Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Artificial Vascular Graft
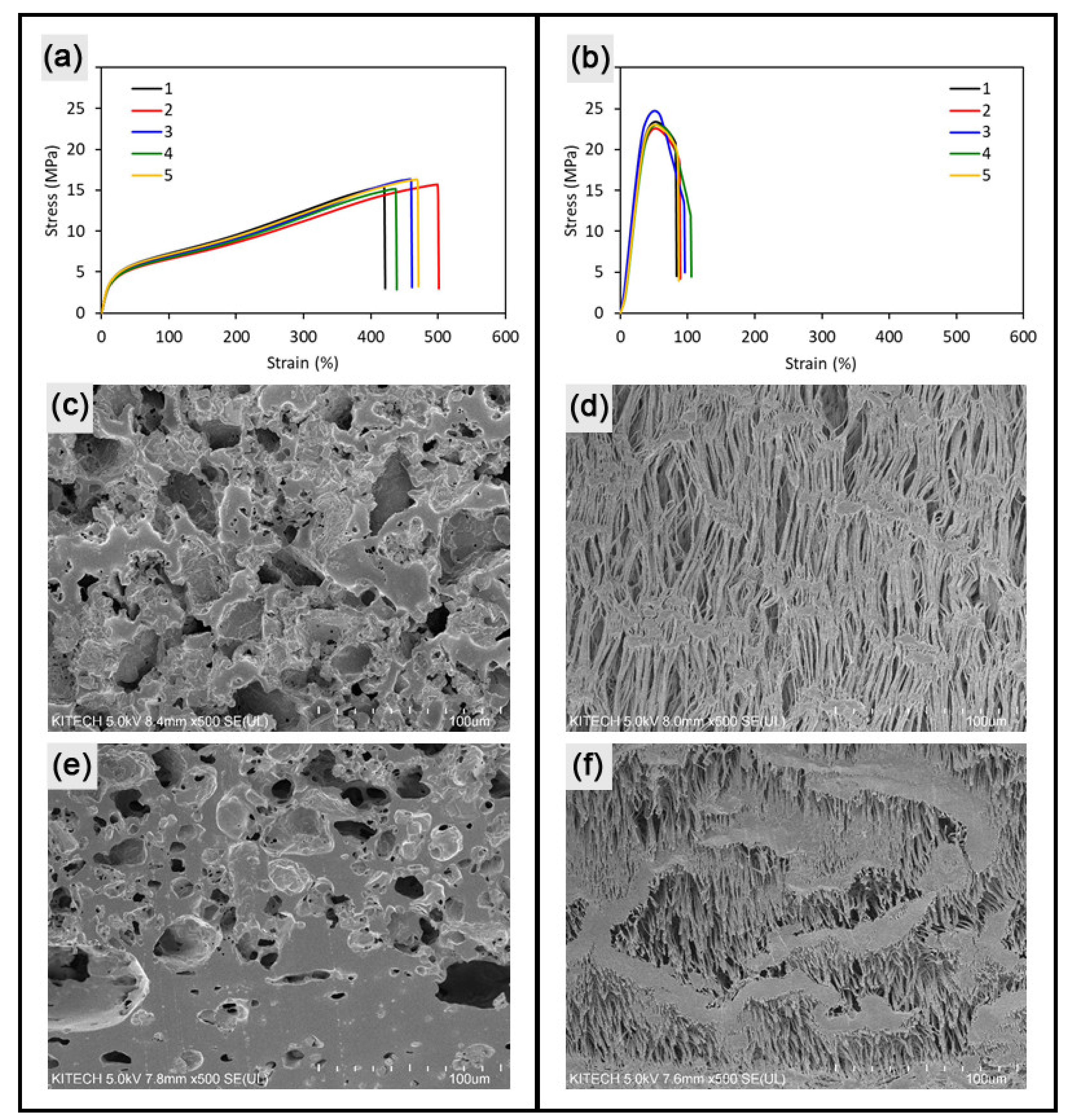
2.2. Mechanical Characterization of Vascular Grafts
2.3. Surface Characterization of Vascular Grafts
2.4. In Vitro Cell Studies
2.4.1. Cell Culture
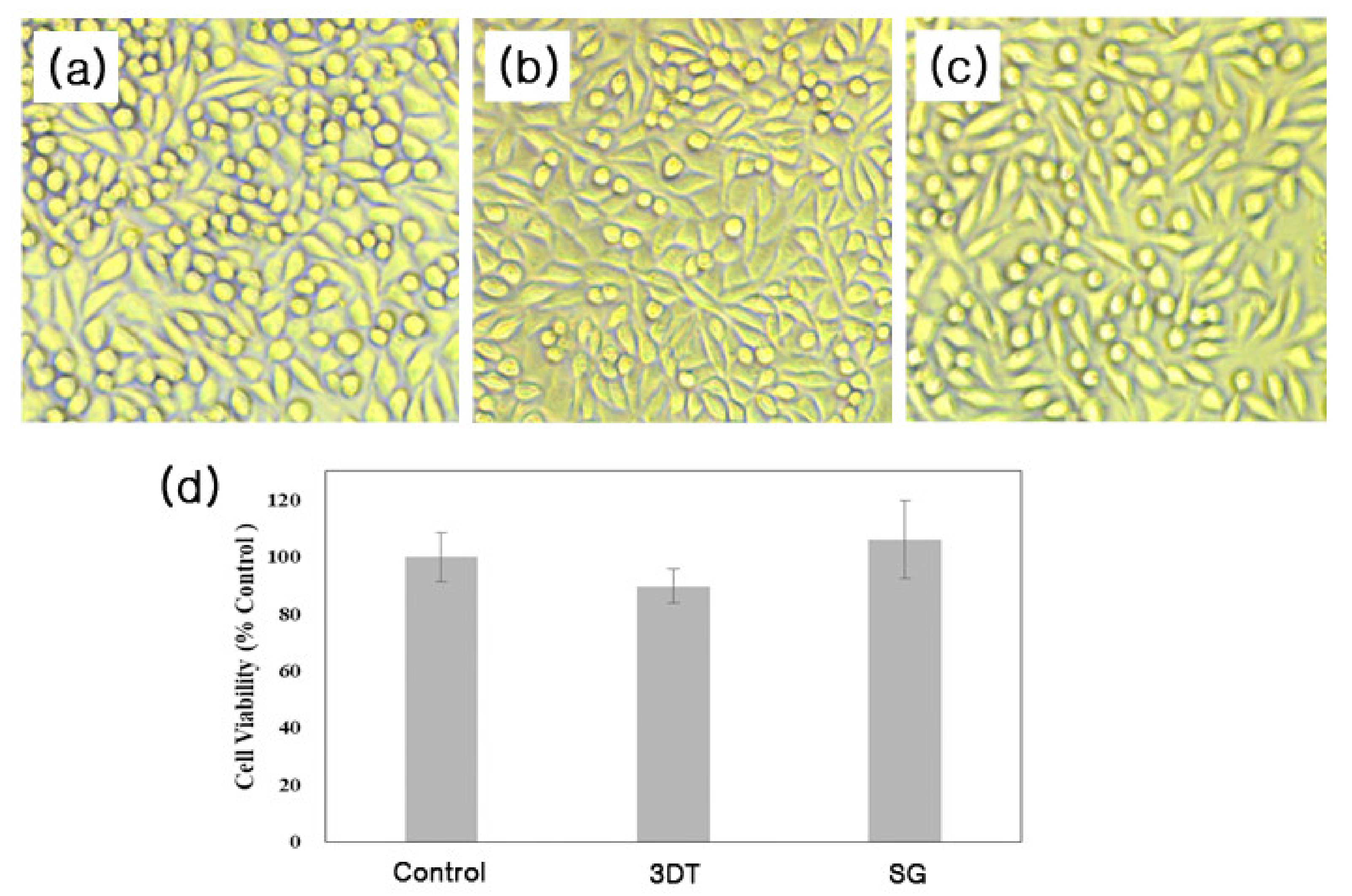
2.4.2. Cytotoxicity
2.5. In Vivo Animal Studies
2.5.1. Animals
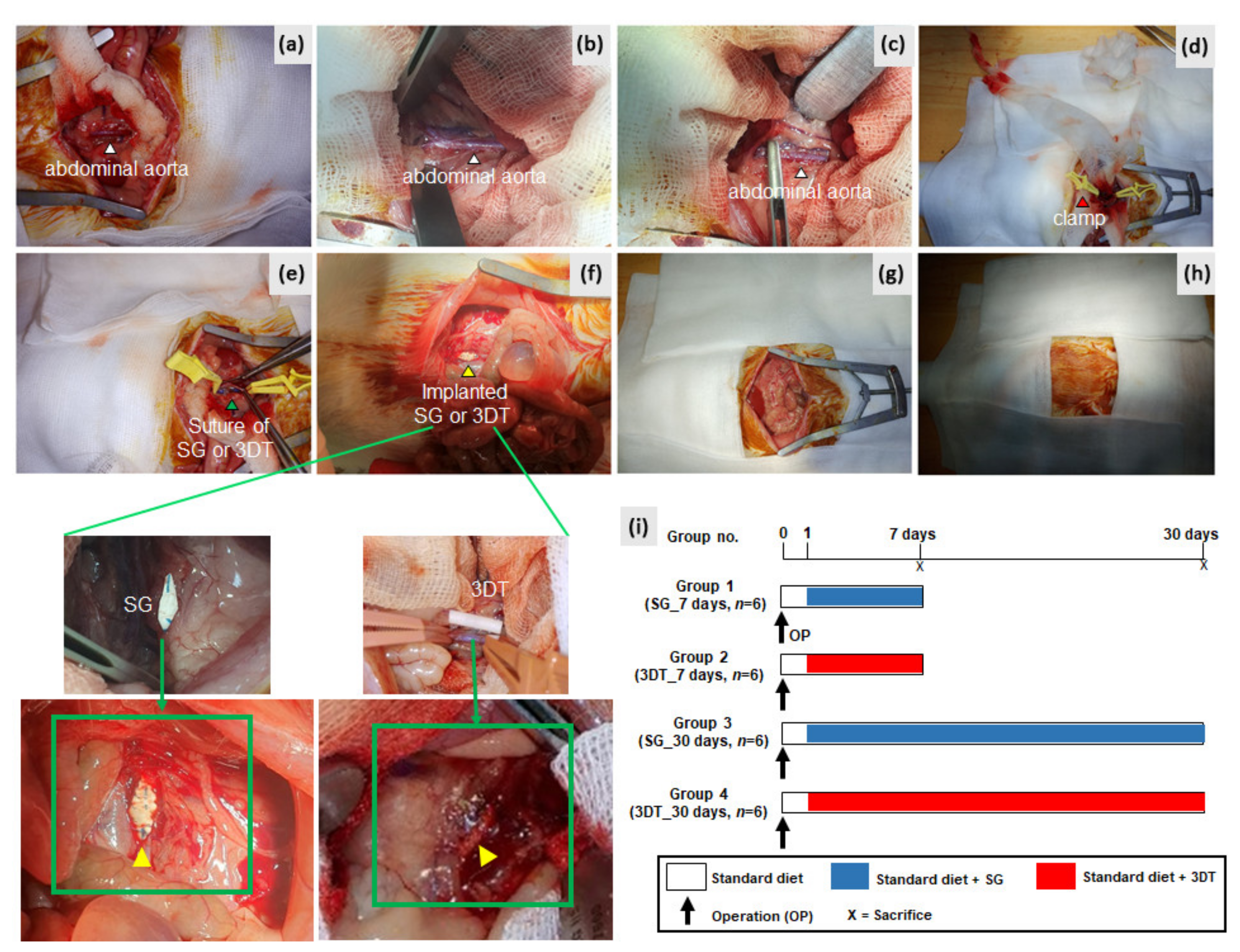
2.5.2. Experimental Design
2.5.3. Tissue Harvesting and Staining
2.5.4. Histopathology Scoring
2.6. Statistical Analysis
3. Results
3.1. 3D Customized Artificial Vascular Grafts
3.2. In Vitro Cytotoxicity
3.3. In Vivo Implantation
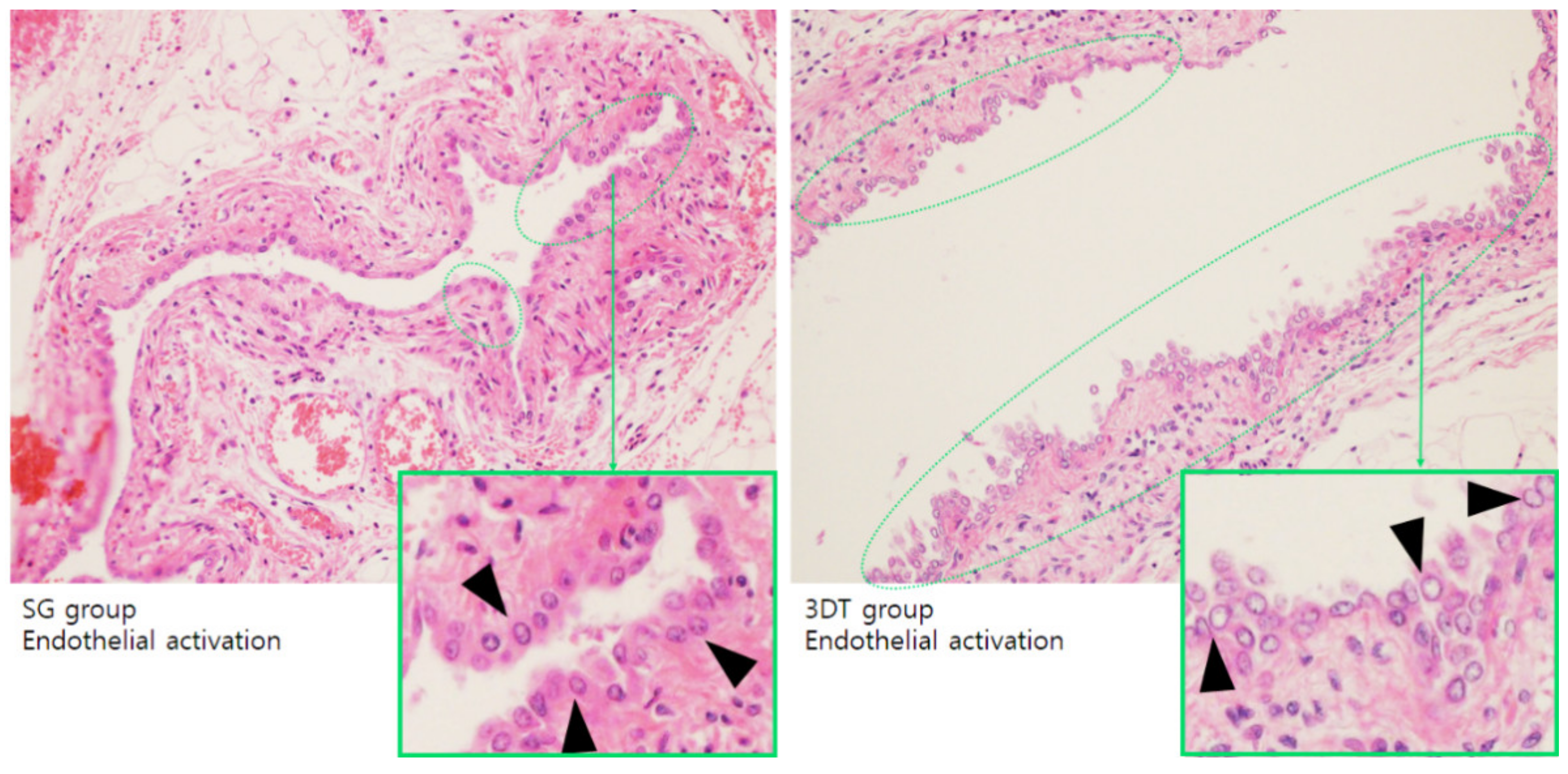
3.3.1. Endothelialization
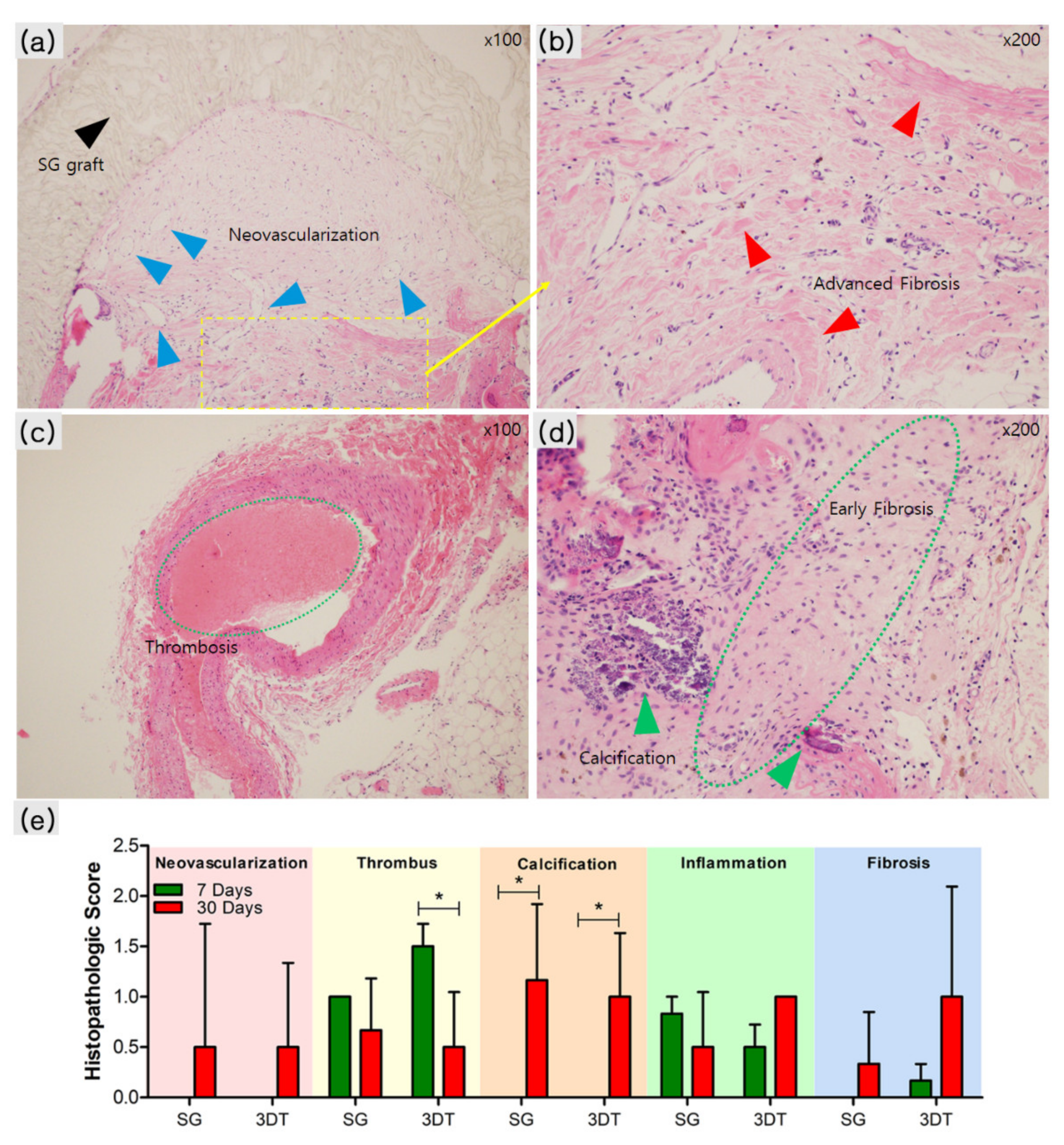
3.3.2. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez, J.C. Down the ataxin-1 track. Nat. Rev. Neurosci. 2000, 1, 154. [Google Scholar] [CrossRef]
- Lord, M.S.; Yu, W.; Cheng, B.; Simmons, A.; Poole-Warren, L.; Whitelock, J.M. The modulation of platelet and endothelial cell adhesion to vascular graft materials by perlecan. Biomaterials 2009, 30, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Vascular Grafts Market Size. In Share & Trends Analysis Report by Product (Peripheral, Endovascular Stent), by Application (Vascular Occlusion, Cardiac Aneurysms), by Raw Material (PTFE, Polyester), and Segment Forecasts, 2019–2026; Grand View Research, Inc.: San Francisco, CA, USA, 2019.
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Wang, Y.; Hu, J.; Wang, C.; Lu, S.; Mo, X. A Method for Preparation of an Internal Layer of Artificial Vascular Graft Co-Modified with Salvianolic Acid B and Heparin. ACS Appl. Mater. Interfaces 2018, 10, 19365–19372. [Google Scholar] [CrossRef]
- Conte, M.S. The ideal small arterial substitute: A search for the Holy Grail? FASEB J. 1998, 12, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.C.; Kligman, F.; Tang, C.; Kottke-Marchant, K.; Marchant, R.E. A biomimetic peptide fluorosurfactant polymer for endothelialization of ePTFE with limited platelet adhesion. Biomaterials 2007, 28, 3537–3548. [Google Scholar] [CrossRef]
- Chlupac, J.; Filova, E.; Bacakova, L. Vascular prostheses: 50 years of advancement from synthetic towards tissue engineering and cell therapy. Rozhledy v Chirurgii Mesicnik Ceskoslovenske Chirurgicke Spolecnosti 2010, 89, 85–94. [Google Scholar]
- Chlupac, J.; Filova, E.; Bacakova, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58 (Suppl. 2), S119–S139. [Google Scholar]
- Ravi, S.; Chaikof, E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2010, 5, 107–120. [Google Scholar] [CrossRef]
- Moby, V.; Boura, C.; Kerdjoudj, H.; Voegel, J.C.; Marchal, L.; Dumas, D.; Schaaf, P.; Stoltz, J.F.; Menu, P. Poly(styrenesulfonate)/poly(allylamine) multilayers: A route to favor endothelial cell growth on expanded poly(tetrafluoroethylene) vascular grafts. Biomacromolecules 2007, 8, 2156–2160. [Google Scholar] [CrossRef]
- Simper, D.; Stalboerger, P.G.; Panetta, C.J.; Wang, S.; Caplice, N.M. Smooth muscle progenitor cells in human blood. Circulation 2002, 106, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Izhar, U.; Schwalb, H.; Borman, J.B.; Hellener, G.R.; Hotoveli-Salomon, A.; Marom, G.; Stern, T.; Cohn, D. Novel synthetic selectively degradable vascular prostheses: A preliminary implantation study. J. Surg. Res. 2001, 95, 152–160. [Google Scholar] [CrossRef]
- Guidoin, R.; Chakfe, N.; Maurel, S.; How, T.; Batt, M.; Marois, M.; Gosselin, C. Expanded polytetrafluoroethylene arterial prostheses in humans: Histopathological study of 298 surgically excised grafts. Biomaterials 1993, 14, 678–693. [Google Scholar] [CrossRef]
- Gangloff, C.; Grimault, O.; Theron, M.; Pichavant, K.; Galinat, H.; Mingant, F.; Ozier, Y. A clinically relevant and bias-controlled murine model to study acute traumatic coagulopathy. Sci. Rep. 2018, 8, 5783. [Google Scholar] [CrossRef]
- Sohn, S.H.; Kim, T.S.; Kim, J.W.; Yoo, S.M.; Jo, W.M. Anti-thrombotic and anti-inflammatory activity of sulodexide compared to aspirin in the rat model. Clin. Hemorheol. Microcirc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Park, S.J.; Yoon, Y.; Son, Y.; Park, S.H. Fabrication of 3D freeform porous tubular constructs with mechanical flexibility mimicking that of soft vascular tissue. J. Mech. Behav. Biomed. Mater. 2019, 91, 193–201. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, B.K.; Lee, J.E.; Chun, S.W.; Jang, K.; Kim, Y.H.; Jeong, M.A.; Kim, Y.; Kang, K.; Lee, N.K.; et al. Design and Fabrication of a Thin-Walled Free-Form Scaffold on the Basis of Medical Image Data and a 3D Printed Template: Its Potential Use in Bile Duct Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 12290–12298. [Google Scholar] [CrossRef]
- Crombez, M.; Chevallier, P.; Gaudreault, R.C.; Petitclerc, E.; Mantovani, D.; Laroche, G. Improving arterial prosthesis neo-endothelialization: Application of a proactive VEGF construct onto PTFE surfaces. Biomaterials 2005, 26, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; Giachelli, C.M. Regulation of cardiovascular calcification. Cardiovasc. Pathol. 2004, 13, 63–70. [Google Scholar] [CrossRef]
- Janzen, J.; Vuong, P.N. Arterial calcifications: Morphological aspects and their pathological implications. Z. Kardiol. 2001, 90 (Suppl. 3), 6–11. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Hermanutz, V.; Wolf, S.E.; Koveker, G.B. Polyurethane vascular prostheses decreases neointimal formation compared with expanded polytetrafluoroethylene. J. Vasc. Surg. 1999, 29, 168–176. [Google Scholar] [CrossRef]
- Cameron, B.L.; Tsuchida, H.; Connall, T.P.; Nagae, T.; Furukawa, K.; Wilson, S.E. High porosity PTFE improves endothelialization of arterial grafts without increasing early thrombogenicity. J. Cardiovasc. Surg. 1993, 34, 281–285. [Google Scholar]
- Sarkar, S.; Schmitz-Rixen, T.; Hamilton, G.; Seifalian, A.M. Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: A review. Med Biol. Eng. Comput. 2007, 45, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.B.F.; Lao, L.L. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Prog. Polym. Sci. 2008, 33, 853–874. [Google Scholar] [CrossRef]
- Uemura, S.; Fathman, C.G.; Rothbard, J.B.; Cooke, J.P. Rapid and efficient vascular transport of arginine polymers inhibits myointimal hyperplasia. Circulation 2000, 102, 2629–2635. [Google Scholar] [CrossRef]
- Teebken, O.E.; Haverich, A. Tissue engineering of small diameter vascular grafts. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 475–485. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Niklason, L.E. Requirements for growing tissue-engineered vascular grafts. Cardiovasc. Pathol. 2003, 12, 59–64. [Google Scholar] [CrossRef]
- Patel, A.; Fine, B.; Sandig, M.; Mequanint, K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc. Res. 2006, 71, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Campbell, J.H. Development of tissue engineered vascular grafts. Curr. Pharm. Biotechnol. 2007, 8, 43–50. [Google Scholar] [CrossRef]
- Ratcliffe, A. Tissue engineering of vascular grafts. Matrix Biol. 2000, 19, 353–357. [Google Scholar] [CrossRef]
- L’Heureux, N.; Paquet, S.; Labbe, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar] [CrossRef]
- Niklason, L.E.; Gao, J.; Abbott, W.M.; Hirschi, K.K.; Houser, S.; Marini, R.; Langer, R. Functional arteries grown in vitro. Science 1999, 284, 489–493. [Google Scholar] [CrossRef]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.N.; Chronos, N.A.; Kyles, A.E.; Gregory, C.R.; Hoyt, G.; et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.; Kim, B.S.; Choi, Y.; Jang, W.B.; Hong, Y.J.; Kwon, S.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Thomas, V.; Donahoe, T.; Nyairo, E.; Dean, D.R.; Vohra, Y.K. Electrospinning of Biosyn((R))-based tubular conduits: Structural, morphological, and mechanical characterizations. Acta Biomater. 2011, 7, 2070–2079. [Google Scholar] [CrossRef]
- Hoerstrup, S.P.; Kadner, A.; Breymann, C.; Maurus, C.F.; Guenter, C.I.; Sodian, R.; Visjager, J.F.; Zund, G.; Turina, M.I. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann. Thorac. Surg. 2002, 74, 46–52. [Google Scholar] [CrossRef]
- Monson, K.L.; Goldsmith, W.; Barbaro, N.M.; Manley, G.T. Significance of source and size in the mechanical response of human cerebral blood vessels. J. Biomech. 2005, 38, 737–744. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Zhang, L.; Sun, W.; Zhou, J. Long-term results of triple-layered small diameter vascular grafts in sheep carotid arteries. Med. Eng. Phys. 2020, 85, 1–6. [Google Scholar] [CrossRef]
- Kang, T.Y.; Lee, J.H.; Kim, B.J.; Kang, J.A.; Hong, J.M.; Kim, B.S.; Cha, H.J.; Rhie, J.W.; Cho, D.W. In vivo endothelization of tubular vascular grafts through in situ recruitment of endothelial and endothelial progenitor cells by RGD-fused mussel adhesive proteins. Biofabrication 2015, 7, 015007. [Google Scholar] [CrossRef]
- Sellers, S.L.; Turner, C.T.; Sathananthan, J.; Cartlidge, T.R.G.; Sin, F.; Bouchareb, R.; Mooney, J.; Norgaard, B.L.; Bax, J.J.; Bernatchez, P.N.; et al. Transcatheter Aortic Heart Valves: Histological Analysis Providing Insight to Leaflet Thickening and Structural Valve Degeneration. JACC Cardiovasc. Imaging 2019, 12, 135–145. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, S.-H.; Kim, T.-H.; Kim, T.-S.; Min, T.-J.; Lee, J.-H.; Yoo, S.-M.; Kim, J.-W.; Lee, J.-E.; Kim, C.-H.; Park, S.-H.; et al. Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease. Materials 2021, 14, 1239. https://doi.org/10.3390/ma14051239
Sohn S-H, Kim T-H, Kim T-S, Min T-J, Lee J-H, Yoo S-M, Kim J-W, Lee J-E, Kim C-H, Park S-H, et al. Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease. Materials. 2021; 14(5):1239. https://doi.org/10.3390/ma14051239
Chicago/Turabian StyleSohn, Sung-Hwa, Tae-Hee Kim, Tae-Sik Kim, Too-Jae Min, Ju-Han Lee, Sung-Mook Yoo, Ji-Won Kim, Ji-Eun Lee, Chae-Hwa Kim, Suk-Hee Park, and et al. 2021. "Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease" Materials 14, no. 5: 1239. https://doi.org/10.3390/ma14051239
APA StyleSohn, S.-H., Kim, T.-H., Kim, T.-S., Min, T.-J., Lee, J.-H., Yoo, S.-M., Kim, J.-W., Lee, J.-E., Kim, C.-H., Park, S.-H., & Jo, W.-M. (2021). Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease. Materials, 14(5), 1239. https://doi.org/10.3390/ma14051239





