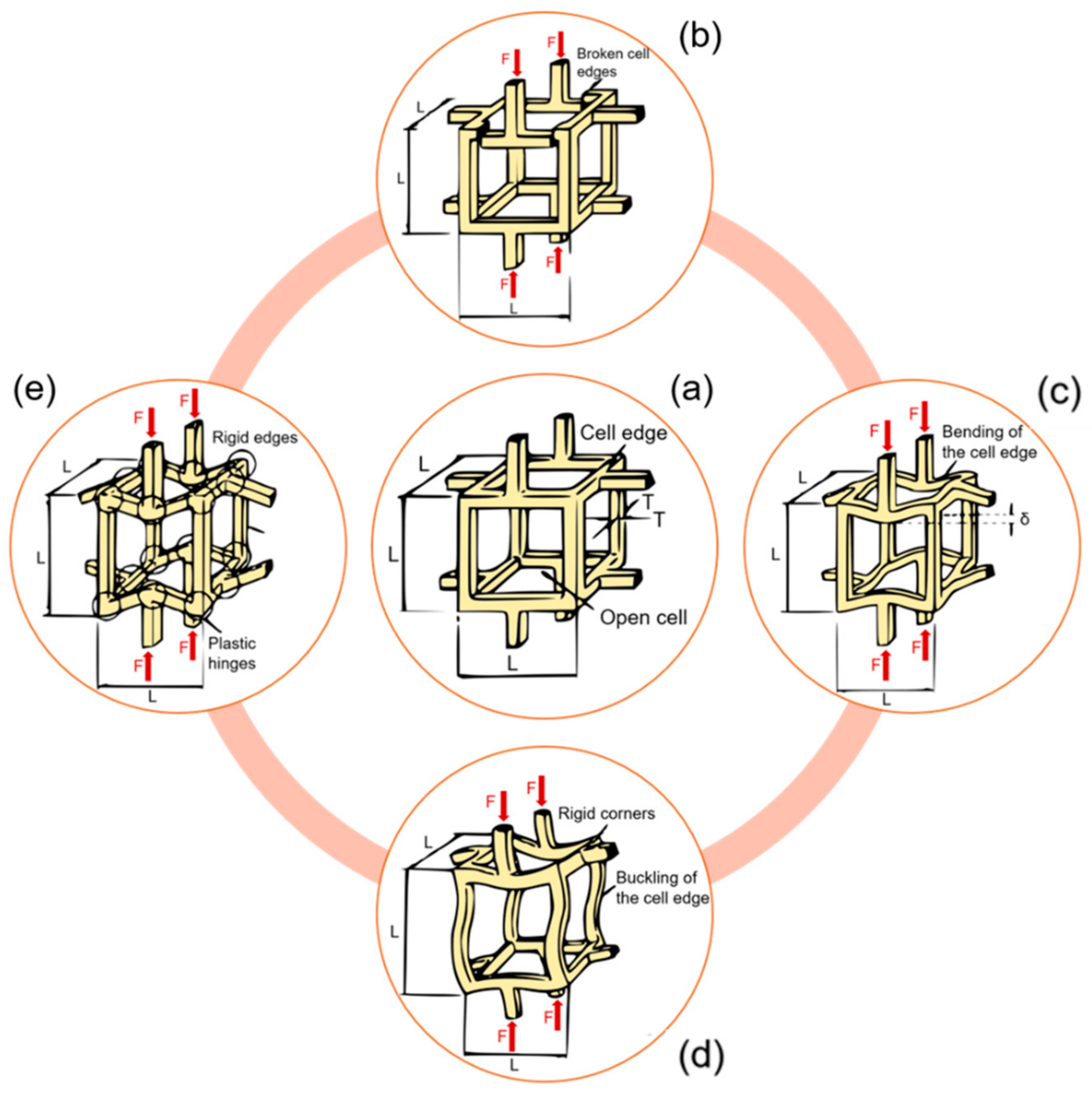
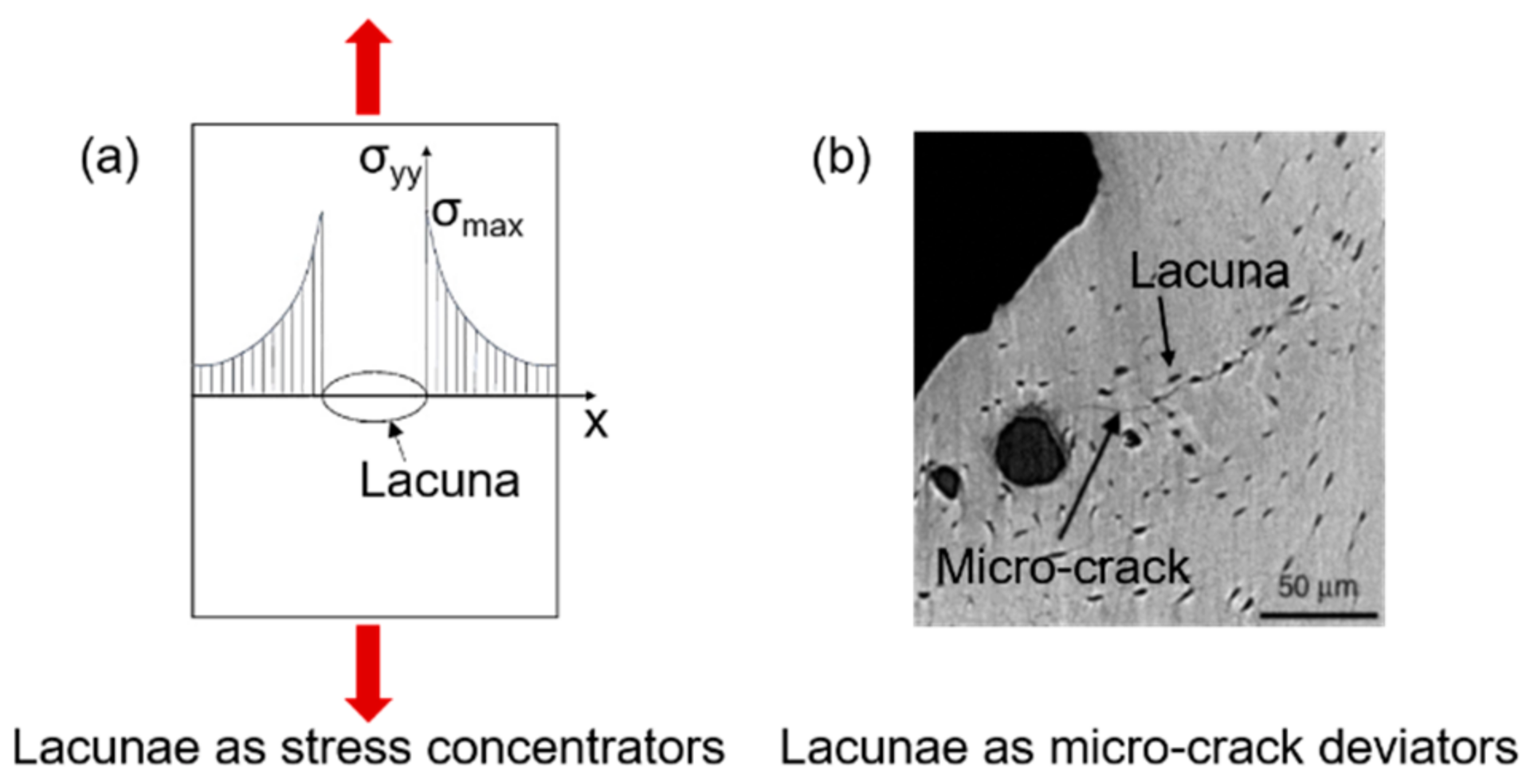
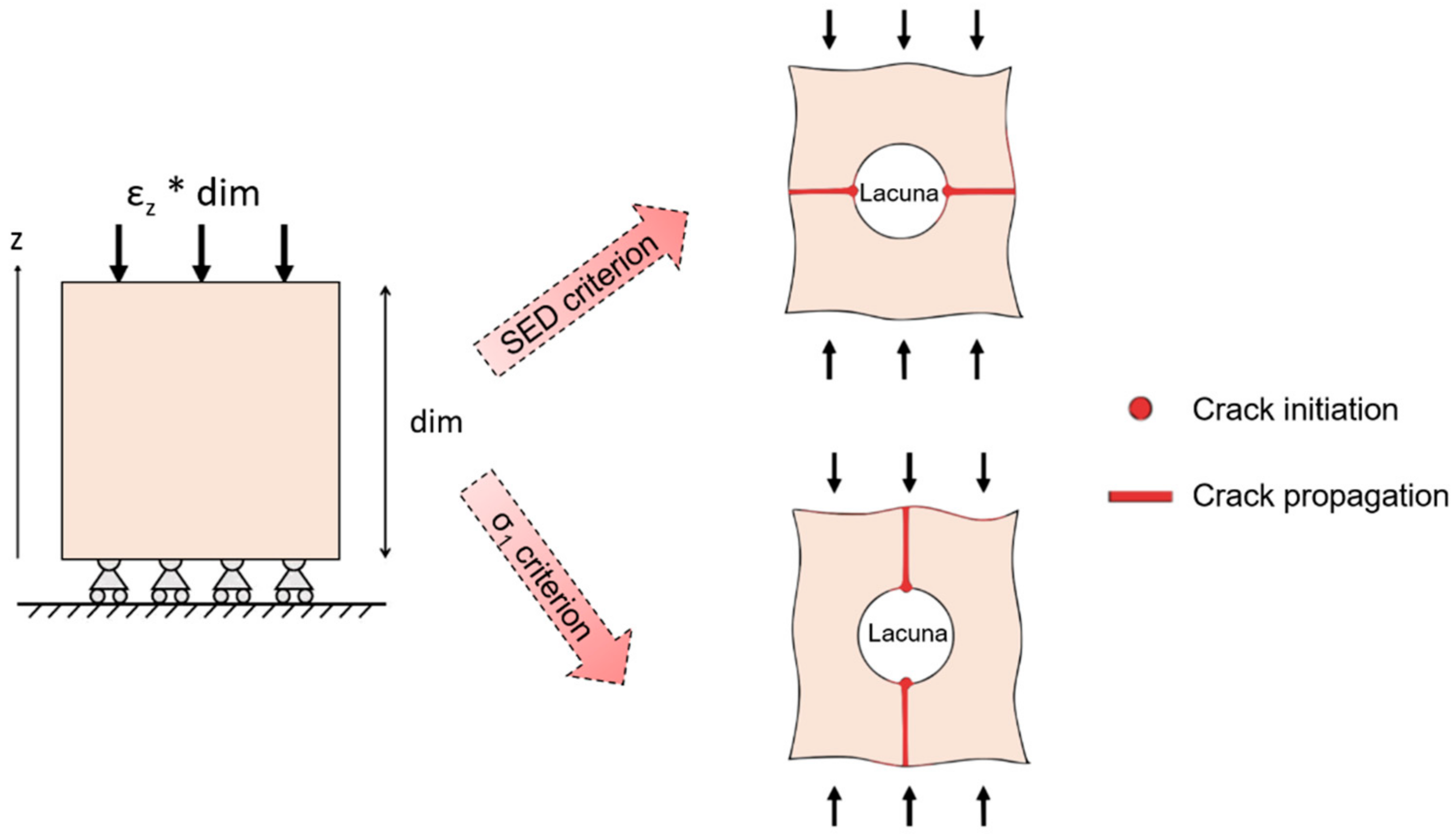
3. Bone Damage Physical Principle
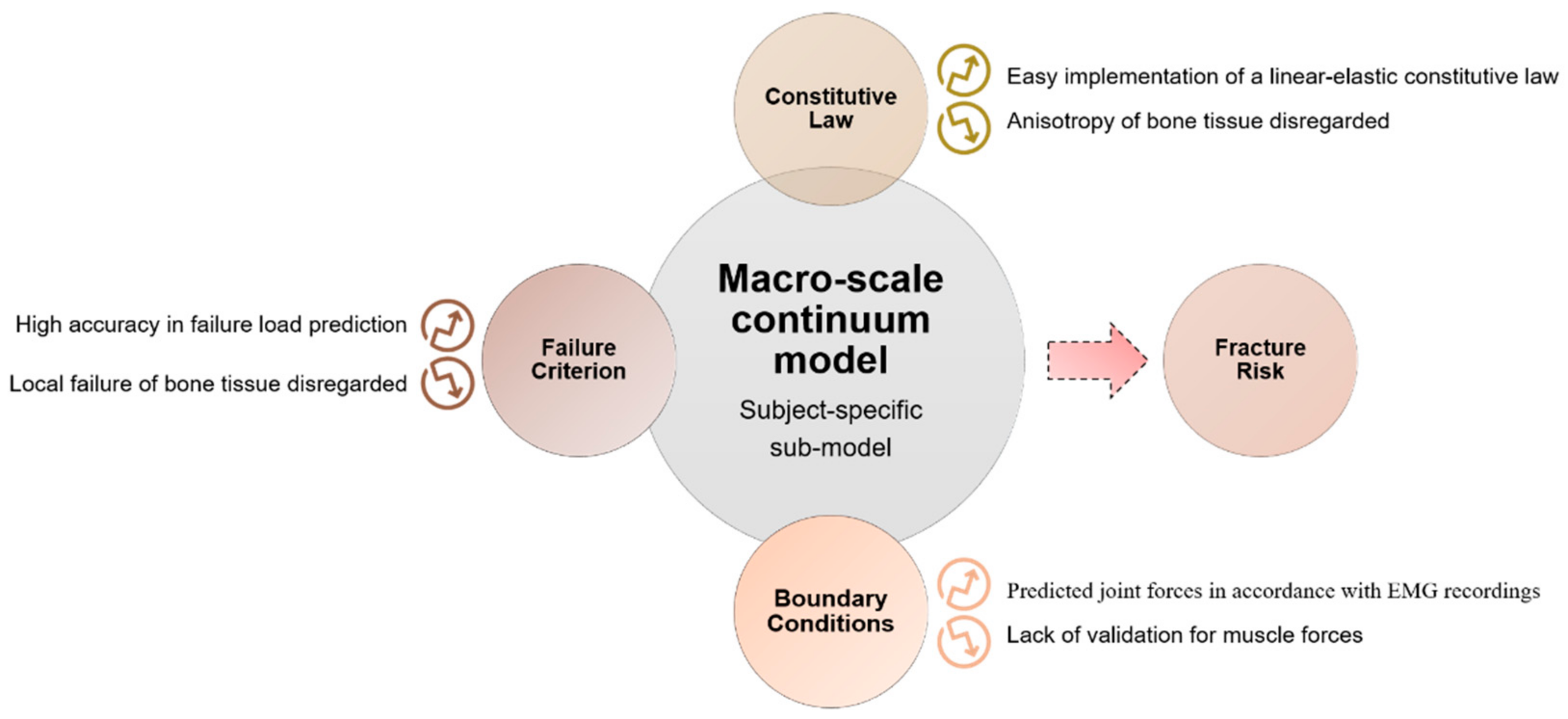
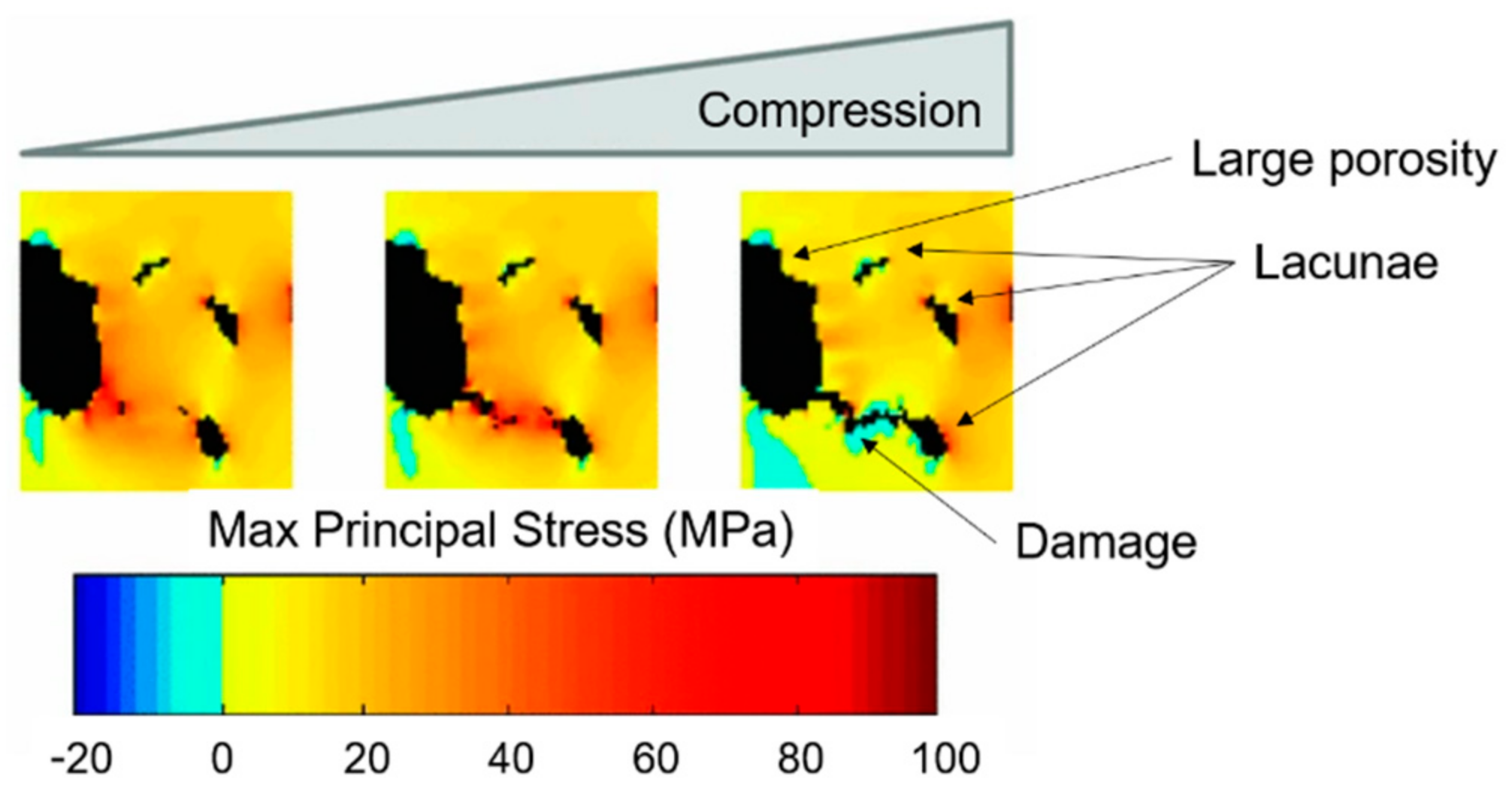
4. Multiscale Computational Damage Models
5. Validation Approaches to Multiscale Computational Damage Models
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Zioupos, P.; Kirchner, H.O.K.; Peterlik, H. Ageing bone fractures: The case of a ductile to brittle transition that shifts with age. Bone 2020, 131. [Google Scholar] [CrossRef]
- Wani, I.M.; Arora, S. Computer-aided diagnosis systems for osteoporosis detection: A comprehensive survey. Med. Biol. Eng. Comput. 2020, 58, 1873–1917. [Google Scholar] [CrossRef]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J. Trends in Fracture Incidence: A Population-Based Study over 20 Years. J. Bone Miner. Res. 2014, 29, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Odén, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef]
- Center, J.R.; Nguyen, T.V.; Schneider, D.; Sambrook, P.N.; Eisman, J.A. Mortality after all major types of osteoporotic fracture in men and women: An observational study. Lancet 1999, 353, 878–882. [Google Scholar] [CrossRef]
- Ji, M.-X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Thabane, L.; Papaioannou, A.; Ioannidis, G.; Levine, M.A.H.; Adachi, J.D. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet. Disord. 2017, 18, 46. [Google Scholar] [CrossRef]
- Tatangelo, G.; Watts, J.; Lim, K.; Connaughton, C.; Abimanyi-Ochom, J.; Borgström, F.; Nicholson, G.C.; Shore-Lorenti, C.; Stuart, A.L.; Iuliano-Burns, S.; et al. The Cost of Osteoporosis, Osteopenia, and Associated Fractures in Australia in 2017. J. Bone Miner. Res. 2019, 34, 616–625. [Google Scholar] [CrossRef] [PubMed]
- IOF. Facts and Statistics International Osteoporosis Foundation. Available online: http://www.iofbonehealth.org/facts-statistics (accessed on 29 January 2020).
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Poundarik, A.A.; Vashishth, D. Multiscale imaging of bone microdamage. Connect. Tissue Res. 2015, 56, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Launey, M.E.; Buehler, M.J.; Ritchie, R.O. On the Mechanistic Origins of Toughness in Bone. Annu. Rev. Mater. Res. 2010, 40, 25–53. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Launey, M.E.; Barth, H.D.; Ritchie, R.O. Mixed-mode fracture of human cortical bone. Biomaterials 2009, 30, 5877–5884. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Ritchie, R.O. Bone as a Structural Material. Adv. Healthc. Mater. 2015, 4, 1287–1304. [Google Scholar] [CrossRef]
- Milne, I.; Ritchie, R.O.; Karihaloo, B.L. Comprehensive Structural Integrity: Cyclic Loading and Fatigue; Elsevier: Amsterdam, The Netherlands, 2003; Volume 4, ISBN 9780080490731. [Google Scholar]
- Ritchie, R.O.; Kinney, J.H.; Kruzic, J.J.; Nalla, R.K. A fracture mechanics and mechanistic approach to the failure of cortical bone. Fatigue Fract. Eng. Mater. Struct. 2005, 28, 345–371. [Google Scholar] [CrossRef]
- Eneh, C.T.M.; Malo, M.K.H.; Karjalainen, J.P.; Liukkonen, J.; Töyräs, J.; Jurvelin, J.S. Effect of porosity, tissue density, and mechanical properties on radial sound speed in human cortical bone. Med. Phys. 2016, 43, 2030–2039. [Google Scholar] [CrossRef]
- Acevedo, C.; Stadelmann, V.A.; Pioletti, D.P.; Alliston, T.; Ritchie, R.O. Fatigue as the missing link between bone fragility and fracture. Nat. Biomed. Eng. 2018, 2, 62–71. [Google Scholar] [CrossRef]
- Ritchie, R.O.; Kinney, J.H.; Kruzic, J.J.; Nalla, R.K. Cortical Bone Fracture. In Wiley Encyclopedia of Biomedical Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hamed, E.; Jasiuk, I. Multiscale damage and strength of lamellar bone modeled by cohesive finite elements. J. Mech. Behav. Biomed. Mater. 2013, 28, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Nalla, R.K.; Kinney, J.H.; Ritchie, R.O. Mechanistic fracture criteria for the failure of human cortical bone. Nat. Mater. 2003, 2, 164–168. [Google Scholar] [CrossRef]
- Bonfoh, N.; Novinyo, E.; Lipinski, P. Modeling of bone adaptative behavior based on cells activities. Biomech. Model. Mechanobiol. 2011, 10, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.O.; Nalla, R.K.; Kruzic, J.J.; Ager, J.W.; Balooch, G.; Kinney, J.H. Fracture and Ageing in Bone: Toughness and Structural Characterization. Strain 2006, 42, 225–232. [Google Scholar] [CrossRef]
- Lespessailles, E.; Chappard, C.; Bonnet, N.; Benhamou, C.L. Imaging techniques for evaluating bone microarchitecture. Jt. Bone Spine 2006, 73, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Molino, G.; Montalbano, G.; Pontremoli, C.; Fiorilli, S.; Vitale-Brovarone, C. Imaging Techniques for the Assessment of the Bone Osteoporosis-Induced Variations with Particular Focus on Micro-CT Potential. Appl. Sci. 2020, 10, 8939. [Google Scholar] [CrossRef]
- Hunt, H.B.; Donnelly, E. Bone Quality Assessment Techniques: Geometric, Compositional, and Mechanical Characterization from Macroscale to Nanoscale. Clin. Rev. Bone Miner. Metab. 2016, 14, 133–149. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Dawson, A.; De Laet, C.; Jonsson, B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos. Int. 2001, 12, 989–995. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Marshall, D.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Libonati, F.; Ferrario, D.; Rinaudo, L.; Messina, C.; Ulivieri, F.M.; Cesana, B.M.; Strano, M.; Vergani, L. Determinants of bone damage: An ex-vivo study on porcine vertebrae. PLoS ONE 2018, 13, e0202210. [Google Scholar] [CrossRef]
- Bouxsein, M.L. Bone quality: Where do we go from here? Osteoporos. Int. 2003, 14, S118–S127. [Google Scholar] [CrossRef]
- Varga, P.; Hesse, B.; Langer, M.; Schrof, S.; Männicke, N.; Suhonen, H.; Pacureanu, A.; Pahr, D.; Peyrin, F.; Raum, K. Synchrotron X-ray phase nano-tomography-based analysis of the lacunar–canalicular network morphology and its relation to the strains experienced by osteocytes in situ as predicted by case-specific finite element analysis. Biomech. Model. Mechanobiol. 2015, 14, 267–282. [Google Scholar] [CrossRef]
- Colombo, C.; Libonati, F.; Rinaudo, L.; Bellazzi, M.; Ulivieri, F.M.; Vergani, L. A new finite element based parameter to predict bone fracture. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Mader, K.S.; Schneider, P.; Müller, R.; Stampanoni, M. A quantitative framework for the 3D characterization of the osteocyte lacunar system. Bone 2013, 57, 142–154. [Google Scholar] [CrossRef]
- D’Elia, G.; Caracchini, G.; Cavalli, L.; Innocenti, P. Bone fragility and imaging techniques. Clin. Cases Miner. Bone Metab. 2009, 6, 234–246. [Google Scholar]
- Ural, A.; Mischinski, S. Multiscale modeling of bone fracture using cohesive finite elements. Eng. Fract. Mech. 2013, 103, 141–152. [Google Scholar] [CrossRef]
- Al-Lamki, L. Radiation Exposure from Medical Imaging: A Wake-up Call for Oman! Sultan Qaboos Univ. Med. J. 2011, 11, 1–4. [Google Scholar] [PubMed]
- Singh, M.; Nagrath, A.R.; Maini, P.S. Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. J. Bone Joint Surg. Am. 1970, 52, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Palermo, L.; Yeung, C.; Black, D.M. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: A prospective analysis from the study of osteoporotic fractures. Osteoporos. Int. 2002, 13, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Brad Abrahams, R. X-ray-based medical imaging and resolution. Am. J. Roentgenol. 2015, 204, W393–W397. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.; Raisz, L.; Martin, T.J. Principles of Bone Biology, Two-Volume Set, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 1–2, ISBN 9780123738844. [Google Scholar]
- Morris, R.M.; Yang, L.; Martín-Fernández, M.A.; Pozo, J.M.; Frangi, A.F.; Wilkinson, J.M. High-Spatial-Resolution Bone Densitometry with Dual-Energy X-ray Absorptiometric Region-free Analysis. Radiology 2015, 274, 532–539. [Google Scholar] [CrossRef]
- IAEA International Atomic Energy Agency. Dual Energy X-ray Absorptiometry—Bone Mineral Densitometry|IAEA. Available online: https://www.iaea.org/resources/rpop/health-professionals/other-specialities-and-imaging-modalities/dxa-bone-mineral-densitometry (accessed on 24 February 2020).
- Zeytinoglu, M.; Jain, R.K.; Vokes, T.J. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone 2017, 104, 54–65. [Google Scholar] [CrossRef] [PubMed]
- El Maghraoui, A.; Roux, C. DXA scanning in clinical practice. QJM 2008, 101, 605–617. [Google Scholar] [CrossRef]
- Schousboe, J.T.; DeBold, C.R. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos. Int. 2006, 17, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E. Quantitative computed tomography. Eur. J. Radiol. 2009, 71, 415–424. [Google Scholar] [CrossRef]
- Brett, A.D.; Brown, J.K. Quantitative computed tomography and opportunistic bone density screening by dual use of computed tomography scans. J. Orthop. Transl. 2015, 3, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Schafer, A.L. Bariatric Surgery, Vitamin D, and Bone Loss. In Vitamin D: Fourth Edition; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 129–150. ISBN 9780128099650. [Google Scholar]
- Yu, E.W.; Thomas, B.J.; Brown, J.K.; Finkelstein, J.S. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2012, 27, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Jahng, J.S.; Kang, K.S.; Park, H.W.; Han, M.H. The Assessment of Bone Mineral Density in Postmenopausal and Senile Osteoporosis Using Quantitative Computed Tomography. J. Korean Orthop. Assoc. 1990, 25, 262. [Google Scholar] [CrossRef]
- Link, T.M.; Majumdar, S.; Augat, P.; Lin, J.C.; Newitt, D.; Lane, N.E.; Genant, H.K. Proximal femur: Assessment for osteoporosis with T2* decay characteristics at MR imaging. Radiology 1998, 209, 531–536. [Google Scholar] [CrossRef]
- Yablonskiy, D.A.; Haacke, E.M. Theory of NMR signal behavior in magnetically inhomogeneous tissues: The static dephasing regime. Magn. Reson. Med. 1994, 32, 749–763. [Google Scholar] [CrossRef]
- Funke, M.; Bruhn, H.; Vosshenrich, R.; Rudolph, O.; Grabbe, E. Bestimmung der T2*-relaxationszeit zur charakterisierung des trabekularen knochens. RoFo 1994, 161, 58–63. [Google Scholar] [CrossRef]
- Van Reeth, E.; Tham, I.W.K.; Tan, C.H.; Poh, C.L. Super-resolution in magnetic resonance imaging: A review. Concepts Magn. Reson. Part A 2012, 40A, 306–325. [Google Scholar] [CrossRef]
- Nothnagle, P.; Chambers, W.; Davidson, M. Introduction to Stereomicroscopy. Available online: http://www.microscopyu.com/articles/stereomicroscopy/stereointro.html (accessed on 23 March 2020).
- Vanderoost, J. From histology to micro-CT: Measuring and modeling resorption cavities and their relation to bone competence. World J. Radiol. 2014, 6, 643. [Google Scholar] [CrossRef]
- Schoenau, E.; Neu, C.M.; Beck, B.; Manz, F.; Rauch, F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J. Bone Miner. Res. 2002, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A. Elettra Sincrotrone Trieste Elettra Sincrotrone Trieste. Available online: https://www.elettra.trieste.it/lightsources/elettra/odac/elettra-parameters/page-3.html?showall= (accessed on 23 March 2020).
- Liu, X.S.; Zhang, X.H.; Rajapakse, C.S.; Wald, M.J.; Magland, J.; Sekhon, K.K.; Adam, M.F.; Sajda, P.; Wehrli, F.W.; Guo, X.E. Accuracy of high-resolution in vivo micro magnetic resonance imaging for measurements of microstructural and mechanical properties of human distal tibial bone. J. Bone Miner. Res. 2010, 25, 2039–2050. [Google Scholar] [CrossRef]
- Fouquet, C.; Gilles, J.F.; Heck, N.; Santos, M.D.; Schwartzmann, R.; Cannaya, V.; Morel, M.P.; Davidson, R.S.; Trembleau, A.; Bolte, S. Improving axial resolution in confocal microscopy with new high refractive index mounting media. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Dufrêne, Y.F. Atomic force microscopy, a powerful tool in microbiology. J. Bacteriol. 2002, 184, 5205–5213. [Google Scholar] [CrossRef]
- Daffner, R.H.; Pavlov, H. Stress fractures: Current concepts. AJR Am. J. Roentgenol. 1992, 159, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D. Fracture and repair of bone: A multiscale problem. J. Mater. Sci. 2007, 42, 8911–8918. [Google Scholar] [CrossRef]
- Shi, X.; Liu, X.; Wang, X.; Guo, X.; Niebur, G.L. Type and orientation of yielded trabeculae during overloading of trabecular bone along orthogonal directions. J. Biomech. 2010, 43, 2460–2466. [Google Scholar] [CrossRef]
- Gibson, L.J. Biomechanics of cellular solids. J. Biomech. 2005, 38, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Fyhrie, D.; Schaffler, M.B. Failure mechanisms in human vertebral cancellous bone. Bone 1994, 15, 105–109. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Busse, B.; Ritchie, R.O. The fracture mechanics of human bone: Influence of disease and treatment. Bonekey Rep. 2015, 4. [Google Scholar] [CrossRef]
- Vashishth, D. Hierarchy of bone microdamage at multiple length scales. Int. J. Fatigue 2007, 29, 1024–1033. [Google Scholar] [CrossRef]
- Voide, R.; Schneider, P.; Stauber, M.; van Lenthe, G.H.; Stampanoni, M.; Müller, R. The importance of murine cortical bone microstructure for microcrack initiation and propagation. Bone 2011, 49, 1186–1193. [Google Scholar] [CrossRef]
- Taylor, D.; Hazenberg, J.G.; Lee, T.C. Living with cracks: Damage and repair in human bone. Nat. Mater. 2007, 6, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Buikstra, J.E. Ortner’s Identification of Pathological Conditions in Human Skeletal Remains; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128097380. [Google Scholar]
- Voide, R.; Schneider, P.; Stauber, M.; Wyss, P.; Stampanoni, M.; Sennhauser, U.; van Lenthe, G.H.; Müller, R. Time-lapsed assessment of microcrack initiation and propagation in murine cortical bone at submicrometer resolution. Bone 2009, 45, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.P.; Kimmel, D.B.; Lappe, J.M.; Recker, R.R. Effect of Macroanatomic Bone Type and Estrogen Loss on Osteocyte Lacunar Properties in Healthy Adult Women. Calcif. Tissue Int. 2017, 100, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. Stress Concentrations in Bone. J. Cell Sci. 1962, s3-103, 111–133. [Google Scholar]
- McNamara, L.M.; Van Der Linden, J.C.; Weinans, H.; Prendergast, P.J. Stress-concentrating effect of resorption lacunae in trabecular bone. J. Biomech. 2006, 39, 734–741. [Google Scholar] [CrossRef]
- Qiu, S.; Rao, S.D.; Fyhrie, D.P.; Palnitkar, S.; Parfitt, M.A. The morphological association between microcracks and osteocyte lacunae in human cortical bone. Bone 2005. [Google Scholar] [CrossRef]
- Courtney, A.C.; Hayes, W.C.; Gibson, L.J. Age-related differences in post-yield damage in human cortical bone. Experiment and model. J. Biomech. 1996, 29, 1463–1471. [Google Scholar] [CrossRef]
- Carter, D.R.; Hayes, W.C.; Schurman, D.J. Fatigue life of compact bone-II. Effects of microstructure and density. J. Biomech. 1976, 9, 211–218. [Google Scholar] [CrossRef]
- Vashishth, D.; Behiri, J.C.; Bonfield, W. Crack growth resistance in cortical bone: Concept of microcrack toughening. J. Biomech. 1997, 30, 763–769. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Radin, E.L.; Burr, D.B. Mechanical and morphological effects of strain rate on fatigue of compact bone. Bone 1989, 10, 207–214. [Google Scholar] [CrossRef]
- Awaji, H.; Ebisudani, M.; Choi, S.-M.; Ohashi, T. Crack Deflection Toughening Mechanism in Brittle Materials. In Fracture Resistance Testing of Monolithic and Composite Brittle Materials; ASTM International: West Conshohocken, PA, USA, 2009; pp. 137–151. [Google Scholar]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Buehler, M.J. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef]
- Travert, C.; Jolivet, E.; Sapin-De Brosses, E.; Mitton, D.; Skalli, W. Sensitivity of patient-specific vertebral finite element model from low dose imaging to material properties and loading conditions. Med. Biol. Eng. Comput. 2011, 49, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Christen, D.; Webster, D.J.; Müller, R. Multiscale modelling and nonlinear.nite element analysis as clinical tools for the assessment of fracture risk. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2653–2668. [Google Scholar] [CrossRef]
- Dagan, D.; Be’ery, M.; Gefen, A. Single-trabecula building block for large-scale finite element models of cancellous bone. Med. Biol. Eng. Comput. 2004, 42, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Van Rietbergen, B.; Odgaard, A.; Kabel, J.; Huiskes, R. Relationships between bone morphology and bone elastic properties can be accurately quantified using high-resolution computer reconstructions. J. Orthop. Res. 1998, 16, 23–28. [Google Scholar] [CrossRef]
- Niebur, G.L.; Feldstein, M.J.; Yuen, J.C.; Chen, T.J.; Keaveny, T.M. High-resolution finite element models with tissue strength asymmetry accurately predict failure of trabecular bone. J. Biomech. 2000, 33, 1575–1583. [Google Scholar] [CrossRef]
- Ilic, S.; Hackl, K.; Gilbert, R. Application of the multiscale FEM to the modeling of cancellous bone. Biomech. Model. Mechanobiol. 2010, 9, 87–102. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodeling; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Sen, C.; Prasad, J. Exploring conditions that make cortical bone geometry optimal for physiological loading. Biomech. Model. Mechanobiol. 2019, 18, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Lengsfeld, M.; Schmitt, J.; Alter, P.; Kaminsky, J.; Leppek, R. Comparison of geometry-based and CT voxel-based finite element modelling and experimental validation. Med. Eng. Phys. 1998, 20, 515–522. [Google Scholar] [CrossRef]
- Webster, D.; Müller, R. In silico models of bone remodeling from macro to nano-from organ to cell. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 241–251. [Google Scholar] [CrossRef]
- Hambli, R. A quasi-brittle continuum damage finite element model of the human proximal femur based on element deletion. Med. Biol. Eng. Comput. 2013, 51, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Jiang, H.; Song, Q.; Yates, C.; Lee, P.V.S.; Wark, J.D. The application of finite element modelling based on clinical pQCT for classification of fracture status. Biomech. Model. Mechanobiol. 2019, 18, 245–260. [Google Scholar] [CrossRef]
- Viceconti, M.; Taddei, F.; Van Sint Jan, S.; Leardini, A.; Cristofolini, L.; Stea, S.; Baruffaldi, F.; Baleani, M. Multiscale modelling of the skeleton for the prediction of the risk of fracture. Clin. Biomech. 2008, 23, 845–852. [Google Scholar] [CrossRef]
- Cowin, S. Bone stress adaptation models. J. Biomech. Eng. 1993, 115, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Schileo, E.; Taddei, F.; Malandrino, A.; Cristofolini, L.; Viceconti, M. Subject-specific finite element models can accurately predict strain levels in long bones. J. Biomech. 2007, 40, 2982–2989. [Google Scholar] [CrossRef] [PubMed]
- Taddei, F.; Schileo, E.; Helgason, B.; Cristofolini, L.; Viceconti, M. The material mapping strategy influences the accuracy of CT-based finite element models of bones: An evaluation against experimental measurements. Med. Eng. Phys. 2007, 29, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Ohnishi, I.; Bessho, M.; Nakamura, K. Nonlinear finite element model predicts vertebral bone strength and fracture site. Spine 2006, 31, 1789–1794. [Google Scholar] [CrossRef]
- Harrison, N.M.; McDonnell, P.; Mullins, L.; Wilson, N.; O’Mahoney, D.; McHugh, P.E. Failure modelling of trabecular bone using a non-linear combined damage and fracture voxel finite element approach. Biomech. Model. Mechanobiol. 2013, 12, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Mosekilde, L. A model of vertebral trabecular bone architecture and its mechanical properties. Bone 1990, 11, 417–423. [Google Scholar] [CrossRef]
- Boutroy, S.; Van Rietbergen, B.; Sornay-Rendu, E.; Munoz, F.; Bouxsein, M.L.; Delmas, P.D. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J. Bone Miner. Res. 2008, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Carbonare, L.D.; Valenti, M.; Bertoldo, F.; Zanatta, M.; Zenari, S.; Realdi, G.; Cascio, V.L.; Giannini, S. Bone microarchitecture evaluated by histomorphometry. Micron 2005, 36, 609–615. [Google Scholar] [CrossRef]
- van Rietbergen, B.; Weinans, H.; Huiskes, R.; Odgaard, A. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J. Mech. 1995, 28, 69–81. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
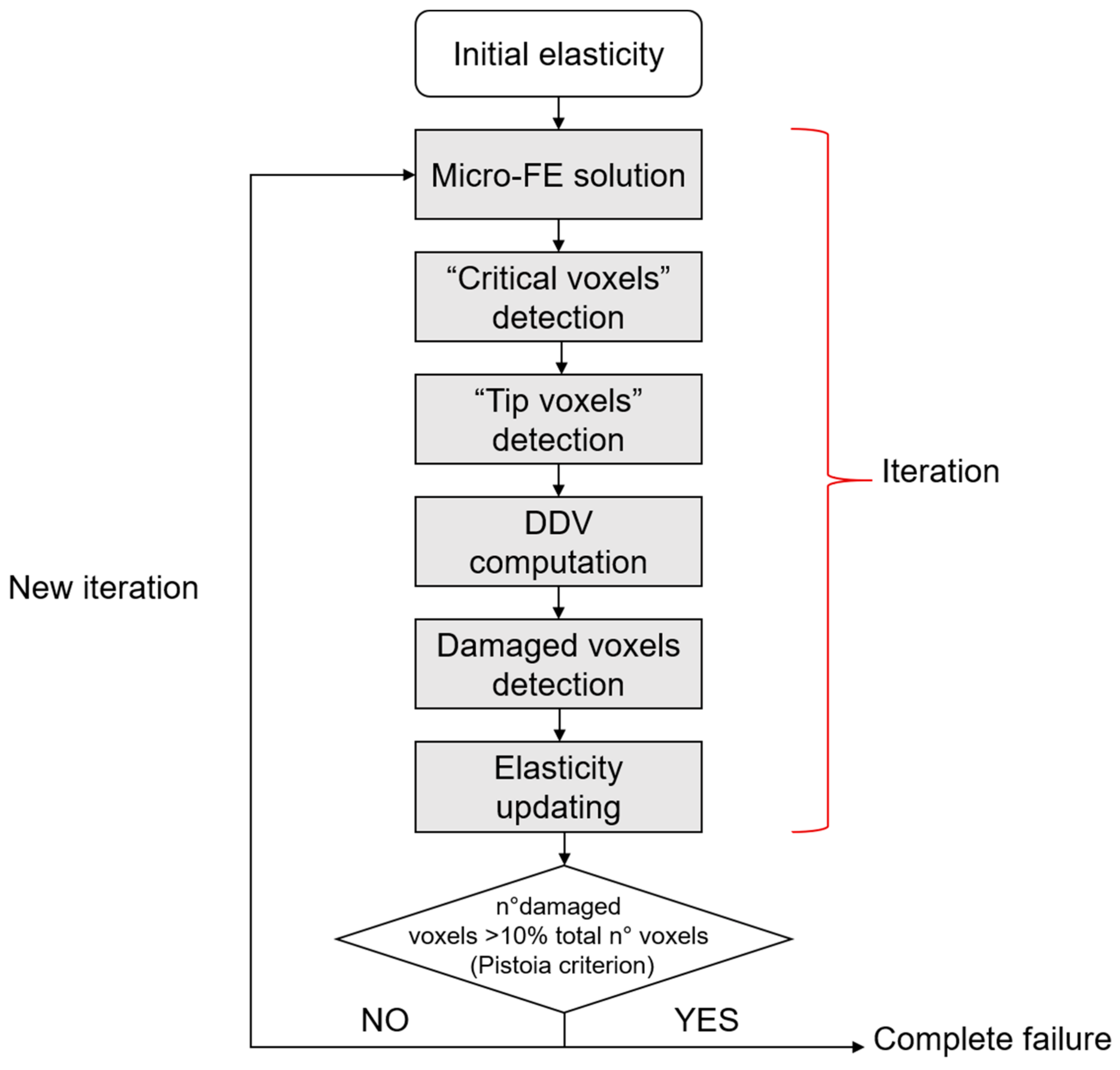
- Pistoia, W.; Lill, C.A.; Eckstein, F.; Rietbergen, B.V.A.N.; Lochmu, E.; Ru, P. Estimation of Distal Radius Failure Load With Micro-Finite Element Analysis Models Based on Three-dimensional Peripheral Quantitative Computed Tomography Images. Bone 2002, 30, 842–848. [Google Scholar] [CrossRef]
- Webster, D.; Schulte, F.A.; Lambers, F.M.; Kuhn, G.; Müller, R. Strain energy density gradients in bone marrow predict osteoblast and osteoclast activity: A finite element study. J. Biomech. 2015, 48, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, F.; Ruffoni, D.; Schneider, P.; Levchuk, A.; Zwahlen, A.; Pankaj, P.; Müller, R. Modeling microdamage behavior of cortical bone. Biomech. Model. Mechanobiol. 2014, 13, 1227–1242. [Google Scholar] [CrossRef]
- Nalla, R.; Kruzic, J.; Kinney, J.; Ritchie, R.O. Mechanistic aspects of fracture and R-curve behavior in human cortical bone. Biomaterials 2005, 26, 217–231. [Google Scholar] [CrossRef]
- Thurner, P.J.; Wyss, P.; Voide, R.; Stauber, M.; Stampanoni, M.; Sennhauser, U.; Müller, R. Time-lapsed investigation of three-dimensional failure and damage accumulation in trabecular bone using synchrotron light. Bone 2006, 39, 289–299. [Google Scholar] [CrossRef]
- Hammond, M.A.; Wallace, J.M.; Allen, M.R.; Siegmund, T. Mechanics of linear microcracking in trabecular bone. J. Biomech. 2019, 83, 34–42. [Google Scholar] [CrossRef]
- Demirtas, A.; Ural, A. Material heterogeneity, microstructure, and microcracks demonstrate differential influence on crack initiation and propagation in cortical bone. Biomech. Model. Mechanobiol. 2018, 17, 1415–1428. [Google Scholar] [CrossRef]
- Stipsitz, M.; Zysset, P.K.; Pahr, D.H. Efficient materially nonlinear μ FE solver for simulations of trabecular bone failure. Biomech. Model. Mechanobiol. 2020, 19, 861–874. [Google Scholar] [CrossRef]
- Dubey, D.K.; Tomar, V. Microstructure dependent dynamic fracture analyses of trabecular bone based on nascent bone atomistic simulations. Mech. Res. Commun. 2008, 35, 24–31. [Google Scholar] [CrossRef]
- Libonati, F.; Nair, A.K.; Vergani, L.; Buehler, M.J. Fracture mechanics of hydroxyapatite single crystals under geometric confinement. J. Mech. Behav. Biomed. Mater. 2013, 20, 184–191. [Google Scholar] [CrossRef]
- Tai, K.; Dao, M.; Suresh, S.; Palazoglu, A.; Ortiz, C. Nanoscale heterogeneity promotes energy dissipation in bone. Nat.Mater. 2007, 6, 454–462. [Google Scholar] [CrossRef]
- Hamed, E.; Jasiuk, I.; Yoo, A.; Lee, Y.H.; Liszka, T. Multi-scale modelling of elastic moduli of trabecular bone. J. R. Soc. Interface 2012, 9, 1654–1673. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Clumpner, B.R. Multiscale modeling of human bone. Multiscale Multidiscip. Model. Exp. Des. 2018, 1, 133–143. [Google Scholar] [CrossRef]
- Oliviero, S.; Giorgi, M.; Laud, P.J.; Dall’Ara, E. Effect of repeated in vivo microCT imaging on the properties of the mouse tibia. PLoS ONE 2019, 14, e0225127. [Google Scholar] [CrossRef]
- Levchuk, A.; Schneider, P.; Meier, M.; Vogel, P.; Donaldson, F.; Müller, R. An automated step-wise micro-compression device for 3D dynamic image-guided failure assessment of bone tissue on a microstructural level using time-lapsed tomography. Front. Mater. 2018, 5. [Google Scholar] [CrossRef]
- Schulte, F.A.; Ruffoni, D.; Lambers, F.M.; Christen, D.; Webster, D.J.; Kuhn, G.; Müller, R. Local Mechanical Stimuli Regulate Bone Formation and Resorption in Mice at the Tissue Level. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Razi, H.; Birkhold, A.I.; Weinkamer, R.; Duda, G.N.; Willie, B.M.; Checa, S. Aging leads to a dysregulation in mechanically driven bone formation and resorption. J. Bone Miner. Res. 2015, 30, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Klinck, R.J.; Campbell, G.M.; Boyd, S.K. Radiation effects on bone architecture in mice and rats resulting from in vivo micro-computed tomography scanning. Med. Eng. Phys. 2008, 30, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Levchuk, A.; Müller, R. Automated Micro-Compression Device for Dynamic Image-Guided Failure Assessment of Bone Ultrastructure and Bone Microdamage. Biomed. Tech. Biomed. Eng. 2010, 8–10. [Google Scholar] [CrossRef]



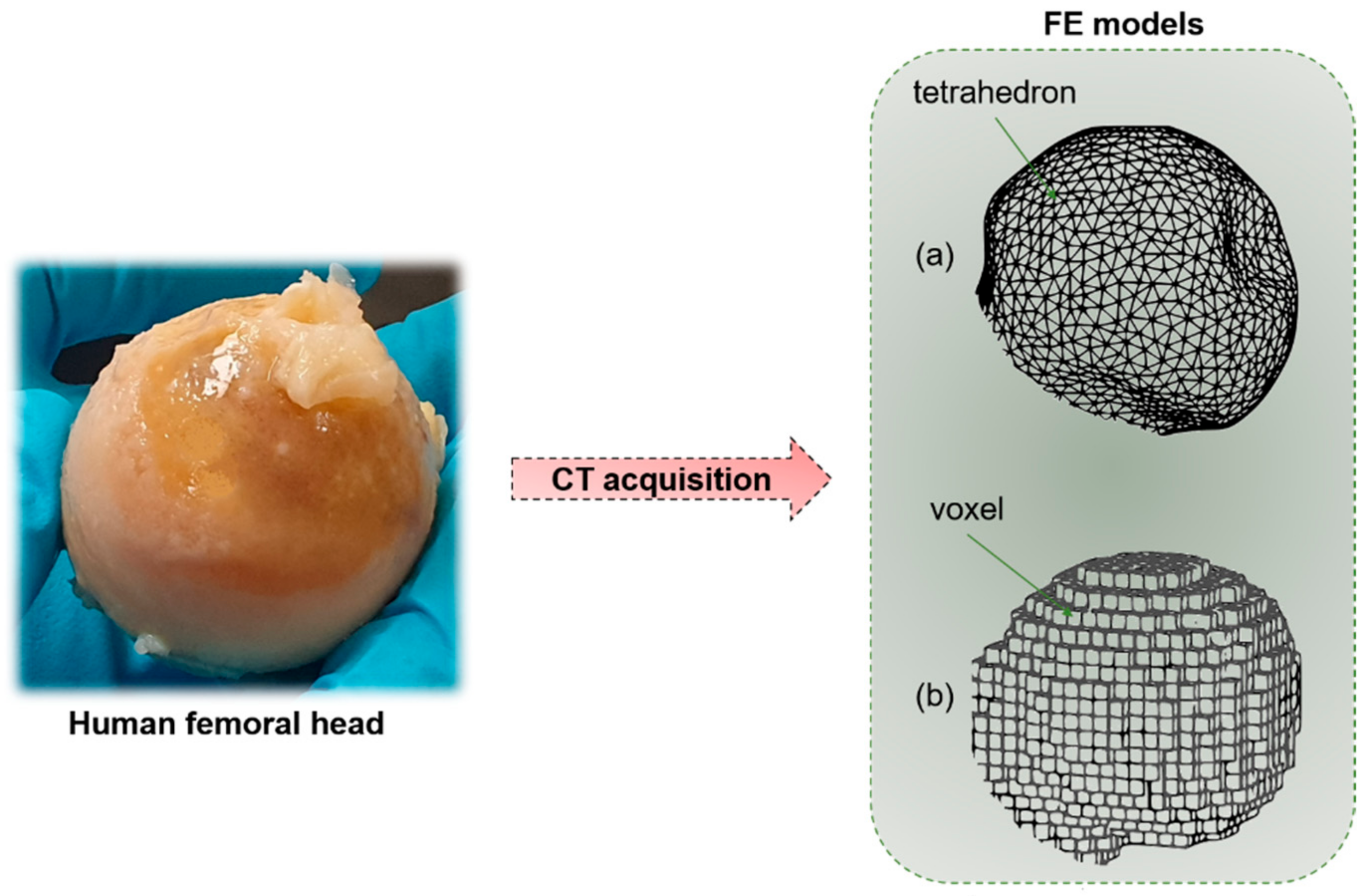
| Macro- and Meso-Scale Imaging Technique | Brief Description of the Technique | Invasiveness | Outcomes | Spatial Resolution | 2D or 3D | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| In Vitro/In Vivo Application | |||||||
| Radiography | Based on the interaction between a beam of photons (X-rays) directed from a source to a receptor. The atoms of the body prevent, in a percentage dependent on their atomic number, some photons from reaching the receptor, reproducing a “negative” image of the body | No Radiation dose: 40–50 times lower, if compared to computed tomography (CT) scans (e.g., radiographs of the abdomen → 0.25 mGy) [39] | Estimation of density variation (fracture risk prediction) by means of two indexes: Singh index [40] for proximal femur and cortical–medullary index [41] for hand radiographs | 0.17 mm/pixel → The size of the monitor screens used in digital radiography is sufficient for 35 × 43 cm2 radiographs to be displayed at a resolution of 2048 × 2560 pixels [42] | 2D | Clear identification of distal radius fractures [43] | Difficult detection of hip and spine fractures Insensitive to changes in Bone Mineral Density (BMD)until 20 to 40% of bone mass lost [43] |
| In vivo | |||||||
| Dual-energy X-ray Absorptiometry (DXA) | Involves the emission of two X-ray beams with different energy levels, that collide with the body of the patient. Once the absorption of the soft tissue has been subtracted, it is possible to determine the absorption of the beam by the bone and therefore the BMD | No Low radiation dose (0.001–0.003 mGy for L-spine, to 0.004 mGy for total body) [37] | Determination of areal BMD in g/cm2 Calculation of bone mineral content (BMC = BMD × area) Calculation of T-score and Z-score (negative for values under the average BMD), that are numerical indexes for the evaluation of osteoporosis. | 1 pixel → ≃ 0.56 × 0.56 mm2. (for a Hologic system) [44] | 2D | Ease of use of the equipment Standardization Short examination time [45] | No bone architecture detection (no difference between cortical and trabecular bone) Sampling errors Incorrect evaluation in obese patients [37] |
| In vivo | |||||||
| Vertebral Fracture Assessment (VFA) | Special DXA analysis that permits the detection of spinal fractures from a lateral image of the spine | No Lower radiation exposure with respect to spine radiography [46] | Spinal fracture detection [47] | Low spatial resolution | 2D | Possibility to add a VFA scan after areal BMD assessment High sensitivity High specificity [48] | Low spatial resolution |
| In vivo | |||||||
| Quantitative Computed Tomography (QCT) | X-ray-based technique that measures BMD. It produces cross-sectional images of X-ray absorption coefficient (measured in Hounsfield units) calibrated to water. It is used to evaluate fracture risk primarily at the lumbar spine and at the hip [49] | Medium–high invasiveness Medium–high radiation dose (0.2–0.4 mGy for a spine exam) [50] | True measurement of BMD assessment (areal BMD does not predict if an individual patient will eventually fracture) | 100× higher resolution with respect to conventional radiologic imaging [51] | 3D (multiple slices are obtained and then reconstructed) | Fracture risk prediction in patients with scoliosis, obesity, etc. without having artificially high BMD values, as in DXA [52] High reproducibility Assessment of cortical and trabecular bone Good accuracy and precision [37] | Relevant radiation dose Low accessibility High cost [53] |
| In vivo | |||||||
| Magnetic Resonance Imaging (MRI) | MRIs employ a magnetic field that forces protons in the body to align with that field. When a radiofrequency current is pulsed through the patient, the protons are strained against the pull of the magnetic field. When the radiofrequency field is turned off, the MRI sensors detect the released energy as the protons realign with the magnetic field. The time it takes for the protons to realign, as well as the amount of energy released, changes depending on the environment and the chemical nature of the molecules | No MRI does not use ionizing radiation | Bone fracture detection Parameters: T2* [54] (effective transverse relaxation time) → a function of the density and orientation of the trabeculae [55] R2* → rate constant of the free induction signal (lower with respect to the control in osteoporotic women’s bone marrow [56]) | MRI scanners used for medical purposes could reach typical resolutions of around 1.5 × 1.5 × 4 mm3 [57] | 3D | Useful in age-related fracture detection (marrow fat increases with age and in osteoporosis, allowing better contrast with the trabecular bone) Investigation of cortical water content [43] | Presence of a magnetic field (risk for patients with pacemakers and all implants containing iron) Noise up to 120 dB Use of contrast agents Claustrophobia side effect |
| In vivo |
| Micro- and Nano-Scale Imaging Technique | Brief Description of the Technique | Invasiveness | Outcomes | Spatial Resolution | 2D or 3D | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| In Vitro/In Vivo Application | |||||||
| Stereomicroscopy Based on Histological Sections | Histology from the bone tissue is obtained and then the sample is properly treated (fixation, dehydration and clearing, embedding, sectioning, staining and mounting). The histological section is then observed by means of an optical microscope | Yes | Traditional technique for the visualization of bone microarchitecture | ~1.6 µm [58] | 2D | Bone remodeling assessment [59] | Destructive and invasive technique Limitations related to the bidimensional output images: the three-dimensional features are lost. High-resolution images (at least 1.4 µm or better) are required to identify and measure individual resorption cavities in the process of bone remodeling [59] |
| In vitro | |||||||
| Micro-Computed Tomography (Micro-CT) and Nano-Computed Tomography (Nano-CT) | Micro- and nano-CT scans use radiographs to generate cross-sections of bone, that are generally processed (image reconstruction) to generate a virtual 3D model without destroying the original bone sample | No Generally, the samples are obtained from surgical wastes that derive from prosthetic treatment | Microarchitectural 3D data for both the cortical and the trabecular sections (tissue volume, bone volume, bone surface, bone volume fraction, bone surface to tissue volume, trabecular/cortical thickness, degree of anisotropy, cortical porosity, etc.) [37]. Local and global parameters related to the lacunar network are obtained [36] | 1.2 µm (micro-CT) ~50–150 nm (nano-CT) | 3D | Large number of obtainable outputs (morphological parameters at different scales) Detailed finite element 3D models could be implemented by using micro-CT images | Static evaluation of micro-scale features Not suitable for in vivo human evaluation due to the high radiation dose No detection of the canalicular network (insufficient resolution for the micro-CT scans) Nano-CT |
| In vitro | |||||||
| Peripheral QCT (pQCT) and High-Resolution pQCT (HR-pQCT) | Dedicated CT scanners for the forearm (radius and ulna) and leg (tibia and fibula) | No Low radiation dose (≈0.003 mGy) [37] | Analysis of the trabecular and cortical sections (BMD, bone mineral content and bone geometrical parameter calculation). Acquisition of biomechanical parameters, such as the cross-sectional moment of inertia. Evaluation of the functional muscle–bone unit [60]. | Isotropic voxel size of 82 μm with HR-pQCT | 3D | High precision and accuracy Low radiation dose Applicable for the study of a large number of diseases, especially pediatric (useful in applications where trabecular and cortical sections are affected in a different way) | Evaluation restricted to the appendicular bone Only transversal data are available for fracture risk prediction Low spatial resolution |
| In vivo | |||||||
| Synchrotron Radiation Imaging (SR) | A high-intensity white beam travels around a fixed closed loop. It permits a high level of detail in bone visualization (ultra-structural porosity detection) | No Generally, the samples are obtained from surgical wastes that derive from prosthetic treatment | Morphological analysis of ultra-structural porosities | Voxel size of 0.9 μm for the white beam [61] | 3D | Visualization of the lacunar and canalicular network Phase contrast permits the clear detection of micro-cracks | Reduced field of view |
| In vitro | |||||||
| Micro-MRI and nano-MRI | The technique generates images by exploiting the nuclear magnetic behavior of different atoms in a sample tissue placed in a magnetic field | No | Structural parameters, such as trabecular bone thickness and mean bone volume fraction, associated with bone biomechanical properties and fracture resistance | Spatial resolution up to 25 µm (micro-MRI) and ~10 nm for the nano-MRI | 3D | Non-destructive technique Good special resolution Good contrast resolution [62] | Long acquisition times High costs [62] |
| In vivo | |||||||
| Laser Scanning Confocal Microscopy (LSCM) | LSCM employs lasers at proper wavelengths to excite fluorochromes that are used to stain bone sections | Yes | Correlation between micro-crack parameters and bone matrix toughness Comparison among damage morphologies [13] | 180 nm laterally and 500 nm axially [63] | 2D/3D images of consecutive planes can be reconstructed into a 3D image in vitro. | Evaluation of bone microdamage | Axial resolution in depth impaired by spherical aberration [63] High costs |
| Scanning Electon Microscopy (SEM) | SEM produces images of the bone sample by scanning the surface with a focused beam of electrons | Yes | Quantitative analysis of fracture surfaces Visualization of microdamage morphology, fiber bridging and interlamellar separation [13] | ~1 nm | 3D In vitro | Significant information related to sub-micro-scale damage | Destructive technique (sample surfaces should be conductive → bone needs to be coated with conductive materials) |
| Atomic Force Microscopy (AFM) | The deflections of a cantilever on the surface of the bone sample are transduced into electrical signals | Yes | Topographical parameters of fractured bone surfaces (mineral particle sizes) Identification of sacrificial bonding | Vertical resolution → up to 0.1 nm Lateral resolution → ~30 nm | 3D In vitro | Versatile imaging technique for the visualization of fracture surfaces High accuracy Non-destructive technique [64] | Small dimensions of the single scan image size (150 × 150 µm, compared with mm for SEM) Slow scan time [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buccino, F.; Colombo, C.; Vergani, L.M. A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective. Materials 2021, 14, 1240. https://doi.org/10.3390/ma14051240
Buccino F, Colombo C, Vergani LM. A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective. Materials. 2021; 14(5):1240. https://doi.org/10.3390/ma14051240
Chicago/Turabian StyleBuccino, Federica, Chiara Colombo, and Laura Maria Vergani. 2021. "A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective" Materials 14, no. 5: 1240. https://doi.org/10.3390/ma14051240
APA StyleBuccino, F., Colombo, C., & Vergani, L. M. (2021). A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective. Materials, 14(5), 1240. https://doi.org/10.3390/ma14051240









