Abstract
The noble, metal-free materials capable of efficiently catalyzing water splitting reactions currently hold a great deal of promise. In this study, we reported the structure and electrochemical performance of new MoS2-based material synthesized with L-cysteine. For this, a facile one-pot hydrothermal process was developed and an array of densely packed nanoplatelet-shaped hybrid species directly on a conductive substrate were obtained. The crucial role of L-cysteine was determined by numerous methods on the structure and composition of the synthesized material and its activity and stability for hydrogen evolution reaction (HER) from the acidic water. A low Tafel slope of 32.6 mV dec−1, close to a Pt cathode, was registered for the first time. The unique HER performance at the surface of this hybrid material in comparison with recently reported MoS2-based electrocatalysts was attributed to the formation of more defective 1T, 2H-MoS2/MoOx, C nanostructures with the dominant 1T-MoS2 phase and thermally degraded cysteine residues entrapped. Numerous stacks of metallic (1T-MoS2 and MoO2) and semiconducting (2H-MoS2 and MoO3) fragments relayed the formation of highly active layered nanosheets possessing a low hydrogen adsorption free energy and much greater durability, whereas intercalated cysteine fragments had a low Tafel slope of the HER reaction. X-ray photoelectron spectroscopy, scanning electron microscopy, thermography with mass spectrometry, high-resolution transmission electron microscopy, Raman spectroscopy techniques, and linear sweep voltammetry were applied to verify our findings.
1. Introduction
Hydrogen gas produced from water electrolysis via catalytic splitting is ascribed to a mostly clean energy carrier. However, to achieve relevance for practical usage, the hydrogen evolution reaction (HER) rate, as at the surface of a Pt-based electrode, which exhibits the best HER performance [1], requires alternative cheaper catalysts.
Over the past decade, numerous reports have been devoted to the synthesis of various nanostructured materials for catalysis of water splitting reactions. Among them, nanoscale MoS2 species have been the most intensively investigated 2D material because of the specific graphene-like layered morphology and unique catalytic, biological, and energy-related properties [2,3,4,5]. However, the intrinsic conductivity, catalytic activity, and stability of the pure and the most thermodynamically sTable 2H-MoS2 nanostructured films are usually poor in comparison with Pt group metals and compounds [6]. In addition, the overvoltage of pristine 2H-MoS2 nanoplatelets for HER is significantly larger, about −0.2 V vs. reference hydrogen electrode, RHE, potential [7,8,9,10] compared with Pt/C [7]. Therefore, much effort has been devoted to the development of novel, more effective hybrid electrocatalysts. In this context, MoS2 films doped by other elements [11]; hybridized with 1T-MoS2 [12,13], WO3 [14], and glycine [15]; decorated with various guest nanoparticles [16,17,18]; deposited onto graphite [19], graphene, and graphene oxides [20,21]; and carbon nanotubes [22] have been proposed. Various methods have been explored to synthesize layered MoS2 nanomaterials, including laser ablation [23], thermal decomposition [24], gas-phase reaction [25], magnetron sputtering [26], and hydrothermal [27] or sonochemical [28] processing. An efficient strategy to enhance HER activity of MoS2-based electrocatalysts is to design highly conducive substrates, such as 3D-structured graphene [29,30], graphene oxide [31], foams [32,33], carbon fiber [34], etc. [22]. Various HER reaction Tafel slopes from 41 to 68 mV dec−1 and overpotentials from −185 to −260 mV were reported for the best samples. As is known, better HER performance is characteristic for the metasTable 1T (1T’) phase compared with 2H-MoS2 atoms and the transformation to the sTable 2H-MoS2 phase. It is crucial to synthesize sTable 1T (1T’)-MoS2-based catalysts [35,36].
It has been both experimentally and theoretically proven that the catalytically active sites for HER are located just at the unsaturated sulfur atoms of the MoS2 nanoplatelet edges because the edge sites in 2D MoS2 have near-zero hydrogen absorption free energy (0.08 eV) [37,38]. Consequently, the engineering of defect-rich MoS2 nanostructures directly influence the activity of MoS2-based electrocatalysts the most. Therefore, various post-treatment methods resulting in the design of highly-defective MoS2 through etching [39], doping [40,41], intercalation [42], and nanoparticulation [36] have been reported. However, the hybridization of MoS2 nanostructures with cysteine amino acid resulting in the formation of an HER electrocatalyst with a surprisingly low Tafel slope of 32.6 mV dec−1, which is the lowest among the reported for MoS2 materials, has not yet been reported.
Here, we reported the synthesis recipe, chemical composition, structure, and electrochemical performance of a new hybrid material composed of the dominant 1T-MoS2 phase heterostructured with 2H-MoS2, Mo(IV), and Mo(VI) oxide fragments and carbon. We showed that the engineered hybrids have enhanced electrochemical performance and significantly higher stability in comparison with pure 2D MoS2, as exhibited by an increase in the HER current density to over 80 mA cm−2 at −0.35 V overvoltage.
The samples were characterized by means of X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), thermogravimetry (TG), and differential thermal spectroscopy (DTA) coupled with mass spectrometry, Raman spectroscopy, and cycling voltammetry.
2. Results and Discussion
Hydrothermal processing of the ammonium heptamolybdate and the thiourea solution at 220–225 °C resulted in the formation of crystalline MoS2 nanoplatelet species in the solution bulk [15]. In this way, uniform and well-attached nanoplatelet films can also be engineered directly at various substrates. The thickness, varying from 0.7 to 2.5 μm, of these black-colored films is mainly dependent on the autoclaving time [15]. The morphology of films formed under the same autoclaving conditions in the solution without and containing L-cysteine was quite similar (Figure 1a).
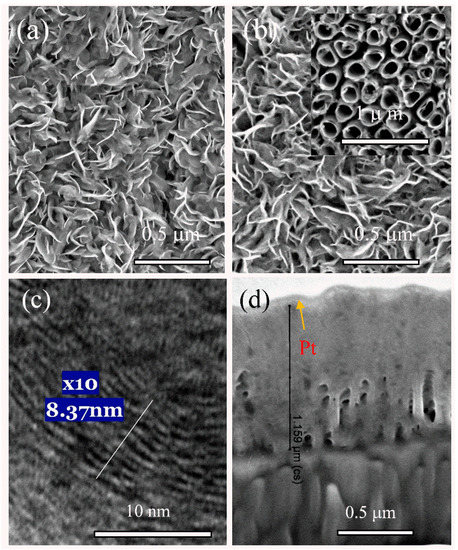
Figure 1.
Top-side (a,b) and cross-sectional (d) scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM) (c) images of film synthesized hydrothermally on the natotubed titania surface (inset) from the solution containing 5.0 (NH4)6Mo7O24 4H2O, 90 thiourea without (a) and containing 2.0 mmo L−1 L-cysteine (b–d) at 220 °C for 5 h.
The HRTEM image (Figure 1b) clearly revealed that the atomic flatness of the sandwiched layers in the composite MoS2-cyst film was greatly disrupted because of a non-periodic atom arrangement and increased to 8.4 Å distance between S-Mo-S lattices compared with the characteristic one of crystalline molybdenite 2H-MoS2 (6.15 Å) [43]. However, a markedly larger variation in the Mo-to-Mo spacing, nonlinear atom distributions, and the presence of numerous twists can be viewed. In many sites, the spacing between neighboring monolayers exceeded 10 Å. According to previous reports, significantly larger distances between the stacked S-Mo-S planes compared with characteristic of pure molybdenite were found, which implied the intercalation of guest molecules.
To further study the intercalation of cysteine or thiourea molecules or fragments inside the film, Raman spectroscopy investigations were performed. Figure 2a displays the Raman spectra of the films designed at the Ti/TiO2 substrate by hydrothermal synthesis in the basic.

Figure 2.
(A) Raman spectra of (a) as-grown MoS2 and (b) MoS2-cys films at the Ti/TiO2 substrate as well as (c) the same MoS2-cys film after the HER processing by potential cycling within a range of 0.05 to −0.35 V vs. the RHE potential for 1000 scans; (B) (1) thermogravimetry (TG) plot and variables of (2) CO2, (3) H2O, (4) NH3, and (5) H2S ionic currents during annealing of L-cysteine in argon determined by mass spectrometry (MS) analysis.
Solution without (a) and with 2 mmol L−1 L-cysteine (b,c) before (a,b) and after (c) the prolonged HER processing. As for film synthesized without cysteine, two clearly resolved peaks at 409.1 cm−1 and 379.0 cm−1, attributable to the A1g and E2g1 longitudinal acoustic phonon modes, respectively, and typical for the crystalline 2H-MoS2 [20] from few-layered flakes, were detected. The Raman spectrum of the as-grown MoS2-cyst film is shown in Figure 2a,b. This spectrum apart of a low intensity A1g mode peaked at 405.4 cm−1. The broad additional vibration modes peaked at 143.9 and 293.7 cm−1 and the very broad mode peaked in the 1100–1650 cm−1 region. These modes can be associated with the presence of molybdenum oxides and organic molecule fragments, respectively, entrapped inside this film [44].
A similar shape of the Raman spectrum is also characteristic for MoS2-cys film after the prolonged usage as an HER catalyst by 1000 potential scans within a 0.05 to −0.35 V window (Figure 2a). However, for this film, a significantly sharper and stronger A1g mode was determined, likely indicating the presence of a larger amount of crystalline MoS2 phase compared with the as-grown film.
Identification of gaseous species with m/z = 44 (CO2), m/z = 17 (NH3), and m/z = 34 (H2S) released during the thermal decomposition of cysteine in an argon atmosphere via evolved gas analytical mass spectrometry revealed that all functional groups, namely -COOH, -SH, and -NH2 ought to be detached from the cysteine molecule at around 220 °C (Figure 2b). Therefore, it was difficult to suspect the intercalation or adsorption of cysteine molecules inside and onto the 2D MoS2 nanoflakes. This allowed us to draw the important conclusion that an increased HER performance at the hybrid MoS2-cyst electrocatalyst cannot be related to adsorption and intercalation of cysteine molecules; from the DTA/MS analysis of all emitted species as well as the XPS data, it could have been COS or CS2 species formed via the thermal splitting of thiourea and cysteine in a synthesis reactor.
To evaluate the electrolytic HER activity, we performed linear sweep voltammetry (LSV) measurements in a typical three-electrode setup. To assess the durability of the hybrid electrocatalyst, up to 2000 potential sweeps were conducted. Figure 3 shows the sets of LSV curves obtained.
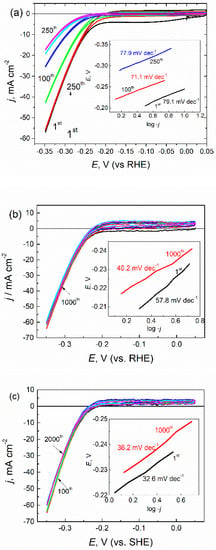
Figure 3.
The sets of cyclic voltammograms recorded in the H2-saturated solution of 0.5 H2SO4 mol L−1 at a 10 mV s−1 potential sweep rate of: (a) pure MoS2 film and the same film hybridized with (b) 1.0 and (c) 3.0 mmol L−1 of L-cysteine. In the insets are the Tafel slopes calculated for the indicated specimen and the potential scan cycle.
At a potential scan rate of 10 mV s−1 in argon-saturated 0.5 mol L−1 H2SO4 solution (pH = 0), films were synthesized without (a) and with L-cysteine (b,c). From these, the onset potential of HER at the cysteine-free as well as the cysteine-used MoS2 electrode approximated to about −0.2 V vs. RHE. Additionally, from the current density variables, only some higher activity within all tested potential window were obtained for electrodes fabricated using L-cysteine. However, the electrochemical performance of HER at the electrodes grown in the L-cysteine-containing reactor differed significantly. The potential cycling within the 0.05 to −0.35 V potentials window of the L-cysteine-free electrode usually resulted in an HER-activity decrease down to 15–18 mA cm−2 just after 250 cycles (Figure 3a), implying about a 70% decay from initial activity. In contrast, the electrodes covered with nanoplatelet-shaped hybrid MoS2/cysteine film showed considerable higher HER activity in the same potentials window, decreasing insignificantly during the subsequent 2000 potential scans (Figure 3b,c). A further benefit is that MoS2/cysteine hybrid HER electrocatalyst possessed significantly lower Tafel slopes (Figure 3b,c) compared with the ones synthesized under the same conditions without cysteine. In the case of films synthesized in the presence of just 1 mmol L−1 cysteine, the prolonged HER processing resulted in the marked decrease in the Tafel slope value from 57.8 to 40.2 mV dec−1, ca. by 30% (Figure 3b). An increase in the cysteine concentration to 3 mmol L−1 resulted in the surprisingly low Tafel slope value (32.6 mV dec−1) of as-grown film, which was the lowest among all reported data for MoS2-based electrocatalysts and was close to the Tafel slope value characteristic for the HER at the Pt and Pt/C substrates. The Tafel slope value of 30 mV dec−1 indicates that the recombination reaction Hads + Hads → H2 is an HER rate-limiting stage followed by a fast discharge reaction: H3O+ + e → Hads + H2O, whereas the Tafel slopes of 40 mV dec−1 indicates that H2 evolution proceeds via a Volmer–Heyrovsky pathway [45].
X-ray photoelectron spectroscopy was used to investigate the chemical states of the elements in the surface region of the synthesized films. The full-range survey spectrum taken from the MoS2-cyst sample is shown in Figure 4a, from which the presence of the Mo, S, O, N, and C elements was evidenced.
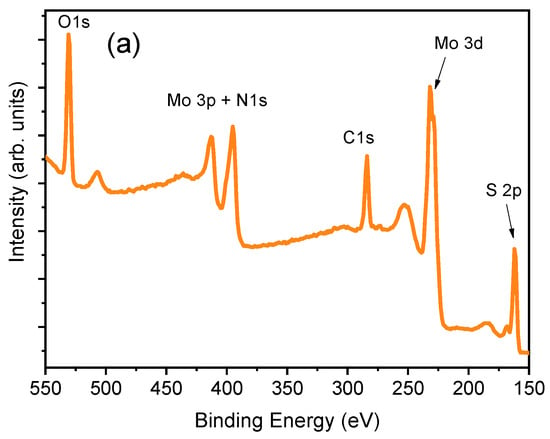
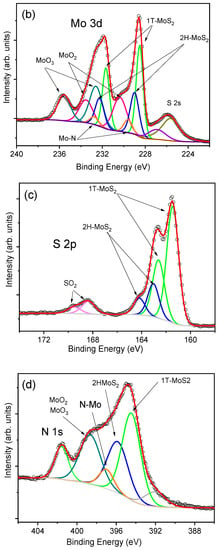
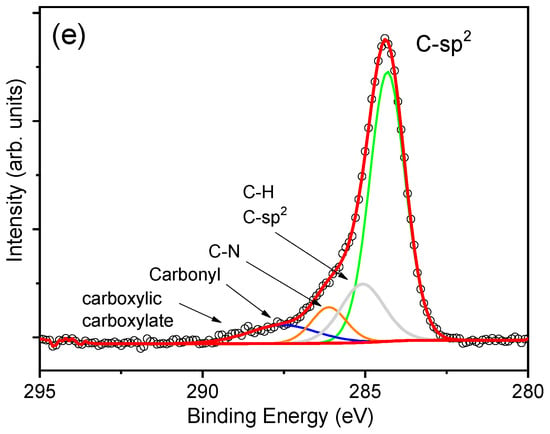
Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of as-grown MoS2-cyst film: (a): survey, (b): Mo3d, (c): S 2p, (d): Mo 3p), and (e): C1s.
Figure 4b revealed the presence of Mo4+ in both the semiconducting 2H-MoS2 and the metallic 1T-MoS2 and MoO2 phonon modes, whose 3d5/2 and 3d3/2 binding energies (BEs) were 229.0 and 232.2 eV for the 2H phase [46], 228.5 and 231.7 eV for 1T-MoS2 [13], and 230.4 and 233.6 eV for MoO2 [13,47], respectively, with the most intensive peaks for the thermodynamically metasTable 1T-MoO2. A similar conclusion can be drawn from the analysis of S 2p BE peaks presented in Figure 4c. For this spectrum, the doublets with components at 161.5 and 162.6 eV correspond to the BEs of S 2p3/2 and 2p1/2 states in the 2H-MoS2, whereas the most intense peaks are characteristic of the octahedral 1T phase. It is worth noticing that some amounts of Mo6+ and S4+ were also determined, for which the binding energies of 232.5 eV and 235.6 eV (Mo 3d5/2 and Mo 3d3/2, respectively), were attributed to MoO3, and 168.4 and 169.6 eV (S 2p3/2 and S 2p1/2, respectively) to SO2 [48]. The analysis of Mo 3p and C 1s modes also revealed the presence of nitrogen and carbon (Figure 4d,) in the hybrid film synthesized with L-cysteine, indicating the formation of multiphasic 1T/2H-MoS2-MoOx/C,N material. With regard to the N 1s spectrum, the peak located at 401.6 eV should be ascribed to the intercalation of NH4+, whereas the BE peaked at 397.0 eV can be attributed to the formation of Mo-N bond. From the XPS analysis, the concentration of elements was C, Mo, S, O, and N equaled to 32.8 at.% for C, 11.5 at.% for Mo, 25.3 at.% for S, 26.4 at.% for O, and 4.0 at.% for N.
The interactions between various amino acids (AA) and single-layer MoS2 nanosheet have been theoretically investigated by Dong et al. [49], concluding that no chemical bonds formed between them. The adsorption strength of AA on MoS2 depended on the AA type influencing the spatial distribution of the HOMO and LUMO orbitals and the MoS2 band gap decreased. As reported by Dong et al., the cysteine adsorption at the single layer 2H-MoS2 substrate reduced the band gap by 0.27 eV because of the enhanced hybridization between the Mo d-orbital and the S p-orbital after oxygen incorporation [49]. Therefore, it is reasonable to suggest that adsorption of cysteine at the nanosheets surface of the hybrid multiple-layered MoS2 film could modulate its catalytic properties towards becoming more active and stable. However, we did not determine the insertion of cysteine molecules, although the XP spectra revealed insertion of C and N atoms inside the MoS2-cyst products. Since cysteine molecules start degrading at around 200 °C, the formation and insertion of MoS2 fragments at 220 °C may be expected. The insertion of quest species, as shown in the study, was also elucidated by the greatly disrupted S-Mo-S layers and the increased distance between two neighboring ones (see Figure 1c). We suggest that insertion of quest species may affect the catalytic stability and activity of 1T-/2H-MoS2/MoOx hybrid films because of the formation of a higher amount of catalytically active sites and the stabilization of the highly conducting 1T-MoS2 phase. The low Tafel slope determined for this HER electrocatalyst (32.6 mV dec−1), which is much smaller than that of the bulk MoS2c, could be explained as follows.
According to Weiss et al. [50], the thermal degradation of cysteine molecules proceeds via this reaction:
2Cyst = C6H14O4S2 → 2CO2 + 2H2S + NH3 + C4H7N
The pathway to the formation of C4H7N could be the ejection of the carboxyl group, –C*OOH, and the –SH group from Cyst. The remaining chain NH2–Cα–C* is short, but still suitable for intercalation, further cyclization, and formation of 2,5-dihydro-1H-pyrole (ChemSpider 13870958) with 69 Da or another pyroline with the double bond elsewhere in the ring. The exact composition of the intercalated species has not been elucidated yet. However, if intercalated cysteine fragments are like NH2–Cα–C*, the processing of HER at the MoS2/NH2–Cα–C* edges may be changed significantly towards a reaction with a low Tafel slope, as established in this study. Further studies are currently being carried out by Furje-transformed infrared (FTIR) spectroscopy and nuclear magnetic resonance (NMR).
3. Conclusions
Here, we report on the one-pot hydrothermal synthesis of the hybrid-type 2D MoS2-based electrocatalyst for efficient hydrogen evolution from the acidic solution. Based on the XPS, SEM, HRTEM, and TG results it was inferred that the increased activity and stability of a novel hybrid MoS2 film are related to the formation of a 2D composite from dominating metallic-type and highly active 1T-MoS2 and MoO2 phases interfaced with the semiconducting 2H-MoS2 and MoO3 phases and carbon. The surprisingly low Tafel slope of 32.6 mV dec−1 for this HER electrocatalyst in the strongly acidic aqueous solution was determined for the first time implying that H2 formation at this electrode proceeds via the Volmer–Tafel pathway. This was attributed to the possible insertion between two neighboring S–Mo–S nanosheets of thermally degraded cysteine residue species, like NH2–Cα–C*. These findings highlight the need to study further the influence of amino acid on the formation mechanism of hybrid films.
4. Materials and Methods
4.1. Materials and Chemicals
Ammonium heptamolybdate tetrahydrate (NH4)6Mo7O24 4H2O (99.5%) was obtained from Reachem (Bratislava, Slovakia), whereas thiourea, (NH2)2CS (99%), and L-cysteine were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received. The Ti specimens with the working surface of 1.0 cm2 (7 × 7 mm2) and a tag (1 × 30 mm2) were cut from Ti foil (99.7 at%, 0.127 mm thick, Aldrich). For Ti surface anodizing, NH4F and H3PO4 were purchased from Reachem, Bratislava, Svovakia. Aqueous solution for hydrogen evolution was prepared from deionized water (18.4 MΩ) and analytically grade sulfuric acid.
4.2. Ti Surface Preparation and Anodizing
The surface of samples was ultrasonically cleaned in acetone, ethanol, and water (6 min in each) and air dried. Ti samples anodizing was conducted in the thermostated Teflon cell containing 2.0 mol L−1 H3PO4 and 0.5 mol L−1 of NH4F at 17 ± 0.3 °C and 20 V for 1 h. Two platinum plates were used as cathodes. After anodizing, the specimens were thoroughly rinsed, air-dried, and calcined at 450 °C for 2 h using a 10 °C min−1 ramp. An anatase TiO2 nanotubed sublayer between Ti and MoS2 was chosen as the substrate for the nanostructured HER catalyst because of its fairy good chemical and thermal stability, huge surface area, low resistance in the hydrogen environment, and because of the adsorption affinity of the MoS2 species to titanium oxide [51,52].
4.3. Synthesis
To cover the Ti/TiO2 samples with catalytically active nanostructured MoS2 film, the hydrothermal processing was conducted in a Teflon line stainless steel autoclave (25 mL in volume) at 220 °C for 5 to 10 h using a 10 °C min−1 ramp. An aqueous solution of 5 mmol L−1 ammonium heptamolybdate and 90 mmol L−1 thiourea without and containing up to 5 mmol L−1 of L-cysteine was used. The synthesized products were collected by centrifugation, rinsed thoroughly, and dried at 60 °C. To obtain densely packed MoS2 nanoplatelet films well-attached to the substrate, the Ti specimen covered with the nanotubed anatase TiO2 layer in a thickness of about 1 μm was inserted inside the reactor.
4.4. Raman Spectra
Raman investigations were performed on an inVia (Renishaw, New Mills, UK) spectrometer equipped with a thermoelectrically cooled (−70 °C) CCD camera. Spectra were excited at 532 nm by a diode-pumped solid-state laser on a ~2 μm diameter spot with power at the sample = 0.06 mW. The accumulation time was 400 s. Raman scattering wavenumber axis was calibrated by the silicon peak at 520.7 nm. To determine the parameters of the bands, the fitting of experimental spectra with the Gaussian–Lorentzian shape components using GRAMS/A1 8.0 (Thermo Scientific) software (version 8.0, Thermo Electron Corp.). was conducted.
4.5. SEM and HRTEM
The morphology and microstructure of the films and species obtained were analyzed using a scanning electron microscope (FEI Helios Nanolab 650, Eindhoven, The Netherlands) and a high-resolution transmission microscope FEI TECNAI F20 (Eindhoven, The Netherlands). To estimate their elemental composition, the products were analysed with a CrossBeam Auriga Workstation (Eindhoven, The Netherlands) equipped with a field emission gun and an energy dispersive X-ray spectrometer.
4.6. XPS
X-ray photoelectron spectroscopy (XPS) was used to evaluate the relative elemental composition of the samples. For the XPS measurements, a spectroscope VERSAPROBE PHI 500 from Physical Electronics (Physical Electronics, Chanhassen, MN, USA) was used; the excitation source was a monochromatized AlK. The energy resolution was 0.6 eV. A dual-beam charge neutralization (electron gun (~1 eV) and Argon Ion gun (<10 eV)) was used for charge compensation. The C1s peak at 284.3 eV was used for binding energy calibration.
4.7. TG/HDSC-MS
A simultaneous thermal analysis STA Pt 1600 (Linseis, Germany) apparatus equipped with a mass spectrometer MS Thermostar GDS 320 (Linseis/Pfeiffer, Germany) was used for research on the thermal decomposition processes of L-cysteine. For this, the specimen of 10 mg weight in PtRh cans was evacuated and then heated at 10 °C min−1 in an argon atmosphere. The data were collected and fitted using the Evaluation and Quadera software (version 4.62, INFICON AG, Bad Ragaz, Switzerland).
4.8. Cyclic Voltammetry
Electrochemical measurements were performed in a three-electrode-configured setup using a Zahner Zennium (Kronach, Germany) electrochemical workstation. The (Ag/AgCl,KClsat) electrode was used as a reference, while a glassy carbon stripe with an area of ~10 cm−2 and Ti/TiO2/MoS2 were used as the counter and the working electrode, respectively. A linear sweep voltammetry with a scan rate of 10 mV s−1 within 0.05 to −0.35 V vs. the RHE potential range was conducted in 0.5 mol L−1 of H2SO4 pre-purged with H2 for 30 min. In the stability tests, up to 2000 cycles were recorded. All potentials in the text refer to RHE.
Author Contributions
Conceptualization, administration and writing, A.J.; syntheses and electrochemical studies, P.G.; XPS investigations and data analysis, C.B.; TG/DTA/MS study and editing, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
CB thanks the Belgian Fund for Scientific Research under the FRFC contract CDR J001019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Romualdas Trusovas for Raman spectra collection, Arnas Naujokaitis for SEM images, and Marija Kurtinaitiene for Ti sample preparation. CB thanks the Belgian Fund for Scientific Research under the FRFC contract CDR J001019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheng, W. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, W.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 field-effect transistor for next-generation label-free biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Tashiro, M.; Sonobe, S.; Takahashi, M. Environmental effect on hysteresis of transfer characteristics in molybdenum disulfide field effect transistors. Sci. Rep. 2016, 6, 30084. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Liu, Y.; Lv, H.; Liu, L.; Rehman, A.U.; Kan, K.; Zhang, W.; He, L.; Wang, Y.; Wang, R.; et al. 3D-multilayer MoS2 nanosheets vertically grown on highly mesoporous cubic In2O3 for high-performance gas sensing at room temperature. Appl. Surf. Sci. 2019, 466, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental applications of 2D molybdenum disulfide (MoS2). Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef] [PubMed]
- Zoller, F.; Luxa, J.; Bein, T.; Fattakhova-Rohlfing, D.; Bouša, D.; Sofer, Z. Flexible freestanding MoS2-based composite paper for energy conversion and storage. Beilstein J. Nanotechnol. 2019, 10, 1488–1496. [Google Scholar] [CrossRef]
- Youn, D.H.; Jang, J.W.; Kim, J.Y.; Jang, J.S.; Choi, S.H.; Lee, J.S. Fabrication of graphene-based electrode in less than a minute through hybrid microwave annealing. Sci. Rep. 2015, 4, 5492. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All the Catalytic Active Sites of MoS2 for Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and Optimizing MoS2 Basal Planes for Hydrogen Evolution through the Formation of Strained Sulphur Vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef]
- Voiry, D.; Fullon, R.; Yang, J.; Silva, C.C.L.; Kappera, R.; Buzkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef]
- Li, R.; Yang, L.; Xiong, T.; Wu, Y.; Cao, L.; Yuan, D.; Zhou, W. Nitrogen doped MoS2 nanosheets synthesized via a low-temperature process as electrocatalysts with enhanced activity for hydrogen evolution reaction. J. Power Source 2017, 356, 133–139. [Google Scholar] [CrossRef]
- Tan, C.; Luo, Z.; Chaturvedi, A.; Cai, Y.; Du, Y.; Gong, Y.; Huang, Y.; Lai, Z.; Zhang, X. Preparation of high-percentage 1T-phase transition metal dichalcogenide nanodots for electrochemical hydrogen evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Huang, W.Z.; Xu, Z.D.; Liu, R.; Zheng, Y.F. Tungstenic acid induced assembly of hierarchical flower-like MoS2 spheres. Mater. Res. Bull. 2008, 43, 2799–2805. [Google Scholar] [CrossRef]
- Naujokaitis, A.; Gaigalas, P.; Bittencourt, C.; Mickevičius, S.; Jagminas, A. 1T/2H MoS2/MoO3 hybrid assembles with glycine as highly efficient and stable electrocatalyst for water splitting. Int. J. Hydrog. Energy 2019, 44, 24237–24245. [Google Scholar] [CrossRef]
- Jagminas, A.; Naujokaitis, A.; Žalnėravičius, R.; Jasulaitiene, V.; Valušis, G. Tuning the activity of nanoplatelet MoS2-based catalyst for efficient hydrogen evolution via electrochemical decoration with Pt nanoparticles. Appl. Surf. Sci. 2016, 385, 56–62. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Bao, S.; Wang, M.; Qi, X.; Fan, Z.; Zhang, H. Solution-phase epitaxial growth of noble metal nanostruc-tures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 2013, 4, 1444. [Google Scholar] [CrossRef] [PubMed]
- Peto, J.; Ollar, T.; Vancso, P.; Popov, Z.; Magda, G.Z.; Dobrik, G.; Hwang, C.; Sorokin, P.B.; Tapaszto, N. Spontaneous Doping of the Basal Plane of MoS2 Single Layers through Oxygen Substitution under Ambient Conditions. Nat. Chem. 2018, 10, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, H.-J.; Shi, X.; Lee, J.-T.; Choi, J.-M.; Song, M.-S.; Park, J.H. Graphene/acid co-assisted synthesis of ultrathin MoS2 nanosheets with outstanding rate capability for lithium battery anode. Inorg. Chem. 2013, 52, 9807–9812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kim, H.-J.; Lee, J.-T.; Chang, G.-W.; Shi, X.; Kim, W.; Ma, M.; Kong, K.-j.; Choi, J.-M.; Song, M.S.; et al. Unconventional pore and defect generation in molybdenum disulfide: Application in high-rate lithium-ion batteries and the hydrogen evolution reaction. ChemSusChem 2014, 7, 2489–2495. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, X.M.; Liu, Z.L.; Wang, J.Y.; Lee, J.M.; Wang, X. Facile synthesis of low crystalline MoS2 nanosheet-coated CNT for enhanced hydrogen evolution reaction. Nanoscale 2013, 5, 7768–7771. [Google Scholar] [CrossRef]
- Hu, J.J.; Zabinski, J.S.; Sanders, J.H.; Bultman, J.E.; Voevodin, A.A. Pulsed Laser Syntheses of Layer-Structured WS2 Nanomaterials in Water. J. Phys. Chem. B 2006, 110, 8914–8916. [Google Scholar] [CrossRef] [PubMed]
- Zelenski, C.M.; Dorhout, P.K. Template Synthesis of Near-Monodisperse1 Microscale Nanofibers and Nanotubules of MoS2. J. Am. Chem. Soc. 1998, 120, 734–742. [Google Scholar] [CrossRef]
- Feldman, Y.; Wasserman, E.; Szolovitz, D.J.; Tenal, R. High-rate, gas phase growth of MoS2 nested inorganic fullerenes and nanotubes. Science 1995, 267, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Ellmer, K.; Mientus, R.; Seegers, S.; Wei, V. Highly (001)-textured WS2−x films prepared by reactive radio frequency magnetron sputtering. Phys. Status Solidi A 2004, 201, R97–R100. [Google Scholar] [CrossRef]
- Wei, R.; Yang, H.; Du, K.; Fu, W.; Zou, G. A facile method to prepare MoS2 with nanoflower-like morphology. Mater. Chem. Phys. 2008, 108, 188–191. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.X.; Li, H.; Xu, Z.D. Synthesis and characterization of MoS2 nanostructures with different morphologies via an ionic liquid-assisted hydrothermal rout. Mater. Chem. Phys. 2009, 116, 400–405. [Google Scholar] [CrossRef]
- Liao, L.; Zhu, J.; Bian, X.; Zhu, L.; Scanlon, M.D.; Girault, H.H. MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv. Funct. Mater. 2013, 23, 5326–5333. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Lin, X.; Li, R.; Li, X.; Fang, Y.; Huang, J. Synthesis of CuMoS2/rGO hybrid as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Power Sources 2015, 292, 15–22. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Wang, S.; Ding, D.; Chen, M.; Liu, C.; Tian, Z.; Novoselov, K.S.; Ma, C.; Deng, D.; et al. Multiscale structural and electronic control of molybdenum disulfide foam for highly efficient hydrogen production. Nat. Commun. 2017, 8, 14430. [Google Scholar]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; So, K. Molybdenum Sulfide/N-Doped CNT Forest Hybrid Catalysts for High-Performance Hydrogen Evolution Reaction. Nano Lett 2014, 14, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, X.; Chen, Y.; Zhang, S.; Gao, P.; Li, C. A strategy to synergistically increase the number of active edge sites and the conductivity of MoS2 nanosheets for hydrogen evolution. Nanoscale 2015, 7, 8731–8738. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, X.; Bao, S.; Zhang, Z.; Fei, H. Phase engineering of multiphasic 1T/2H MoS2catalyst for highly efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 2681–2687. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kwon, I.S.; Abbas, H.G.; Jung, G.; Lee, Y.; Park, J.; Kang, H.S. Stable methylammonium-intercalated 1T-MoS2 for efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 5613–5617. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nilsen, J.H.; Horch, S.; Chorkendorff, I.; Norskov, J.K. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.B.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS 2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Xu, H.; Dahl-Petersen, C.; Yang, Q.; Cheng, D.; Cao, D.; Besenbacher, F.; Lauritsen, J.V.; Helveg, S.; et al. Controllable etching of MoS basal planes for enhanced hydrogen evolution through the formation of active edge sites. Nano Energy 2018, 49, 634–643. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Abroshan, H.; Zhang, J.; Guo, J.; Siahrostami, S.; Zheng, X. Enhancing catalytic activity of MoS2 based plane S-vacancy by Co cluster addition. ACS Energy Lett. 2018, 3, 2685–2693. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; Sun, Y.; Chen, X.; Zhong, W.; Du, Y. Monolayer MoS2 quantum dots as catalysts for efficient hydrogen evolution. RSC Adv. 2015, 5, 97696–97701. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Choi, H.C. Synthesis and properties of molybdenum disulphide: From bulk to atomic layers. RSC Adv. 2015, 2, 7495–74514. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disordered Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Watzele, S.; Fichtner, J.; Garlyyev, B.; Schwammlein, J.N.; Bandarenka, A.S. On the dominating mechanism of the hydrogen evolution reaction of polycrystalline Pt electrodes in acidic media. ACS Cat. 2018, 8, 9456–9462. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, J.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Xie, S.; Chen, K.; Bell, A.T.; Iglesia, E. Structural characterization of molybdenum oxide supported on zirconia. J. Phys. Chem. B 2000, 104, 10059–10068. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, J.; Kim, H.U.; Bak, H.; Jeon, M.; Ryn, G.H.; Lee, Z.; Yeom, G.Y.; Kim, K.; Jang, J. Lower temperature synthesis of large-scale molybdenum disulfide films directly on a plastic substrate. Adv. Mater. 2015, 27, 5223–5229. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Liu, L.; Klausen, L.H.; Wang, Y.; Mi, J.; Dong, M. Modulation the electronic property of 2D monolayer MoS2 by amino acid. Appl. Mater. Today 2019, 14, 151–158. [Google Scholar] [CrossRef]
- Weis, I.M.; Muth, C.; Drumm, R.; Kirchner, O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, X.; Guan, L.; Chai, J.; Zhang, H.; Wang, S.; Pan, J.; Tao, J. Defect assisted coupling of MoS2/TiO2 interface and tuning of its electronic structure. Nanotechnology 2016, 27, 355203. [Google Scholar] [CrossRef] [PubMed]
- Jagminas, A.; Naujokaitis, A.; Gaigalas, P.; Ramanavičius, S.; Kurtinaitienė, M.; Trusovas, R. Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water. Metals 2020, 10, 1251. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).