Lab-Scale Study of Temperature and Duration Effects on Carbonized Solid Fuels Properties Produced from Municipal Solid Waste Components
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Low-Temperature Pyrolysis
2.2.2. Regression Modeling
- Model I—linear equation y(T,t) = a1 + a2T + a3 × t;
- Model II—second-order polynomial equation y(T,t) = a1 + a2 × T + a3 × T2 + a4 × t + a5 × t2;
- Model III—factorial regression equation y(T,t) = a1 + a2 × T + a3 × t + a4 × T × t;
- Model IV—response surface regression equation y(T,t) = a1 + a2 × T + a3 × t + a4 × T2 + a5 × t2 + a6 × T × t.
- y(T,t)—the variable that depends on process temperature and time.
- T—low-temperature pyrolysis process temperature (°C), (300–500 °C).
- t—low-temperature pyrolysis process residence time (min) (20–60 min).
- a1—intercept
- a2–a6—regression coefficients
- determination coefficient;
- —repeated observations;
- —value of the dependent variable predicted by the regression model;
- —mean value of the dependent variable (measured);
- —value of the dependent variable (measured).
- AIC—a value of Akaike analysis;
- n—the number of measurements;
- e—the value of the residuals of the model (defined as the difference between predicted and experimental value);
- K—number of regressions coefficients (including the intercept).
2.2.3. General Model
- —the estimated value of the studied parameter of CSF from a mixture of MSW/RDF at T & t conditions, MJ∙kg−1;
- —the estimated value of the studied parameter of i-CSF from individual MSW/RDF component under T & t conditions, MJ∙kg−1;
- —percentage mass share of i-CSF from individual MSW/RDF component in the total mass of CSF from MSW/RDF mixture, %.
3. Results and Discussion
3.1. Regression Models
3.1.1. Mass Yield of CSF
3.1.2. Energy Densification Ratio of CSF
3.1.3. Energy Yield of CSF
3.1.4. Moisture Content of CSF
3.1.5. Organic Matter Content of CSF
3.1.6. Ash Content of CSF
3.1.7. Combustible Parts of CSF
3.1.8. Carbon Content of CSF
3.1.9. Hydrogen Content of CSF
3.1.10. Nitrogen Content of CSF
3.1.11. Sulfur Content of CSF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| OECD—Organization for Economic Co-operation and Development |
| GDP—gross domestic product |
| MSW—municipal solid waste |
| MBT—mechanical biological treatment plants |
| RDF—refuse-derived fuel |
| CSF—carbonized solid fuel |
| MY—mass yield |
| EDr—energy densification ratio |
| EY—energy yield |
| MC—moisture content |
| VM—volatile matter content |
| FC—fixed carbon content |
| AC—ash content |
| CP—combustible parts content |
| VS—volatile solids content |
| C, H, N, S—elemental composition, carbon, hydrogen, nitrogen, and sulfur, respectively |
| T—pyrolysis temperature |
| t—pyrolysis duration |
| R2—determination coefficient |
| AIC—Akaike Information Criterion |
Appendix A
| Material | Assessment Criterion | Models of MY | Models of EDr | Models of EY | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | I | II | III | IV | ||
| Carton | R2 | 0.63 | 0.69 | 0.69 | 0.77 | 0.02 | 0.16 | 0.02 | 0.16 | 0.64 | 0.68 | 0.69 | 0.76 |
| AIC | 269.52 | 259.99 | 259.80 | 242.24 | −76.53 | −76.14 | −74.64 | −75.86 | 271.89 | 265.23 | 261.11 | 247.88 | |
| Fabric | R2 | 0.64 | 0.70 | 0.68 | 0.78 | 0.52 | 0.54 | 0.53 | 0.55 | 0.66 | 0.73 | 0.71 | 0.81 |
| AIC | 299.28 | 289.46 | 293.17 | 278.17 | −2.44 | −0.07 | −1.00 | 1.34 | 290.23 | 276.34 | 283.67 | 261.86 | |
| Kitchen waste | R2 | 0.66 | 0.72 | 0.74 | 0.85 | 0.52 | 0.54 | 0.53 | 0.55 | 0.66 | 0.71 | 0.73 | 0.81 |
| AIC | 245.66 | 235.55 | 237.73 | 220.75 | 0.07 | 0.06 | 0.05 | 0.04 | 253.88 | 247.01 | 251.22 | 242.35 | |
| Paper | R2 | 0.63 | 0.70 | 0.68 | 0.79 | 0.33 | 0.41 | 0.33 | 0.41 | 0.65 | 0.73 | 0.70 | 0.81 |
| AIC | 257.79 | 248.73 | 245.51 | 226.40 | −72.45 | −72.63 | −70.52 | −70.70 | 271.30 | 260.61 | 262.42 | 243.58 | |
| Plastic | R2 | 0.47 | 0.62 | 0.51 | 0.72 | 0.21 | 0.43 | 0.30 | 0.57 | 0.18 | 0.24 | 0.18 | 0.24 |
| AIC | 312.00 | 302.08 | 302.03 | 283.52 | −15.05 | −21.19 | −16.56 | −28.02 | 320.15 | 319.00 | 322.13 | 320.97 | |
| Rubber | R2 | 0.84 | 0.85 | 0.84 | 0.85 | 0.84 | 0.86 | 0.86 | 0.88 | 0.84 | 0.85 | 0.84 | 0.85 |
| AIC | 256.65 | 259.79 | 257.96 | 261.08 | −44.35 | −44.54 | −46.08 | −46.81 | 273.34 | 275.75 | 274.75 | 277.13 | |
| PAP/AL/PE composite packaging pack | R2 | 0.81 | 0.82 | 0.82 | 0.83 | 0.01 | 0.52 | 0.21 | 0.73 | 0.69 | 0.77 | 0.70 | 0.78 |
| AIC | 253.00 | 243.88 | 253.84 | 252.39 | 8.40 | −11.52 | 2.63 | −28.25 | 281.43 | 274.41 | 281.81 | 274.12 | |
| Wood | R2 | 0.71 | 0.75 | 0.75 | 0.81 | 0.79 | 0.81 | 0.81 | 0.82 | 0.71 | 0.75 | 0.73 | 0.79 |
| AIC | 250.71 | 253.41 | 244.85 | 234.38 | −89.20 | −87.94 | −90.20 | −85.50 | 246.50 | 242.16 | 242.58 | 236.32 | |
| I, II, III, IV—represents linear, second-order polynomial, factorial regression, and quadratic regression equation, respectively | |||||||||||||
| Bold font means model chosen to further analysis | |||||||||||||
| R2—higher = better | |||||||||||||
| AIC—lower = better | |||||||||||||
| Material | Assessment Criterion | Models of MC | Models of OM | Models of AC | Models of CP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | I | II | III | IV | I | II | III | IV | ||
| Carton | R2 | 0.04 | 0.06 | 0.09 | 0.11 | 0.68 | 0.76 | 0.70 | 0.78 | 0.73 | 0.82 | 0.76 | 0.84 | 0.34 | 0.82 | 0.76 | 0.84 |
| AIC | 425.41 | 427.25 | 422.39 | 424.12 | 777.69 | 753.01 | 773.20 | 746.24 | 711.09 | 678.84 | 703.21 | 666.26 | 711.09 | 678.84 | 703.21 | 666.26 | |
| Fabric | R2 | 0.12 | 0.13 | 0.12 | 0.13 | 0.28 | 0.31 | 0.28 | 0.31 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| AIC | 572.20 | 575.01 | 573.90 | 576.71 | 394.74 | 394.50 | 396.29 | 396.03 | 248.23 | 251.34 | 249.77 | 252.87 | 248.23 | 251.34 | 249.77 | 252.87 | |
| Kitchen waste | R2 | 0.08 | 0.09 | 0.09 | 0.10 | 0.41 | 0.42 | 0.48 | 0.49 | 0.34 | 0.35 | 0.38 | 0.40 | 0.34 | 0.35 | 0.38 | 0.40 |
| AIC | 552.21 | 555.48 | 553.32 | 556.59 | 779.34 | 781.83 | 769.64 | 771.94 | 701.54 | 703.77 | 696.17 | 698.26 | 701.54 | 703.77 | 696.17 | 698.26 | |
| Paper | R2 | 0.10 | 0.18 | 0.12 | 0.21 | 0.65 | 0.67 | 0.70 | 0.72 | 0.57 | 0.60 | 0.60 | 0.63 | 0.57 | 0.59 | 0.60 | 0.63 |
| AIC | 474.00 | 468.17 | 473.05 | 466.91 | 835.09 | 832.68 | 821.55 | 818.02 | 795.10 | 793.79 | 789.61 | 787.88 | 795.78 | 794.28 | 790.13 | 788.18 | |
| Plastic | R2 | 0.13 | 0.32 | 0.15 | 0.35 | 0.36 | 0.56 | 0.49 | 0.69 | 0.42 | 0.62 | 0.57 | 0.77 | 0.42 | 0.62 | 0.57 | 0.77 |
| AIC | 228.08 | 207.06 | 227.11 | 205.21 | 1019.27 | 986.02 | 998.46 | 952.73 | 906.47 | 869.28 | 877.36 | 819.24 | 906.47 | 869.28 | 877.36 | 819.24 | |
| Rubber | R2 | 0.02 | 0.03 | 0.04 | 0.05 | 0.81 | 0.83 | 0.82 | 0.84 | 0.76 | 0.77 | 0.76 | 0.78 | 0.76 | 0.77 | 0.76 | 0.78 |
| AIC | 278.67 | 281.69 | 278.24 | 281.23 | 795.10 | 790.14 | 792.82 | 787.44 | 761.22 | 757.62 | 761.65 | 757.92 | 761.22 | 757.62 | 761.65 | 757.92 | |
| PAP/AL/PE composite packaging pack | R2 | 0.09 | 0.49 | 0.09 | 0.49 | 0.72 | 0.82 | 0.73 | 0.83 | 0.79 | 0.85 | 0.79 | 0.86 | 0.79 | 0.85 | 0.79 | 0.86 |
| AIC | 440.29 | 387.26 | 441.96 | 388.67 | 790.44 | 748.72 | 790.20 | 747.14 | 753.19 | 719.22 | 752.86 | 717.78 | 753.19 | 719.22 | 752.86 | 717.78 | |
| Wood | R2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.44 | 0.49 | 0.44 | 0.49 | 0.30 | 0.32 | 0.31 | 0.32 | 0.31 | 0.32 | 0.31 | 0.32 |
| AIC | 526.03 | 529.91 | 528.00 | 531.88 | 602.39 | 596.66 | 604.39 | 598.66 | 556.27 | 558.76 | 557.84 | 560.32 | 556.52 | 558.97 | 558.05 | 560.49 | |
| I, II, III, IV—represents linear, second-order polynomial, factorial regression, and quadratic regression equation, respectively | |||||||||||||||||
| Bold font means model chosen to further analysis | |||||||||||||||||
| R2—higher = better | |||||||||||||||||
| AIC—lower = better | |||||||||||||||||
| Material | Assessment Criterion | Models of C | Models of H | Models of N | Models of S | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | I | II | III | IV | I | II | III | IV | ||
| Carton | R2 | 0.28 | 0.31 | 0.43 | 0.46 | 0.81 | 0.91 | 0.82 | 0.92 | 0.24 | 0.33 | 0.32 | 0.41 | 0.19 | 0.20 | 0.25 | 0.26 |
| AIC | 155.21 | 157.48 | 149.58 | 151.40 | 74.66 | 53.18 | 75.56 | 52.75 | 6.00 | 10.00 | 8.00 | 12.00 | −103.41 | −99.50 | −103.93 | −100.03 | |
| Fabric | R2 | 0.50 | 0.59 | 0.53 | 0.62 | 0.69 | 0.75 | 0.73 | 0.79 | 0.48 | 0.50 | 0.49 | 0.51 | 0.17 | 0.38 | 0.18 | 0.39 |
| AIC | 250.53 | 247.93 | 250.81 | 247.81 | 111.48 | 108.26 | 109.36 | 105.06 | 41.59 | 44.39 | 43.32 | 46.10 | −73.76 | −79.35 | −72.44 | −78.26 | |
| Kitchen waste | R2 | 0.08 | 0.25 | 0.10 | 0.26 | 0.80 | 0.83 | 0.80 | 0.83 | 0.40 | 0.44 | 0.42 | 0.46 | 0.15 | 0.33 | 0.17 | 0.35 |
| AIC | 205.12 | 202.63 | 206.69 | 204.10 | 62.00 | 59.98 | 63.68 | 61.59 | 38.23 | 40.25 | 39.44 | 41.40 | −127.42 | −131.31 | −126.36 | −130.51 | |
| Paper | R2 | 0.05 | 0.08 | 0.21 | 0.24 | 0.79 | 0.85 | 0.82 | 0.88 | 0.53 | 0.68 | 0.54 | 0.69 | 0.08 | 0.43 | 0.14 | 0.49 |
| AIC | 174.17 | 177.10 | 170.06 | 172.75 | 72.24 | 65.33 | 69.46 | 60.55 | −134.97 | −142.61 | −133.25 | −141.02 | −101.24 | −112.91 | −101.59 | −114.79 | |
| Plastic | R2 | 0.43 | 0.55 | 0.59 | 0.72 | 0.39 | 0.49 | 0.55 | 0.66 | 0.09 | 0.12 | 0.10 | 0.13 | 0.44 | 0.64 | 0.58 | 0.78 |
| AIC | 283.75 | 279.63 | 274.50 | 266.42 | 186.60 | 184.37 | 178.12 | 173.23 | 38.18 | 41.14 | 39.94 | 42.90 | −99.17 | −109.64 | −107.19 | −124.84 | |
| Rubber | R2 | 0.68 | 0.68 | 0.68 | 0.69 | 0.79 | 0.90 | 0.79 | 0.91 | 0.26 | 0.29 | 0.35 | 0.38 | 0.19 | 0.19 | 0.26 | 0.28 |
| AIC | 228.13 | 231.84 | 229.86 | 233.57 | 71.31 | 50.69 | 72.62 | 47.99 | −79.03 | −76.19 | −80.69 | −78.01 | −100.17 | −96.35 | −101.41 | −97.92 | |
| PAP/AL/PE composite packaging pack | R2 | 0.00 | 0.34 | 0.30 | 0.63 | 0.54 | 0.67 | 0.58 | 0.72 | 0.17 | 0.21 | 0.17 | 0.21 | 0.13 | 0.51 | 0.24 | 0.61 |
| AIC | 238.66 | 229.32 | 229.18 | 212.18 | 132.35 | 124.97 | 131.10 | 122.22 | −73.69 | −71.25 | −71.78 | −69.34 | −135.93 | −150.21 | −137.99 | −155.79 | |
| Wood | R2 | 0.63 | 0.64 | 0.70 | 0.72 | 0.63 | 0.64 | 0.70 | 0.72 | 0.32 | 0.35 | 0.44 | 0.46 | 0.07 | 0.32 | 0.08 | 0.33 |
| AIC | 171.08 | 173.86 | 166.00 | 168.49 | 171.08 | 173.86 | 166.00 | 168.49 | −2.69 | 0.29 | −6.17 | 3.18 | −170.48 | −176.61 | −168.86 | −175.00 | |
| I, II, III, IV—represent linear, second-order polynomial, factorial regression, and quadratic regression equation, respectively | |||||||||||||||||
| Bold font means the model chosen to further analysis | |||||||||||||||||
| R2—higher = better | |||||||||||||||||
| AIC—lower = better | |||||||||||||||||
References
- Mavropoulos, A. Waste Management 2030+. Available online: https://waste-management-world.com/a/waste-management (accessed on 13 December 2020).
- Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300. [Google Scholar] [CrossRef]
- Bagheri, M.; Esfilar, R.; Sina Golchi, M.; Kennedy, C.A. Towards a circular economy: A comprehensive study of higher heat values and emission potential of various municipal solid wastes. Waste Manag. 2020, 101, 210–221. [Google Scholar] [CrossRef]
- Kaczor, Z.; Buliński, Z.; Werle, S. Modelling approaches to waste biomass pyrolysis: A review. Renew. Energy 2020, 159, 427–443. [Google Scholar] [CrossRef]
- Stępień, P.; Pulka, J.; Serowik, M.; Białowiec, A. Thermogravimetric and calorimetric characteristics of alternative fuel in terms of its use in low-temperature pyrolysis. Waste Biomass Valori. 2019, 10, 1669–1677. [Google Scholar] [CrossRef]
- Ayeleru, O.O.; Dlova, S.; Akinribide, O.J.; Ntuli, F.; Kupolati, W.K.; Marina, P.F.; Blencowe, A.; Olubambi, P.A. Challenges of plastic waste generation and management in sub-Saharan Africa: A review. Waste Manag. 2020, 110, 24–42. [Google Scholar] [CrossRef]
- Buczma, S.R. Fighting waste crime: Legal and practical challenges: What lesson has been learned more than ten years after the adoption of Directive 2008/99? Era Forum 2020. [Google Scholar] [CrossRef]
- Levoyannis, C. The EU Green Deal and the Impact on the Future of Gas and Gas Infrastructure in the European Union. In Aspects of the Energy Union: Application and Effects of European Energy Policies in SE Europe and Eastern Mediterranean; Mathioulakis, M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 201–224. ISBN 978-3-030-55981-6. [Google Scholar] [CrossRef]
- Herrero, M.; Laca, A.; Laca, A.; Díaz, M. Application of life cycle assessment to food industry wastes. Food Ind. Wastes 2020, 331–353. [Google Scholar] [CrossRef]
- Pires, A.; Martinho, G.; Rodrigues, S.; Gomes, M.I. Preparation for Reusing, Recycling, Recovering, and Landfilling: Waste Hierarchy Steps After Waste Collection. In Sustainable Solid Waste Collection and Management; Springer International Publishing: Cham, Switzerland, 2019; pp. 45–59. ISBN 978-3-319-93200-2. [Google Scholar] [CrossRef]
- Stȩpień, P.; Serowik, M.; Koziel, J.A.; Białowiec, A. Waste to carbon: Estimating the energy demand for production of carbonized refuse-derived fuel. Sustainability 2019, 11, 5685. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J. The RDF/SRF torrefaction: An effect of temperature on characterization of the product—Carbonized refuse derived fuel. Waste Manag. 2017, 70, 91–100. [Google Scholar] [CrossRef]
- Jewiarz, M.; Mudryk, K.; Wróbel, M.; Frączek, J.; Dziedzic, K. Parameters affecting RDF-based pellet quality. Energies 2020, 13, 910. [Google Scholar] [CrossRef]
- Świechowski, K.; Syguła, E.; Koziel, J.A.; Stępień, P.; Kugler, S.; Manczarski, P.; Białowiec, A. Low-temperature pyrolysis of municipal solid waste components and refuse-derived fuel—Process efficiency and fuel properties of carbonized solid fuel. Data 2020, 5, 48. [Google Scholar] [CrossRef]
- Swiechowski, K.; Stegenta-Dabrowska, S.; Liszewski, M.; Babelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree pruned biomass torrefaction: Process kinetics. Materials. 2019, 12, 3334. [Google Scholar] [CrossRef] [PubMed]
- Stępień, P.; Świechowski, K.; Hnat, M.; Kugler, S.; Stegenta-Dąbrowska, S.; Koziel, J.A.; Manczarski, P.; Białowiec, A. Waste to carbon: Biocoal from elephant dung as new cooking fuel. Energies 2019, 12, 4344. [Google Scholar] [CrossRef]
- Syguła, E.; Koziel, J.A.; Białowiec, A. Proof-of-concept of spent mushrooms compost torrefaction—Studying the process kinetics and the influence of temperature and duration on the calorific value of the produced biocoal. Energies 2019, 12, 3060. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Longo, A.; Vilarinho, C.; Gonçalves, M. Torrefaction and carbonization of refuse derived fuel: Char characterization and evaluation of gaseous and liquid emissions. Bioresour. Technol. 2019, 285, 121325. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Lin, J.; Ma, R.; Luo, J.; Sun, S.; Cui, C.; Fang, L.; Huang, H. Microwave pyrolysis of food waste for high-quality syngas production: Positive effects of a CO2 reaction atmosphere and insights into the intrinsic reaction mechanisms. Energy Convers. Manag. 2020, 206, 112490. [Google Scholar] [CrossRef]
- Wang, L.; Ni, H.; Zhang, J.; Shi, Q.; Zhang, R.; Yu, H.; Li, M. Enzymatic treatment improves fast pyrolysis product selectivity of softwood and hardwood lignin. Sci. Total Environ. 2020, 717, 137241. [Google Scholar] [CrossRef]
- Basu, P. Chapter 2—Biomass Characteristics. In Biomass Gasification and Pyrolysis; Basu, P., Ed.; Academic Press: Boston, MA, USA, 2010; pp. 27–63. ISBN 978-0-12-374988-8. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; De Oliveira Matias, J.C.; Da Silva Catalão, J.P. Chapter 1—Introduction. In Torrefaction of Biomass for Energy Applications; Nunes, L.J.R., De Oliveira Matias, J.C., Da Silva Catalão, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–43. ISBN 978-0-12-809462-4. [Google Scholar] [CrossRef]
- Municipal Solid Waste. Analysis of Combustible And Non-Combustible Content, PN-Z-15008-04:1993 Standard. Available online: https://sklep.pkn.pl/pn-z-15008-03-1993p.html (accessed on 13 December 2020).
- Waste Characteristics. Determination of Organic Matter Content for Waste, Slurry and Sludge, PN-EN 15169:2011 Standard. Available online: https://sklep.pkn.pl/pn-en-15169-2011p.html?options=cart (accessed on 13 December 2020).
- Determination of the Higher Heating Value and the Lower Heating Value, PN-G-04513:1981 Standard. Solid Fuels. Available online: https://sklep.pkn.pl/pn-z-15008-04-1993p.html?options=cart (accessed on 13 December 2020).
- Caillat, S.; Vakkilainen, E. Large-scale biomass combustion plants: An overview. Sci. Technol. Eng. 2013, 189–224. [Google Scholar] [CrossRef]
- Stegenta, S.; Kałdun, B.; Białowiec, A. Model selection and estimation of kinetic parameters of oxygen consumption during biostabilization of under-size fraction of municipal solid waste. Rocz. Ochr. Środowiska 2016, 18, 800–814. Available online: https://www.researchgate.net/publication/328601322_Model_selection_and_estimation_of_kinetic_parameters_of_oxygen_consumption_during_biostabilization_of_under-size_fraction_of_municipal_solid_waste (accessed on 13 December 2020).
- Białowiec, A. Innovations in Waste Management, Selected Issues; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2018; ISBN 9788377172780. [Google Scholar]
- Özyuǧ Uran, A.; Yaman, S. Prediction of Calorific Value of Biomass from Proximate Analysis. Energy Procedia 2017, 107, 130–136. [Google Scholar] [CrossRef]
- Hosokai, S.; Matsuoka, K.; Kuramoto, K.; Suzuki, Y. Modification of Dulong’s formula to estimate heating value of gas, liquid and solid fuels. Fuel Process. Technol. 2016, 152, 399–405. [Google Scholar] [CrossRef]
- Demirbas, A.; Ak, N.; Aslan, A.; Sen, N. Calculation of higher heating values of hydrocarbon compounds and fatty acids. Pet. Sci. Technol. 2018, 36, 712–717. [Google Scholar] [CrossRef]
- Akkaya, E.; Demir, A. Predicting the heating value of municipal solid waste-based materials: An artificial neural network model. Energy Sources Part A Recover. Util. Environ. Eff. 2010, 32, 1777–1783. [Google Scholar] [CrossRef]
- Tiikma, L.; Tamvelius, H.; Luik, L. Coprocessing of heavy shale oil with polyethylene waste. J. Anal. Appl. Pyrolysis 2007, 79, 191–195. [Google Scholar] [CrossRef]
- Nobre, C.; Vilarinho, C.; Alves, O.; Mendes, B.; Goncalves, M. Upgrading of refuse derived fuel through torrefaction and carbonization: Evaluation of RDF char fuel properties. Energy 2019, 181, 66–76. [Google Scholar] [CrossRef]
- Hanif, M.U.; Capareda, S.C.; Iqbal, H.; Arazo, R.O.; Anwar Baig, M. Effects of pyrolysis temperature on product yields and energy recovery from co-feeding of cotton gin trash, cow manure, and microalgae: A simulation study. PLoS ONE 2016, e0152230. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Haykiri, H.; Kurt, G.; Yaman, S. Properties of biochars obtained from RDF by carbonization: Influences of devolatilization severity. Waste Biomass Valorization 2017, 8, 539–547. [Google Scholar] [CrossRef]
- Huo, H.; Ma, Y. TG/DTA—FTIR study on total resource recovery from Tetra Pak waste by pyrolysis under a CO2 environment. Prog. React. Kinet. Mech. 2018, 43, 229–235. [Google Scholar] [CrossRef]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-specific enhancement of the carbon−carbon dioxide (Boudouard) reaction. J. Phys. Chem. 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Muley, P.D.; Henkel, C.; Abdollahi, K.K.; Marculescu, C.; Boldor, D. A critical comparison of pyrolysis of cellulose, lignin, and pine sawdust using an induction heating reactor. Energy Convers. Manag. 2016, 117, 273–280. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Cheung, W.H.; Mckay, G. Tyre char preparation from waste tyre rubber for dye removal from effluents. J. Hazard. Mater. 2010, 175, 151–158. [Google Scholar] [CrossRef]
- Acosta, R.; Tavera, C.; Gauthier-Maradei, P.; Nabarlatz, D. Production of oil and char by intermediate pyrolysis of scrap tyres: Influence on yield and product characteristics. Int. J. Chem. React. Eng. 2015, 13, 189–200. [Google Scholar] [CrossRef]
- Zeng, K.; Pham Minh, D.; Gauthier, D.; Weiss-Hortala, E.; Nzihou, A.; Flamant, G. The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood. Bioresour. Technol. 2015, 182, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Ho Kim, K.; Kim, J.; Cho, T.; Weon Choi, J. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.; Chen, W.; Xie, Y.; Liu, Z.; Chang, J.-S. Torrefaction performance and energy usage of biomass wastes and their correlations with torrefaction severity index. Appl. Energy 2018, 220, 598–604. [Google Scholar] [CrossRef]
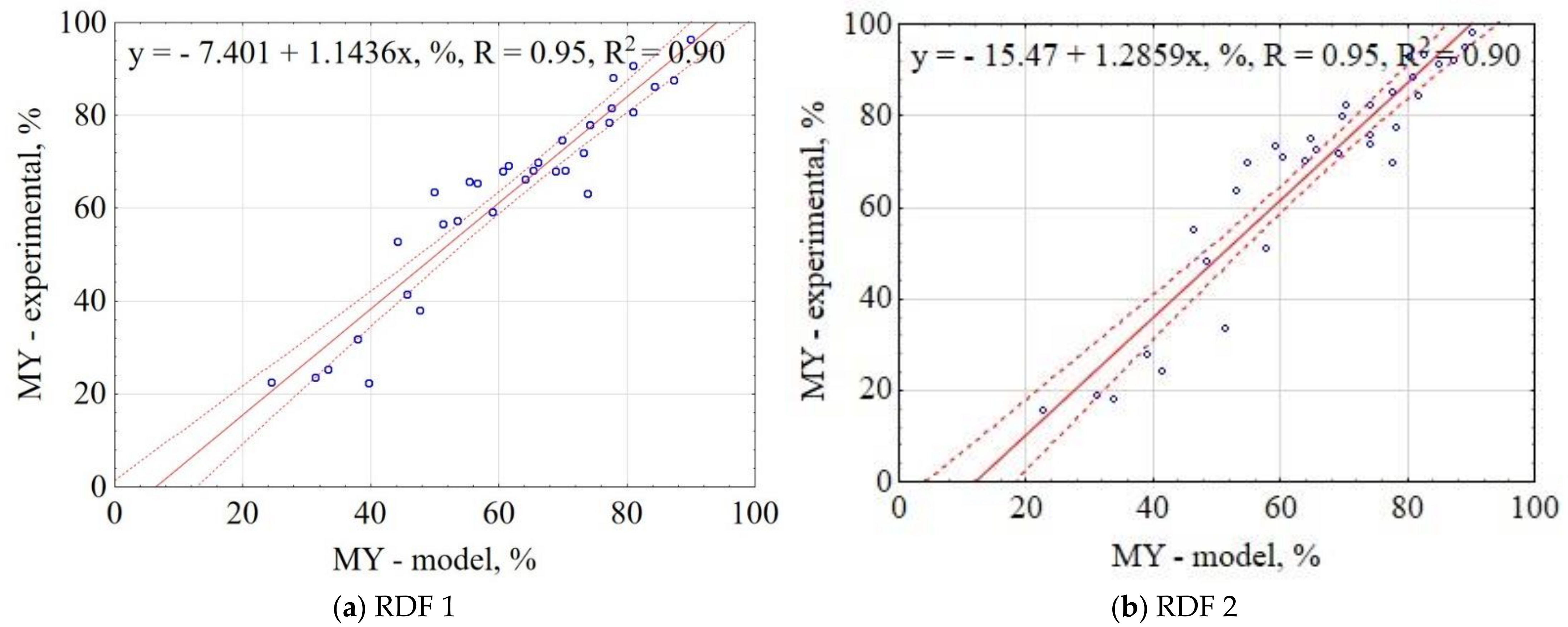

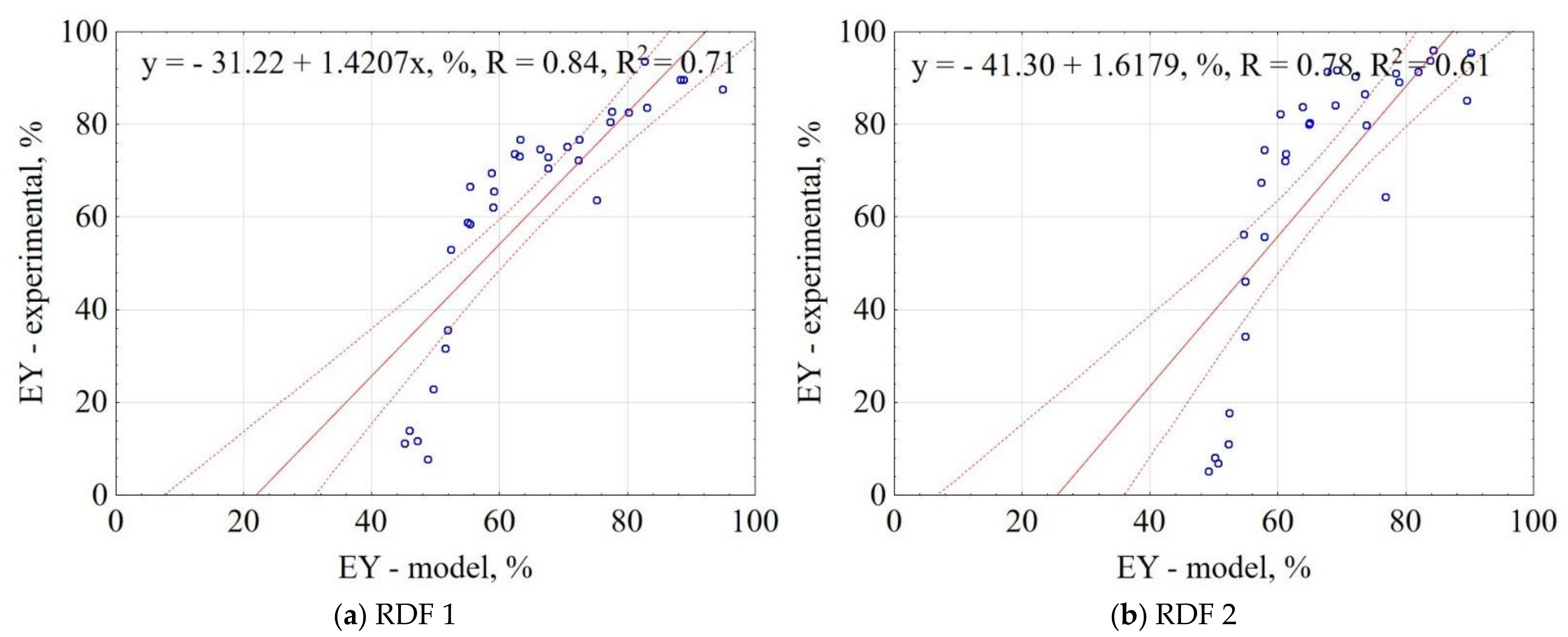
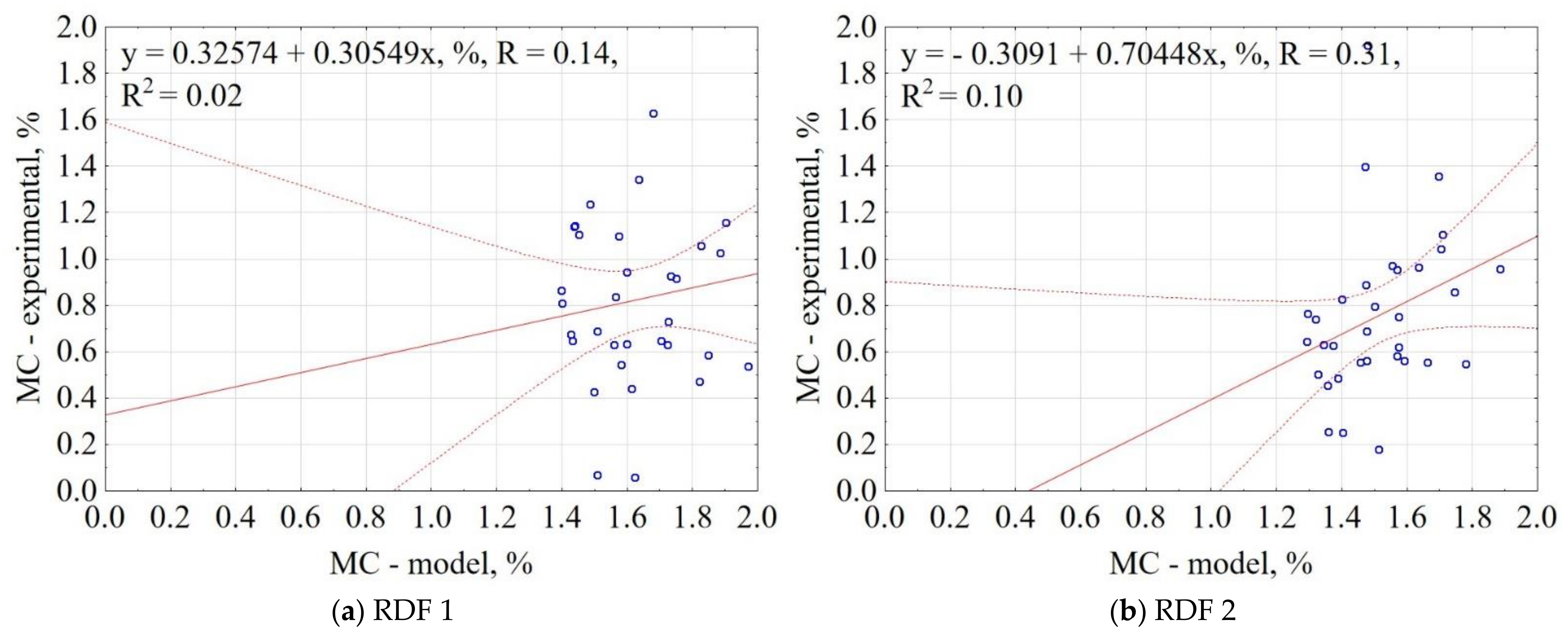

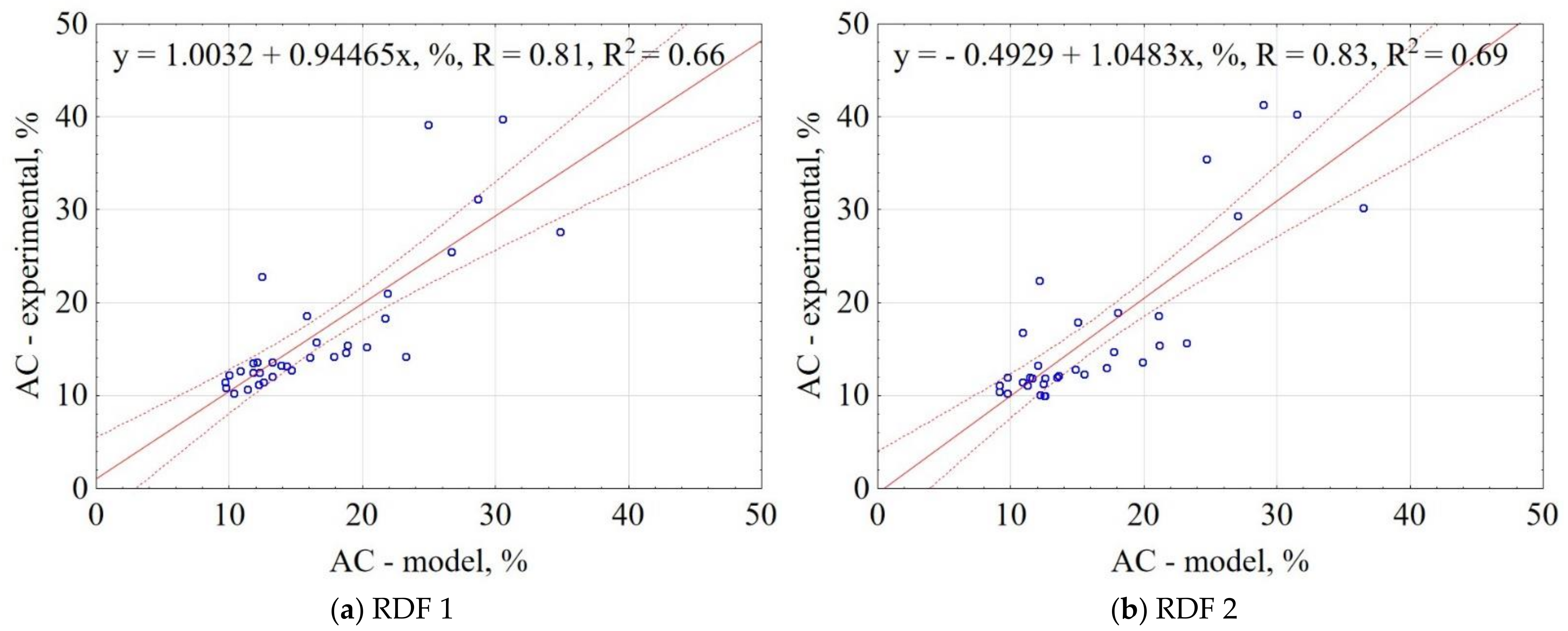

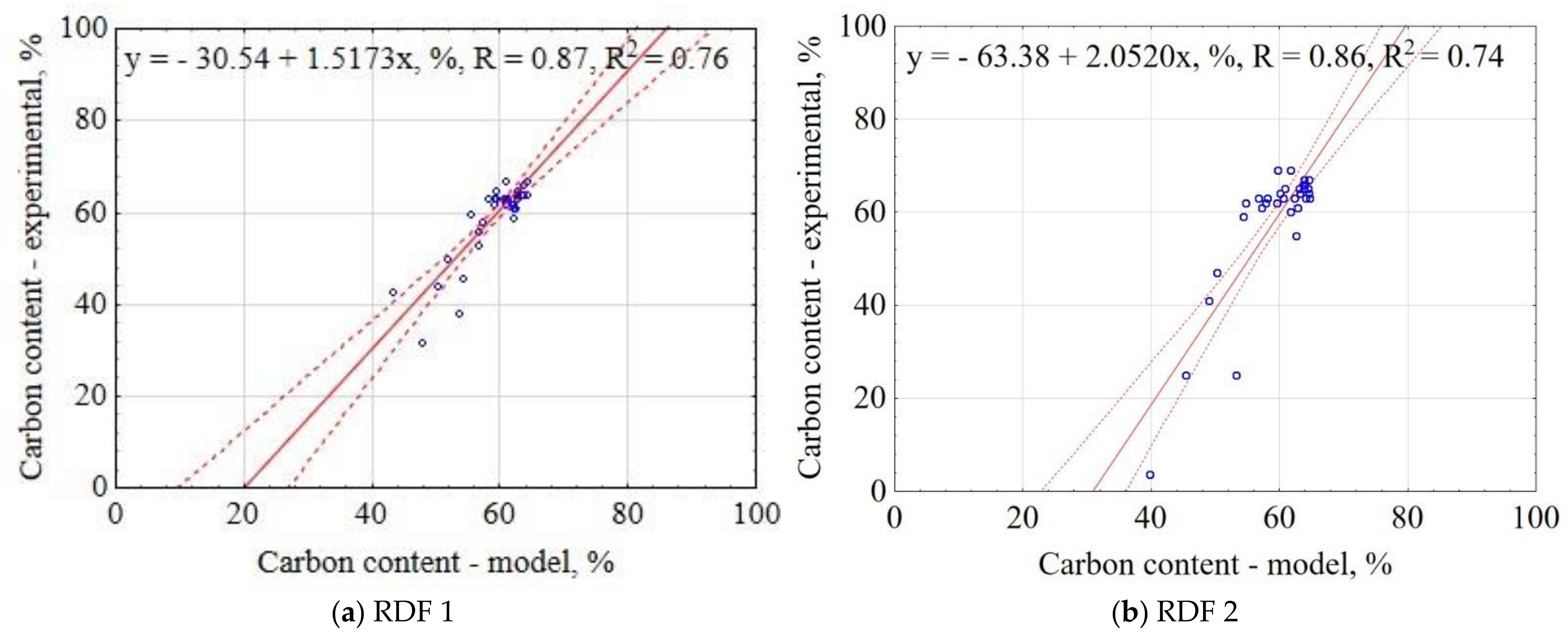
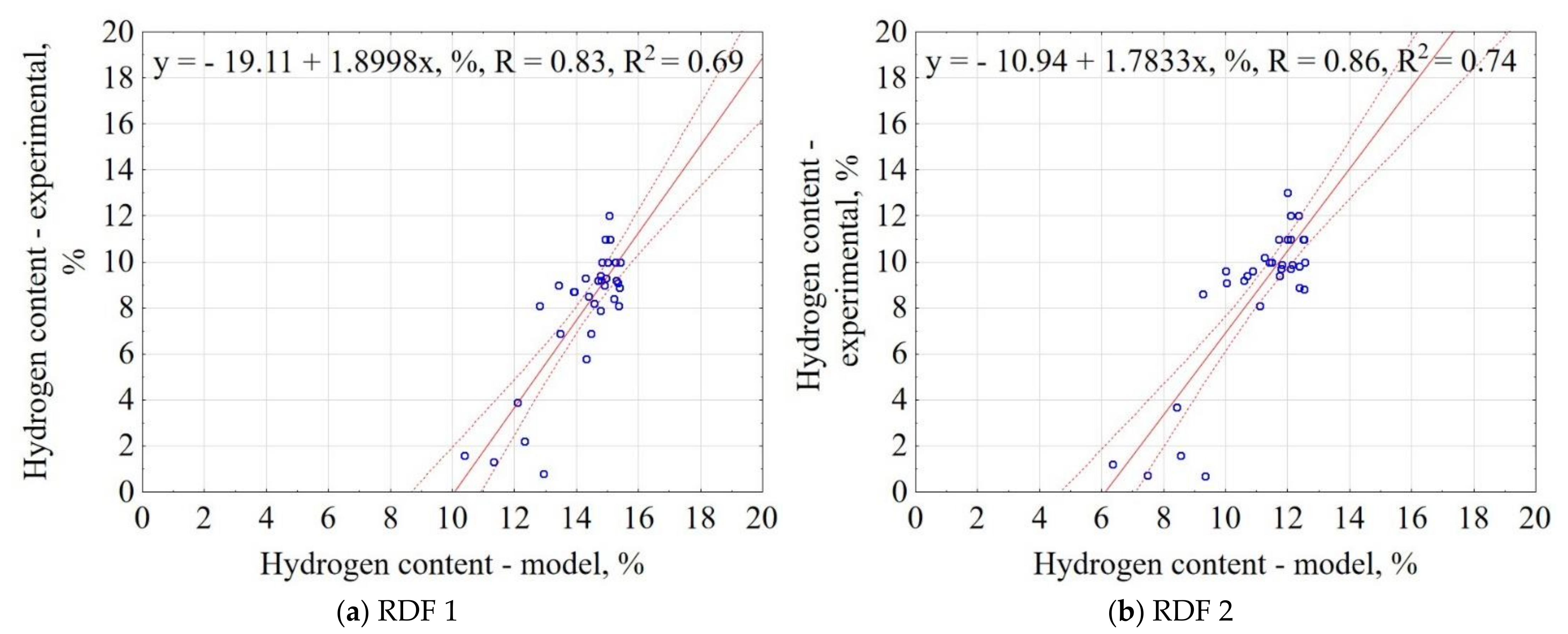

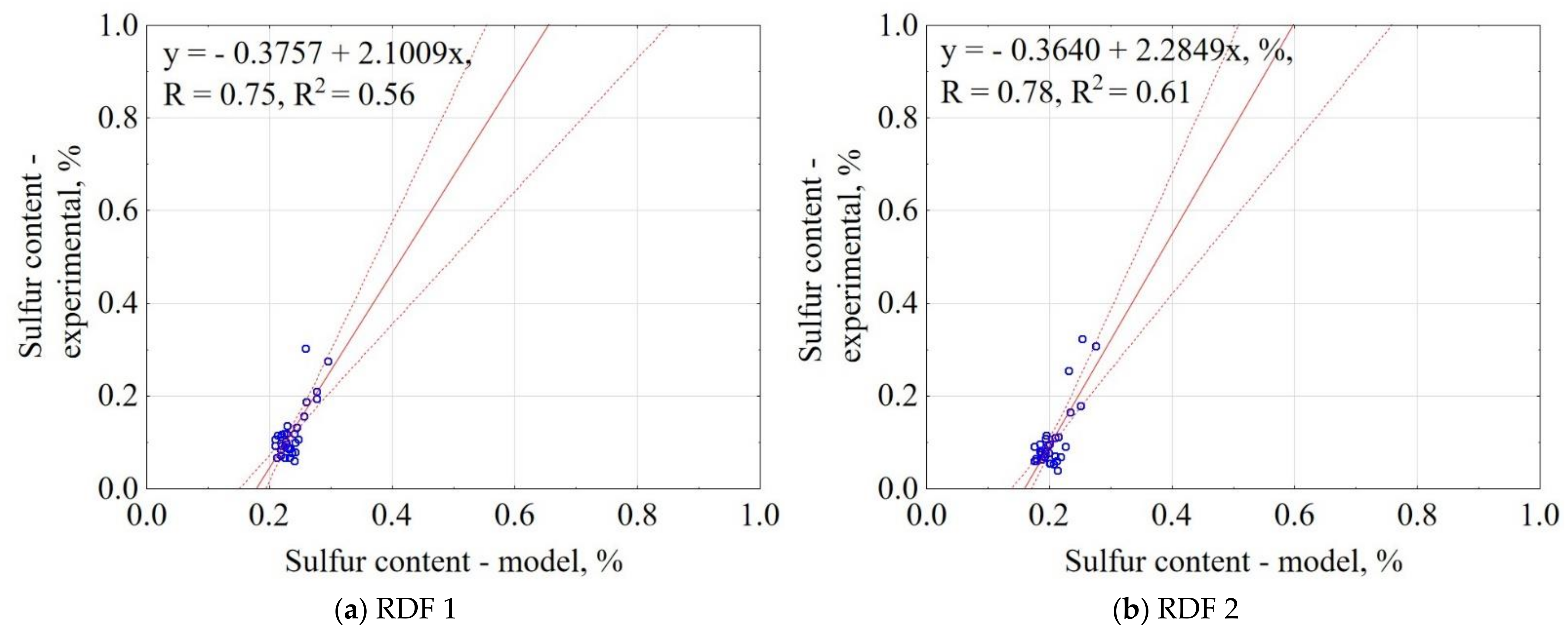
| Material | Equation | R2 |
|---|---|---|
| Carton | MY(T,t) = 453.833 − 1.49491 × T − 3.47577 × t + 0.00137504 × T2 + 0.0133644 × t2 + 0.00499467 × T × t | 0.77 |
| Fabric | MY(T,t) = 661.088 − 2.36376 × T − 4.65892 × t + 0.00223887 × T2 + 0.0187874 × t2 + 0.00669526 × T × t | 0.78 |
| Kitchen waste | MY(T,t) = 337.338 − 1.1419 × T − 1.70984 × t + 0.00109174 × T2 + 0.00214157 × t2 + 0.00324575 × T × t | 0.85 |
| Paper | MY(T,t) = 395.322 − 1.30458 × T − 2.76693 × t + 0.00120622 × T2 + 0.0079086 × t2 + 0.00453242 × T × t | 0.79 |
| Plastic | MY(T,t) = −390.144 + 2.47977 × T + 2.62558 × t − 0.00295447 × T2 + 0.0075406 × t2 − 0.00959024 × T × t | 0.72 |
| Rubber | MY(T,t) = 216.783 − 0.27841 × T − 5.73357∙10−5 × T2 − 0.961876 × t + 0.00652372 × t2 | 0.85 |
| PAP/AL/PE composite packaging pack | MY(T,t) = 268.722 − 0.611037 × T − 1.96903 × t + 0.000373715 × T2 + 0.0131861 × t2 + 0.00132244 × T × t | 0.83 |
| Wood | MY(T,t) = 341.093 − 1.08665 × T − 2.14508 × t + 0.000978011 × T2 + 0.00776588 × t2 + 0.00316288 × T × t | 0.81 |
| Material | Equation | R2 |
|---|---|---|
| Carton | EDr(T,t) = 0.521138 + 0.0024532 × T + 0.00542369 × t − 3.07672 × 10−6 × T2 − 8.29299 × 10−5 × t2 + 2.76737 × 10−6 × T × t | 0.16 |
| Fabric | EDr(T,t) = −0.00672325 + 0.00222108 × T + 0.0223182 × t + 1.32688 × 10−6 × T2 − 0.000176585 × t2 − 1.9214 × 10−5 × T × t | 0.55 |
| Kitchen waste | EDr(T,t) = 0.119321 + 0.00462755 × T + 0.00777896 × t − 4.13357∙10−6 × T2 + 3.05844∙10−5 × t2 − 2.39844∙10−5 × T × t | 0.55 |
| Paper | EDr(T,t) = 1.9716 − 0.00364838 × T − 0.00723548 × t + 3.79197 × 10−6 × T2 + 6.59619∙10−5 × t2 + 2.2538 × 10−6 × T × t | 0.41 |
| Plastic | EDr(T,t) = −2.65302 + 0.0183674 × T + 0.0136888 × t − 2.16719 × 10−5 × T2 + 9.6406∙10−5 × t2 − 6.10462 × 10−5 × T × t | 0.57 |
| Rubber | EDr(T,t) = 0.862326 + 0.00302199 × T − 0.00264502 × t − 5.80671∙10−6 × T2 + 0.000101288 × t2 − 2.56826 × 10−5 × T × t | 0.88 |
| PAP/AL/PE composite packaging pack | EDr(T,t) = −5.71804 + 0.0327167 × T + 0.0298075 × t − 3.66779 × 10−5 × T2 + 2.0243 × 10−5 × t2 − 8.0066 × 10−5 × T × t | 0.73 |
| Wood | EDr(T,t) = 0.0728126 + 0.0040324 × T + 0.00837479 × t − 2.97147 × 10−6 × T2 − 2.61616 × 10−5 × t2 − 1.17289 × 10−5 × T × t | 0.82 |
| Material | Equation | R2 |
|---|---|---|
| Carton | EY(T,t) = 463.255 − 1.50599 × T − 3.38498 × t + 0.00135398 × T2 + 0.00995947 × t2 + 0.00536615 × T × t | 0.76 |
| Fabric | EY(T,t) = 644.004 − 2.29865 × T − 4.12665 × t + 0.00222253 × T2 + 0.0151381 × t2 + 0.00597733 × T × t | 0.81 |
| Kitchen waste | EY(T,t) = 350.058 − 1.14163 × T − 1.55505 × t + 0.0011033 × T2 + 0.00316085 × t2 + 0.00261416 × T × t | 0.81 |
| Paper | EY(T,t) = 473.744 − 1.61526 × T − 3.26723 × t + 0.0015321 × T2 + 0.0111973 × t2 + 0.00497287 × T × t | 0.81 |
| Plastic | EY(T,t) = −9.37476 + 0.90073 × T − 0.00130925 × T2 − 2.83805 × t + 0.0328112 × t2 | 0.24 |
| Rubber | EY(T,t) = 264.858 − 0.388561 × T − 5.14248∙10−5 × T2 − 1.56949 × t + 0.0114869∙t2 | 0.85 |
| PAP/AL/PE composite packaging pack | EY(T,t) = −53.6102 + 1.01489 × T − 0.678159 × t − 0.00150398 × T2 + 0.0150335 × t2 − 0.00239616 × T × t | 0.78 |
| Wood | EY(T,t) = 311.037 − 0.913675 × T − 1.84836 × t + 0.00080429 × T2 + 0.00715877 × t2 + 0.00261271 × T × t | 0.79 |
| Material | Equation | R2 |
|---|---|---|
| Carton | MC(T,t) = 4.45356 − 0.0162423 × T + 0.0509884 × t + 2.81284∙10−5 × T2 + 0.000382481 × t2 − 0.000179923 × T × t | 0.11 |
| Fabric | MC(T,t) = −9.21332 + 0.0470837 × T − 5.06548∙10−5 × T2 + 0.0599597 × t − 0.000364179 × t2 | 0.13 |
| Kitchen waste | MC(T,t) = −3.55952 + 0.0185074 × T + 0.116747 × t − 6.46219 × 10−6 × T2 − 0.000707797 × t2 − 0.000144938 × T × t | 0.10 |
| Paper | MC(T,t) = 21.8367 − 0.0859335 × T − 0.0675578 × t + 9.25108∙10−5 × T2 − 0.000173046 × t2 + 0.000177062 × T × t | 0.21 |
| Plastic | MC(T,t) = 8.3711 − 0.0361188 × T − 0.0326103 × t + 4.02833 × 10−5 × T2 + 0.00016858 × t2 + 5.13878 × 10−5 × T × t | 0.35 |
| Rubber | MC(T,t) = 2.14313 − 0.00478142 × T − 0.0373156 × t + 3.77281 × 10−6 × T2 + 0.000197849 × t2 + 6.01358 × 10−5 × T × t | 0.05 |
| PAP/AL/PE composite packaging pack | MC(T,t) = 29.7302 − 0.135813 × T + 0.000164348 × T2 − 0.053865 × t + 0.000720295 × t2 | 0.49 |
| Wood | MC(T,t) = 0.992658 + 0.00451654 × T + 0.00649111∙t | 0.05 |
| Material | Equation | R2 |
|---|---|---|
| Carton | OM(T,t) = 211.614 − 0.475308 × T − 1.51295 × t + 0.000401745 × T2 + 0.0108101 × t2 + 0.00120767 × T × t | 0.78 |
| Fabric | OM(T,t) = 99.7307 + 0.00951611 × T − 2.04132 × 10−5 × T2 − 0.0610921 × t + 0.000676729 × t2 | 0.31 |
| Kitchen waste | OM(T,t) = 114.173 − 0.0560588 × T − 0.923412 × t − 9.10507 × 10−5 × T2 + 0.00277531 × t2 + 0.00161421 × T × t | 0.49 |
| Paper | OM(T,t) = 228.645 − 0.608106 × T − 1.07174 × t + 0.000466622 × T2 − 0.000843949 × t2 + 0.00244208 × T × t | 0.72 |
| Plastic | OM(T,t) = −370.952 + 2.24812 × T + 2.39959 × t − 0.00265716 × T2 + 0.00200287 × t2 − 0.00736942 × T × t | 0.69 |
| Rubber | OM(T,t) = 54.0888 + 0.236418 × T + 0.269375 × t − 0.000443141 × T2 − 0.00150753 × t2 − 0.00107728 × T × t | 0.84 |
| PAP/AL/PE composite packaging pack | OM(T,t) = −9.35866 + 0.604654 × T − 0.329619 × t − 0.000867447 × T2 + 0.00510722 × t2 − 0.000765698 × T × t | 0.83 |
| Wood | OM(T,t) = 104.571 − 0.00912015 × T − 1.92896 × 10−5 × T2 − 0.321397 × t + 0.00329522 × t2 | 0.49 |
| Material | Equation | R2 |
|---|---|---|
| Carton | AC(T,t) = −95.544 + 0.392259 × T + 1.24361 × t − 0.000333039 × T2 − 0.00830639 × t2 − 0.0010555 × T × t | 0.84 |
| Fabric | AC(T,t) = 0.127194 − 0.00312043 × T + 8.32241 × 10−6 × T2 + 0.012343 × t − 7.1599 × 10−5 × t2 | 0.35 |
| Kitchen waste | AC(T,t) = −10.3584 + 0.0434269 × T + 0.612167 × t + 2.97628 × 10−5 × T2 − 0.00231362 × t2 − 0.000874366 × T × t | 0.40 |
| Paper | AC(T,t) = −93.6731 + 0.428111 × T + 0.630943 × t − 0.000347455 × T2 + 0.000742667 × t2 − 0.00141276 × T × t | 0.63 |
| Plastic | AC(T,t) = 285.315 − 1.33859 × T − 1.72824 × t + 0.00156606 × T2 + 0.00119026 × t2 + 0.00477206 × T × t | 0.77 |
| Rubber | AC(T,t) = 31.3507 − 0.160593 × T − 0.224709 × t + 0.000313069 × T2 + 0.00295488 × t2 + 0.000553607 × T × t | 0.78 |
| PAP/AL/PE composite packaging pack | AC(T,t) = 68.7329 − 0.416816 × T + 0.333945 × t + 0.000643374 × T2 − 0.00512668 × t2 + 0.000646058 × T × t | 0.86 |
| Wood | AC(T,t) = −0.0557718 + 0.000621897 × T + 1.61345 × 10−5 × T2 + 0.120489 × t − 0.00101243 × t2 | 0.32 |
| Material | Equation | R2 |
|---|---|---|
| Carton | CP(T,t) = 195.544 − 0.392259 × T−1.24361 × t + 0.000333039 × T2 + 0.00830639 × t2 + 0.0010555 × T × t | 0.84 |
| Fabric | CP(T,t) = 99.8728 + 0.00312028 × T − 8.32223 × 10−6 × T2 − 0.012343 × t + 7.1599∙10−5 × t2 | 0.35 |
| Kitchen waste | CP(T,t) = 110.358 − 0.0434266 × T − 0.612168 × t − 2.97632 × 10−5 × T2 + 0.00231363 × t2 + 0.000874366 × T × t | 0.40 |
| Paper | CP(T,t) = 194.858 − 0.433983 × T − 0.632625 × t + 0.000354099 × T2 − 0.000849486 × t2 + 0.0014323 × T × t | 0.63 |
| Plastic | CP(T,t) = −185.315 + 1.33859 × T + 1.72824 × t − 0.00156606 × T2 − 0.00119026 × t2 − 0.00477206 × T × t | 0.77 |
| Rubber | CP(T,t) = 68.6493 + 0.160593 × T + 0.224709 × t − 0.000313069 × T2 − 0.00295489 × t2 − 0.000553607 × T × t | 0.78 |
| PAP/AL/PE composite packaging pack | CP(T,t) = 31.2671 + 0.416816 × T − 0.333945 × t − 0.000643374 × T2 + 0.00512668 × t2 − 0.000646058 × T × t | 0.86 |
| Wood | CP(T,t) = 100.096 − 0.000577691 × T−1 × 62655∙10−5 × T2 − 0.122004 × t + 0.00102758 × t2 | 0.32 |
| Material | Equation | R2 |
|---|---|---|
| Carton | C(T,t) = 14.9394 + 0.110664 × T + 0.27 × t − 9.22688 × 10−5 × T2 + 0.001 × t2 − 0.000734091 × T × t | 0.46 |
| Fabric | C(T,t) = −139 + 0.776403 × T + 1.32727 × t − 0.000759518 × T2 − 0.00727273 × t2 − 0.00154545 × T × t | 0.62 |
| Kitchen waste | C(T,t) = 111.158 − 0.329169 × T + 0.342273 × t + 0.00041453 × T2 − 0.00179546 × t2 − 0.000393182 × T × t | 0.26 |
| Paper | C(T,t) = 43.697 − 0.0542969 × T + 0.429545 × t + 0.00010878 × T2 − 0.000568175 × t2 − 0.000886363 × T × t | 0.24 |
| Plastic | C(T,t) = −207.545 + 1.34185 × T + 2.12727 × t − 0.0015472 × T2 + 9.26989 × 10−10 × t2 − 0.00609091 × T × t | 0.72 |
| Rubber | C(T,t) = 118.755 − 0.131543 × T + 0.118908 × t + 6.15577 × 10−5 × T2 − 0.00221363 × t2 − 0.000437044 × T × t | 0.69 |
| PAP/AL/PE composite packaging pack | C(T,t) = −144.424 + 0.858967 × T + 1.63864 × t − 0.000925602 × T2 − 0.00511364 × t2 − 0.00310227 × T × t | 0.63 |
| Wood | C(T,t) = 43.6364 − 0.0147981 × T + 0.523864 × t + 0.000107323 × T2 − 0.00073864 × t2 − 0.000903409 × T × t | 0.72 |
| Material | Equation | R2 |
|---|---|---|
| Carton | H(T,t) = 25.7364 − 0.0817727 × T − 0.156818 × t + 7.95455 × 10−5 × T2 + 0.00120455 × t2 + 8.63636 × 10−5 × T × t | 0.92 |
| Fabric | H(T,t) = 34.3758 − 0.114567 × T − 0.164091 × t + 0.000107906 × T2 + 6.81809 × 10−5 × t2 + 0.000285227 × T × t | 0.79 |
| Kitchen waste | H(T,t) = 15.6939 − 0.041657 × T + 3.73932 × 10−5 × T2 − 0.0625 × t + 0.000534091 × t2 | 0.83 |
| Paper | H(T,t) = 23.429 − 0.0742717 × T − 0.145876 × t + 6.72703 × 10−5 × T2 + 0.000605284 × t2 + 0.000175301 × T × t | 0.88 |
| Plastic | H(T,t) = −44.8006 + 0.276956 × T + 0.432934 × t − 0.000314235 × T2 + 0.00050216 × t2 − 0.00135652 × T × t | 0.66 |
| Rubber | H(T,t) = 26.1644 − 0.0922177 × T − 0.177385 × t + 9.00828 × 10−5 × T2 + 0.00123522 × t2 + 0.000127838 × T × t | 0.91 |
| PAP/AL/PE composite packaging pack | H(T,t) = −20.7405 + 0.15232 × T + 0.0595576 × t − 0.000193613 × T2 + 0.000570871 × t2 − 0.000377273 × T × t | 0.72 |
| Wood | H(T,t) = 4.36363 − 0.00147975 × T + 0.0523864 × t + 1.07323 × 10−5 × T2 − 7.38636 × 10−5 × t2 − 9.03409 × 10−5 × T × t | 0.72 |
| Material | Equation | R2 |
|---|---|---|
| Carton | N(T,t) = 0.353788 − 0.00163594 × T + 0.0102094 × t + 2.79794 × 10−6 × T2 − 4.875 × 10−5 × t2 − 1.24001 × 10−5 × T × t | 0.41 |
| Fabric | N(T,t) = −0.538518 − 0.00249781 × T + 0.0363215 × t + 1.09306 × 10−5 × T2 − 0.000272625 × t2 − 3.04533 × 10−5 × T × t | 0.51 |
| Kitchen waste | N(T,t) = 7.29638 − 0.0187967 × T + 0.0120734 × t + 2.08782 × 10−5 × T2 + 8.75 × 10−5 × t2 − 4.88262 × 10−5 × T × t | 0.46 |
| Paper | N(T,t) = 0.56423 − 0.00232121 × T + 0.000358041 × t + 2.63612 × 10−6 × T2 + 8.12501 × 10−6 × t2 − 1.61463 × 10−6 × T × t | 0.69 |
| Plastic | N(T,t) = −1.5433 + 0.00661536 × T + 0.035132 × t − 8.45851 × 10−6 × T2 − 0.000255625 × t2 − 2.67845 × 10−5 × T × t | 0.13 |
| Rubber | N(T,t) = 0.183955 − 0.000655605 × T + 0.0100913 × t + 1.64191 × 10−6 × T2 − 3.25 × 10−5 × t2 − 1.45105 × 10−5 × T × t | 0.38 |
| PAP/AL/PE composite packaging pack | N(T,t) = 0.277347 − 0.00120315 × T + 0.00538399 × t + 1.20488 × 10−6 × T2 − 5.275 × 10−5 × t2 − 2.4975 × 10−6 × T × t | 0.21 |
| Wood | N(T,t) = −0.743849 + 0.00728439 × T + 0.0366098 × t − 7.02211 × 10−6 × T2 + − 6.75002 × 10−5 × t2 − 6.24626 × 10−5 × T × t | 0.46 |
| Material | Equation | R2 |
|---|---|---|
| Carton | S(T,t) = −0.177334 + 0.000937873 × T + 0.00420794 × t − 4.95826 × 10−7 × T2 − 8.52128 × 10−7 × t2 − 8.69886 × 10−6 × T × t | 0.26 |
| Fabric | S(T,t) = 1.24721 − 0.00598883 × T + 0.00300454 × t + 7.38831∙10−6 × T2 − 1.59088 × 10−6 × t2 − 7.19318 × 10−6 × T × t | 0.39 |
| Kitchen waste | S(T,t) = 0.76394 − 0.00268124 × T − 0.000843186 × t + 3.00117 × 10−6 × T2 − 4.43179 × 10−6 × t2 + 3.75 × 10−6 × T × t | 0.35 |
| Paper | S(T,t) = 0.852576 − 0.00438192 × T + 0.00400454 × t + 5.96737 × 10−6 × T2 + 9.09103 × 10−7 × t2 − 8.71591 × 10−6 × T × t | 0.49 |
| Plastic | S(T,t) = 1.04115 − 0.00503069 × T − 0.00623409 × t + 5.96639 × 10−6 × T2 + 5.68193 × 10−7 × t2 + 1.75341 × 10−5 × T × t | 0.78 |
| Rubber | S(T,t) = 1.3005 + 0.00161075 × T + 0.00576999 × t − 1.16336 × 10−6 × T2 − 6.05901 × 10−6 × t2 − 1.13023 × 10−5 × T × t | 0.28 |
| PAP/AL/PE composite packaging pack | S(T,t) = 0.672402 − 0.00299589 × T − 0.00228128 × t + 3.56719 × 10−6 × T2 − 1.87036 × 10−6 × t2 + 6.33409 × 10−6 × T × t | 0.61 |
| Wood | S(T,t) = 0.278057 − 0.00127582 × T + 0.00100456 × t + 1.59488 × 10−6 × T2 − 6.11576 × 10−6 × t2 − 1.04858 × 10−6 × T × t | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świechowski, K.; Stępień, P.; Syguła, E.; Koziel, J.A.; Białowiec, A. Lab-Scale Study of Temperature and Duration Effects on Carbonized Solid Fuels Properties Produced from Municipal Solid Waste Components. Materials 2021, 14, 1191. https://doi.org/10.3390/ma14051191
Świechowski K, Stępień P, Syguła E, Koziel JA, Białowiec A. Lab-Scale Study of Temperature and Duration Effects on Carbonized Solid Fuels Properties Produced from Municipal Solid Waste Components. Materials. 2021; 14(5):1191. https://doi.org/10.3390/ma14051191
Chicago/Turabian StyleŚwiechowski, Kacper, Paweł Stępień, Ewa Syguła, Jacek A. Koziel, and Andrzej Białowiec. 2021. "Lab-Scale Study of Temperature and Duration Effects on Carbonized Solid Fuels Properties Produced from Municipal Solid Waste Components" Materials 14, no. 5: 1191. https://doi.org/10.3390/ma14051191
APA StyleŚwiechowski, K., Stępień, P., Syguła, E., Koziel, J. A., & Białowiec, A. (2021). Lab-Scale Study of Temperature and Duration Effects on Carbonized Solid Fuels Properties Produced from Municipal Solid Waste Components. Materials, 14(5), 1191. https://doi.org/10.3390/ma14051191










