Designing Spinel Li4Ti5O12 Electrode as Anode Material for Poly(ethylene)oxide-Based Solid-State Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Li Metal–LTO Solid-State Cells
2.2. Microscopical Features of the Electrodes
2.3. Electrochemical Evaluation of the Electrodes
3. Results
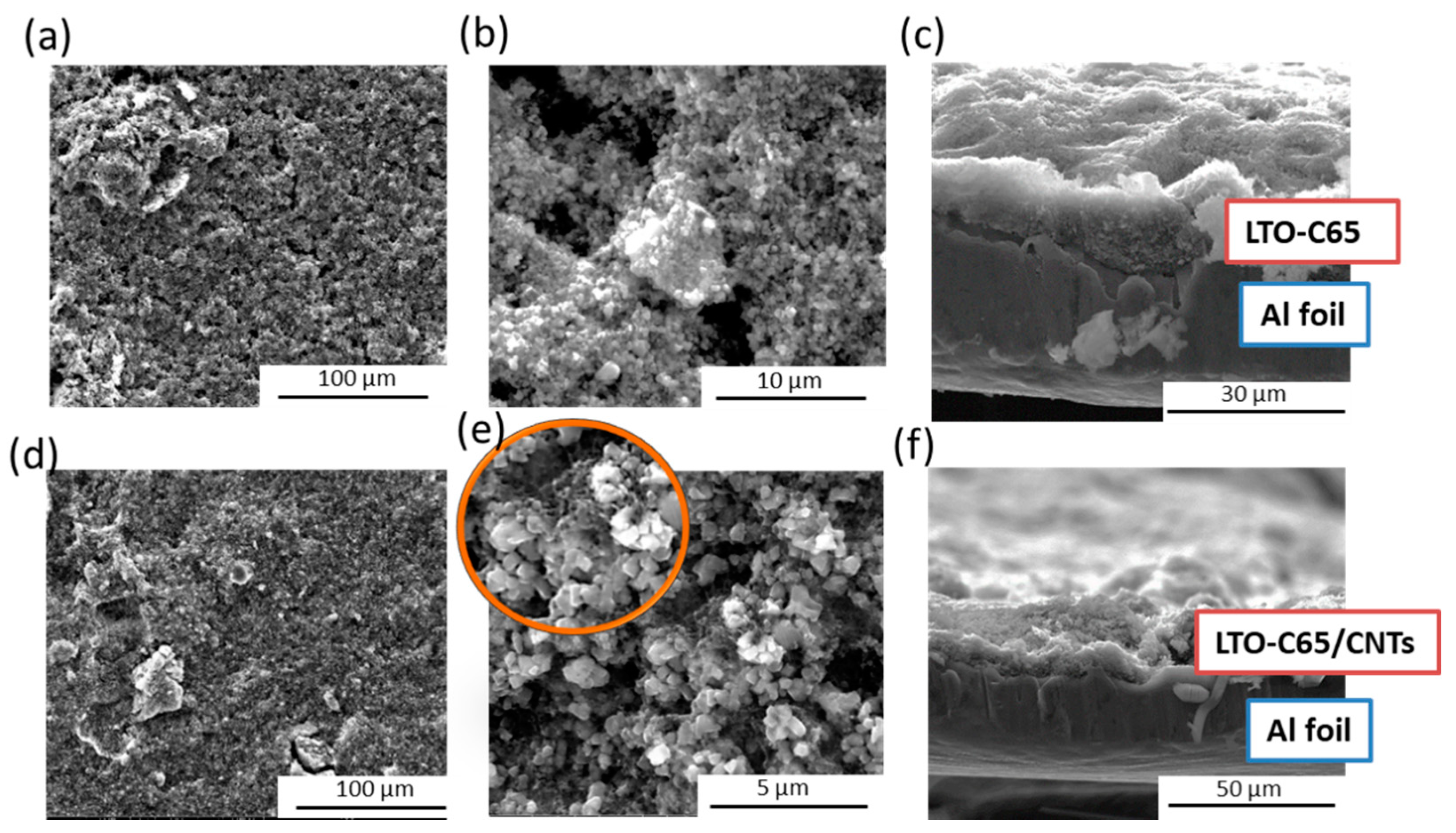
3.1. Microscopical Features and Composition of the Electrode Laminates
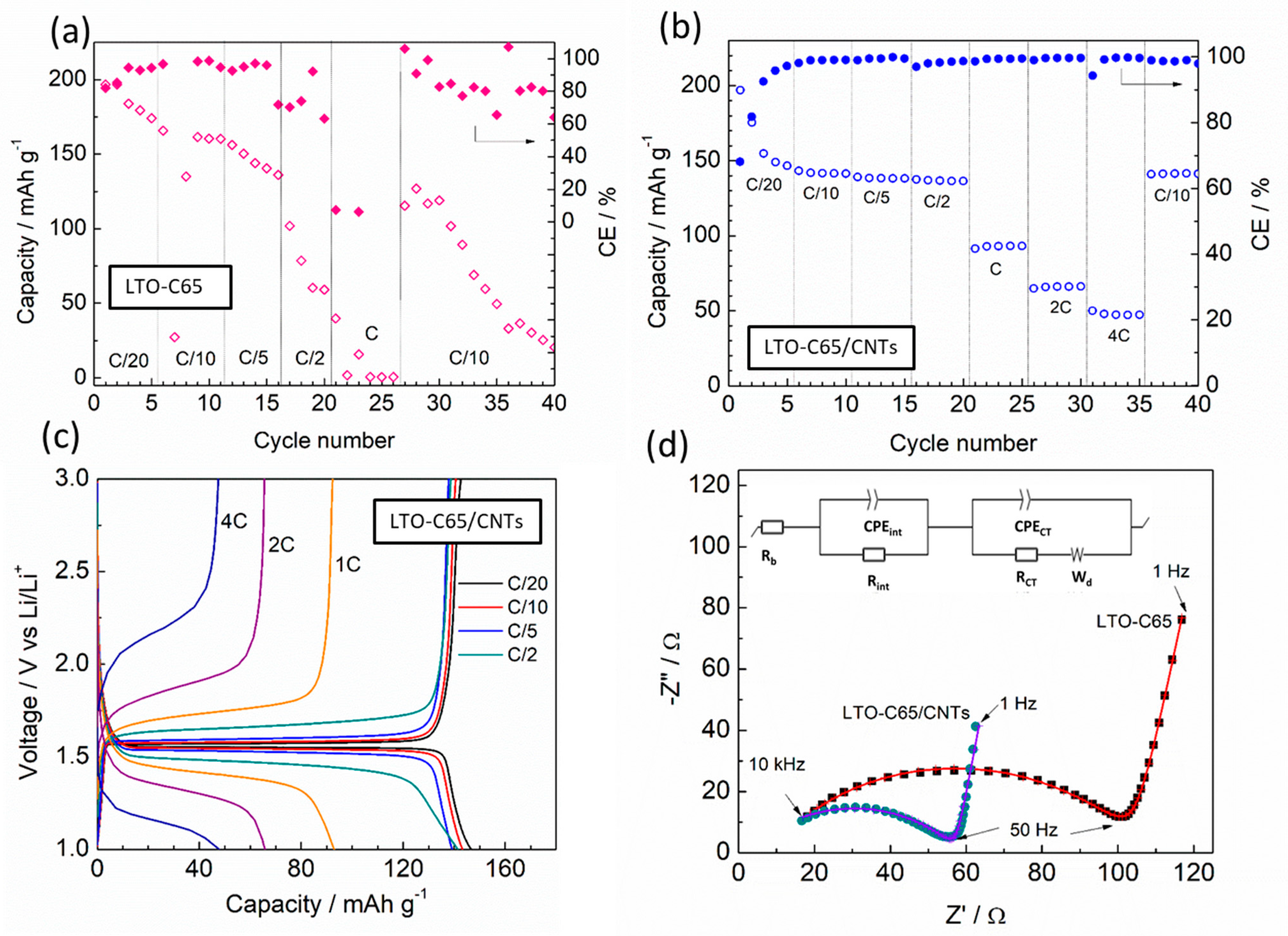
3.2. Electrochemical Performance of the Full Cells
3.2.1. Rate Capability
3.2.2. Long-Term Cycling and Postmortem Analysis
4. Discussion on the Electrode Design for Solid-State Batteries
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A Solid Future for Battery Development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Pasta, M.; Armstrong, D.; Brown, Z.L.; Bu, J.; Castell, M.R.; Chen, P.; Cocks, A.; Corr, S.A.; Cussen, E.J.; Darnbrough, E.; et al. 2020 Roadmap on Solid-State Batteries. J. Phys. Energy 2020, 2, 032008. [Google Scholar] [CrossRef]
- Sun, Y.-K. Promising All-Solid-State Batteries for Future Electric Vehicles. ACS Energy Lett. 2020, 5, 3221–3223. [Google Scholar] [CrossRef]
- Xia, Y.; Fujieda, T.; Tatsumi, K.; Prosini, P.P.; Sakai, T. Thermal and Electrochemical Stability of Cathode Materials in Solid Polymer Electrolyte. J. Power Source 2001, 92, 234–243. [Google Scholar] [CrossRef]
- Building Better Batteries in the Solid State: A Review. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6926585/ (accessed on 21 March 2020).
- Hatzell, K.B.; Chen, X.C.; Cobb, C.L.; Dasgupta, N.P.; Dixit, M.B.; Marbella, L.E.; McDowell, M.T.; Mukherjee, P.P.; Verma, A.; Viswanathan, V.; et al. Challenges in Lithium Metal Anodes for Solid-State Batteries. ACS Energy Lett. 2020, 5, 922–934. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693. [Google Scholar] [CrossRef]
- Cao, D.; Sun, X.; Li, Q.; Natan, A.; Xiang, P.; Zhu, H. Lithium Dendrite in All-Solid-State Batteries: Growth Mechanisms, Suppression Strategies, and Characterizations. Matter 2020, 3, 57–94. [Google Scholar] [CrossRef]
- Aguesse, F.; Manalastas, W.; Buannic, L.; Lopez del Amo, J.M.; Singh, G.; Llordés, A.; Kilner, J. Investigating the Dendritic Growth during Full Cell Cycling of Garnet Electrolyte in Direct Contact with Li Metal. ACS Appl. Mater. Interfaces 2017, 9, 3808–3816. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhou, Z. Towards Practical Lithium-Metal Anodes. Chem. Soc. Rev. 2020, 49, 3040–3071. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Q.; Ma, W.; Yang, L. Polyethylene Oxide-Based Composites as Solid-State Polymer Electrolytes for Lithium Metal Batteries: A Mini Review. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(Ethylene Oxide)-Based Electrolytes for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef]
- Boaretto, N.; Meabe, L.; Martinez-Ibañez, M.; Armand, M.; Zhang, H. Review—Polymer Electrolytes for Rechargeable Batteries: From Nanocomposite to Nanohybrid. J. Electrochem. Soc. 2020, 167, 070524. [Google Scholar] [CrossRef]
- Pitillas Martinez, A.I.; Aguesse, F.; Otaegui, L.; Schneider, M.; Roters, A.; Llordés, A.; Buannic, L. The Cathode Composition, a Key Player in the Success of Li-Metal Solid-State Batteries. J. Phys. Chem. C 2019, 123, 3270–3278. [Google Scholar] [CrossRef]
- BlueSolution—The LMP Technology. Available online: https://www.blue-solutions.com/en/blue-solutions/technology/batteries-lmp/ (accessed on 3 March 2021).
- Yang, Q.; Huang, J.; Li, Y.; Wang, Y.; Qiu, J.; Zhang, J.; Yu, H.; Yu, X.; Li, H.; Chen, L. Surface-Protected LiCoO2 with Ultrathin Solid Oxide Electrolyte Film for High-Voltage Lithium Ion Batteries and Lithium Polymer Batteries. J. Power Source 2018, 388, 65–70. [Google Scholar] [CrossRef]
- Cui, G. A Strategy to Make High Voltage LiCoO2 Compatible with Polyethylene Oxide Electrolyte in All-Solid-State Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A3454. [Google Scholar]
- Liang, J.; Sun, Y.; Zhao, Y.; Sun, Q.; Luo, J.; Zhao, F.; Lin, X.; Li, X.; Li, R.; Zhang, L.; et al. Engineering the Conductive Carbon/PEO Interface to Stabilize Solid Polymer Electrolytes for All-Solid-State High Voltage LiCoO2 Batteries. J. Mater. Chem. A 2020, 8, 2769–2776. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Judez, X.; Chakir, M.; Armand, M.; Buannic, L. High Voltage Solid State Batteries: Targeting High Energy Density with Polymer Composite Electrolytes. J. Electrochem. Soc. 2020, 167, 020548. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Ma, J.; Xu, G.; Dong, T.; Cui, G. Polymer Electrolytes for High Energy Density Ternary Cathode Material-Based Lithium Batteries. Electrochem. Energy Rev. 2019, 2, 128–148. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, J.; Chen, H.; Yu, Q.; Li, B. High Electrochemical Stability of a 3D Cross-Linked Network PEO@nano-SiO2 Composite Polymer Electrolyte for Lithium Metal Batteries. J. Mater. Chem. A 2019, 7, 6832–6839. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, Z.; Ma, C.; Yang, J.; Ma, Z.-F.; Zheng, S. Challenges of Spinel Li4Ti5O12 for Lithium-Ion Battery Industrial Applications. Adv. Energy Mater. 2017, 7, 1601625. [Google Scholar] [CrossRef]
- Xu, G.; Han, P.; Dong, S.; Liu, H.; Cui, G.; Chen, L. Li4Ti5O12-Based Energy Conversion and Storage Systems: Status and Prospects. Coord. Chem. Rev. 2017, 343, 139–184. [Google Scholar] [CrossRef]
- Tian, R.; Park, S.-H.; King, P.J.; Cunningham, G.; Coelho, J.; Nicolosi, V.; Coleman, J.N. Quantifying the Factors Limiting Rate Performance in Battery Electrodes. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Jiang, F.; Peng, P. Elucidating the Performance Limitations of Lithium-Ion Batteries Due to Species and Charge Transport through Five Characteristic Parameters. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.; Lee, H.; Park, S.; Cha, H.; Kim, J.; Cho, J. Improvements to the Overpotential of All-Solid-State Lithium-Ion Batteries during the Past Ten Years. Adv. Energy Mater. 2020, 10, 2000904. [Google Scholar] [CrossRef]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-Ion Batteries: Science and Technologies; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; ISBN 978-0-387-34445-4. [Google Scholar]
- Frackowiak, E.; Béguin, F. Electrochemical Storage of Energy in Carbon Nanotubes and Nanostructured Carbons. Carbon 2002, 40, 1775–1787. [Google Scholar] [CrossRef]
- Che, G.; Lakshmi, B.B.; Fisher, E.R.; Martin, C.R. Carbon Nanotubule Membranes for Electrochemical Energy Storage and Production. Nature 1998, 393, 346–349. [Google Scholar] [CrossRef]
- Fang, S.; Shen, L.; Zhang, X. Application of Carbon Nanotubes in Lithium-Ion Batteries. In Industrial Applications of Carbon Nanotubes; Peng, H., Li, Q., Chen, T., Eds.; Micro and Nano Technologies; Elsevier: Boston, MA, USA, 2017; Chapter 9; pp. 251–276. ISBN 978-0-323-41481-4. [Google Scholar]
- Rynne, O.; Dubarry, M.; Molson, C.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Rochefort, D.; Dollé, M. Designs of Experiments for Beginners—A Quick Start Guide for Application to Electrode Formulation. Batteries 2019, 5, 72. [Google Scholar] [CrossRef]
- He, Y.-B.; Liu, M.; Huang, Z.-D.; Zhang, B.; Yu, Y.; Li, B.; Kang, F.; Kim, J.-K. Effect of Solid Electrolyte Interface (SEI) Film on Cyclic Performance of Li4Ti5O12 Anodes for Li Ion Batteries. J. Power Source 2013, 239, 269–276. [Google Scholar] [CrossRef]
- Westerhoff, U.; Kurbach, K.; Lienesch, F.; Kurrat, M. Analysis of Lithium-Ion Battery Models Based on Electrochemical Impedance Spectroscopy. Energy Technol. 2016, 4, 1620–1630. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, Y.-J.; Wang, G.X. Electrochemical Properties of Carbon Nanotube-Dispersed PEO-LiX Electrolytes. Met. Mater. Int. 2006, 12, 69–73. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
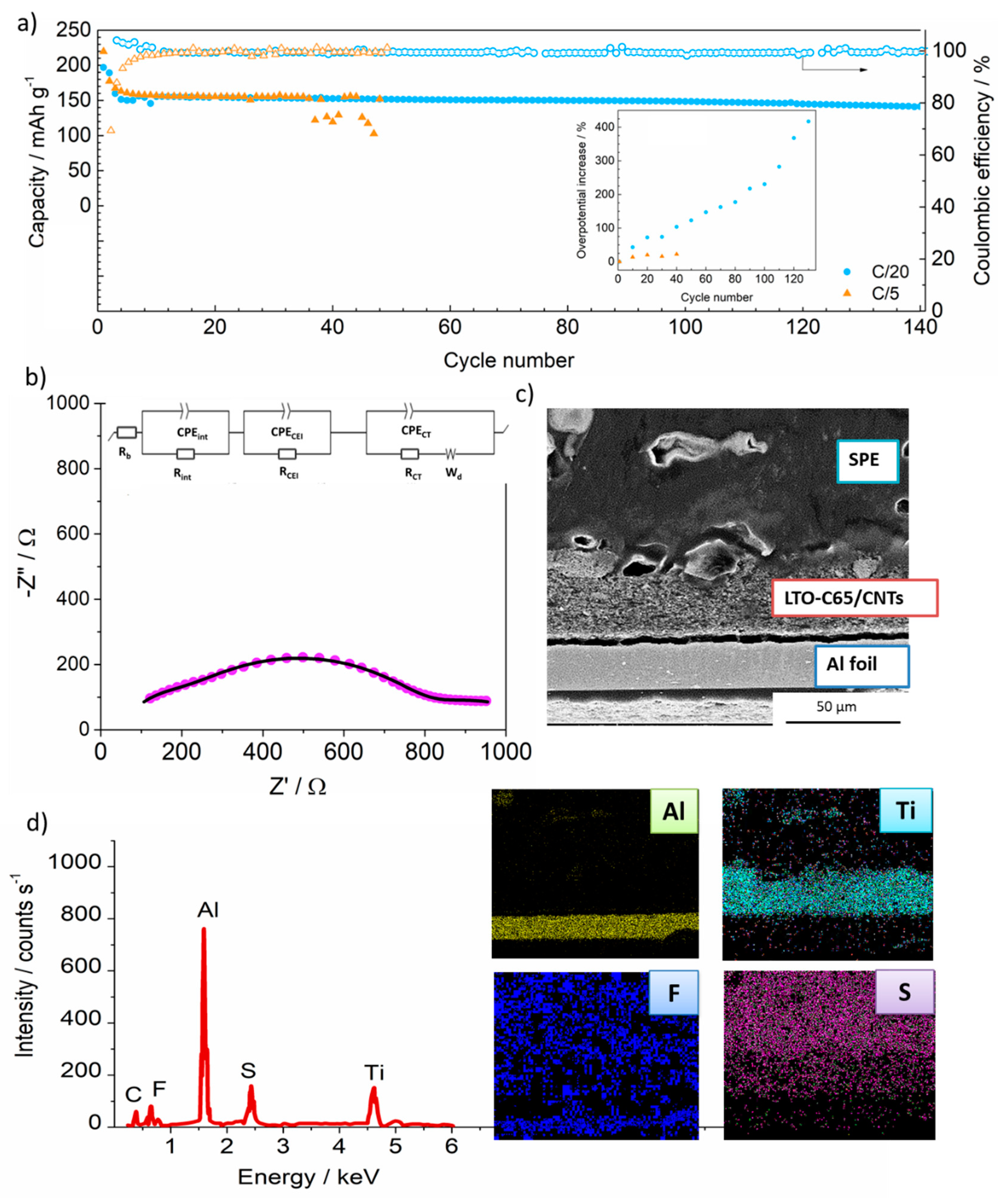
| Sample | Rb | Rint | RCEI | RCT |
|---|---|---|---|---|
| LTO-C65 | 8 | 97 | - | <1 |
| LTO-C65/CNTs | 3 | 54 | - | <1 |
| Cycled (C/20)-LTO-C65/CNTs | 15 | 298 | 394 | 307 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orue Mendizabal, A.; Gomez, N.; Aguesse, F.; López-Aranguren, P. Designing Spinel Li4Ti5O12 Electrode as Anode Material for Poly(ethylene)oxide-Based Solid-State Batteries. Materials 2021, 14, 1213. https://doi.org/10.3390/ma14051213
Orue Mendizabal A, Gomez N, Aguesse F, López-Aranguren P. Designing Spinel Li4Ti5O12 Electrode as Anode Material for Poly(ethylene)oxide-Based Solid-State Batteries. Materials. 2021; 14(5):1213. https://doi.org/10.3390/ma14051213
Chicago/Turabian StyleOrue Mendizabal, Ander, Nuria Gomez, Frédéric Aguesse, and Pedro López-Aranguren. 2021. "Designing Spinel Li4Ti5O12 Electrode as Anode Material for Poly(ethylene)oxide-Based Solid-State Batteries" Materials 14, no. 5: 1213. https://doi.org/10.3390/ma14051213
APA StyleOrue Mendizabal, A., Gomez, N., Aguesse, F., & López-Aranguren, P. (2021). Designing Spinel Li4Ti5O12 Electrode as Anode Material for Poly(ethylene)oxide-Based Solid-State Batteries. Materials, 14(5), 1213. https://doi.org/10.3390/ma14051213






