Structural and Secondary Electron Yield Properties of Titanium–Palladium Films with Laser-Treated Copper Substrate for Application in Neutron Generators
Abstract
1. Introduction
2. Experiments and Methods
2.1. Sample Preparation
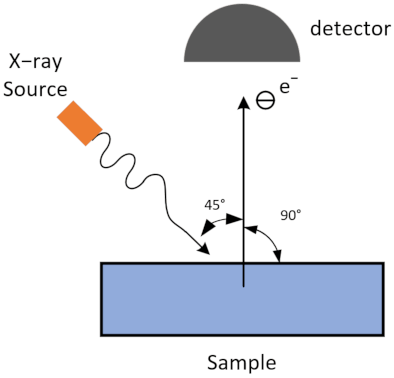
2.2. Characterization Methods
3. Results and Discussion
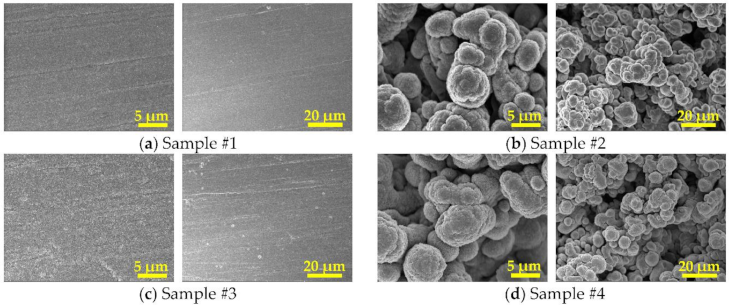
3.1. Surface and Cross-Section Morphologies
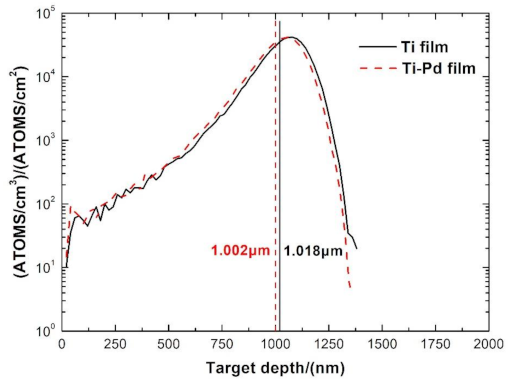
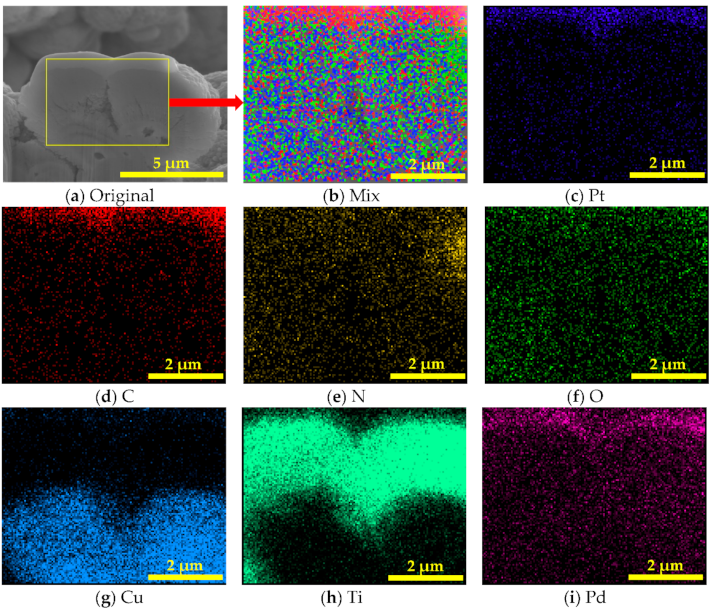
3.2. Surface Chemical Composition
3.3. SEY
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; Wang, J.; Ma, Z.; Lu, X.; Wei, Z.; Zhang, S.; Liu, Y.; Zhang, Z.; Zhang, Y.; Yao, Z. Design of a compact D–D neutron generator. Nucl. Instrum. Methods Phys. Res. Sect. A-Accel. Spectrom. Dect. Assoc. Equip. 2018, 904, 107–112. [Google Scholar] [CrossRef]
- Reijonen, J.; Leung, K.-N.; Firestone, R.B.; English, J.A.; Perry, D.L.; Smith, A.; Gicquel, F.; Sun, M.; Koivunoro, H.; Lou, T.-P.; et al. First PGAA and NAA experimental results from a compact high intensity D–D neutron generator. Nucl. Instrum. Methods Phys. Res. Sect. A-Accel. Spectrom. Dect. Assoc. Equip. 2004, 522, 598–602. [Google Scholar] [CrossRef][Green Version]
- Marchese, N.; Cannuli, A.; Caccamo, M.T.; Pace, C. New generation non-stationary portable neutron generators for biophysical applications of Neutron Activation Analysis. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 3661–3670. [Google Scholar] [CrossRef]
- Bishnoi, S.; Sarkar, P.S.; Thomas, R.G.; Patel, T.; Pal, M.; Adhikari, P.S.; Sinha, A.; Saxena, A.; Gadkari, S.C. Preliminary Experimentation of Fast Neutron Radiography with D-T Neutron Generator at BARC. J. Nondestruct. Eval. 2019, 38, 13. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wang, Y.; Li, T.; Zhang, Z. Design and Optimization of a Fast Neutron Radiography System Based on a High-Intensity D-T Fusion Neutron Generator. Nucl. Technol. 2019, 205, 978–986. [Google Scholar] [CrossRef]
- Bergaoui, K.; Reguigui, N.; Gary, C.K.; Cremer, J.T.; Vainionpaa, J.H.; Piestrup, M.A. Design, testing and optimization of a neutron radiography system based on a Deuterium–Deuterium (D–D) neutron generator. J. Radioanal. Nucl. Chem. 2014, 299, 41–51. [Google Scholar] [CrossRef]
- Sahoo, G.S.; Sharma, S.D.; Tripathy, S.P.; Bandyopadhyay, T. Design and dosimetric evaluation of beam shaping assembly for BNCT of compact D–T neutron generator by Monte Carlo simulation. Biomed. Phys. Eng. Express 2017, 4, 015018. [Google Scholar] [CrossRef]
- Kasesaz, Y.; Karimi, M. A novel design of beam shaping assembly to use D-T neutron generator for BNCT. Appl. Radiat. Isot. 2016, 118, 317–325. [Google Scholar] [CrossRef]
- Remetti, R.; Lepore, L.; Cherubini, N. Development and experimental validation of a monte carlo modeling of the neutron emission from a dt generator. Nucl. Instrum. Methods Phys. Res. Sect. A-Accel. Spectrom. Dect. Assoc. Equip. 2017, 842, 7–13. [Google Scholar] [CrossRef]
- Metwally, W.A.; Taqatqa, O.A.; Ballaith, M.M.; Chen, A.X.; Piestrup, M.A. Neutron and photon dose mapping of a DD neutron generator. Radiat. Prot. Dosim. 2017, 176, 258–263. [Google Scholar] [CrossRef]
- Song, G.; Wang, G.; Yu, Q.; Wang, W.; Chen, C.; Wu, Y. Design and analyses of rotating tritium target system for high intensity D–T fusion neutron generator. J. Fusion Energy. 2015, 34, 183–190. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Zhang, Y.; Li, J.; Xia, L.; Zhang, J.; Ding, Y.; Jiang, B.; Huang, Z.; Ma, Z.; et al. Design of a high-current low-energy beam transport line for an intense DT/DD neutron generator. Nucl. Instrum. Methods Phys. Res. Sect. A-Accel. Spectrom. Dect. Assoc. Equip. 2016, 811, 76–81. [Google Scholar] [CrossRef]
- Fantidis, J.; Antoniadis, A. Optimization study for BNCT facility based on a DT neutron generator. Int. J. Radiat. Res. 2015, 13, 13–24. [Google Scholar]
- Taylor, M.; Sengbusch, E.; Seyfert, C.; Moll, E.; Radel, R. Thermal neutron radiography using a high-flux compact neutron generator. Phys. Procedia. 2017, 88, 175–183. [Google Scholar] [CrossRef]
- Kromer, H.; Adams, R.; Soubelet, B.; Zboray, R.; Prasser, H.M. Thermal analysis, design, and testing of a rotating beam target for a compact DD fast neutron generator. Appl. Radiat. Isot. 2019, 145, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Bai, X.; Liu, C.; Wang, J.; Ma, Z.; Lu, X.; Wei, Z.; Zhang, Z.; Zhang, Y.; Yao, Z. Study on secondary electron suppression in compact D–D neutron generator. Nucl. Sci. Tech. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Qiao, Y. Progress in studies and Applications of neutron tube. Nucl. Electron. Detect. Technol. 2008, 28, 1134–1139. [Google Scholar]
- Meng, X.; Dong, Z. Dynamic Study on Effect of Secondary Electron on Ion Beam Quality. High Power Laser Part. Beams 2018, 30, 112–116. [Google Scholar]
- Jin, D. The Formation Process of the Electron Current in a Sealed Neutron Tube. Acta Sci. Nat. Univ. Jilin. 1998, 1, 40–44. [Google Scholar]
- Yang, Z.; Peng, Y.; Long, J.; Lan, C.; Dong, P.; Shi, J. Suppression secondary electrons from target surface under pulsed ion beams bombardment. High Power Laser Part. Beams 2012, 24, 2198–2202. [Google Scholar] [CrossRef]
- Jin, D.Z.; Yang, Z.H.; Dai, J.Y. Simulation for Suppressing of Secondary Electrons in Neutron Generator. J. Univ. Electron. Sci. Technol. China 2009, 38, 83–86. [Google Scholar]
- Waltz, C.; Ayllon, M.; Becker, T.; Bernstein, L.; Leung, K.N.; Kirsch, L.; Renne, P.; Van Bibber, K. Beam-induced back-streaming electron suppression analysis for an accelerator type neutron generator designed for 40Ar/39Ar geochronology. Appl. Radiat. Isot. 2017, 125, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, R.; Malyshev, O.B.; Wang, S.; Zolotovskaya, S.A.; Gillespie, W.A.; Abdolvand, A. Low secondary electron yield engineered surface for electron cloud mitigation. Appl. Phys. Lett. 2014, 105, 231605. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Fan, J.; You, Z.; Wang, S.; Xu, Z. Study on the effect of laser parameters on the SEY of aluminum alloy. IEEE Trans. Nucl. Sci. 2019, 66, 609–615. [Google Scholar] [CrossRef]
- Bajek, D.; Wackerow, S.; Zanin, D.A.; Baudin, L.; Bogdanowicz, K.; Valdivieso, E.G.-T.; Calatroni, S.; Girolamo, B.D.; Sitko, M.; Himmerlich, M.; et al. Role of surface microgeometries on electron escape probability and secondary electron yield of metal surfaces. Sci. Rep. 2020, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; You, Z.; Fan, J.; Zhang, J.; Yang, S.; Guo, S.; Wang, S.; Xu, Z. Laser Processed Oxygen-Free High-Conductivity Copper with Ti and Ti–Zr–V–Hf Films Applied in Neutron Tube. Appl. Sci. 2019, 9, 4940. [Google Scholar] [CrossRef]
- Baudin, L.; Sitko, M.; Garion, C.; Chiggiato, P.; Delloro, F.; Gaslain, F.; Sennour, M.; Jeandin, M.; Bajek, D.; Wackerow, S.; et al. Morphological and Chemical Characterization of Laser Treated Surface on Copper. Key Eng. Mater. 2019, 813, 254–260. [Google Scholar] [CrossRef]
- Calatroni, S.; Valdivieso, E.G.T.; Neupert, H.; Nistor, V.; Fontenla, A.T.P.; Taborelli, M.; Chiggiato, P.; Malyshev, O.B.; Valizadeh, R.; Wackerow, S.; et al. First accelerator test of vacuum components with laser-engineered surfaces for electron-cloud mitigation. Phys. Rev. Accel. Beams 2017, 20, 113201. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; You, Z.; Fan, J.; Zhang, J.; Qiao, Z.; Wang, S.; Xu, Z. Non-Evaporable Getter Ti-V-Hf-Zr Film Coating on Laser-Treated Aluminum Alloy Substrate for Electron Cloud Mitigation. Coatings 2019, 9, 839. [Google Scholar] [CrossRef]
- Williams, D.L.; Vainionpaa, J.H.; Jones, G.; Piestrup, M.A.; Gary, C.K.; Harris, J.L.; Fuller, M.J.; Cremer, J.T.; Ludewigt, B.A.; Kwan, J.W.; et al. High Intensity, Pulsed, D-D Neutron Generator. In Proceedings of the 20th International Conference on Application of Accelerators in Research and Industry, Ft Worth, TX, USA, 10–15 August 2008; Volume 1099, pp. 936–939. [Google Scholar]
- Bergaoui, K.; Reguigui, N.; Gary, C.K.; Brown, C.; Cremer, J.T.; Vainionpaa, J.H.; Piestrup, M.A. Development of a new deuterium-deuterium (D-D) neutron generator for prompt gamma-ray neutron activation analysis. Appl. Radiat. Isot. 2014, 94, 319–327. [Google Scholar] [CrossRef]
- Liu, Y.; Byrne, P.; Wang, H.; Koltick, D.; Zheng, W.; Nie, L. A compact DD neutron generator-based NAA system to quantify manganese (Mn) in bone in vivo. Physiol. Meas. 2014, 35, 1899–1911. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benvenuti, C.; Chiggiato, P.; Cicoira, F.; L’Aminot, Y.; Ruzinov, V. Vacuum properties of palladium thin film coatings. Vacuum 2004, 73, 139–144. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xu, Y.; Zhang, B.; Wei, W. Research on the secondary electron yield of TiZrV-Pd thin film coatings. Vacuum 2016, 131, 81–88. [Google Scholar] [CrossRef][Green Version]
- Lim, H.R.; Eom, N.S.A.; Cho, J.-H.; Cho, H.-B.; Choa, Y.-H. Hydrogen gettering of titanium palladium/palladium nanocomposite films synthesized by cosputtering and vacuum-annealing. Int. J. Hydrog. Energy 2018, 43, 19990–19997. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Xu, Y.; Wang, Y. Research on deposition rate of TiZrV/Pd film by DC magnetron sputtering method. Nucl. Sci. Tech. 2017, 28, 50. [Google Scholar] [CrossRef]
- Shugard, A.D.; Walters, R.T.; Blarigan, P.V. Titanium tritide radioisotope heat source development: Palladium-coated titanium hydriding kinetics and tritium loading tests. Energy Conv. Manag. 2012, 64, 371–377. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM-The stopping and range of ions in matter (2010). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of Functional Nanoengineering and Nanosecond Laser Texturing for Design of Superhydrophobic Aluminum Alloy with Exceptional Mechanical and Chemical Properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef]
- Wu, X.; Lai, F.; Lin, L.; Lin, L.; Qu, Y. Influence of thickness on the structural and optical properties of vanadium oxide thin films. Chin. J. Lasers 2008, 35, 311. [Google Scholar]
- Toyoshima, R.; Yoshida, M.; Monya, Y.; Kousa, Y.; Suzuki, K.; Abe, H.; Mun, B.S.; Mase, K.; Amemiya, K.; Kondoh, H. In Situ Ambient Pressure XPS Study of CO Oxidation Reaction on Pd(111) Surfaces. J. Phys. Chem. C 2012, 116, 18691–18697. [Google Scholar] [CrossRef]
- Wang, J.; Sian, T.; Valizadeh, R.; Wang, Y.; Wang, S. The Effect of Air Exposure on SEY and Surface Composition of Laser Treated Copper Applied in Accelerators. IEEE Trans. Nucl. Sci. 2018, 65, 2620–2627. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tanaka, A. Alteration of Ti 2p XPS spectrum for titanium oxide by low-energy Ar ion bombardment. Surf. Interface Anal. 2002, 34, 262–265. [Google Scholar] [CrossRef]
- Chastain, J.; King, J.R.C. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corporation 1992, 40, 221. [Google Scholar]
- Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. XPS/STM study of model bimetallic Pd–Au/HOPG catalysts. Appl. Surf. Sci. 2016, 367, 214–221. [Google Scholar] [CrossRef]
- Ohya, K. Comparative study of target atomic number dependence of ion induced and electron induced secondary electron emission. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 206, 52–56. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; You, Z.; Fan, J.; Zhang, J.; Wang, S.; Xu, Z. Laser Induced Nano and Micro Structures of Molybdenum Surface Applied in Multistage Depressed Collector for Secondary Electron Suppression. Appl. Sci.-Basel 2019, 9, 4374. [Google Scholar] [CrossRef]
- Lin, Y.; Joy, D.C. A new examination of secondary electron yield data. Surf. Interface Anal. 2005, 37, 895–900. [Google Scholar] [CrossRef]
- Hilleret, N.; Scheuerlein, C.; Taborelli, M. The secondary-electron yield of air-exposed metal surfaces. Appl. Phys. A-Mater. Sci. Process. 2003, 76, 1085–1091. [Google Scholar] [CrossRef]
- Iyasu, T.; Inoue, M.; Yoshikawa, H.; Shimizu, R. Experimental studies of secondary electron emission of TiO2 and Ti. Jpn. J. Appl. Phys. 2006, 45, 7879. [Google Scholar] [CrossRef]



| Sample | Film Coating | Substrate | δmax1 | Emax2/eV | Ra 3/μm |
|---|---|---|---|---|---|
| # 1 | Ti | Untreated OFHC | 1.36 | 200 | 0.201 |
| # 2 | Ti | Laser-treated OFHC | 0.93 | 300 | 7.502 |
| # 3 | Ti-Pd | Untreated OFHC | 1.19 | 400 | 0.154 |
| # 4 | Ti-Pd | Laser-treated OFHC | 0.87 | 400 | 6.543 |
| Sample | C | N | O | Ti | Pd |
|---|---|---|---|---|---|
| #2 | 30.54% | 0.15% | 49.70% | 19.61% | 0 |
| #4 | 19.22% | 3.00% | 52.27% | 0 | 25.51% |
| Sample | C-C/C-H | C-OH/C-O-C | C=O | O-C=O |
|---|---|---|---|---|
| #2 | 79.27% | 14.18% | 1.28% | 5.27% |
| #4 | 72.20% | 16.48% | 6.92% | 4.40% |
| Sample | O element | Metal | Metal Suboxide | Metal Oxide | ||
|---|---|---|---|---|---|---|
| Lattice Oxide | Hydroxides/Defect Oxides | Water/Organic O | ||||
| #2 | 88.15% | 9.43% | 2.42% | Ti metal | Ti suboxide | TiO2 |
| 7.11% | 4.19% | 88.70% | ||||
| #4 | 27.53% | 48.53% | 23.94% | Pd metal | PdO | PdO2 |
| 68.67% | 21.31% | 10.02% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, S.; Wang, J.; You, Z.; Zhang, J.; Hu, Y.; Wu, Y.; Fan, J.; Li, H.; Zhan, Q.; et al. Structural and Secondary Electron Yield Properties of Titanium–Palladium Films with Laser-Treated Copper Substrate for Application in Neutron Generators. Materials 2021, 14, 1222. https://doi.org/10.3390/ma14051222
Gao Y, Wang S, Wang J, You Z, Zhang J, Hu Y, Wu Y, Fan J, Li H, Zhan Q, et al. Structural and Secondary Electron Yield Properties of Titanium–Palladium Films with Laser-Treated Copper Substrate for Application in Neutron Generators. Materials. 2021; 14(5):1222. https://doi.org/10.3390/ma14051222
Chicago/Turabian StyleGao, Yong, Sheng Wang, Jie Wang, Zhiming You, Jing Zhang, Yaocheng Hu, Yue Wu, Jiakun Fan, Haipeng Li, Qin Zhan, and et al. 2021. "Structural and Secondary Electron Yield Properties of Titanium–Palladium Films with Laser-Treated Copper Substrate for Application in Neutron Generators" Materials 14, no. 5: 1222. https://doi.org/10.3390/ma14051222
APA StyleGao, Y., Wang, S., Wang, J., You, Z., Zhang, J., Hu, Y., Wu, Y., Fan, J., Li, H., Zhan, Q., Yang, H., & Xu, Z. (2021). Structural and Secondary Electron Yield Properties of Titanium–Palladium Films with Laser-Treated Copper Substrate for Application in Neutron Generators. Materials, 14(5), 1222. https://doi.org/10.3390/ma14051222








